Abstract
The aim of the present study was to formulate serratiopeptidase (SER)-loaded chitosan (CS) nanoparticles for oral delivery. SER is a proteolytic enzyme which is very sensitive to change in temperature and pH. SER-loaded CS nanoparticles were fabricated by ionic gelation method using tripolyphosphate (TPP). Nanoparticles were characterized for its particle size, morphology, entrapment efficiency, loading efficiency, percent recovery, and in vitro dissolution study. SER-CS nanoparticles had a particle size in the range of 400–600 nm with polydispersity index below 0.5. SER association was up to 80 ± 4.2%. SER loading and CS/TPP mass ratio were the primary parameters having direct influence on SER-CS nanoparticles. SER-CS nanoparticles were freeze dried using trehalose (20%) as a cryoprotectant. In vitro dissolution showed initial burst followed by sustained release up to 24 h. In vivo anti-inflammatory activity was carried out in rat paw edema model. In vivo anti-inflammatory activity in rat paw edema showed prolonged anti-inflammatory effect up to 32 h relative to plain SER.
KEY WORDS: anti-inflammatory activity, chitosan, nanoparticle, serratiopeptidase, TPP
INTRODUCTION
Oral route for drug administration is the preferred route because of its noninvasive nature. However, administration of peptides and proteins through oral route remains a challenging task due to poor bioavailability (1). Incorporation of protein and peptide into polymeric nanoparticles and microparticles has gained a lot of interest in the last two decades to improve their oral bioavailability and stability (2–5). Chitosan is one of the polymers which have gained a lot of attention in nanoparticulate drug delivery system of protein and peptide (5–9).
Serratiopeptidase (SER) is a proteolytic enzyme used in pain management, particularly for its anti-inflammatory action (10). The mechanism of anti-inflammatory effect of SER is because of hydrolysis of histamine, bradykinin, and serotonin. Serratiopeptidase also has a proteolytic and fibrinolytic effect. This is achieved by dissolving the complement (specific proteins responsible for inflammation) and increasing the plasmin activity by inhibiting the plasmin inactivators (11). This anti-inflammatory activity of SER can be evaluated by Rat Paw Edema model, wherein edema is induced by Aerosil and the decrease in the thickness of the inflamed rat paw is measured using plethysmometer (12). SER is an extracellular metalloprotease enzyme isolated from Serratia marcescens Serratia sp. (strain E-15). It contains 450 amino acids cleaved with the peptide bond having molecular weight of approximately 60 kDa. SER is labile to heat, moisture, and pH of the environment. The enzyme has maximal activity at pH 9.0 and at temperature 40°C. SER shows excellent stability at lower temperatures in the pH range from 5 to 10.
It is unstable at 37°C in alkaline conditions. The enzyme is completely inactivated by heating at 55°C for 15 min. The isoelectric point lies between pH 5.0 and 5.5 (13). Encapsulation of such sensitive active into the matrix of nanoparticles would be an effective approach for delivering it via oral route. Formulation of a nanoparticulate system for such a sensitive peptide is a challenging task. The objective of the present study was thus to develop a stable nanoparticulate system for oral SER delivery.
Chitosan (CS) is a polysaccharide, structurally similar to cellulose, obtained from the deacetylation of chitin, which is a naturally occurring and copiously available (in aquatic crustaceans). Both CS and cellulose are made by linear (1–4) β-linked monosaccharides. A noteworthy difference between CS and cellulose is that CS is poised of 2-amino-2-deoxy-β-d-glucan combined with glycosidic linkages. The presence of primary amine groups provide special properties to CS and make it very useful in pharmaceutical industry. Along with its abundant availability and biocompatible nature, CS has a positive charge, good mucoadhesive property, and permeation-enhancing capability across cell lines (14). Literature results reveal various methods for preparation of CS nanoparticles like complex coacervation/precipitation technique (15), emulsion-droplet coalescence method (16), and reverse micellar method (17).
However, preparation of CS nanoparticles by reversible physical cross-linking owing to electrostatic interaction called ionic gelation, instead of chemical cross-linking, has gained a lot of interest (18). Sodium tripolyphosphate (TPP) is a polyanion, which can interact with the cationic CS by electrostatic forces and produce nanoparticles. The ionic gelation method is a mild method, which does not induce any physical and chemical stress on sensitive molecules like protein and peptide (19–24). Therefore, ionic gelation method was opted for fabrication of SER-loaded CS nanoparticles.
MATERIALS AND METHODS
Materials
Chitosan (deacetylation degree min. 95%) was obtained from A & E Connock (perfumery and cosmetic) Ltd. England. Tripolyphosphate (TPP) was purchased from Sigma Chemical Co., USA. Serratiopeptidase was obtained from Biocon Ltd., India. Ultrapure water was obtained from Milli-Q equipment Millipore, USA. Casein was obtained from BDH, India. All other chemicals and reagents were of analytical grades.
Methods
Preparation of Chitosan Solution
Chitosan (CS) was dissolved in acetic acid solution (2% w/v) in double-distilled water under magnetic stirring (Remi, India). pH of this solution was adjusted to 5.5 using 4 N sodium hydroxide solution. The solution was filtered through muslin cloth and centrifuged (Remi, India) at 5,000 rpm. The supernatant was collected and used for fabrication of nanoparticles.
