Abstract
Self-emulsifying pellets were prepared using microcrystalline cellulose, emulsions of caprylic/capric triglyceride, and three Cremophors (ELP, RH40, and RH60) at 1.5 and 2.3 weight ratios, and two drugs (furosemide and propranolol) of different lipophilicity. Droplet size, zeta potential (ζ) and viscosity of emulsions, and pellet size, shape, friability, tensile strength, disintegration, and drug migration in pellets were determined. Evaluation of reconstituted emulsions was based on droplet size and ζ. Factorial design and 3-way ANOVA was applied to estimate the significance of the effects of the drug, surfactant and oil/surfactant ratio. It was found that droplet size, viscosity and ζ of emulsions, and size, shape, and friability of pellets were affected by the studied factors and were significant interactions between their effects on pellet size and friability. Migration of drug towards the pellet surface was higher for the less lipophilic furosemide and higher oil content. Linear relationships were found between the emulsion viscosity and the shape parameters of the pellets (for the aspect ratio R2 = 0.796 for furosemide and R2 = 0.885 for propranolol and for the shape factor, eRR2 = 0.740 and R2 = 0.960, respectively). For all the formulations examined, an exponential relationship was found between migration (M%) and the product of viscosity (η) and solubility of drug in oil/surfactant mixture (S) (M% = 98.1e-0.016 [η•S], R2 = 0.856), which may be useful in formulation work.
KEY WORDS: drug distribution, emulsion and pellet characterization, friability and tensile strength, furosemide and propranolol, self-emulsifying pellets
INTRODUCTION
Self-emulsifying pharmaceutical pellets combine the advantages of emulsions and multi-particulate solid dosage forms, namely improved absorption of lipophilic drugs with lower variability in gastric transit time (independently of nutrition state) and better stability in the gastric fluids and easier application of coatings for GI track targeting (1–7). It has been reported that self-emulsifying pellets do release the drug in the dog GI tract, as if they were the emulsion liquid itself (8). Furthermore, it has been found that bioavailability of poorly water soluble drugs is improved when they are administered as self-emulsifying pellets, although there are no such products yet on the market (9,10).
Self-emulsifying pellets can be prepared by incorporating self-emulsifying mixtures (oil/surfactant/drug) in the form of o/w emulsions in microcrystalline cellulose (MCC) during the process of wet massing, before extrusion–spheronization. It has been reported that with surfactants of higher hydrophilicity the massing liquid requirements for pelletization increase and faster disintegration of the self-emulsifying MCC pellets is achieved, while the reconstitution of emulsion is facilitated with higher oil/surfactant ratios (11,12).
Until now, the effect of the drug nature on the properties of self-emulsifying drug delivery systems has received relatively little attention and even fewer literature reports appear for self-emulsifying pellets (13,14). From the available reports it appears that the presence of drug in the oily phase of o/w emulsions affects their droplet size and stability, with maximum destabilization occurring near drug saturation (15,16). Furthermore, the presence of the drug may affect the viscosity of the emulsions through its effect on the droplet diameter and its presence on the oil/water interface (17) which in turn may affect agglomeration during wet massing of MCC with the emulsion, the rheological properties of the resulting wet mass, and the properties of the final pellets (18). In addition, the presence of any free drug in the aqueous phase of the emulsion and its migration during drying, towards the surface of the pellets, may alter the distribution of the drug in the pellet (19–21). So far, there is little bibliographic information on this point and no data at all for the distribution (migration) of drugs in self-emulsifying pellets, although previously published results on the kinetics of drug release and emulsion reconstitution, have indicated the operation of a bi-phasic release process, a fast initial and a slow terminal release attributed to migration of oil/surfactant/drug mixture during drying of pellets (22).
