Abstract
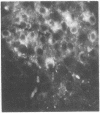
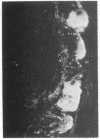
The localization of the vasoactive intestinal polypeptide (VIP) has been studied with immunohistochemistry and radioimmunoanalysis. VIP immunoreactivity is present in gastrointestinal nerves, which constitute a quantitatively important nerve population that may be intrinsic to the gut wall. VIP-immunoreactive neurons are also found within the ventromedial hypothalamus and give off processes that travel latteral to the third ventricle. Results of radioimmunoanalysis strongly indicate that the immunoreactive material represents true VIP. Thus VIP, at present a gastrointestinal hormone candidate, appears to represent a new neuronal peptide occurring in both the central and peripheral nervous system.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayliss W. M., Starling E. H. The mechanism of pancreatic secretion. J Physiol. 1902 Sep 12;28(5):325–353. doi: 10.1113/jphysiol.1902.sp000920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodanszky M., Klausner Y. S., Said S. I. Biological activities of synthetic peptides corresponding to fragments of and to the entire sequence of the vasoactive intestinal peptide. Proc Natl Acad Sci U S A. 1973 Feb;70(2):382–384. doi: 10.1073/pnas.70.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura S., Yoshida T., Said S. I. Vasoactive intestinal polypoptide: inactivation in liver and potentiation in lung of anesthetized dogs (384699). Proc Soc Exp Biol Med. 1975 Jan;148(1):25–29. doi: 10.3181/00379727-148-38469. [DOI] [PubMed] [Google Scholar]
- Mutt V., Said S. I. Structure of the porcine vasoactive intestinal octacosapeptide. The amino-acid sequence. Use of kallikrein in its determination. Eur J Biochem. 1974 Mar 1;42(2):581–589. doi: 10.1111/j.1432-1033.1974.tb03373.x. [DOI] [PubMed] [Google Scholar]
- Nilsson A. Isolation, amino acid composition and terminal amino acid residues of the vasoactive actacosapeptide from chicken intestine. Partial purification of chicken secretin. FEBS Lett. 1974 Oct 15;47(2):284–289. doi: 10.1016/0014-5793(74)81031-8. [DOI] [PubMed] [Google Scholar]
- Nilsson A. Structure of the vasoactive intestinal octacosapeptide from chicken intestine. The amino acid sequence. FEBS Lett. 1975 Dec 15;60(2):322–326. doi: 10.1016/0014-5793(75)80740-x. [DOI] [PubMed] [Google Scholar]
- Nilsson G., Hökfelt T., Pernow B. Distribution of substance P-like immuno-reactivity in the rat central nervous system as revealed by immunohistochemistry. Med Biol. 1974 Dec;52(6):424–427. [PubMed] [Google Scholar]
- Nilsson G., Larsson L. I., Håkanson R., Brodin E., Pernow B., Sundler F. Localization of substance P-like immunoreactivity in mouse gut. Histochemistry. 1975;43(1):97–99. doi: 10.1007/BF00490158. [DOI] [PubMed] [Google Scholar]
- Pearse A. G., Polak J. M. Bifunctional reagents as vapour- and liquid-phase fixatives for immunohistochemistry. Histochem J. 1975 Mar;7(2):179–186. doi: 10.1007/BF01004561. [DOI] [PubMed] [Google Scholar]
- Pearse A. G., Polak J. M. Immunocytochemical localization of substance P in mammalian intestine. Histochemistry. 1975;41(4):373–375. doi: 10.1007/BF00490081. [DOI] [PubMed] [Google Scholar]
- Polak J. M., Pearse A. G., Garaud J. C., Bloom S. R. Cellular localization of a vasoactive intestinal peptide in the mammalian and avian gastrointestinal tract. Gut. 1974 Sep;15(9):720–724. doi: 10.1136/gut.15.9.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak J. M., Pearse A. G., Grimelius L., Bloom S. R. Growth-hormone release-inhibiting hormone in gastrointestinal and pancreatic D cells. Lancet. 1975 May 31;1(7918):1220–1222. doi: 10.1016/s0140-6736(75)92198-4. [DOI] [PubMed] [Google Scholar]
- Rufener C., Dubois M. P., Malaisse Lagae F., Oric L. Immuno-fluorescent reactivity to anti-somatostatin in the gastro-intestinal mucosa of the dog. Diabetologia. 1975 Aug;11(4):321–324. doi: 10.1007/BF00422398. [DOI] [PubMed] [Google Scholar]
- Said S. I., Mutt V. Polypeptide with broad biological activity: isolation from small intestine. Science. 1970 Sep 18;169(3951):1217–1218. doi: 10.1126/science.169.3951.1217. [DOI] [PubMed] [Google Scholar]
- Vanderhaeghen J. J., Signeau J. C., Gepts W. New peptide in the vertebrate CNS reacting with antigastrin antibodies. Nature. 1975 Oct 16;257(5527):604–605. doi: 10.1038/257604a0. [DOI] [PubMed] [Google Scholar]