Abstract
Objective
To examine reasons why rheumatoid arthritis patients discontinued subcutaneous (SQ) anti-tumor necrosis factor (anti-TNF) treatment in the past 12 months, so as to help inform successful, uninterrupted therapy.
Methods
Data were collected in March and April 2011 using self-reported, internet-based questionnaires. Study inclusion criteria comprised: rheumatoid arthritis diagnosis; discontinuation of SQ anti-TNF medication (adalimumab, certolizumab, etanercept, or golimumab) within the past 12 months; aged ≥18 years; United States residency; and consent to participate. Patients reported primary and other reasons for discontinuation of their most recently discontinued anti-TNF.
Results
Questionnaires from 250 patients were analyzed; 72.8% were female, 80.8% were white, and median age was 51 years. Patients had discontinued etanercept (n=109), adalimumab (n=98), certolizumab (n=24), or golimumab (n=19) within the past 12 months. When prompted about their primary reason for discontinuation, lack of effectiveness (40.8%) was cited most often, followed by injection experience (18.4%). Combining prompted primary and other reasons for discontinuation, 60.8% of patients reported lack of effectiveness, while 40.8% reported injection experience, which included: pain/burning/discomfort after injection (14.4%); pain/burning/discomfort during injection (13.2%); injection reactions such as redness/swelling after injection (12.4%); dislike of self-injection (11.6%); dislike of frequency of injection (10.4%); and fear of injection/needles (6.8%).
Conclusion
From the patient perspective, there are unmet needs with regard to the effectiveness and injection experience associated with SQ anti-TNF medications, which may lead to discontinuation. Treatment options with a better injection experience may address these needs. These results demonstrate the importance of including the patient perspective when making prescribing decisions or payer access and coverage decisions.
Keywords: persistence, subcutaneous injection, anti-TNF
Introduction
Rheumatoid arthritis (RA) is a systemic, inflammatory condition that can affect multiple organs,1 but which is primarily associated with the destruction and deformity of synovial joints.2 The prevalence of RA is reported to be 0.5%–1% in developed countries, with twice as many women affected as men.3
Patients with RA report severe discomfort, fatigue, physical disability, and a general deficit in their health-related quality of life relative to the general population.4 In addition, many patients with RA eventually require surgeries such as hip and knee replacements.3,5 Traditionally, these outcomes were regarded as the extent of the disease. However, large population studies now recognize that patients with RA have a 1.5- to 1.6-fold higher mortality rate than unaffected individuals.6 This risk of mortality increases with the severity of clinical outcomes that are often used to measure this chronic, progressive disease, such as scores of physical functioning and levels of circulating rheumatoid factor.6
Current therapy for RA includes anti-inflammatory drugs and disease-modifying antirheumatic drugs (DMARDs), the latter group comprising two classes: biologics and nonbiologics. Nonbiologic DMARDs include hydroxychloroquine, sulfasalazine, and methotrexate, the last of which is the current standard of care. Treatment guidelines advocate that those patients with an inadequate response to methotrexate be switched to biologic DMARDs.7 Anti-tumor necrosis factors (anti-TNFs) are currently predominant among the biologics used in the management of disease, but other options include inhibitors of T-cell co-stimulation and CD20 inhibitors.8
Prior to the introduction of biologics, remission from RA was thought to be rare and unpredictable.9 However, therapy with anti-TNFs has demonstrated that remission is an achievable treatment target,8,9 and so the successful delivery of anti-TNF therapy is important to ensure that such a positive clinical outcome is achieved by the maximum number of patients.5
For many chronic diseases, discontinuation of treatment is a substantial concern,10 undermining the success of long-term therapy in conditions as diverse as coronary artery disease and osteoporosis and causing otherwise preventable morbidity and mortality.11 Similarly, discontinuation from RA therapy is a substantial problem – it has been reported that 21%–35% of patients who are administered anti-TNF therapy discontinue within their first year of treatment.12 In cases where patients discontinue anti-TNF for reasons other than effectiveness, patients may be exposed to otherwise preventable symptoms of pain and fatigue as well as irreversible joint damage.13
Improved understanding of the reasons behind discontinuation will help to inform strategies that improve persistence and, in turn, clinical outcomes. To date, studies have highlighted poor tolerability, inadequate effectiveness,14 and out-of-pocket costs15 as common reasons for discontinuation. As head-to-head trials differentiating the efficacy of anti-TNFs from one another are lacking, therapy selection on this basis is not a forthright decision when initiating treatment. However, anti-TNFs are clearly differentiated from one another by other factors, such as route of administration (intravenous infusion or subcutaneous [SQ] injection), frequency of dosing, and cost, which may affect persistence.15,16 Among SQ therapies, patient injection experience may also be significant and is an attribute that may have lacked comprehensive scrutiny to date.17 The aforementioned factors may serve as a more practical basis on which to select therapy. The current study explores which of these attributes contribute to the discontinuation of SQ anti-TNF treatment (specifically of adalimumab, certolizumab, etanercept, and golimumab) from the patient’s perspective. A patient-reported approach was taken since the decision to discontinue treatment may be a very personal one. Moreover, there may be significant disconnect between patient and physician perceptions surrounding this decision, as has been reported for other aspects of RA management, such as patient versus physician priorities for symptom improvement18 and assessment of disease severity.16 As such, there may be unique value in determining the patient perspective. With these data, this study aims to provide information that may help guide the development of treatment strategies that improve persistence.
