Abstract
Background
Recent studies have shown that C-reactive protein (CRP) is a useful predictive factor in several cancers; however, its role in esophageal cancer (EC) is controversial.
Methods
A systematic literature search was performed using Medline, PubMed, and Web of Science to analyze the prognostic value of serum CRP in patients with EC. A meta-analysis was performed to assess the association between serum CRP and overall survival (OS) in patients with EC.
Results
A total of eight studies involving 1,471 patients were included in our study. Our pooled results demonstrated that a high level of serum CRP was associated with poor OS (hazard ratio [HR]: 1.40, 95% confidence interval [CI]: 1.25–1.57, I2=81.3%, P<0.0001). Subgroup analyses were performed in further investigations. When the patients were segregated according to treatment, pathological type, and cut-off level, high levels of serum CRP were found to be significantly correlated with OS.
Conclusion
Our meta-analysis revealed that high levels of serum CRP were significantly associated with poor OS in patients with EC.
Keywords: CRP, esophageal cancer, meta-analysis
Introduction
Esophageal cancer (EC) is the eighth most frequent cancer and the sixth leading cause of cancer death worldwide.1 Surgical resection remains the modality of choice, however, the prognosis is poor.2 The reason for this may be the relatively late stage of diagnosis and rapid progression.3,4 Thus, it is important for us to identify predictive factors for the prognosis in patients with EC.
In an attempt to better estimate the prognosis, many prognostic factors have been investigated, such as depth of invasion, nodal metastasis, tumor-node-metastasis (TNM) stage, and other miscellaneous factors.5,6 Recently, systemic inflammatory response (SIR) has been found to play an important role in cancer.7,8 Serum C-reactive protein (CRP) is a very sensitive indicator of SIR, and was initially identified in the plasma of patients as a substance reacting with pneumococcal C-polysaccharide, which appeared in patients with inflammation.9–11 CRP is synthesized mainly by hepatocytes as a part of the SIR.12,13 CRP is a representative acute-phase reactant and is the most widely used systemic biomarker of inflammation.14,15
Previously published studies have shown that serum CRP is a predictor of survival in several cancers, such as gastric cancer,16,17 colorectal cancer,18,19 pancreatic cancer,20 hepatocellular cancer,21 urological cancer,22 ovarian cancer,23 lymphoma,24 and osteosarcoma.25 Similar results have also been found in patients with EC.26–33 Nozoe et al26 and Ikeda et al27 demonstrated that CRP can be an independent marker in patients with EC. Gockel et al29 investigated 291 EC patients who underwent curative resection, and they concluded that a high level of CRP is associated with tumor progression and poor overall survival (OS). This observation is in line with data from Wang et al,30 Feng et al32 and Song et al33 but is contrary to the results of Shimada et al28 and Zingg et al31 who suggested that CRP is not an independent factor. Due to the inconsistent results, the role of serum CRP in EC is still controversial. In this study, therefore, we conduct a systematic review and meta-analysis to analyze the prognostic value of serum CRP for OS in patients with EC.
Materials and methods
Literature search
A systematic literature search was performed using Medline, PubMed, and Web of Science to analyze the prognostic value of CRP in patients with EC from 1984 to 2013. The search strategies included the keywords “C-reactive protein” or “CRP” combined with “esophageal cancer” or “esophageal carcinoma”. All selected studies were English language articles. Only human research was included. In addition, the reference lists of the identified studies were checked for further relevant studies.
Inclusion/exclusion criteria
Inclusion criteria for this meta-analysis were as follows: 1) patients were diagnosed as having EC by pathology; 2) the serum CRP was measured before treatment; 3) the relationship between CRP and OS was reported. The exclusion criteria were: 1) review, letter, case report, or nonhuman research; 2) insufficient data to extract the hazard ratio (HR) and the 95% confidence interval (CI).
Data extraction
Three authors (Huang, Feng, and Liu) reviewed the included studies and extracted the data independently. If the results reported overlapped, only the most recent or the most complete study was used in the meta-analysis. The extracted data included “authors”, “year”, “sample”, “country”, “treatment”, “pathological type”, “cut-off level”, “TNM stage”, and HR and 95% CI for the correlation between serum CRP and OS.