Preparation of Serratiopeptidase Loaded Chitosan Nanoparticles
SER-loaded CS nanoparticles were prepared by ionic gelation method (22,24). Different concentrations of CS solutions (0.8, 1.2, and 1.6 mg/ml), TPP solutions (0.06, 0.09, 0.12, 0.18, and 0.24 mg/ml), and SER solution (1.25 mg/ml) were prepared in double-distilled water. TPP and SER solutions were filtered through 0.22-μm nylon filter. Forty milliliters of CS solution was taken in a beaker; to this, 4 ml of SER solution was added and stirred for 5 min under magnetic stirring (Remi, India). Furthermore, TPP solution (6 ml) was added dropwise to the CS-SER solution under magnetic stirring. Spontaneous crosslinking of TPP with CS leads to the formation of nanoparticles. The nanoparticulate dispersion was stirred at room temperature for 10 min before further processing. Final concentration of SER was maintained at 0.1 mg/ml. Effect of SER concentration on nanoparticle size, drug entrapment, and loading capacity was evaluated.
Effect of SER Loading
SER-loaded chitosan nanoparticles were formulated with different concentration of SER ranging from 0.10, 0.20, 0.30, to 0.40 mg/ml. SER-CS nanoparticles were characterized for different physicochemical properties (Fig. 1a). Blank CS nanoparticles (without drug) were also fabricated. Nanoparticles were centrifuged at 40,000 rpm (112,000×g) at 20°C for 30 min. The supernatant was decanted to collect nanoparticles which were further redispersed in double-distilled water. pH of the dispersion was maintained at 5.5. Nanoparticles were loaded with SER in a ratio of 1:1, 1.5:1, and 3:1 and stirred for 1 h. Five milliliters of sample was withdrawn at 15, 30, and 60 min. Sample was centrifuged and the supernatant was evaluated for their SER activity. Percent drug entrapment was calculated as follows:
| 1 |
Fig. 1.
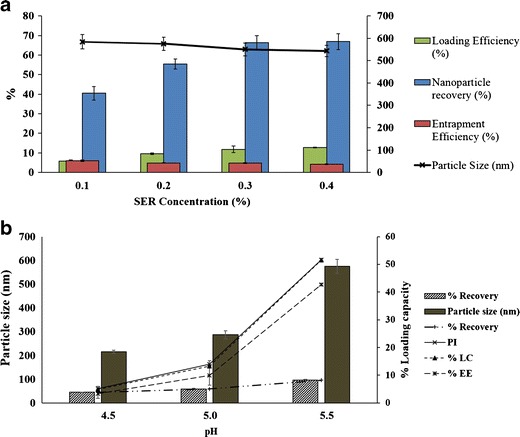
a Effect of SER loading on SER-CS nanoparticles; b percent loading of SER on blank nanoparticles
Effect of pH on Nanoparticle Formation
Effect of pH on particle size, drug loading, and entrapment efficiency was studied. CS solution (1.0 mg/ml) was prepared in 2% acetic acid solution. pH of CS solution was adjusted to 4.5, 5.0, and 5.5 using 4 N NaOH. Effect of pH on SER-CS nanoparticles was evaluated.
Physicochemical Characterization of SER-CS Nanoparticles
Particle Size
Particle size (Z-average mean) and polydispersity index (size distribution) of freshly prepared SER-CS nanoparticles were analyzed by photon correlation spectroscopy (Beckman counter, USA). Samples were analyzed after dilution with double-distilled water (n = 3) and the mean value of the three was adopted.
SEM Analysis
Morphological features of SER-CS nanoparticles were evaluated using scanning electron microscopy (SEM) (JEOL JSM-840 SEM HITACHI, Japan). Nanoparticles were diluted 1:1,000 times with double-distilled water. The dispersion was fixed on an adhesive carbon tape and dried at room temperature. The tape was further coated with platinum under vacuum and its microscopical examination was performed (25).
Assay of Serratiopeptidase Proteolytic Activity
Proteolytic activity was assayed by the Kunitz methods with a slight modification (26). This method is based on enzymatic hydrolysis of casein by SER. SER breaks casein into tyrosine, whose concentration is determined by UV spectroscopy (Jasco, Japan) at 275 nm. Briefly; casein solution (0.6% w/v) was prepared in borate buffer (pH 9.0). Two milliliters of borate buffer was mixed with 5 ml of casein solution and 2 ml of enzyme solution (equivalent to 10 μg/ml) was added to this mixture followed by incubation at 37°C for 30 min. Reaction was terminated by addition of 5.0 ml of 5.0% trichloroacetic acid and the mixture was allowed to stand for 30 min at 37°C. The supernatant was filtered through Whatman filter no. 42 and subjected to spectrophotometric determination at 275 nm. One unit of proteolytic activity (PU) was defined as the amount of the enzyme giving optical density equivalent to 1.0 μg of tyrosine in 1 min at 275 nm. Specific activity of the enzyme was expressed as units per milligrams of casein.