Patients and methods
Study design
This study used a quantitative, cross-sectional, self- administered, internet-based survey to examine patients’ reasons for discontinuing SQ anti-TNF therapy. The study protocol and survey design were reviewed and approved by the Essex Institutional Review Board (Lebanon, NJ, USA).
Study population
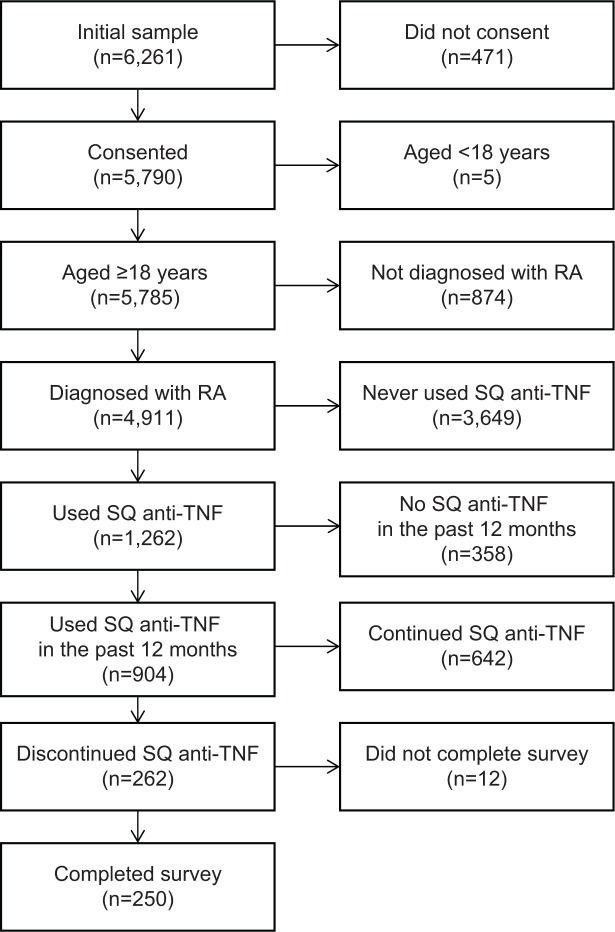
Patients self-reporting experience with RA were identified from four databases: the 2008, 2009, and 2010 National Health and Wellness Surveys, and the Lightspeed Research Ailment Panel. Patients were also recruited over the telephone by the market research company, Inviting Ideas. A total of 6,261 potential participants were identified. Patients from the National Health and Wellness Surveys and Lightspeed Research Ailment Panel were emailed invitations to participate and were screened for inclusion criteria and consent at the beginning of the online survey. For patients recruited over the telephone, an interviewer first asked for consent and then screened patients for inclusion criteria before directing them to the online questionnaire for completion. Inclusion criteria ensured that patients: were diagnosed with RA by a health care provider; had discontinued use of an SQ anti-TNF medication in the past 12 months (specifically adalimumab, certolizumab, etanercept, or golimumab); were aged ≥18 years; were US residents; and could read and write English. A total of 250 patients met the inclusion criteria for the study (Figure 1).
Figure 1.
Study inclusion and exclusion criteria.
Abbreviations: RA, rheumatoid arthritis; SQ, subcutaneous; TNF, tumor necrosis factor.
Data collection
The patient survey took approximately 15 minutes to complete. Demographic variables such as age, sex, ethnicity, employment status, and education level were captured. Patients were asked background questions concerning their disease and historical treatment, such as length of time since diagnosis and RA treatments previously used. With respect to discontinuation, participants were asked which treatment they had discontinued, length of time on therapy prior to discontinuation, and length of time since discontinuing.
Patients were asked why they discontinued their most recently administered SQ anti-TNF treatment in two sections. In the first section, patients were asked two unprompted questions, for which they had to type a response. These questions were: “What is the ONE main reason you stopped taking [the discontinued SQ anti-TNF]?” and “What other reasons, if any, caused you to stop taking [the discontinued SQ anti-TNF]?”. In the second section, patients were prompted with a list of reasons from which to choose an answer to the questions “Which of the following best categorizes the main reason you stopped taking [the discontinued SQ anti-TNF]?” (for which patients could only select one answer) and “Which of the following best categorizes any secondary reasons you stopped taking [the discontinued SQ anti-TNF]?” (for which patients could select as many reasons as they felt appropriate). The prompted section was added to detect whether specific issues, such as “injection experience”, were lost within broader categories such as “safety” and “tolerability” within the unprompted section of the questionnaire.
For purposes of analysis, unprompted/prompted responses were grouped into categories, as indicated in Table 1. For secondary reasons, each patient could contribute a maximum of once to each category outlined in Table 1. For example, if a patient selected all three safety-related reasons as secondary causes for discontinuation, this only counted once toward the tally for the overall category of safety.
Table 1.