Statistical analysis
All statistical analyses were performed using Stata version 12.0 (StataCorp LP, College Station, TX, USA). The pooled HR and 95% CI were used to analyze the relationship between CRP and OS. A significant heterogeneity was defined as P<0.10 or I2>50%.34 A combined HR>1 indicated a worse OS, and it was considered statistically significant if the 95% CI did not overlap.
Results
According to the search strategies described, a total of 82 studies were identified. The search results are shown in Figure 1. After reviewing all 82 studies carefully, eight eligible studies, including 1,471 patients with EC, were included in the meta-analysis.26–33 The main characteristics of the eight included studies are listed in Table 1. Among these studies, three were performed in Japan,26–28 two in the People’s Republic of China,32,33 one in Germany,29 one in Taiwan,30 and one in Australia.31 Five studies set a cut-off value of 5 mg/L,26,27,29,30,33 and three studies set a cut-off value of 10 mg/L.28,31,32
Figure 1.
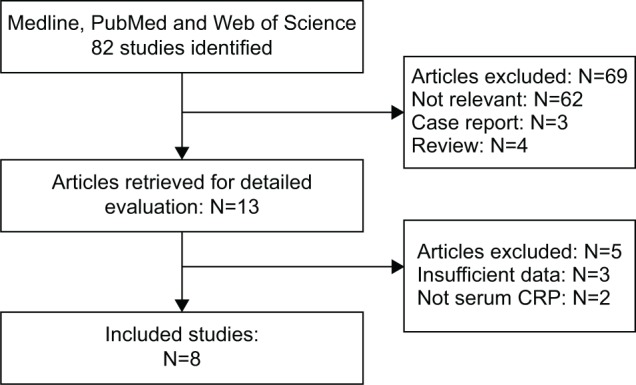
Flow chart of the meta-analysis.
Abbreviation: CRP, C-reactive protein.
Table 1.
Characteristics of included studies
| Author | Year | Country | Sample (M/F) | Treatment | Survival analysis | Pathological type | HR (95% CI) | Cut-off level (mg/L) | TNM stage I–II/III–IV |
|---|---|---|---|---|---|---|---|---|---|
| Nozoe et al26 | 2001 | Japan | 262 (227/35) | C/R + S | OS | SCC + AC | 3.300 (2.174–5.000) | 5 | 157/105 |
| Ikeda et al27 | 2003 | Japan | 356 (327/29) | C/R + S + C/R | OS | SCC + AC + OT | 1.52 (1.05–2.21) | 5 | 186/170 |
| Shimada et al28 | 2003 | Japan | 150 (128/22) | S | OS | SCC | 1.42 (0.83–2.41) | 10 | 67/83 |
| Gockel et al29 | 2006 | Germany | 291 (NA) | S | OS | SCC + AC + OT | 1.182 (1.030–1.356) | 5 | NA |
| Wang et al30 | 2009 | Taiwan | 123 (120/3) | R | OS | SCC + AC | 12.116 (3.449–42.567) | 5 | 22/101 |
| Zingg et al31 | 2010 | Australia | 90 (74/16) | C/R + S | OS | SCC + AC | 1.42 (0.71–2.85) | 10 | 75/15 |
| Feng et al32 | 2013 | People’s Republic of China | 43 (30/13) | S + C/R | OS | SCCE | 2.756 (1.115–6.813) | 10 | NA |
| Song et al33 | 2013 | People’s Republic of China | 156 (134/22) | S + C/R | OS | SCC | 2.131 (1.213–4.451) | 5 | NA |
Abbreviations: M/F, male/female; S, surgery; C/R, chemotherapy/radiotherapy; R, radiotherapy; OS, overall survival; SCC, squamous cell carcinoma; AC, adenocarcinoma; OT, others; SCCE, small cell carcinoma of the esophagus; NA, not available; HR, hazard ratio; CI, confidence interval; TNM, tumor-node-metastasis.