Therefore, the aim of the present work was to expand the already studied effects of formulation variables (drug lipophilicity, surfactant type, and oil/surfactant ratio) on the properties of the emulsions used for the preparation of MCC pellets (droplet size, ζ, and viscosity) and elucidate possible relationships between them and the properties of the produced self-emulsifying pellets (micromeritic, mechanical, and drug migration). Furosemide and propranolol that belong to BCS class IV and II, respectively, and of similar aqueous solubility (0.063 and 0.051 mg/ml at 25°C) but different lipophilicity (log P 2.0 and 3.5) were the drugs, medium chain triglycerides the oil and Cremophors ELP, RH40, and RH60, the surfactants, employed at oil/surfactant ratios of 1.5 and 2.3 previously found to give good reconstitution of emulsions from the self-emulsifying pellets (12,22).
MATERIALS AND METHODS
Materials
Microcrystalline cellulose (Avicel® PH-101, lot 6950C, FMC Ireland) was used as the pellet forming material. Furosemide (FS) and propranolol base (PR) were selected as the active pharmaceutical ingredients. Furosemide (Batch 9033HRII) from Ipca (Mumbai, India) was donated by Help Hellas (Athens, Greece) and propranolol base was prepared by reacting 20% w/w propranolol hydrochloride with 3% w/w sodium bicarbonate water solutions, followed by filtration and drying (23). Medium chain triglycerides of caprylic/capric esters; C8: 59.6%, C10: 39.9%, C14 0.4% (Radia 7104, Oleon N.V., Oelegen, Belgium), and glyceryl polyethylene glycol ricinoleate (Cremophor ELP) or glyceryl polyethylene glycol oxystearate (Cremophors RH 40 and RH 60), donated by BASF (Ludwigshafen, Germany), were used as the oil and the surfactant components of the self-emulsifying liquid mixture. Distilled water was used as the external phase of the emulsions employed as massing liquids during pellet preparation.
Emulsion Preparation and Characterization
Appropriate amounts of oil and surfactant were mixed at ratios 1.5 and 2.3 and then drug (2% w/w) was added to them. The mixtures were heated at 50°C, until clear solutions were formed. Then, o/w emulsions used as massing liquids for the preparation of pellets were made by 1/4 dilution containing 25% (w/w) of oil/surfactant/drug mixture and 75% deionized water and their viscosity was measured. For the determination of droplet size and ζ of the emulsions, a higher dilution of oil/surfactant mixture in water (1/10) was applied to avoid multiple scattering effects.
Droplet Size
The droplet size was measured in triplicate using the Dynamic Light Scattering sizer (Zetasizer, ZEN3600, Malvern Instruments Ltd, Worcestershire, UK) and expressed as the average hydrodynamic diameter derived from the change of intensity of scattered light with time (laser 633 nm, detection angle 173°).
Zeta Potential (ζ)
Emulsions were placed in the electrophoretic cell of the above mentioned Zetasizer and ζ, which represents the charge on the surface of the droplets, was obtained from the electrophoretic mobility using the M3-PALS technique which is a combination of Malvern’s laser Doppler velocimetry method (M3 measurement technique) and the Phase Analysis Light Scattering technique (PALS).
Viscosity
Kinematic viscosity was measured with an Ubbelohde glass capillary viscometer (Schott Geräte, Mainz, Germany) of internal diameter 0.63 mm, taking as reference the viscosity of water at 25°C (η = 0.89 cST (centistokes, mm2/s)) and the efflux time for water (81 ± 0.1 s). Measurement was applied to inert emulsions without drug, which were not used for pellet preparation, and, for the twelve emulsions containing drug. To convert the values to cP (centipoise, g·s/cm2), multiplication by the average density, calculated from the density of the oil (0.95 g/cm3) and that of the surfactants (1.05 g/cc, Handbook of Pharmaceutical Excipients), was applied.