Surveyed reasons for discontinuation
| Category | Reason |
|---|---|
| Unprompted section | |
| Lack of effectiveness | – |
| Miscellaneous | Prefer other medication |
| Doctor’s advice | |
| Remission | |
| Other | |
| Administration | Injection |
| Miscellaneous | |
| Cost/insurance coverage | – |
| Safety/side effects/tolerability | – |
| Comorbidity/contraindication | – |
| Did not know | – |
| Prompted section | |
| Effectiveness | Loss of effectiveness after initial relief |
| Took too long to relieve symptoms | |
| Therapy never relieved symptoms | |
| Injection experience | Pain/burning/discomfort during injection |
| Pain/burning/discomfort after injection | |
| Injection reactions | |
| Dislike of self-injection | |
| Dislike of injection frequency | |
| Fearful of injection | |
| Cost/insurance coverage | Could no longer afford |
| Difficulty obtaining approval | |
| Safety | Experienced infection |
| Fearful of infection | |
| Other safety concern | |
| Other | – |
| Remission | – |
In a final section, the questionnaire explored if participants experienced injection issues regardless of whether they had been identified as a factor in their discontinuation at an earlier stage of the survey. All patients were asked whether they experienced pain/burning/discomfort during or after injection and also whether they experienced injection reactions (redness/swelling). Those patients who had experienced any of these issues were asked to rate their symptoms on a scale of ascending severity from 1–5. Participants were then asked whether their symptoms provoked them to contact their doctor and whether they did so immediately, at their next appointment, or not at all.
Data analysis
Descriptive and summary statistics were generated for patient demographic variables and for discontinuation data. In the final analysis, all patients who reported a negative injection experience were stratified into five categories: patients who cited injection experience as a primary reason for discontinuing treatment; those who cited it as a secondary reason for discontinuing treatment; those who cited it as either a secondary or primary reason; those who cited they experienced injection issues, but did not perceive this as a factor when discontinuing therapy; and an overall category that summarized all patients who experienced injection issues, regardless of that experience being perceived as a cause for discontinuation of therapy or not.
Results
Patient sample
The majority of the 250 RA patients in the sample were female (72.8%, n=182), as expected from prior prevalence data,3 and were predominantly white (80.8%, n=202) (Table 2). The mean (median) age of patients was 51 (51) years. The mean (median) number of years since initial diagnosis of RA was 11 (7).
Table 2.
Patient characteristics
| Patient characteristics | Patients, % (n=250) |
|---|---|
| Sex | |
| Female | 72.8 |
| Male | 27.2 |
| Ethnicity | |
| Non-white | 19.2 |
| White | 80.8 |
| Relationship status | |
| Married/committed relationship | 70.0 |
| Not married/no committed relationship | 30.0 |
| Education status | |
| College or further educated | 58.0 |
| Less than college educated | 42.0 |
| Employment status | |
| Full-time, part-time, or self-employed | 61.2 |
| Unemployed, homemaker, retired, disabled, student | 38.8 |
| Income | |
| < US$50,000 | 30.4 |
| > US$50,000 | 63.2 |
| Information missing | 6.4 |
| Age, years | |
| 21–40 | 20.4 |
| 41–50 | 26.0 |
| 51–55 | 19.2 |
| 56–65 | 24.4 |
| 66–85 | 10.0 |
| BMI | |
| Underweight | 1.2 |
| Normal weight | 28.4 |
| Overweight | 30.0 |
| Obese | 34.0 |
| Information missing | 6.4 |
| Health insurance | |
| Managed care (HMO, PPO) | 54.4 |
| Medicare | 3.2 |
| Medicaid | 23.6 |
| Individual/family insurance plan | 26.4 |
| VA CHAMPUS | 2.0 |
| US region | |
| Northeast | 29.6 |
| Midwest | 21.2 |
| South | 24.4 |
| West | 24.4 |
Abbreviations: BMI, body mass index; HMO, Health Maintenance Organization; PPO, Preferred Provider Organization; VA, Veterans’ Affairs; CHAMPUS, Civilian Health and Medical Program of the Uniformed Services.
Discontinuation characteristics
Table 3 summarizes the discontinuation characteristics of the sample population. Participants used anti-TNF therapy for a mean (median) of 2.51 (1.25) years prior to discontinuation, and the median time elapsed between discontinuation and patients’ participation in this study was 6 months.
Table 3.
Patient discontinuation characteristics
| Discontinuation characteristics | Patients, % (n=250) |
|---|---|
| Subcutaneous anti-TNF discontinued | |
| Adalimumab | 39.2 |
| Certolizumab | 9.6 |
| Etanercept | 43.6 |
| Golimumab | 7.6 |
| Other RA therapy taken at the time of discontinuation | |
| NSAIDs | 43.6 |
| Steroids | 25.2 |
| Nonbiologic DMARDs | 38.0 |
| Other | 2.8 |
| Severity of RA at initiation of treatment | |
| Mild | 8.4 |
| Moderate | 53.2 |
| Severe | 38.4 |
| Change in RA condition while taking anti-TNF therapy | |
| Worsened | 12.4 |
| Stable | 44.4 |
| Improved | 43.2 |
| Switching | |
| Discontinued therapy without switching | 48.0 |
| Discontinued and switched therapy | 52.0 |
| The decision to discontinue | |
| Physician suggested immediate stop | 17.6 |
| Physician asked patient to consider stop | 27.6 |
| Patient initiated discussion, and physician agreed | 43.6 |
| Patient initiated discussion, but physician disagreed | 3.6 |
| Patient did not discuss with physician | 7.6 |
Abbreviations: RA, rheumatoid arthritis; NSAIDs, nonsteroidal anti-inflammatory drugs; DMARDs, disease-modifying antirheumatic drugs; TNF, tumor necrosis factor.