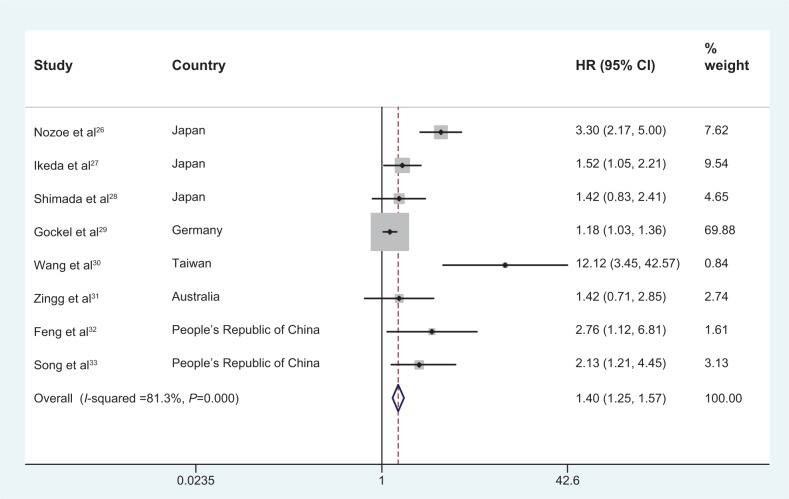
Our pooled results demonstrated that high levels of serum CRP were associated with poor OS in patients with EC (HR: 1.40, 95% CI: 1.25–1.57). The analysis showed a significant heterogeneity for OS (I2=81.3%, P<0.0001) (Figure 2). In further investigations, subgroup analyses were also performed in our meta-analysis. When the patients were segregated according to treatment, pathological type, and cut-off level, high levels of serum CRP were also found to be significantly correlated with OS (Table 2; Figure 3A–F).
Figure 2.
Forest plot of the association between serum CRP and OS in EC.
Abbreviations: CRP, C-reactive protein; OS, overall survival; EC, esophageal cancer; HR, hazard ratio; CI, confidence interval.
Table 2.
Subgroup analyses of pooled HRs for increased serum CRP and OS in EC
| Subgroup | Number of cohorts | Number of patients | References | HR (95% CI) | Heterogeneity
|
|
|---|---|---|---|---|---|---|
| I2 (%) | P-value | |||||
| Treatment | ||||||
| S | 2 | 441 | 28, 29 | 1.20 (1.05–1.37) | 0.0 | 0.514 |
| S + AT | 5 | 907 | 26, 27, 31, 32, 33 | 2.08 (1.65–2.62) | 55.5 | 0.061 |
| Pathological type | ||||||
| SCC | 2 | 306 | 28, 33 | 1.67 (1.11–2.52) | 0.0 | 0.344 |
| SCC + AC/OT | 5 | 1,122 | 26, 27, 29, 30, 31 | 1.36 (1.20–1.53) | 88.0 | <0.0001 |
| Cut-off level | ||||||
| 5 mg/L | 5 | 1,188 | 19, 20, 23, 24, 27 | 1.38 (1.22–1.56) | 88.6 | <0.0001 |
| 10 mg/L | 3 | 283 | 22, 25, 26 | 1.60 (1.09–2.35) | 0.0 | 0.429 |
Abbreviations: S, surgery; AT, adjuvant therapy; SCC, squamous cell carcinoma; AC, adenocarcinoma; OT, others; HR, hazard ratio; CI, confidence interval; CRP, C-reactive protein; OS, overall survival; EC, esophageal cancer.
Figure 3.
Forest plot of the association between serum CRP and OS in EC by subgroup analyses.
Notes: Subgroup analysis according to treatment (A) surgery only; (B) surgery combined with chemotherapy/radiotherapy, pathological type; (C) squamous cell carcinoma; (D) squamous cell carcinoma, adenocarcinoma and others and cut-off level (E) cut-off =10 mg/L; (F) cut-off =5 mg/L.
Abbreviations: CRP, C-reactive protein; OS, overall survival; EC, esophageal cancer; HR, hazard ratio; CI, confidence interval.
Discussion
In this meta-analysis, our pooled results demonstrated that high levels of serum CRP were associated with poor OS in patients with EC (HR: 1.40, 95% CI: 1.25–1.57). There was a significant heterogeneity for OS (I2=81.3%, P<0.0001). We did further subgroup analyses to evaluate the correlation between serum CRP and OS in the included studies.
In the subgroup analyses, with data stratified by treatment and pathological type, both subgroups showed significant HRs and 95% CIs. These results, on the other hand, indicated that no matter what treatment or pathological type the patients were in, the high levels of serum CRP could significantly predict the poor OS in patients with EC. In addition, when stratified by cut-off value, the results were also significant no matter what cut-off level was used: 5 mg/L (HR: 1.38, 95% CI: 1.22–1.56, I2=88.6%) or 10 mg/L (HR: 1.60, 95% CI: 1.09–2.35, I2=0).