Pellet Preparation and Characterization
For the preparation of pellets by extrusion/spheronization, 30 g batches of MCC powder were mixed with 30 g emulsions (75% water and 25% w/w oil/surfactant/drug self-emulsifying mixture) for about 5 min in a 1-L cylindrical mixing vessel, fitted with a three-blade impeller (22). The emulsions had milky appearance except for those containing FS and oil/surfactant ratio 1.5, which gave clear (RH40) or slightly turbid, translucent emulsions. Further addition of small amounts of 2 to 5 g of water was required to obtain most spherical pellets. The resulting wet mass was extruded in a radial extruder (Model 20, Caleva Process Solutions, Dorset, UK) operated at 25 rpm and fitted with a 1-mm circular orifice and 1.75 mm thickness screen. The extrudate was immediately processed for 10 min at 1,360 rpm corresponding to a linear perimeter speed of 8.55 m/s in a spheronizer (Model 120, Caleva Process Solutions, Dorset, UK) fitted with a cross-hatch friction plate. The produced pellets were dried for 12 h in an air-circulation tray oven (UT6, Heraeus Instruments, Hanau, Germany) at 40°C. The final dry pellets contained 0.4% drug, 19.6% oil/surfactant mixture, and 80% MCC and their properties were evaluated as follows:
Size
Size distribution was determined by placing approximately 10 g of pellets on a stack of 10 cm diameter sieves of 300, 425, 600, 850, and 1,200 μm aperture (DIN/ISO3310-1, Retch, Haan, Germany) and vibrating for 10 min (Fritsch Analysette 3, Oberstein, Germany). Median pellet diameter was derived from cumulative weight plots of the weight of remaining pellets on each sieve.
Shape
Pellet shape was determined by using an image processing and analysis system comprised of stereomicroscope, top cold light source (Olympus SZX9, Japan and Highlight 3,100, Olympus Optical), video camera (VC-2512, Sanyo Electric, Japan), and software (Quantimet 500, Cambridge, England). About 100 pellets were examined, in 3–4 optical fields, at a total magnification of 6.5 × 5 = 32.5. The shape was expressed as fullness index [FI% = 100 × (actual surface area − convex surface area)/actual surface area] which represents the surface irregularity, as aspect ratio (AR = length/breadth) and as shape factor eR which combines both geometrical and surface irregularity (24). All shape parameters tend towards unity with increasing sphericity.
Friability
Friability was determined in a granule friabilator (Copley Scientific, type FRV 2000, Nottingham, UK) rotated at a speed of 25 rpm, for 10 min. Pellets (10 g) of the size fraction 600–1,200 μm were placed in the drum together with 20 g, 4 mm, glass beads. After rotation, the fines produced due to the shocks of the falling pellets were separated by sieving through a 600-μm sieve, for 5 min at 2 mm vibration amplitude (Fritsch Analysette, Oberstein, Germany). Friability, FR%, was calculated from the weight difference of the pellets remaining on the sieve (Wf) compared to initial pellet weight (Wo) expressed as percentage:
| 1 |
Tensile Strength
Pellets from the 1,000–1,200 μm fraction were diametrically loaded by using a modified CT-5 testing machine (Engineering System, Nottingham, UK), at an upper platen speed of 1 mm/min. The tester was fitted with a 25 N load cell (model ELFM-T2M, Entran, USA) connected to a signal amplifier (RDP E308, UK). The signal was collected with a polymeter (Handyscope, Holland) and recorded in Microsoft Excel files. The tensile strength, σt, was calculated from the breaking force, F, and the pellet radius, R, by using the equation (25):
| 2 |
Disintegration
The disintegration time of pellets, 1,000–1,200 μm in diameter, was determined in a modified reciprocating cylinder USP Apparatus 3 (Bio-Dis RRT9, G.B. Caleva, UK) as described previously (12).
Drug Migration
Pellets (5 g) of the size fraction 850–1,200 μm were placed together with 20 g, 4 mm glass beads, in 250 mL specially modified cylindrical glass jars in order to separate surface layers from the pellets and estimate drug content. The interior surface of the jars was covered by glued sand paper type P800 (FEPA classification) corresponding to about 16 μm grain size, which was selected after preliminary trials on the basis of the yield of outer layer removal from pellets. The jars were mounted on a Turbula mixer (Type T2C, Willy Bachofen AG, Basel Switzerland) and tumbled at a speed of 90 rpm. At 3-min intervals, the fines (<212 μm) were removed by sieving the jar content and tumbling was continued until collecting more than 250 mg fine powder.