Entrapment Efficiency and Loading Capacity
SER-loaded CS nanoparticles were separated from the aqueous dispersion medium by ultracentrifugation (Thermo Sorvall, USA) at 40,000 rpm (112,000×g) at 20°C for 30 min. Nanoparticles were settled in the form of pellets and the supernatant containing free drug was analyzed for SER activity. Loading capacity (LC) and entrapment efficiency (EE) were calculated using following formula.
| 2 |
Production Yield of Nanoparticles
Production yield of nanoparticles was determined by centrifugation. Briefly, 8 ml of nanoparticles suspension was ultracentrifuged at 45,000 rpm for 30 min. The supernatant was discarded and the centrifuge tube containing nanoparticle pellets was kept at 50°C until constant weight was achieved. The production yield was calculated by comparing actual weight with the theoretical weight of nanoparticles.
Freeze Drying of SER-CS Nanoparticles
Different cryoprotectants like mannitol, trehalose, sucrose, dextrose, hydroxypropyl beta-cyclodextrin, and lactose were screened for freeze drying of SER-CS nanoparticles using freeze thaw study (n = 3). Freeze-thaw study is a quick approach used for screening of cryoprotectants. In this, a mixture of nanoparticles and cryoprotectant is freezed at −80°C (freezing stage) for 16 h. The freezed sample is allowed to come to room temperature (thaw stage) and subjected to particle size analysis (27,28). Different cryoprotectants were added in a concentration of 5, 10, 20, and 30 times w/w with respect to total solid content. Nanoparticles were subjected to freezing at −80°C for 16 h in a deep freezer (Eclipse 400, RS Biotech, UK) followed by thawing at room temperature. Particle size and redispersibility of the nanoparticles before freezing and after thawing was determined. Trehalose and sucrose showed reproducible particle size and had good redispersibility. Therefore, nanoparticles were subjected to freeze drying (n = 3) (Labconco freeze-dryer, Free-Zone 4.5, USA) using trehalose (20% w/w) and sucrose (10% w/w) as cryoprotectants. Sublimation was carried out for 24 h at vacuum pressure of 54 × 10−3 bar with the surface temperature of condenser maintained at −54°C. Samples were collected and stored in desiccators after drying. The lyophilized samples were redispersed in double-distilled water and its particle size was analyzed.
In Vitro Dissolution Study
In vitro drug release study was performed in pH 7.4 phosphate buffer. Eight hundred milliliters of pH 7.4 phosphate buffer was taken in USP type II dissolution apparatus. The steering element speed was kept at 50 rpm and the temperature of the medium was maintained at 37 ± 0.5°C. Freeze-dried nanoparticles equivalent to 5 mg of SER were added to the dissolution medium. Two milliliters of sample was withdrawn at 1-, 2-, 4-, 8-, 12-, and 24-h intervals. Samples were centrifuged and the supernatant was analyzed for drug release. SER being unstable in solution state undergoes degradation in dissolution medium giving false results. Therefore, its degradation pattern into the dissolution media was studied to get corrected release pattern. Five milligrams of plain SER was added in a dissolution vessel containing 800 ml of pH 7.4 phosphate buffer. Sample was withdrawn at 1-, 2-, 4-, 8-, 12-, and 24-h intervals. Dissolution profile of SER in nanoparticles was calculated by correcting with plain SER activity during dissolution studies to get actual drug release.
In Vitro Stability of SER-CS Nanoparticles
SER-CS nanoparticles (n = 3) were incubated with pH 1.2, simulated gastric fluid, simulated intestinal fluid, pH 6.8 buffer for 2 h. Samples were withdrawn after 0, 2 h, and SER activity was evaluated.
In Vivo Pharmacodynamic Evaluation in Rat Paw Edema Model
In vivo anti-inflammatory activity of the SER-CS nanoparticles was evaluated in rats. Animals were maintained in accordance with the guidelines given by Control for the Prevention and Care of Scientific and Experimental Animal (CPCSEA). The experiment was approved by institutional animal ethics committee (IAEC) (ICT/IAEC/0909/06). Sprague Dawley rats of either sex weighing between 150 and 200 g were housed in cages. Animals were acclimatized to the laboratory conditions for at least 7 days prior to the test and fed with laboratory animal standard diet feed with tap water ad libitum. Animals were starved overnight before starting the study. Animals were categorized in IV groups (Table I).
Table I.
Distribution of Animals
| Group | Description | No. of animals |
|---|---|---|
| I | Serratiopeptidase solution | 06 |
| II | Serratiopeptidase nanoparticulate formulation I (CS8) | 06 |
| III | Serratiopeptidase nanoparticulate formulation II (CS3) | 06 |
| IV | Control (blank solvent) | 06 |
One hour before induction of paw edema, group I was fed with SER solution (0.9 mg/kg) by oral gavage. Groups II and III received SER-CS nanoparticles CS 8 and CS 3 (equivalent to dose of 0.9 mg/kg of SER dispersed in water), respectively, by oral gavage. Group IV received water by oral gavage (control).
