Abstract
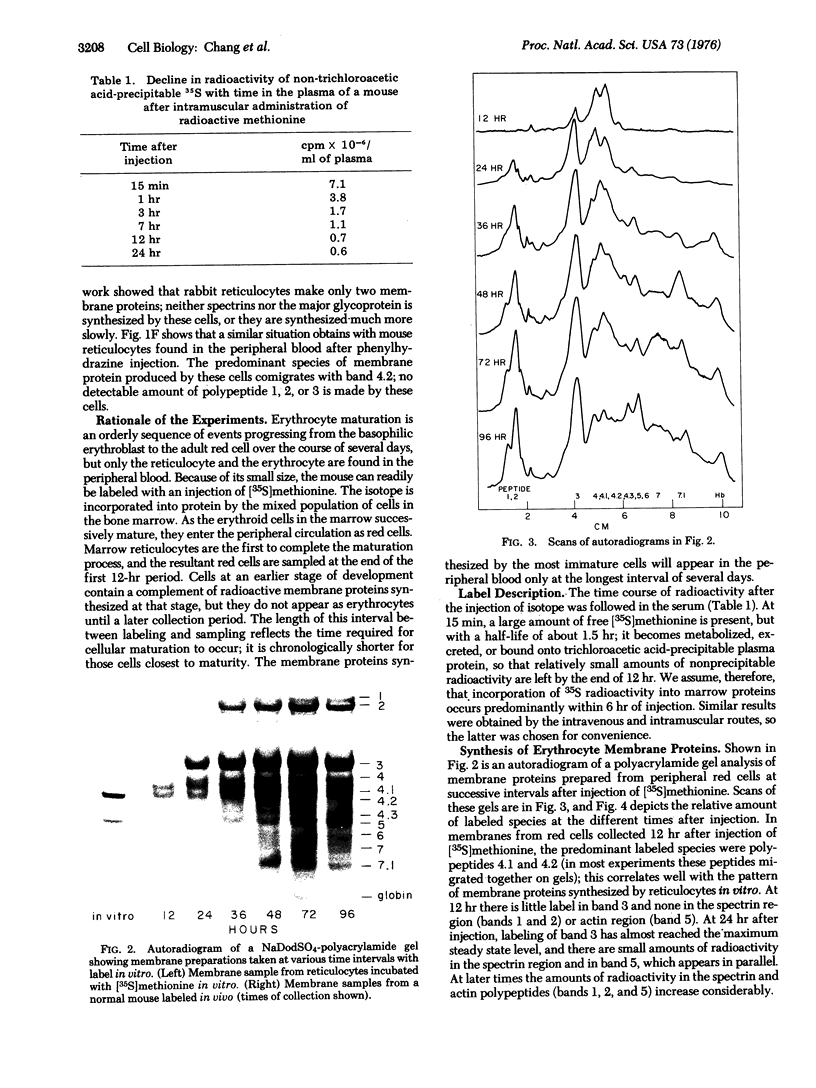
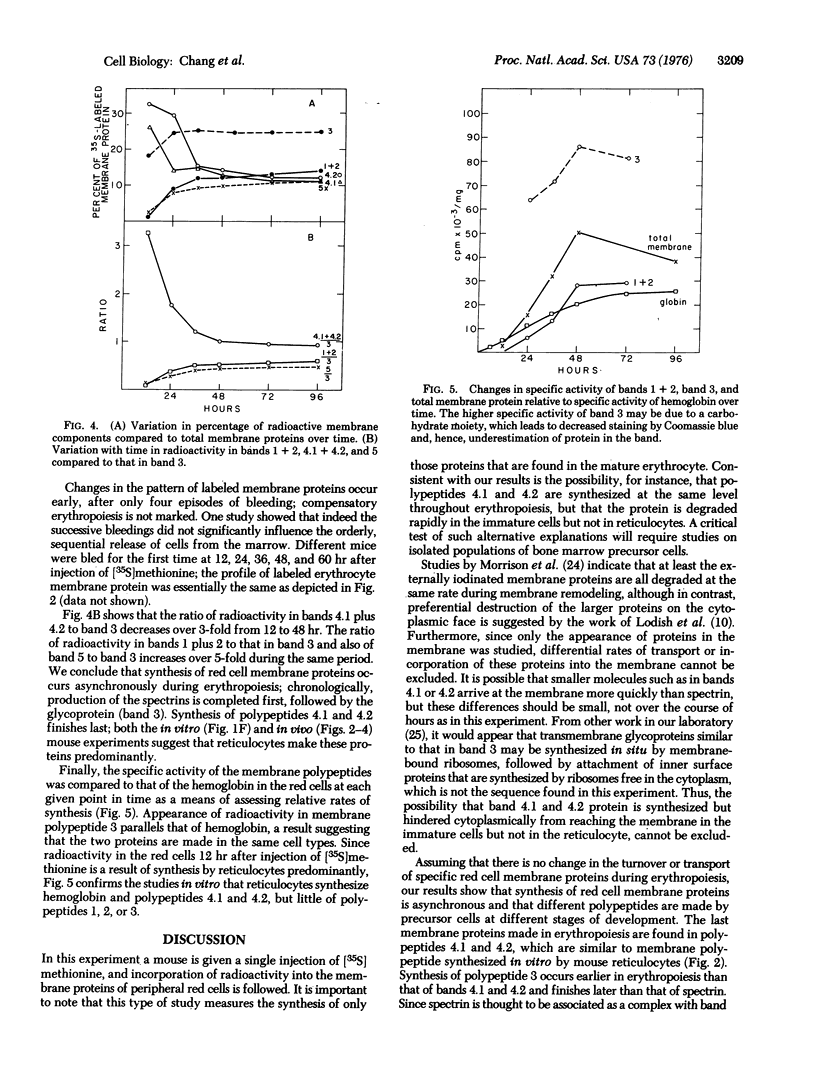
The synthesis of membrane proteins of the mature mouse erythrocyte is asynchronous. During erythropoiesis, synthesis of the bulk of the spectrin and actin polypeptides is completed before that of the major transmembrane glycoprotein. Synthesis of the glycoprotein ceases before that of several minor proteins found on the inner surface of the red cell membrane, and one of these minor proteins is made predominantly by reticulocytes. These findings were the result of experiments in which a normal mouse was given a single injection of [35S]methionine. The appearance of radioactivity in the membrane proteins of circulating mature erythrocytes was followed. The earliest labeled proteins to emerge into the blood represent those synthesized at the last stages of erythropoiesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. Membrane structure: some general principles. Science. 1973 Aug 17;181(4100):622–629. doi: 10.1126/science.181.4100.622. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Guidotti G. Membrane proteins. Annu Rev Biochem. 1972;41:731–752. doi: 10.1146/annurev.bi.41.070172.003503. [DOI] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. The enzymatic iodination of the red cell membrane. J Cell Biol. 1972 Nov;55(2):390–405. doi: 10.1083/jcb.55.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano R. L. The proteins of the erythrocyte membrane. Biochim Biophys Acta. 1973 Dec 28;300(4):341–378. doi: 10.1016/0304-4157(73)90013-0. [DOI] [PubMed] [Google Scholar]
- Koch P. A., Gardner F. H., Carter J. R., Jr Red cell maturation: loss of a reticulocyte-specific membrane protein. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1296–1299. doi: 10.1016/0006-291x(73)91128-5. [DOI] [PubMed] [Google Scholar]