The molecular mechanism by which CRP can be correlated with a prognosis of cancer remains to be determined. One possible explanation is interleukin-6 (IL-6). CRP is synthesized mainly by hepatocytes.12,13 Production of CRP by hepatocytes is induced mainly by IL-6, although other cytokines, such as tumor necrosis factor α (TNFα), transforming growth factor β (TGFβ), and IL-1, can also synthesize this protein.35,36 It has been reported that IL-6 is able to contribute to the cancer growth and progression.37,38 Moreover, IL-6 probably links the inflammation and angiogenesis via the expression of vascular endothelial growth factor (VEGF).39 On the other hand, as with other proteic biomarkers of SIR, CRP has several biological functions, including complement activation and platelet activation.40,41
There are several limitations in our meta-analysis. First, the number of included studies was limited. Second, significant heterogeneity was observed. Third, the pooled predictive HR between serum CRP and OS were not strong (HR=1.40, P<0.0001). Empirically, HR>2 is considered strongly predictive.42 Therefore, the results should be used with caution when using CRP to predict OS in EC. Moreover, our meta-analysis showed that CRP is an independent predictive factor in patients with EC; however, it should be kept in mind that CRP alone, without other variables, may not be associated with postoperative survival in patients with EC. In addition, in our study, variation in the types of treatment may have had an influence on OS, and this can possibly introduce bias into the study. The results should therefore be interpreted with caution. Further large prospective studies should be performed to confirm whether serum CRP has a prognostic value in patients with EC.
In conclusion, our meta-analysis revealed that high levels of serum CRP were significantly associated with poor OS in patients with EC. We conclude that serum CRP might serve as a useful biomarker for EC.
Footnotes
Disclosure
The authors declare no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Eloubeidi MA, Desmond R, Arguedas MR, Reed CE, Wilcox CM. Prognostic factors for the survival of patients with esophageal carcinoma in the US: the importance of tumor length and lymph node status. Cancer. 2002;95(7):1434–1443. doi: 10.1002/cncr.10868. [DOI] [PubMed] [Google Scholar]
- 3.Feng JF, Zhao Q, Chen QX. Prognostic value of subcarinal lymph node metastasis in patients with esophageal squamous cell carcinoma. Asian Pac J Cancer Prev. 2013;14(5):3183–3186. doi: 10.7314/apjcp.2013.14.5.3183. [DOI] [PubMed] [Google Scholar]
- 4.Tachibana M, Kinugasa S, Hirahara N, Yoshimura H. Lymph node classification of esophageal squamous cell carcinoma and adenocarcinoma. Eur J Cardiothorac Surg. 2008;34(2):427–431. doi: 10.1016/j.ejcts.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 5.Peyre CG, Hagen JA, DeMeester SR, et al. The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann Surg. 2008;248(4):549–556. doi: 10.1097/SLA.0b013e318188c474. [DOI] [PubMed] [Google Scholar]
- 6.Feng JF, Huang Y, Zhao Q. Tumor length in elderly patients with esophageal squamous cell carcinoma: is it a prognostic factor? Ups J Med Sci. 2013;118(3):145–152. doi: 10.3109/03009734.2013.792887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 8.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 9.Tillett WS, Francis T., Jr Serological reactions in pneumonia with a nonprotein somatic fraction of pneumococcus. J Exp Med. 1930;52(4):561–585. doi: 10.1084/jem.52.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diehl EE, Haines GK, III, Radosevich JA, Potempa LA. Immunohistochemical localization of modified C-reactive protein antigen in normal vascular tissue. Am J Med Sci. 2000;319(2):79–83. doi: 10.1097/00000441-200002000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Potempa LA, Siegel JN, Fiedel BA, Potempa RT, Gewurz H. Expression, detection, and assay of neoantigen (neo-CRP) associated with a free, human C-reactive subunit. Mol Immunol. 1987;24(5):531–541. doi: 10.1016/0161-5890(87)90028-9. [DOI] [PubMed] [Google Scholar]