Rat paw edema was induced by subcutaneous injection of 0.1 ml of 2.5% suspension of aerosol into the plantar side of left hind paw. The paw was marked with ink at the level of the lateral malleolus and immersed into mercury up to this mark. The paw volume was measured plethysmographically before and after injection at 0, 7, 8, 9, 10, 12, 26, and 32 h. All the results were evaluated by applying statistical significance test (25).
RESULTS AND DISCUSSION
Preparation of Serratiopeptidase Loaded Chitosan Nanoparticles
Effect of Polyelectrolyte Concentration
SER-loaded nanoparticles were prepared with different concentrations of CS and TPP. Increasing ratio of CS to TPP showed twofold increase in particles size (Table II). TPP is incorporated as a crosslinking and condensing agent, which forms hydrogen bonds with free amine groups on both SER and CS molecules, resulting in formation of compact SER-CS nanoparticles. Increasing TPP concentration facilitates formation of compact nanoparticles structure resulting in decrease in particle size of the nanoparticles. Entrapment efficiency of SER in nanoparticles was also increased from 2.68 ± 1.14 to 78.75 ± 1.46. Furthermore, effect of TPP concentration on loading capacity of SER was evaluated. TPP showed initial increase followed by a decrease in drug loading. This was due to mechanism of protein association with polyelectrolyte nanoparticles mediated by ionic interactions. Increasing polyelectrolytes concentration shifts the ionic equilibrium between SER and polyelectrolytes towards the associated complexes. Loading capacity of SER showed a typical initial increase followed by a decrease in loading of SER. Increasing electrolyte concentration increases density of nanoparticles which ultimately increases drug loading. However, further increase in electrolyte concentration showed increase in concentration of total nanoparticle with constant amount of drug which ultimately decreased the ratio of SER to total nanoparticles thus decreasing drug loading.
Table II.
Influence of Cs: Tpp Mass Ratio on Nanoparticle Properties
| Batch no. | CS Conca. (mg/ml) | TPP Concb (mg/ml) | Entrapment efficiency (%) c | Loading capacity (%) | Nanoparticle recovery (%) | Particle size (nm) c | PI |
|---|---|---|---|---|---|---|---|
| CS1 | 0.8 | 0.06 | 49.89 ± 0.59 | 4.01 ± 0.32 | 13.67 ± 1.84 | 422.0 ± 25.46 | 0.23 ± 0.04 |
| CS2 | 0.09 | 58.92 ± 2.38 | 4.62 ± 1.36 | 25.88 ± 3.57 | 448.6 ± 57.35 | 0.39 ± 0.05 | |
| CS3 | 0.12 | 56.53 ± 2.80 | 1.56 ± 0.36 | 53.31 ± 2.80 | 479.5 ± 5.23 | 0.48 ± 0.01 | |
| CS4 | 0.18 | 62.33 ± 1.49 | 1.62 ± 0.10 | 51.79 ± 3.32 | 683.0 ± 5.66 | 0.39 ± 0.03 | |
| CS5 | 0.24 | 78.75 ± 1.46 | 1.04 ± 0.03 | 78.12 ± 1.16 | 801.5 ± 17.68 | 0.73 ± 0.06 | |
| CS6 | 1.2 | 0.06 | 37.27 ± 2.85 | 10.38 ± 1.60 | 45.01 ± 0.03 | 480.7 ± 1.34 | 0.59 ± 0.02 |
| CS7 | 0.09 | 42.81 ± 0.88 | 8.51 ± 0.09 | 54.18 ± 3.50 | 619.2 ± 4.87 | 0.38 ± 0.01 | |
| CS8 | 0.12 | 64.77 ± 3.34 | 6.05 ± 0.04 | 70.64 ± 0.93 | 632.6 ± 19.37 | 0.34 ± 0.06 | |
| CS9 | 0.18 | 64.14 ± 4.53 | 5.05 ± 0.73 | 85.73 ± 3.97 | 688.1 ± 2.05 | 0.26 ± 0.04 | |
| CS10 | 0.24 | 70.63 ± 5.07 | 4.98 ± 0.45 | 92.33 ± 3.73 | 913.6 ± 49.21 | 0.34 ± 0.02 | |
| CS11 | 1.6 | 0.06 | 2.68 ± 1.14 | 0.53 ± 0.13 | 10.56 ± 1.37 | 474.3 ± 54.80 | 0.13 ± 0.07 |
| CS12 | 0.09 | 43.39 ± 3.67 | 5.38 ± 0.42 | 62.45 ± 1.73 | 523.9 ± 18.53 | 0.13 ± 0.02 | |
| CS13 | 0.12 | 71.67 ± 2.56 | 5.52 ± 0.16 | 73.66 ± 2.19 | 578.9 ± 13.01 | 0.06 ± 0.05 | |
| CS14 | 0.18 | 70.48 ± 3.19 | 4.48 ± 0.29 | 83.11 ± 0.47 | 608.5 ± 21.35 | 0.30 ± 0.30 | |
| CS15 | 0.24 | 75.60 ± 3.15 | 4.45 ± 0.08 | 96.49 ± 1.14 | 814.0 ± 36.49 | 0.20 ± 0.10 |
aFinal concentration of CS in nanoparticle suspension
bFinal concentration of TPP in nanoparticle suspension
cMean entrapment efficiency (%). Data shown are the mean ± standard deviation (n = 3)
Influence of Drug Loading
Effect of SER concentration on SER-CS nanoparticles was evaluated. Increasing SER concentration decreased particle size of the nanoparticles from 584.7 ± 31.54 to 543.5 ± 24.89 nm. Anionic SER efficiently cross links with the cationic CS and forms a compact nanoparticles structure, producing dense nanoparticles with less size. Nanoparticle recovery and loading capacity was increased with increasing SER due to increased ionic interactions. However, there was significant decrease in entrapment efficiency which might be due to the presence of free SER.
