Abstract
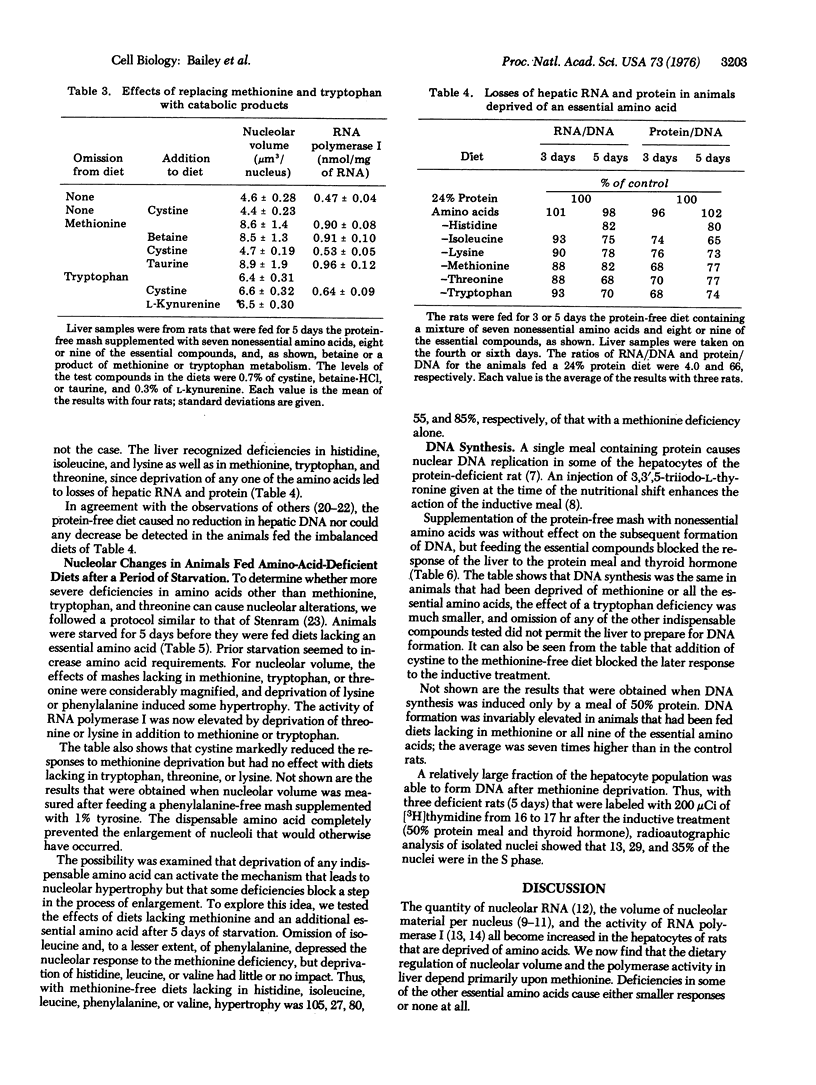
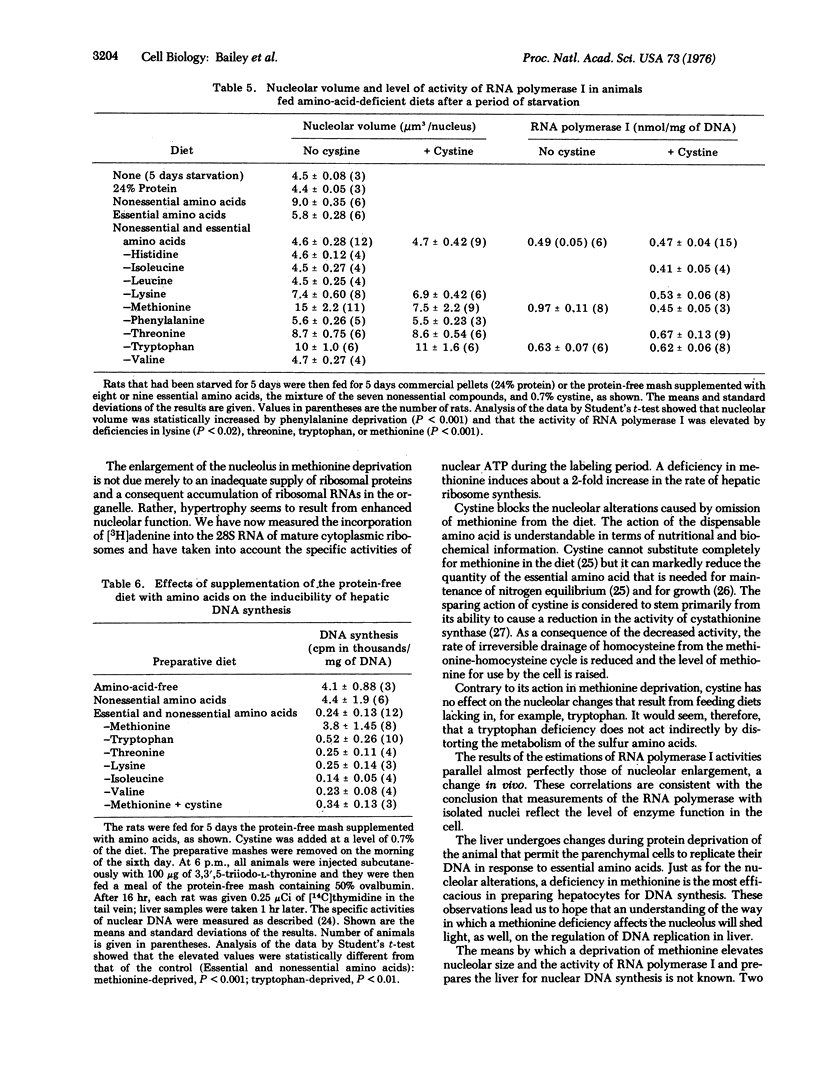
The volume of nucleolar material per nucleus and the activity of RNA polymerase I (RNA nucleotidyltransferase I) become doubled in the liver cells of rats that are fed for several days a diet that lacks essential amino acids. Omission of methionine from a fully supplemented diet is equivalent to leaving out all the amino acids, and the responses to a deficiency of tryptophan are about 40% as great. Deprivation of one of the remaining essential amino acids gives either small responses or none at all. Supplementation of the methionine-free diet with cystine blocks the nucleolar enlargement and the enhancement of the polymerase activity that would otherwise take place, but the dispensable amino acid does not affect the responses to a deprivation of one of the other essential amino acids. After deprivation of all the essential amino acids or only methionine, hepatocytes make DNA when the rat is fed a meal with protein. A preparatory diet lacking in tryptophan is much less effective; a deficiency in any of the other indispensable compounds tested fails to prepare the liver for DNA synthesis. The results give hope that elucidation of the means by which methionine deprivation affects the nucleolus will also provide information on the regulation of nuclear DNA replication in liver. One attractive possibility is that the amino acid deficiency acts by producing some imbalance in protein metabolism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony L. E., Edozien J. C. Experimental protein and energy in the rat. J Nutr. 1975 Jun;105(6):631–648. doi: 10.1093/jn/105.6.631. [DOI] [PubMed] [Google Scholar]