- 12.Egenhofer C, Alsdorff K, Fehsel K, Kolb-Bachofen V. Membrane associated C-reactive protein on rat liver macrophages is synthesized within the macrophages, expressed as neo-C-reactive protein and bound through a C-reactive protein-specific membrane receptor. Hepatology. 1995;18(5):1216–1223. [PubMed] [Google Scholar]
- 13.Weinhold B, Rüther U. Interleukin-6-dependent and independent regulation of the human C-reactive protein gene. Biochem J. 1997;327:425–429. doi: 10.1042/bj3270425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gabay C, Kushner I. Acute phase protein and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 15.Volanakis JE. Human C-reactive protein: expression, structure, and function. Mol Immunol. 2001;38(2–3):189–197. doi: 10.1016/s0161-5890(01)00042-6. [DOI] [PubMed] [Google Scholar]
- 16.Shimura T, Kitagawa M, Yamada T, et al. C-reactive protein is a potential prognostic factor for metastatic gastric cancer. Anticancer Res. 2012;32(2):491–496. [PubMed] [Google Scholar]
- 17.Nozoe T, Iguchi T, Adachi E, Matsukuma A, Ezaki T. Preoperative elevation of serum C-reactive protein as an independent prognostic indicator for gastric cancer. Surg Today. 2011;41(4):510–513. doi: 10.1007/s00595-009-4297-x. [DOI] [PubMed] [Google Scholar]
- 18.Koike Y, Miki C, Okugawa Y, et al. Preoperative C-reactive protein as a prognostic and therapeutic marker for colorectal cancer. J Surg Oncol. 2008;98(7):540–544. doi: 10.1002/jso.21154. [DOI] [PubMed] [Google Scholar]
- 19.Nozoe T, Matsumata T, Kitamura M, Sugimachi K. Significance of preoperative elevation of serum C-reactive protein as an indicator for prognosis in colorectal cancer. Am J Surg. 1998;176(4):335–338. doi: 10.1016/s0002-9610(98)00204-9. [DOI] [PubMed] [Google Scholar]
- 20.Pine JK, Fusai KG, Young R, et al. Serum C-reactive protein concentration and the prognosis of ductal adenocarcinoma of the head of pancreas. Eur J Surg Oncol. 2009;35(6):605–610. doi: 10.1016/j.ejso.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Hashimoto K, Ikeda Y, Korenaga D, et al. The impact of preoperative serum C-reactive protein on the prognosis of patients with hepatocellular carcinoma. Cancer. 2005;103(9):1856–1864. doi: 10.1002/cncr.20976. [DOI] [PubMed] [Google Scholar]
- 22.Karakiewicz PI, Hutterer GC, Trinh QD, et al. C-reactive protein is an informative predictor of renal cell carcinoma-specific mortality: a European study of 313 patients. Cancer. 2007;110(6):1241–1247. doi: 10.1002/cncr.22896. [DOI] [PubMed] [Google Scholar]
- 23.Hefler LA, Concin N, Hofstetter G, et al. Serum C-reactive protein as independent prognostic variable in patients with ovarian cancer. Clin Cancer Res. 2008;14(3):710–714. doi: 10.1158/1078-0432.CCR-07-1044. [DOI] [PubMed] [Google Scholar]
- 24.Herishanu Y, Perry C, Braunstein R, et al. Early-mid treatment C-reactive protein level is a prognostic factor in aggressive non-Hodgkin’s lymphoma. Eur J Haematol. 2007;79(2):150–154. doi: 10.1111/j.1600-0609.2007.00894.x. [DOI] [PubMed] [Google Scholar]
- 25.Zahlten-Hinguranage A, Goldschmidt H, Cremer FW, et al. Preoperative elevation of serum C-reactive protein is predictive for prognosis in myeloma bone disease after surgery. Br J Cancer. 2006;95(7):782–787. doi: 10.1038/sj.bjc.6603329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nozoe T, Saeki H, Sugimachi K. Significance of pre-operative elevation of serum C-reactive protein as an indicator of prognosis. Am J Surg. 2001;182(2):197–201. doi: 10.1016/s0002-9610(01)00684-5. [DOI] [PubMed] [Google Scholar]