The drug content was determined in the two size fraction (<212 and >212 μm) applying a validated UV spectrophotometric method in five samples as follows. Samples (50 mg) corresponding to 200 μg drug (nominal amount) were mixed for 5 min with 40 mL methanol, centrifuged for 3 min at 4,500 rpm (Heraeus Labofouge 400R, Thermo Electron Corporation, Osterode, Germany), and the concentration of extracted drug in the supernatant was determined at 272 and 290 nm for FS and PR, respectively. The extraction process and UV determination was repeated once with 20 and then with 10 mL methanol until absorbance lower than 0.003 was obtained.
Validation of the method was based on linearity between absorbance, A, and concentration, C, (R2 > 0.999, in the range 0.5–20.0 μg/mL and 1.0–50.0 μg/mL for FS and PR, respectively). The relationships between concentration and absorbance were: C = 16.8067 × A + 0.4403 and C = 42.9185 × A + 0.4206. Considering the sensitivity of the spectrophotometer as A = 0.001, the lower limit of detection (LLOD) was 0.45 and 0.55 μg/mL and the lower limit of quantification LLOQ was 1.0 and 1.5 μg/mL for FS (A = 0.034) and PR (A = 0.026), respectively, which corresponds to coefficient of variation <2% (for five samples).
From the drug content in the two fractions (Douter and Dinner) the drug migration (M%) was expressed as:
| 3 |
On the basis of LLOQ (1.0 μg/mL for FS and 1.5 μg/mL for PR), any difference (Douter − Dinner) greater than 0.5% for FS and 0.75% for PR will be valid.
Reconstituted Emulsions
The evaluation of reconstituted emulsions was based on the determination of droplet size and zeta potential as already described for the emulsions used as wet massing liquids in the preparation of pellets. The reconstitution was evaluated using a USP II dissolution apparatus (Pharma-Test, Hainburg, Germany) by adding 3 g pellets corresponding to 0.6 g of oil/surfactant/drug mixture in 200 mL distilled water, at 37 ± 0.5°C, and stirring for 3 h at 100 rpm. These conditions ensured complete reconstitution for most pellet batches (22).
Experimental Design and Statistical Analysis
A mixed level full factorial, completely randomized design (12 runs) with two factors at two levels (drug lipophilicity and oil/surfactant ratio) and one factor at three (type of surfactant), was employed to study their effects on the properties of emulsions and pellets. The prepared batches according to the experimental design are shown in Table I.
Table I.
Mixed Level Full Factorial Experimental Design
| Formulation | Drug | Surfactant | Oil/surfactant ratio |
|---|---|---|---|
| 1 | FS | ELP | 1.5 |
| 2 | FS | ELP | 2.3 |
| 3 | FS | RH40 | 1.5 |
| 4 | FS | RH40 | 2.3 |
| 5 | FS | RH60 | 1.5 |
| 6 | FS | RH60 | 2.3 |
| 7 | PR | ELP | 1.5 |
| 8 | PR | ELP | 2.3 |
| 9 | PR | RH40 | 1.5 |
| 10 | PR | RH40 | 2.3 |
| 11 | PR | RH60 | 1.5 |
| 12 | PR | RH60 | 2.3 |
For the statistical analysis, a 3-way analysis of variance (ANOVA) was applied and the statistical index adjusted coefficient of determination (Ra2) was used, representing the proportion of the variability in the data explained by the ANOVA, after adjusting for the number of experiments and the number of variables (26). Furthermore, pairwise comparison of means (2-tailed t test) was applied for the estimation of the significance of the differences in the properties of emulsions due to the addition of drugs. For the analysis of the data, the SPSS 20.0 statistical software was used (IBM SPSS Statistics, Inc., Chicago, IL USA). Effects were considered significant at p value <0.05, less significant at p between 0.05 and 0.10, and without significance at p > 0.10.