- BURTON K. The relation between the synthesis of deoxyribonucleic acid and the synthesis of protein in the multiplication of bacteriophage T2. Biochem J. 1955 Nov;61(3):473–483. doi: 10.1042/bj0610473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey R. P., Rudert W. A., Short J., Lieberman I. Nucleolar changes in liver before the onset of deoxyribonucleic acid replication. J Biol Chem. 1975 Jun 10;250(11):4305–4309. [PubMed] [Google Scholar]
- Finkelstein J. D., Mudd S. H. Trans-sulfuration in mammals. The methionine-sparing effect of cystine. J Biol Chem. 1967 Mar 10;242(5):873–880. [PubMed] [Google Scholar]
- Kosterlitz H. W. The effects of changes in dietary protein on the composition and structure of the liver cell. J Physiol. 1947 Jun 2;106(2):194–210.1. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mandel P., Quirin-Stricker C. Effect of protein deprivation on soluble and "aggregate" RNA polymerase in rat liver. Life Sci. 1967 Jun 15;6(12):1299–1303. doi: 10.1016/0024-3205(67)90025-2. [DOI] [PubMed] [Google Scholar]
- Parsa I., Marsh W. H., Fitzgerald P. J. Pancreas acinar cell differentiation. 8. Effect of methionine on DNA synthesis of pancreas anlage in organ culture. Exp Cell Res. 1973 Dec;82(2):466–468. doi: 10.1016/0014-4827(73)90369-8. [DOI] [PubMed] [Google Scholar]
- RATHER L. J. Experimental alteration of nuclear and cytoplasmic components of the liver cell with thioacetamide. I. Early onset and reversibility of volume changes of the nucleolus, nucleus and cytoplasm. Bull Johns Hopkins Hosp. 1951 Jan;88(1):38–58. [PubMed] [Google Scholar]
- ROSE W. C., WIXOM R. L. The amino acid requirements of man. XIII. The sparing effect of cystine on the methionine requirement. J Biol Chem. 1955 Oct;216(2):753–773. [PubMed] [Google Scholar]
- Reddy J., Chiga M., Svoboda D. Initiation of the division cycle of rat hepatocytes following a single injection of thioacetamide. Lab Invest. 1969 May;20(5):405–411. [PubMed] [Google Scholar]