Reasons for discontinuation
Unprompted reasons
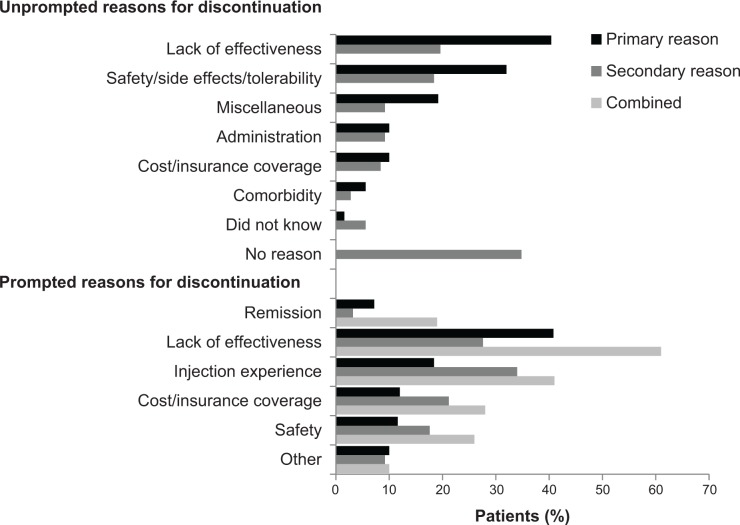
Lack of effectiveness was the most frequently reported primary, unprompted reason for discontinuing treatment (40%, n=101), followed by concerns about safety/tolerability (32%, n=80) (Figure 2). A substantial portion of patients reported no secondary reason for discontinuation (35%, n=87), although lack of effectiveness and safety/tolerability also rated highly (20% [n=49] and 18% [n=46], respectively). Table 4 outlines how each of the individual reasons reported were categorized into these broader categories, and the relative frequency with which each was cited by patients.
Figure 2.
Relative frequency of reasons for discontinuation.
Notes: Combined data report the proportion of patients citing each reason as either a primary or secondary cause of discontinuation. This applies only to the prompted section of the questionnaire, as unprompted primary/secondary reasons were not mutually exclusive.
Table 4.
Reasons for discontinuation
| Reasons | Patients, %a | ||
|---|---|---|---|
| Unprompted reasons | Primary (n=297) | Secondary (n=270) | |
| Lack of effectiveness | 40.4 | 19.6 | |
| Safety/side effects/tolerability | 32.0 | 18.4 | |
| Miscellaneous | 19.2 | 9.2 | |
| Prefer other medication | 4.8 | 2.8 | |
| Doctor’s advice | 8.8 | 4.0 | |
| Remission | 2.8 | 0.8 | |
| Other | 3.2 | 2.0 | |
| Administration | 10.0 | 9.2 | |
| Injection | 8.4 | 8.0 | |
| Miscellaneous | 1.6 | 2.0 | |
| Cost/insurance coverage | 10.0 | 8.4 | |
| Comorbidity/contraindication | 5.6 | 2.8 | |
| Did not know | 1.6 | 5.6 | |
| No reason | 0 | 34.8 | |
| Prompted reasons | Primary (n=250) | Secondary (n=331) | Combinedb (n=462) |
| Remission | 7.2 | 3.2 | 10.4 |
| Effectiveness | 40.8 | 27.6 | 60.8 |
| Loss of effectiveness after initial relief | 20.0 | 12.4 | 32.4 |
| Took too long to relieve symptoms | 4.0 | 10.0 | 14.0 |
| Therapy never relieved symptoms | 16.8 | 7.2 | 24.0 |
| Injection experience | 18.4 | 34.0 | 40.8 |
| P/B/D during injection | 4.4 | 8.8 | 13.2 |
| P/B/D after injection | 3.6 | 10.8 | 14.4 |
| Injection reactions | 4.0 | 8.4 | 12.4 |
| Dislike of self-injection | 1.6 | 10.0 | 11.6 |
| Dislike of injection frequency | 3.6 | 6.8 | 10.4 |
| Fearful of injection | 1.2 | 5.6 | 6.8 |
| Cost/insurance coverage | 12.0 | 21.2 | 27.6 |
| Could no longer afford | 6.8 | 11.2 | 18.0 |
| Difficulty obtaining approval | 5.2 | 11.2 | 16.4 |
| Safety | 11.6 | 17.6 | 26.0 |
| Experienced infection | 4.0 | 3.6 | 7.6 |
| Fearful of infection | 1.6 | 6.8 | 8.4 |
| Other safety concern | 6.0 | 7.2 | 13.2 |
| Other | 10.0 | 9.2 | 19.2 |
Notes:
Some participants gave unprompted responses that covered more than one category for their primary reason (n=297), hence percentages do not sum to 100%. However, prompted primary reasons were mutually exclusive and do sum to 100%. Patients could select multiple secondary reasons for both sections.
The percentage of patients citing the reason as either a primary or secondary cause of discontinuation, which were mutually exclusive in the prompted section.
Abbreviation: P/B/D, pain/burning/discomfort.