RESULTS AND DISCUSSION
The physicochemical properties of drugs, surfactants and oil are summarized in Table II. From the physicochemical properties, it can be seen that the aqueous solutions of FS and PR have high surface tensions (64 and 66 mN/m) indicating minor surface activity compared to the surface tensions of surfactants (41.3–44.8 mN/m). The molecular size of drugs is similar (330.8 and 259.3), but they have different lipophilicities (log P 2.0 and 3.5, respectively) and different solubilities in the oil/surfactant mixtures (24.7–63.2 mg/mL; ref. 22). Therefore, the drugs are expected to be distributed differently between the droplets and the external aqueous phase (15). Similarly, the surfactants employed are expected to be distributed differently in the oil/water interface, due to their different hydrophilicity (HLB 13.9–15.7), and also to present different excess surface concentration in the water/air interface at the pellet surface during drying (Fig. 1). Consequently, the combinations of different drug and surfactant types in altered proportions are expected to influence the formation of emulsions and the properties of the self-emulsifying pellets (12,13).
Table II.
Physicochemical properties of drugs, surfactants and oil
| Material | Molecular weight | Log P | HLBe | m.p. (°C) | Surface tension (mN/m) |
|---|---|---|---|---|---|
| Drugs | |||||
| Furosemide | 330.8 a | 2.0 d | n/a | 219 a | 64.0 (1.0) g |
| Propranolol | 259.3 a | 3.5 e | n/a | 95 a | 66.0 (1.0) g |
| Surfactants | |||||
| Cremophor ELP | 2,500 b | n/a | 13.9 | 20 c | 41.3 (0.3) g |
| Cremophor RH40 | 2,500 b | n/a | 14.3 | 30 c | 44.0 (0.1) g |
| Cremophor RH60 | 2,800 b | n/a | 15.7 | 30c | 44.8 (0.3) g |
| Oil | |||||
| Medium chain triglyceride | 498 c | n/a | n/a | −20 c | 31.1 c |
aMerck index
bBASF Technical Information May 2010
cHandbook of Pharmaceutical Excipients
dAlvarez-Nunez and Yalkowsky (31)
eAvdeef et al. (29)
fMatsaridou et al. (12)
gSaturated drug and 0.1% w/v surfactant solutions in distilled water measured after filtration (0.1 μm) with a du Nuoy balance (Krüss 8600, Germany)
Fig. 1.
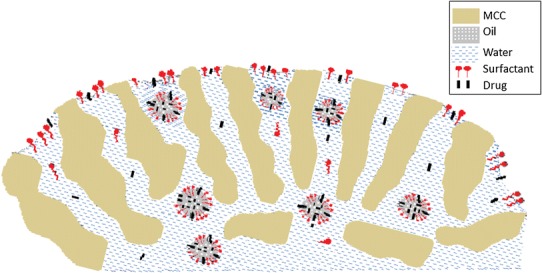
Schematic representation of drug, surfactant and oil in the MCC pellets
Effect of Drug Incorporation on Emulsion Properties
In Fig. 2a–c, the properties of emulsions without (inert) and with drug are compared. It can be seen (Fig. 2a) that the presence of FS decreases the droplet diameter in all cases (pairwise comparison of means, t test, p = 0.009), whereas that of PR decreases the diameter of emulsions with ELP and RH40 but not with RH60. Since the surface activity of the drugs is minor compared to the surfactants, the decrease in droplet diameter caused by the presence of drug implies a co-surfactant drug effect.
Fig. 2.

Comparison of a droplet diameter, b zeta potential, and c viscosity for inert and drug-loaded emulsions used for the preparation of pellets
Regarding the zeta potential (ζ), it can be seen in Fig. 2b that for inert emulsions it is negative due to the free fatty acids present in the oil which confirms previously reported data for self-emulsifying systems with nonionic surfactants (13,27). In the case of inert emulsions with RH60, the zeta potential is less negative compared to the other surfactants probably due to their generally greater droplet diameter and its influence on droplet mobility (Fig. 1a and ref. 12). Interestingly, in Fig. 2b, it can be seen that ζ changes significantly due to the presence of drug, negatively for furosemide and positively for propranolol. These ζ changes should be related to the presence of drug in the oil/water interface affected by both, the surfactant type and the oil/surfactant ratio.