Drug loading was increased by twofold with varying concentration of SER from 0.1 to 0.4 mg/ml, which was attributed to ionic interaction between CS and SER which facilitated SER loading into nanoparticles.
Effect of pH on Nanoparticle Formation
SER-loaded CS nanoparticles were prepared using ionic gelation method. Effect of pH on physicochemical properties of nanoparticles and drug entrapment was evaluated (Fig. 1b). There was increase in particle size, entrapment, and drug loading with increasing pH of the CS solution. Maximum drug loading was obtained with pH 5.5 CS solution which can be attributed to electrostatic interaction of anionic SER with cationic CS molecule. SER has isoelectric pH value between 5.0 and 5.5. At pH 5.5, it has a net anionic charge which facilitates its interaction with cationic chitosan molecule increasing SER entrapment.
Freeze Drying of Serratiopeptidase Loaded Chitosan Nanoparticles
Selection of Cryoprotectant Using Freeze Thaw Study
Freeze thaw study was carried out with different concentration of various cryoprotectants and effect of freezing stresses on redispersibilty and particle size of nanoparticles was evaluated.
Freeze-dried samples showed concentration-dependent cryoprotection and revealed inverse relation between particle size and concentrations. Large aggregates were clearly observed in nanoparticles frozen without cryoprotectant. Mannitol and lactose failed to protect nanoparticles to the freezing stresses and hence were not used for further freeze drying. Lactose showed formation of reddish brown color due to its Millard reaction with the free amino group of SER. Intensity of reddish brown color increased with lactose concentration. Dextrose (20% w/w) and HP-βCD (10% w/w) showed cryoprotectant effect but nanoparticles had poor redispersibility and showed significant increase in particle size above 700 nm. Trehalose (20% w/w) and sucrose (10% w/w) were found to be better cryoprotectant with reproducible particle size and good redispersibility (Table III).
Table III.
Effect of Concentration of Cryoprotectant (a n = 3) on Particle Size of Nanoparticle
| Cryoprotectant | Cryoprotectant concentration (w/w) | Particle size (nm) | ||
|---|---|---|---|---|
| Before centrifugation | After centrifugation | After freeze thaw | ||
| Plain druga | NA | 570.6 ± 3.4 | 618.12 ± 2.15 | Very large aggregates |
| Mannitol | 5 | 570.6 ± 3.2 | 618.12 ± 2.6 | Very large aggregates |
| 10 | ||||
| 20 | ||||
| 30 | ||||
| Trehalose | 5 | 570.6 ± 3.4 | 618.12 ± 2.32 | 1120.3 ± 4.5 |
| 10 | 675.9 ± 2.7 | |||
| 20 | 641.3 ± 4.3 | |||
| 30 | 776.4 ± 3.2 | |||
| Sucrose | 5 | 570.6 ± 3.4 | 618.12 ± 2.42 | 1135.6 ± 2.5 |
| 10 | 655.5 ± 2.7 | |||
| 20 | 684.2 ± 2.5 | |||
| 30 | 687.9 ± 3.2 | |||
| Dextrose | 5 | 570.6 ± 3.2 | 618.12 ± 2.1 | 1320.3 ± 1.6 |
| 10 | 934.5 ± 2.1 | |||
| 20 | 730.1 ± 2.3 | |||
| 30 | 815.6 ± 2.7 | |||
| HP-BCD | 5 | 570.6 ± 3.6 | 618.12 ± 2.7 | 1105.6 ± 4.2 |
| 10 | 706.8 ± 3.7 | |||
| 20 | 733.8 ± 3.2 | |||
| 30 | 759.5 ± 3.6 | |||
| Lactose | 5 | 570.6 ± 3.2 | 618.12 ± 2.1 | Very large aggregates with reddish brown color. |
| 10 | ||||
| 20 | ||||
| 30 | ||||
aIndicates samples were analyzed in triplicates (n = 3)
Freeze Drying of SER-Loaded CS Nanoparticles
SER-loaded CS nanoparticles were freeze dried using sucrose (10 times w/w) and trehalose (20 times w/w). Nanoparticles lyophilized with sucrose absorbed atmospheric moisture and showed twofold increase in particle size which is attributed to aggregation of nanoparticles caused by dehydration stresses during freeze drying. Inversely, nanoparticles dried using trehalose were less hygroscopic, showed easy redispersibility with no significant increase in the particle size (p < 0.05) on reconstitution (Table IV). The property of trehalose as a preferable cryoprotectant for biomolecules is attributed to its numerous advantages over other sugars such as less hygroscopicity, absence of internal hydrogen bonds allowing flexible formation of hydrogen bonds with nanoparticles during freeze-drying, very low chemical reactivity, and higher glass transition temperature (13,27,28). Therefore, trehalose was selected as a suitable cryoprotectant for solidification of SER-CS nanoparticles.