- Koch P. A., Gardner F. H., Gartrell J. E., Jr, Carter J. R., Jr Biogenesis of erythrocyte membrane proteins. In vitro studies with rabbit reticulocytes. Biochim Biophys Acta. 1975 Apr 21;389(1):177–187. doi: 10.1016/0005-2736(75)90395-8. [DOI] [PubMed] [Google Scholar]
- Koch P. A., Gartrell J. E., Jr, Gardner F. H., Carter J. R., Jr Biogenesis of erythrocyte membrane proteins. In vivo studies in anemic rabbits. Biochim Biophys Acta. 1975 Apr 21;389(1):162–176. doi: 10.1016/0005-2736(75)90394-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lodish H. F. Biosynthesis of reticulocyte membrane proteins by membrane-free polyribosomes. Proc Natl Acad Sci U S A. 1973 May;70(5):1526–1530. doi: 10.1073/pnas.70.5.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F., Small B. Membrane proteins synthesized by rabbit reticulocytes. J Cell Biol. 1975 Apr;65(1):51–64. doi: 10.1083/jcb.65.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison M., Michaels A. W., Phillips D. R., Choi S. I. Life span of erythrocyte membrane protein. Nature. 1974 Apr 26;248(5451):763–p. doi: 10.1038/248763a0. [DOI] [PubMed] [Google Scholar]
- Morrison T. G. Site of synthesis of membrane and nonmembrane proteins of vesicular stomatitis virus. J Biol Chem. 1975 Sep 10;250(17):6955–6962. [PubMed] [Google Scholar]
- SCHOELLMANN G., SHAW E. Direct evidence for the presence of histidine in the active center of chymotrypsin. Biochemistry. 1963 Mar-Apr;2:252–255. doi: 10.1021/bi00902a008. [DOI] [PubMed] [Google Scholar]
- Singer S. J. The molecular organization of membranes. Annu Rev Biochem. 1974;43(0):805–833. doi: 10.1146/annurev.bi.43.070174.004105. [DOI] [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., Detmers P. Actin in erythrocyte ghosts and its association with spectrin. Evidence for a nonfilamentous form of these two molecules in situ. J Cell Biol. 1975 Sep;66(3):508–520. doi: 10.1083/jcb.66.3.508. [DOI] [PMC free article] [PubMed] [Google Scholar]