Prompted reasons
Lack of effectiveness remained the most common primary reason for discontinuation when patients were prompted with the list of predefined, potential contributory factors (41%, n=102) (Figure 2). The relative frequency with which safety was cited as a primary reason for discontinuation (12%, n=29) was reduced compared to when patients were left unprompted; the second most frequently reported reason under prompted conditions was patient injection experience (18%, n=46).
The most commonly reported, prompted secondary reason for discontinuation was injection experience (34%, n=85), followed by lack of effectiveness (28%, n=69). This contrasted with patients’ unprompted answers, in which it was more common for patients to cite no secondary reason for discontinuation. Table 4 outlines the main discontinuation categories and the frequency with which each individual reason was cited by participants.
Overall, the median length of time that patients reported had passed from the first occurrence of their primary reason for discontinuation to their actual time of discontinuation was 3.85 months, while the mean (standard deviation) was 10.16 (19.31) months.
Injection experience
Overall, 41% (n=102) of patients reported the injection experience as either a primary or secondary reason for discontinuation. When asked directly whether they had experienced injection issues, 74% (n=186) of the study population reported that they had, regardless of whether they considered this a factor in their eventual discontinuation. These issues were categorized as pain/burning/discomfort during injection; as pain/burning/discomfort after injection; or as an injection reaction. Table 5 outlines the patient-reported severity of these symptoms, stratifying these data according to whether the issue was regarded as a cause for discontinuation or not. These data indicated that there may be a trend for more severe symptoms to be regarded as a more prominent reason for discontinuation. Patient responses also indicated that there may be a relationship between regarding injection issues as a primary/secondary cause for discontinuation and being provoked to discuss these issues with a physician (Table 5). Nevertheless, a substantial portion of patients reported never consulting a physician about their injection issues (ranging from 14% [n=4] to 27% [n=38], depending on the role of the reason in the decision to discontinue).
Table 5.
Patient-rated severity of injection issues and timing of physician consultations
| Patient experience | Injection issue (patients, %)
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P/B/D during injection
|
P/B/D after injection
|
Injection reactions
|
|||||||||||||
| Primary reason (n=38) |
Secondary reason (n=68) |
Primary/secondary reason (n=79) |
Experienced but not a reason (n=133) |
Overall experienced (n=143) |
Primary reason (n=37) |
Secondary reason (n=69) |
Primary/secondary reason (n=81) |
Experienced but not a reason (n=114) |
Overall experienced (n=125) |
Primary reason (n=29) |
Secondary reason (n=62) |
Primary/secondary reason (n=69) |
Experienced but not a reason (n=100) |
Overall experienced (n=112) |
|
| Severity of injection issue | |||||||||||||||
| Very severe | 7.9 | 5.9 | 5.1 | 2.3 | 3.5 | 2.7 | 5.8 | 4.9 | 9.6 | 8.8 | 10.3 | 9.7 | 10.1 | 19.0 | 17.0 |
| Severe | 28.9 | 19.1 | 24.1 | 15.0 | 15.4 | 16.2 | 20.3 | 22.2 | 31.6 | 30.4 | 27.6 | 32.3 | 31.9 | 35.0 | 34.8 |
| Moderate | 42.1 | 44.1 | 43.0 | 42.9 | 43.4 | 62.2 | 55.1 | 55.6 | 45.6 | 45.6 | 37.9 | 41.9 | 40.6 | 30.0 | 32.1 |
| Mild | 18.4 | 26.5 | 24.1 | 32.3 | 30.8 | 13.5 | 15.9 | 14.8 | 12.3 | 13.6 | 17.2 | 12.9 | 14.5 | 15.0 | 14.3 |
| Very mild | 2.6 | 4.4 | 3.8 | 7.5 | 7.0 | 5.4 | 2.9 | 2.5 | 0.9 | 1.6 | 6.9 | 3.2 | 2.9 | 1.0 | 1.8 |
| Consultation with physician | |||||||||||||||
| Immediate | 23.7 | 30.9 | 27.8 | 21.8 | 21.0 | 32.4 | 36.2 | 32.1 | 27.2 | 26.4 | 48.3 | 45.2 | 43.5 | 34.0 | 33.9 |
| At next appointment | 57.9 | 52.9 | 53.2 | 51.9 | 52.4 | 51.4 | 46.4 | 49.4 | 50.9 | 51.2 | 37.9 | 40.3 | 42.0 | 46.0 | 44.6 |
| Never | 18.4 | 16.2 | 19.0 | 26.3 | 26.6 | 16.2 | 17.4 | 18.5 | 21.9 | 22.4 | 13.8 | 14.5 | 14.5 | 20.0 | 21.4 |
Note: Each injection experience was reported by patients in multiple ways to ensure full capture of the experience: 1) as the main reason for discontinuing in either the prompted or unprompted sections; 2) as another reason for discontinuing in either the prompted or unprompted sections; 3) as either the main reason or another reason for discontinuing in either the prompted or unprompted sections; 4) as experienced when previously using the discontinued medication outside the context of a reason for discontinuation; 5) as a combination of all of the above categories.
Abbreviation: P/B/D, pain/burning/discomfort.
Discussion
Chronic disease management is undermined by widespread treatment discontinuation.10,11 Persistence with SQ anti-TNF therapy for RA is associated with improved clinical outcomes,13 but long-term therapy is nevertheless beset by problems with discontinuation.14 Indeed, many patients with RA discontinue within their first year on SQ anti-TNF therapy;12 in the current study population, the median time reported between initiation and discontinuation was 1.25 years.