Table IV.
Particle Size SER-Loaded CS Nanoparticles (n = 3) Before and After Freeze Drying
| Cryoprotectant | Particle size (nm) | |||
|---|---|---|---|---|
| Before centrifugea | After centrifugea | After freeze thawa | After freeze dryinga | |
| Sucrose | 570.6 ± 5.4 | 618.12 ± 3.4 | 655.5 ± 4.76 | 1125.3 ± 5.3 |
| Trehalose | 570.6 ± 6.7 | 618.12 ± 5.3 | 641.3 ± 6.2 | 654.3 ± 5.7 |
aIndicates samples were analyzed in triplicates (n = 3)
In Vitro Dissolution Study
SER-loaded CS nanoparticles showed initial burst for 2 h followed by a sustained release up to 24 h (Fig. 2a). Free peptide adsorbed on the surface of the nanoparticles is released immediately on contact with the dissolution media. SER release was driven by concentration of chitosan polymer and SER. Formulations with CS to TPP weight ratio of 0.8:0.12 mg/ml showed highest initial burst release of 52.24%. Formulations with higher CS and TPP concentration showed more prolonged release of SER. Increase in concentration of CS facilitates its complexation with SER and forms denser CS-SER particles upon interaction with TPP. This high crosslink density has less swelling ability and thus retards the release of SER from the nanoparticles. SER which did not release even after 24 h was due to formation of stable complex of SER with CS.
Fig. 2.
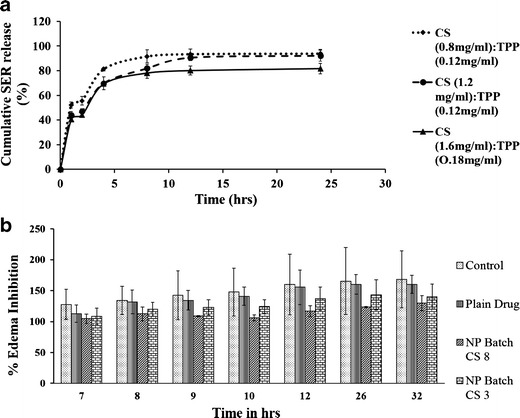
a In vitro release of SER; b % rat paw inhibition
Morphology of SER-CS Nanoparticles
Morphology of SER-loaded CS nanoparticles is depicted in Figs 3 and 4. SEM micrograph of nanoparticles showed clusters of spherical, easily dispersible particles with smooth surfaces and uniform size in agreement with photon spectroscopy analysis.
Fig. 3.
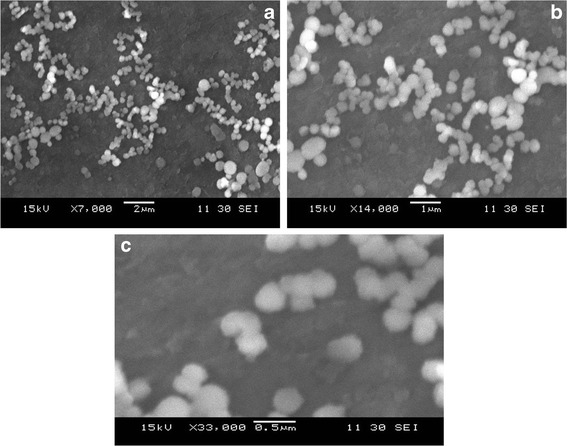
SEM of SER-CS nanoparticles
Fig. 4.
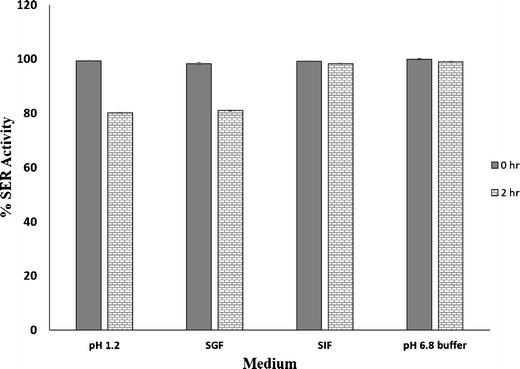
Stability of SER-CS nanoparticles in different pH media (n = 3 with % RSD value of less than 2% in all the media)
In Vitro Stability of SER-CS Nanoparticles
Activity of SER-CS nanoparticles was determined after 0 and 2 h. Assay of SER was performed by Kunitz method. Activity of SER and its stability was determined to investigate effect of various body fluids in vivo. There was no loss of SER activity at 0 h of incubation. SER-CS nanoparticles showed more than 80% activity after 2 h in all the incubation media. This suggested stability of SER when encapsulated into chitosan nanoparticles. SER-CS showed more than 98% activity in pH 6.8 buffer and simulated intestinal fluid (SIF).