- 27.Ikeda M, Natsugoe S, Ueno S, Baba M, Aikou T. Significant host- and tumor-related factors for predicting prognosis in patients with esophageal carcinoma. Ann Surg. 2003;238(2):197–202. doi: 10.1097/01.sla.0000080822.22415.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimada H, Nabeya Y, Okazumi S, et al. Elevation of pre-operative serum C-reactive protein level is related to poor prognosis in esophageal squamous cell carcinoma. J Surg Oncol. 2003;83(4):248–252. doi: 10.1002/jso.10275. [DOI] [PubMed] [Google Scholar]
- 29.Gockel I, Dirksen K, Messow CM, Junginger T. Significance of preoperative C-reactive protein as a parameter of the perioperative course and long-term prognosis in squamous cell carcinoma and adenocarcinoma of the oesophagus. World J Gastroenterol. 2006;12(23):3746–3750. doi: 10.3748/wjg.v12.i23.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang CY, Hsieh MJ, Chiu YC, et al. Higher serum C-reactive protein concentration and hypoalbuminemia are poor prognostic indicators in patients with esophageal cancer undergoing radiotherapy. Radiother Oncol. 2009;92(2):270–275. doi: 10.1016/j.radonc.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Zingg U, Forberger J, Rajcic B, Langton C, Jamieson G. Association of C-reactive protein levels and long-term survival after neoadjuvant therapy and esophagectomy for esophageal cancer. J Gastrointest Surg. 2010;14(3):462–469. doi: 10.1007/s11605-009-1113-2. [DOI] [PubMed] [Google Scholar]
- 32.Feng JF, Zhao HG, Liu JS, Chen QX. Significance of preoperative C-reactive protein as a parameter in patients with small cell carcinoma of the esophagus. Onco Targets Ther. 2013;6:1147–1151. doi: 10.2147/OTT.S50039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song ZB, Lin BC, Li B, et al. Preoperative elevation of serum C-reactive protein as an indicator of poor prognosis for early-stage esophageal squamous cell carcinoma. Kaohsiung J Med Sci. 2013;29(12):662–666. doi: 10.1016/j.kjms.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gresser I, Delers F, Tran Quangs N, et al. Tumor necrosis factor induces acute phase protein in rats. J Biol Regul Homeost Agents. 1987;1(4):173–176. [PubMed] [Google Scholar]
- 36.Kuta AE, Baum LL. C-reactive protein is produced by a small number of normal human peripheral blood lymphocytes. J Exp Med. 1986;164(1):321–326. doi: 10.1084/jem.164.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takizawa H, Ohtoshi T, Ohta K, et al. Growth inhibition of human lung cancer cell lines by interleukin-6 in vitro: a possible role in tumor growth via an autocrine mechanism. Cancer Res. 1993;53(18):4175–4181. [PubMed] [Google Scholar]
- 38.Nachbaur DM, Herold M, Maneschg A, Huber H. Serum levels of interleukin-6 in multiple myeloma and other hematological disorders: correlation with disease activity and other prognostic parameters. Ann Hematol. 1991;62(2–3):54–58. doi: 10.1007/BF01714900. [DOI] [PubMed] [Google Scholar]
- 39.Huang S, Wu M, Wang HP, Yang CS, Kuo ML, Lin JT. Correlation between serum levels of interleukin-6 and vascular endothelial growth factor in gastric carcinoma. J Gastroenterol Hepatol. 2002;17(11):1165–1169. doi: 10.1046/j.1440-1746.2002.02873.x. [DOI] [PubMed] [Google Scholar]
- 40.Allin KH, Nordestgaard BG. Elevated C-reactive protein in the diagnosis prognosis, and cause of cancer. Crit Rev Clin Lab Sci. 2011;48(4):155–170. doi: 10.3109/10408363.2011.599831. [DOI] [PubMed] [Google Scholar]
- 41.Groblewska M, Mroczko B, Sosnowska D, Szmitkowski M. Interleukin-6 and C-reactive protein in esophageal cancer. Clin Chim Acta. 2012;413(19–20):1583–1590. doi: 10.1016/j.cca.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 42.Hayes DF, Isaacs C, Stearns V. Prognostic factors in breast cancer: current and new predictors of metastasis. J Mammary Gland Biol Neoplasia. 2001;6(4):375–392. doi: 10.1023/a:1014778713034. [DOI] [PubMed] [Google Scholar]