As reported in a previous study examining the reasons for discontinuing anti-TNF therapy,14 patients frequently perceived lack of effectiveness as a reason for discontinuing treatment. This is interesting, as relatively few patients in the current study reported that their symptoms worsened while on therapy (12%), whereas 44% of patients reported stable symptoms and 43% reported improved symptoms. However, at the time of discontinuation, only 38% of patients reported using a nonbiologic DMARD. Concomitant use of nonbiologic DMARDs, especially methotrexate, with anti-TNF therapy has demonstrated greater efficacy in clinical trials than anti-TNF therapy alone.19–21 Of note, golimumab is indicated only for use with methotrexate and not as a monotherapy.22 In the unprompted responses, concerns about safety/tolerability were the second most frequently reported class of reason for discontinuation. This may be surprising, as, in clinical trials, SQ anti-TNFs are generally thought to be well tolerated and to share good benefit-to-risk profiles.23 However, as the current study involved only those patients who had discontinued, it may be biased toward those who have experienced safety/tolerability problems; thus, a high prevalence of such concerns in this patient population may not be an unexpected finding.
When patients were prompted with a choice of predefined reasons for discontinuation, concerns about the injection experience replaced safety issues as the second most common answer (18% of patients reported injection problems when prompted, compared with 8.4% when patients were not). This may suggest that patients automatically categorized their problems with the injection experience as a more serious safety concern and that, from the patient perspective, injection issues may be a more important determinant of discontinuation than has previously been appreciated.17 Interestingly, in a study of risk trade-offs, Fraenkel et al reported that patients would prefer a treatment with an unknown long-term safety profile and no adverse short-term effects over a treatment with a confirmed, persistent, short-term side effect such as chronic injection reaction – even if this problem was easily reversed.24
Secondary reasons for discontinuing therapy, for which patients could list multiple factors, displayed a different trend. Unprompted, 35% of patients cited no secondary reason for discontinuation. However, when prompted with categories from which to choose, 34% of patients cited the injection experience as a factor in their discontinuation. This contrasts with the unprompted section, in which only 8% of patients reported concerns with the injection experience. This difference suggests that patients may lack the terminology to express, independently, certain influential aspects of their therapeutic experience. However, when these topics are broached, then they may be considered a substantial concern to patients. This highlights the importance of comprehensive patient–physician discourse during the initiation and maintenance of therapy that establishes common terminology and treatment priorities for both patients and physicians. Indeed, other articles report how these attributes of RA management can be lacking,16,18 and deficits in communication may have an impact upon long-term persistence if patients’ concerns are inadequately addressed.25
Overall, 74% of patients who discontinued an SQ anti-TNF therapy experienced some kind of injection issue. A recent Wall Street Journal article reported that the most commonly reported adverse events to the US Food and Drug Administration across all available prescription medications for all conditions between January 2004 and November 2011 were related to injection site pain with adalimumab (75,049 reported events) and etanercept (83,750 reported events).26 It is likely that the real incidence of injection site pain is higher since there are likely patients who are not reporting their experiences.
Results of this study show that a majority of patients chose to discuss their injection issue with their physician. However, 14%–18% of those patients who regarded injection problems as their primary reason for discontinuation did not engage in a discussion with their doctor as a result. Again, these findings highlight the importance of establishing a productive, considered patient–physician discourse, as some of these patients’ issues may have been resolved after consultation. Indeed, underreporting of this nature may be one reason why the importance of the injection experience has been underemphasized in the literature to date, despite such issues being found to be widely prevalent upon targeted examination.17
There are many factors which may further influence patient injection experience based on the unique attributes of individual SQ anti-TNF therapy for the treatment of moderately to severely active RA. SQ anti-TNF agents vary in frequency of dosing, with weekly dosing for etanercept, 2-weekly or weekly dosing for adalimumab, 4-weekly or 2-weekly dosing for certolizumab, and monthly dosing for golimumab. Agents may be delivered in a number of ways, including as reconstituted solutions in vials drawn and injected by health care providers for certolizumab and etanercept, single-use prefilled syringes available for self-administration for all agents, a pen device for adalimumab, and autoinjector devices for etanercept and golimumab. The pH of solutions being injected also vary by agent and delivery system, ranging from pH of approximately 4.7 for the prefilled syringe of certolizumab to 7.4 for the multiuse vial of etanercept.19–22 Injection experience with individual agents, especially as it relates to frequency of dosing, type of device including the associated needle size, and pH of the solution being injected should be considered in future research of patient experience and persistence with SQ anti-TNF therapy.
According to American College of Rheumatology guidelines, use of nonbiologic and biologic DMARDs should be for the purpose of targeting remission or low disease activity, though there are no recommendations for changes in treatment regimens if remission is achieved.7 Of patients discontinuing SQ anti-TNF therapy in the past year, 10% reported that remission was a reason for discontinuation, though only 3% reported remission as the primary reason without being prompted and 7% reported remission as a primary reason when prompted. Studies of therapy discontinuation after remission have generally found that biologics may be withdrawn successfully from some patients who experience remission after intensive treatment with methotrexate and anti-TNF agents. However, these patients should be monitored and biologics reinitiated if remission is not sustained. When this occurs, there does not appear to be any negative consequences from the “drug holiday”.27 As this is a cross-sectional study, it is not possible to know if patients who discontinued because of remission will eventually reinitiate biologic therapy in the future.