In Vivo Pharmacodynamic Evaluation of SER-CS Nanoparticles
Anti-inflammatory properties of SER-CS nanoSER-CS nanoparticulate system showed significantly enhanced anti-inflammatory activity over plain drug (p < 0.05). Plain drug solution and controlled group did not differ in inhibiting edema (p < 0.05). CS 8 and CS 3 formulations were administered to groups III and IV, respectively, (Fig. 2b). Both the CS-based nanoparticles showed enhanced anti-inflammatory activity over plain SER solution. Immediate anti-inflammatory effect of nanoparticulate system was attributed to transmucosal permeation enhancer effect of chitosan, its mucoadhesive property, paracellular and transcelluar transport of the nanoparticles through intestinal mucosa (1,19).
Controlled release of SER, improved stability, mucoadhesive properties, and permeation enhancer effect of chitosan are responsible for improved anti-inflammatory of SER in the form of nanoparticles. Nanoparticles CS 8 and CS 3 were compared for edema inhibition. CS 8 showed increased inhibition of edema, which was attributed to high drug loading, more sustained release, and long-term residence of drug in circulation.
CONCLUSION
Successful encapsulation of serratiopeptidase, a sensitive proteolytic enzyme, was done into chitosan nanoparticles. Ionic gelation method was found to be a simple, mild, and reproducible method for fabrication of nanoparticles. Particle size of the SER-CS nanoparticles can be modulated by varying concentration of CS and TPP. Particle size of 400–600 nm with narrow distribution with more than 80% serratiopeptidase association could be obtained. The electrochemical interaction study showed ionic interaction between CS and serratiopeptidase. Nanoparticles were successfully freeze dried with trehalose (10% w/w) with reproducible particle size on reconstitution. The in vitro dissolution showed initial burst followed by a sustained release up to 32 h. SER-loaded CS nanoparticles showed more pronounced and long-term anti-inflammatory effect. Thus, chitosan nanoparticles can be a novel and efficient alternative to the sensitive peptide molecules like serratiopeptidase.
Acknowledgments
The authors want to acknowledge DBT and UGC for fellowship and AICTE/NAFETIC and Kusum HealthCare for proving laboratory facilities.
Conflict of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.des Rieux A, Fievez V, Garinot M, Schneider Y-J, Préat V. Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. J Control Release. 2006;116(1):1–27. doi: 10.1016/j.jconrel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 2.Sarmento B, Ribeiro A, Veiga F, Sampaio P, Neufeld R, Ferreira D. Alginate/chitosan nanoparticles are effective for oral insulin delivery. Pharm Res. 2007;24(12):2198–206. doi: 10.1007/s11095-007-9367-4. [DOI] [PubMed] [Google Scholar]
- 3.Fd C, Aj T, Dm C, Lq Z, Shi K. Preparation of insulin loaded PLGA-Hp55 nanoparticles for oral delivery. J Pharm Sci. 2007;96(2):421–7. doi: 10.1002/jps.20750. [DOI] [PubMed] [Google Scholar]
- 4.Tasset C, Barette N, Thysman S, Ketelslegers J-M, Lemoine D. Polyisobutylcyanoacrylate nanoparticles as sustained release system for calcitonin. J Control Release. 1995;33(1):23–30. doi: 10.1016/0168-3659(94)00060-8. [DOI] [Google Scholar]
- 5.Santander-Ortega MJ, Csaba N, González L, Bastos-González D, Ortega-Vinuesa JL, Alonso MJ. Protein-loaded PLGA-PEO blend nanoparticles: encapsulation, release and degradation characteristics. Colloid Polym Sci. 2010;288(2):141–50. doi: 10.1007/s00396-009-2131-z. [DOI] [Google Scholar]
- 6.Lin Y-H, Chang C-H, Wu Y-S, Hsu Y-M, Chiou S-F, Chen Y-J. Development of pH-responsive chitosan/heparin nanoparticles for stomach-specific anti- < i > Helicobacter pylori</i > therapy. Biomaterials. 2009;30(19):3332–42. doi: 10.1016/j.biomaterials.2009.02.036. [DOI] [PubMed] [Google Scholar]
- 7.Santander-Ortega M, Bastos-Gonzalez D, Ortega-Vinuesa J, Alonso M. Insulin-loaded PLGA nanoparticles for oral administration: an in vitro physico-chemical characterization. J Biomed Nanotechnol. 2009;5(1):45–53. doi: 10.1166/jbn.2009.022. [DOI] [PubMed] [Google Scholar]
- 8.Patel AR, Kulkarni S, Nandekar TD, Vavia PR. Evaluation of alkyl polyglucoside as an alternative surfactant in the preparation of peptide-loaded nanoparticles. J Microencapsul. 2008;25(8):531–40. doi: 10.1080/02652040802075526. [DOI] [PubMed] [Google Scholar]
- 9.Agnihotri SA, Mallikarjuna NN, Aminabhavi TM. Recent advances on chitosan-based micro-and nanoparticles in drug delivery. J Control Release. 2004;100(1):5–28. doi: 10.1016/j.jconrel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Mazzone A, Catalani M, Costanzo M, Drusian A, Mandoli A, Russo S, et al. Evaluation of Serratia peptidase in acute or chronic inflammation of otorhinolaryngology pathology: a multicentre, double-blind, randomized trial versus placebo. J Int Med Res. 1989;18(5):379–88. doi: 10.1177/030006059001800506. [DOI] [PubMed] [Google Scholar]