- STENRAM U. Interferometric determinations of the ribose nucleic acid concentration in liver nucleoli of protein-fed and protein-deprived rats. Exp Cell Res. 1958 Aug;15(1):174–183. doi: 10.1016/0014-4827(58)90073-9. [DOI] [PubMed] [Google Scholar]
- STENRAM U. Nucleolar size in the liver of rats fed diets deficient in essential amino acids. Acta Pathol Microbiol Scand. 1956;38(5):364–374. [PubMed] [Google Scholar]
- STENRAM U. The nucleolar size in the liver cell of rats fed high and non protein diets. Exp Cell Res. 1953 Dec;5(2):539–541. doi: 10.1016/0014-4827(53)90241-9. [DOI] [PubMed] [Google Scholar]
- SVOBODA D., HIGGINSON J. ULTRASTRUCTURAL CHANGES PRODUCED BY PROTEIN AND RELATED DEFICIENCIES IN THE RAT LIVER. Am J Pathol. 1964 Sep;45:353–379. [PMC free article] [PubMed] [Google Scholar]
- Shaw C., Fillios L. C. RNA polymerase activities and other aspects of hepatic protein synthesis during early protein depletion in the rat. J Nutr. 1968 Nov;96(3):327–336. doi: 10.1093/jn/96.3.327. [DOI] [PubMed] [Google Scholar]
- Short J., Armstrong N. B., Zemel R., Lieberman I. A role for amino acids in the induction of deoxyribonucleic acid synthesis in liver. Biochem Biophys Res Commun. 1973 Jan 23;50(2):430–437. doi: 10.1016/0006-291x(73)90858-9. [DOI] [PubMed] [Google Scholar]
- Short J., Tsukada K., Rudert W. A., Lieberman I. Cyclic adenosine 3':5'-monophosphate and the induction of deoxyribonucleic acid synthesis in liver. J Biol Chem. 1975 May 25;250(10):3602–3606. [PubMed] [Google Scholar]
- TSUKADA K., LIEBERMAN I. METABOLISM OF NUCLEOLAR RIBONUCLEIC ACID AFTER PARTIAL HEPATECTOMY. J Biol Chem. 1964 May;239:1564–1568. [PubMed] [Google Scholar]
- Tata J. R., Baker B. Sub-nuclear fractionation. II. Intranuclear compartmentation of transcription in vivo and in vitro. Exp Cell Res. 1974 Jan;83(1):125–138. doi: 10.1016/0014-4827(74)90695-8. [DOI] [PubMed] [Google Scholar]