Studies have shown that out-of-pocket payments for RA therapy have a negative impact upon persistence. It has also been suggested that, in the long-term, out-of-pocket payments increase the overall health care costs resulting from RA due to the worsened clinical outcomes associated with the discontinuation they may provoke.15 This study may contribute to this evidence, suggesting that patients’ cost concerns form part of the decision to discontinue – approximately one-tenth of patients spontaneously cited cost issues as either a primary or secondary factor contributing to the cessation of their treatment. When prompted, a greater proportion of patients (21%) reported cost as a secondary reason, indicating that concerns with cost are relatively prevalent, if not foremost, among patients’ concerns. This may be compared to a hierarchy of patient-perspective treatment preferences proposed in the risk trade-off study by Fraenkel et al in which determinants such as route of administration, safety, and cost concerns were evaluated.24
As 55% of patients who discontinued SQ anti-TNF reported that they initiated the decision to discontinue, either through initiating discussions with their physician or making the decision on their own without consulting their physician, there may be much value in the patient-reported data this study reports. Indeed, prior studies note there can be substantial discord between patients’ and physicians’ perspectives elsewhere in the management of RA, such as in treatment priorities18 and in perceptions of disease severity,16 or even in the injection experience.17
However, it should be noted that the sample used in this study may not fully represent the natural population of patients with RA. For instance, patients in this study generally had a higher socioeconomic status, according to average income and education, than the general population. Biases in the sample also may have been introduced by the study design, which recruited some patients over the telephone and required all patients to have access to an internet-based questionnaire. Those patients recruited by telephone were more likely to be employed and to live in the US Northeast versus the Midwest or South. They were also less likely to use other DMARDs, more likely to rate their severity at discontinuation as moderate, and were diagnosed with RA for a shorter period of time.
This study was designed to identify patients with RA who discontinued an SQ anti-TNF therapy in the past 12 months and to explore the patients’ reasons for the discontinuation in a cross-sectional manner. Due to the study design, it is not possible to assess the proportion of patients with poor injection experience who discontinue versus those who simply tolerate the experience and continue treatment. Future research should consider longitudinal study designs, which would add clarity to this question. Additionally, as a median of 6 months passed between patients’ time of discontinuation and their participation in this study, the accuracy of patients’ recall may also limit the reliability of these data. Indeed, formal medical records were not used to corroborate survey results; therefore, RA diagnosis and treatment discontinuation characteristics cannot be confirmed. Comparison between patients’ accounts and medical records would test the reliability of the study’s findings, as well as perhaps enable further analysis of the degree of discord that exists in the patient–physician discourse. The study design also precluded the use of statistical tests to compare prompted and unprompted sections of the questionnaire, as patients’ responses in each section could not be accurately sorted to identical sets of categories.
This study’s findings have highlighted that there is substantial unmet need in current therapy. Although multiple SQ anti-TNFs are available, a population of patients perceive them to be ineffective and discontinue therapy, potentially resulting in suboptimal outcomes. Moreover, patients have safety concerns that appear to extend to, and that may be conflated with, aspects of the injection experience – including perceptions of injection pain, frequency of administration, and fear of needles.
Choice of optimal SQ anti-TNF therapy may not be obvious from a perspective of effectiveness, as head-to-head trials that differentiate these agents are lacking. In terms of safety, clinical trials reviewing the relative safety of different SQ anti-TNF therapies are also inconclusive, other than to note that these drugs generally have good risk–benefit profiles. Prior research has recommended that the choice of SQ anti-TNF should be based on clinical experience, convenience, patient preference, route of administration, and cost.19 The present study highlights that a neglected aspect of therapy that may have a substantial impact on discontinuation is the patient injection experience, including: pain, burning, and discomfort during or after injection; injection reactions; frequency of injections; and dislike or fear of self-injection. Anti-TNF medications with improved attributes related to the injection experience may benefit patients by maximizing persistency.
Acknowledgments
The authors would like to thank the team at Kantar Health for their help in finalizing the study design and conducting the data collection. The authors would also like to thank the team at Medaxial, a Covance Company, for their help in developing the manuscript.
Footnotes
Disclosure
This study was funded by Janssen Scientific Affairs, LLC, Horsham, PA, USA. Susan C Bolge and Neeta Tandon are employees of Janssen Scientific Affairs, LLC and stockholders of the parent company Johnson & Johnson. Amir Goren is an employee of Kantar Health, New York, NY, USA, whom Janssen Scientific Affairs, LLC contracted for research services.