- 11.Jadav SP, Patel NH, Shah TG, Gajera MV, Trivedi HR, Shah BK. Comparison of anti-inflammatory activity of serratiopeptidase and diclofenac in albino rats. J Pharmacol Pharmacother. 2010;1(2):116. doi: 10.4103/0976-500X.72362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fereidoni M, Ahmadiani A, Semnanian S, Javan M. An accurate and simple method for measurement of paw edema. J Pharmacol Toxicol Methods. 2000;43(1):11–4. doi: 10.1016/S1056-8719(00)00089-7. [DOI] [PubMed] [Google Scholar]
- 13.MAEJIMA K, MIYATA K, TOMODA K. A manganese superoxide dismutase from Serratia marcescens. Agric Biol Chem. 1983;47(7):1537–43. doi: 10.1271/bbb1961.47.1537. [DOI] [Google Scholar]
- 14.Rinaudo M. Chitin and chitosan: properties and applications. Prog Polym Sci. 2006;31(7):603–32. doi: 10.1016/j.progpolymsci.2006.06.001. [DOI] [Google Scholar]
- 15.Mao H-Q, Roy K, Troung-Le VL, Janes KA, Lin KY, Wang Y, et al. Chitosan-DNA nanoparticles as gene carriers: synthesis, characterization and transfection efficiency. J Control Release. 2001;70(3):399–421. doi: 10.1016/S0168-3659(00)00361-8. [DOI] [PubMed] [Google Scholar]
- 16.Tokumitsu H, Ichikawa H, Fukumori Y. Chitosan-gadopentetic acid complex nanoparticles for gadolinium neutron-capture therapy of cancer: preparation by novel emulsion-droplet coalescence technique and characterization. Pharm Res. 1999;16(12):1830–5. doi: 10.1023/A:1018995124527. [DOI] [PubMed] [Google Scholar]
- 17.Mitra S, Gaur U, Ghosh P, Maitra A. Tumour targeted delivery of encapsulated dextran-doxorubicin conjugate using chitosan nanoparticles as carrier. J Control Release. 2001;74(1):317–23. doi: 10.1016/S0168-3659(01)00342-X. [DOI] [PubMed] [Google Scholar]
- 18.Calvo P, Remunan-Lopez C, Vila-Jato J, Alonso M. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J Appl Polym Sci. 1997;63(1):125–32. doi: 10.1002/(SICI)1097-4628(19970103)63:1<125::AID-APP13>3.0.CO;2-4. [DOI] [Google Scholar]
- 19.Gan Q, Wang T. Chitosan nanoparticle as protein delivery carrier—systematic examination of fabrication conditions for efficient loading and release. Colloids Surf B: Biointerfaces. 2007;59(1):24–34. doi: 10.1016/j.colsurfb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Aktaş Y, Andrieux K, Alonso MJ, Calvo P, Gürsoy RN, Couvreur P, et al. Preparation and in vitro evaluation of chitosan nanoparticles containing a caspase inhibitor. Int J Pharm. 2005;298(2):378–83. doi: 10.1016/j.ijpharm.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 21.Katas H, Alpar HO. Development and characterisation of chitosan nanoparticles for siRNA delivery. J Control Release. 2006;115(2):216–25. doi: 10.1016/j.jconrel.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 22.Rudzinski WE, Aminabhavi TM. Chitosan as a carrier for targeted delivery of small interfering RNA. Int J Pharm. 2010;399(1):1–11. doi: 10.1016/j.ijpharm.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 23.Gan Q, Wang T, Cochrane C, McCarron P. Modulation of surface charge, particle size and morphological properties of chitosan—TPP nanoparticles intended for gene delivery. Colloids Surf B: Biointerfaces. 2005;44(2):65–73. doi: 10.1016/j.colsurfb.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Csaba N, Köping-Höggård M, Alonso MJ. Ionically crosslinked chitosan/tripolyphosphate nanoparticles for oligonucleotide and plasmid DNA delivery. Int J Pharm. 2009;382(1):205–14. doi: 10.1016/j.ijpharm.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 25.Kumbhar D, Wavikar P, Vavia P. Niosomal Gel of Lornoxicam for topical delivery: in vitro assessment and pharmacodynamic activity. AAPS PharmSciTech. 2013;14(3):1072–82. doi: 10.1208/s12249-013-9986-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crystalline KM, Desoxyribonuclease I. Isolation and general properties spectrophotometric method for the measurement of desoxyribonuclease activity. J Gen Physiol. 1950;33(4):349–62. doi: 10.1085/jgp.33.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdelwahed W, Degobert G, Stainmesse S, Fessi H. Freeze-drying of nanoparticles: formulation, process and storage considerations. Adv Drug Deliv Rev. 2006;58(15):1688–713. doi: 10.1016/j.addr.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 28.Date PV, Samad A, Devarajan PV. Freeze thaw: a simple approach for prediction of optimal cryoprotectant for freeze drying. AAPS PharmSciTech. 2010;11(1):304–13. doi: 10.1208/s12249-010-9382-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