References
- 1.Young A, Koduri G. Extra-articular manifestations and complications of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2007;21(5):907–927. doi: 10.1016/j.berh.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 3.Kvien TK. Epidemiology and burden of illness of rheumatoid arthritis. Pharmacoeconomics. 2004;22(2 Suppl 1):1–12. doi: 10.2165/00019053-200422001-00002. [DOI] [PubMed] [Google Scholar]
- 4.Salaffi F, Carotti M, Gasparini S, Intorcia M, Grassi W. The health-related quality of life in rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis: a comparison with a selected sample of healthy people. Health Qual Life Outcomes. 2009;7:25. doi: 10.1186/1477-7525-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.David G, Tandon N, Waters HC, Gunnarsson C, Kavanaugh A. Rheumatoid arthritis and total hip and knee replacement: impact of biologics. Am J Pharm Benefits. 2014 [Google Scholar]
- 6.Sokka T, Abelson B, Pincus T. Mortality in rheumatoid arthritis: 2008 update. Clin Exp Rheumatol. 2008;26(5 Suppl 51):S35–S61. [PubMed] [Google Scholar]
- 7.Singh JA, Furst DE, Bharat A, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64(5):625–639. doi: 10.1002/acr.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tak PP, Kalden JR. Advances in rheumatology: new targeted therapeutics. Arthritis Res Ther. 2011;13(Suppl 1):S5. doi: 10.1186/1478-6354-13-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breedveld FC, Combe B. Understanding emerging treatment paradigms in rheumatoid arthritis. Arthritis Res Ther. 2011;13(Suppl 1):S3. doi: 10.1186/1478-6354-13-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Geest S, Sabaté E. Adherence to long-term therapies: evidence for action. Eur J Cardiovasc Nurs. 2003;2(4):323. doi: 10.1016/S1474-5151(03)00091-4. [DOI] [PubMed] [Google Scholar]
- 11.Yeaw J, Benner JS, Walt JG, Sian S, Smith DB. Comparing adherence and persistence across 6 chronic medication classes. J Manag Care Pharm. 2009;15(9):728–740. doi: 10.18553/jmcp.2009.15.9.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blom M, Kievit W, Fransen J, et al. The reason for discontinuation of the first tumor necrosis factor (TNF) blocking agent does not influence the effect of a second TNF blocking agent in patients with rheumatoid arthritis. J Rheumatol. 2009;36(10):2171–2177. doi: 10.3899/jrheum.090054. [DOI] [PubMed] [Google Scholar]
- 13.Ingham M, Reed G, Bolce R, et al. Patterns of anti-TNF treatment in the CORRONA registry: observed responses to maintenance, switching and discontinuation [abstract] Ann Rheum Dis. 2011;70(Suppl 3):415. [Google Scholar]
- 14.Markenson JA, Gibofsky A, Palmer WR, et al. Persistence with anti-tumor necrosis factor therapies in patients with rheumatoid arthritis: observations from the RADIUS registry. J Rheumatol. 2011;38(7):1273–1281. doi: 10.3899/jrheum.101142. [DOI] [PubMed] [Google Scholar]
- 15.Curkendall S, Patel V, Gleeson M, Campbell RS, Zagari M, Dubois R. Compliance with biologic therapies for rheumatoid arthritis: do patient out-of-pocket payments matter? Arthritis Rheum. 2008;59(10):1519–1526. doi: 10.1002/art.24114. [DOI] [PubMed] [Google Scholar]
- 16.Barton JL, Imboden J, Graf J, Glidden D, Yelin EH, Schillinger D. Patient-physician discordance in assessments of global disease severity in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2010;62(6):857–864. doi: 10.1002/acr.20132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curtis JR, Hobar C, Hansbrough K. Injection-site burning and stinging in patients with rheumatoid arthritis using injectable biologics. Curr Med Res Opin. 2011;27:71–78. doi: 10.1185/03007995.2010.534959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.da Silva JA, Ramiro S, Pedro S, Rodrigues A, Vasconcelos JC, Benito-Garcia E. Patients- and physicians-priorities for improvement. The case of rheumatic diseases. Acta Reumatol Port. 2010;35(2):192–199. [PubMed] [Google Scholar]
- 19.Enbrel® (etanercept) [package insert] Thousand Oaks, CA: Immunex Corporation; 2013. [Google Scholar]
- 20.Humira® (adalimumab) [package insert] North Chicago, IL: AbbVie, Inc; 2014. [Google Scholar]
- 21.Cimzia® (certolizumab) [package insert] Smyrna, GA: UCB, Inc; 2013. [Google Scholar]
- 22.Simponi® (golimumab) [package insert] Horsham, PA: Janssen Biotech, Inc; 2014. [Google Scholar]
- 23.Fleischmann R, Yocum D. Does safety make a difference in selecting the right TNF antagonist? Arthritis Res Ther. 2004;6(Suppl 2):S12–S18. doi: 10.1186/ar995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fraenkel L, Bogardus S, Concato J, Felson D. Risk communication in rheumatoid arthritis. J Rheumatol. 2003;30(3):443–448. [PubMed] [Google Scholar]
- 25.van den Bemt BJ, van Lankveld WG. How can we improve adherence to therapy by patients with rheumatoid arthritis? Nat Clin Pract Rheumatol. 2007;3(12):681. doi: 10.1038/ncprheum0672. [DOI] [PubMed] [Google Scholar]
- 26.Beck M. Searching for Side Effects [webpage on the Internet] New York, NY: The Wall Street Journal. 2012. [Accessed February 6, 2013]. Available from: http://online.wsj.com/article/SB10001424052970203920204577193052426275904.html?KEYWORDS=Searching+for+Side+Effects.
- 27.Tanaka Y, Hirata S. Is it possible to withdraw biologics from therapy in rheumatoid arthritis? Clin Ther. 2013;35(12):2028–2035. doi: 10.1016/j.clinthera.2013.10.008. [DOI] [PubMed] [Google Scholar]