More specifically, for furosemide which is a weak acid (pKa = 4.25), its carboxylic acid ionizes in the aqueous solution giving negatively charged carboxylate and negative overall droplet surface charge, which explains the decrease of zeta potential. Conversely, the characteristic turning to positive values by propranolol should arise from its basic character due to the presence of amino group (−NH, pKa = 9.53) which neutralize the free fatty acids present in the oil of the self-emulsifying mixture (28).
Regarding the emulsion viscosity, it can be seen from Fig. 2c that in all cases, incorporation of drugs causes an increase. This can be attributed to the presence of drugs in the oil/water interface and the consequent alteration in the surrounding the droplet hydrated layer and hence in the shearing resistance (29). In general, FS addition gave greater viscosity than PR, which is in agreement with its higher hydrophilicity and therefore increased presence in the oil/water interface (15). Pairwise comparison of means, t test, gave p = 0.012 for FS and p = 0.006 for PR. The higher viscosity seen in Fig. 2c for formulation with Cremophor RH40 with oil/surfactant ratio 1.5 and furosemide in comparison with the formulation with ratio 2.3 is associated with the transparent appearance of the emulsion and its lower average droplet diameter (part of Pellet preparation in Materials and Methods and Table III). Formation of transparent dispersions of RH40 with medium chain triglycerides was also previously observed for emulsions without drug (12).
Table III.
Droplet diameter, polydispersity index, and zeta potential of added and reconstituted emulsions together with the viscosity of added emulsions [mean, (SD), n = 3]
| Cremophor/ratio | Added emulsions | Reconstituted emulsions | Viscosity (cP) | ||||
|---|---|---|---|---|---|---|---|
| dv (nm) | PDI | ζ (mV) | dv (nm) | PDI | ζ (mV) | ||
| Furosemide | |||||||
| ELP/1.5 | 240 (4) | 0.264 | −1.4 (2.7) | 494 (43) | 0.406 | −25.7 (0.9) | 4.94 (0.00) |
| ELP/2.3 | 291 (4) | 0.285 | −1.3 (0.9) | 562 (40) | 0.468 | −27.9 (1.8) | 3.98 (0.03) |
| RH40/1.5 | 182 (1) | 0.283 | −1.6 (3.3) | 303 (16) | 0.488 | −24.3 (0.6) | 7.75 (0.08) |
| RH40/2.3 | 302 (4) | 0.411 | −1.7 (0.4) | 632 (74) | 0.498 | −27.8 (0.4) | 4.98 (0.02) |
| RH60/1.5 | 227 (4) | 0.262 | −2.4 (1.4) | 533 (30) | 0.447 | −28.5 (1.1) | 4.86 (0.03) |
| RH60/2.3 | 310 (18) | 0.290 | −1.8 (0.5) | 643 (69) | 0.487 | −28.2 (0.8) | 4.25 (0.01) |
| Propranolol | |||||||
| ELP/1.5 | 222 (4) | 0.236 | 0.6 (0.2) | 467 (52) | 0.381 | −19.2 (0.4) | 3.89 (0.00) |
| ELP/2.3 | 259 (4) | 0.205 | 0.5 (0.2) | 596 (119) | 0.447 | −22.6 (0.2) | 2.93 (0.03) |
| RH40/1.5 | 200 (2) | 0.236 | 0.4 (1.0) | 552 (8) | 0.407 | −11.6 (0.3) | 4.94 (0.02) |
| RH40/2.3 | 263 (6) | 0.212 | 1.2 (1.4) | 349 (10) | 0.452 | −13.4 (0.4) | 3.97 (0.01) |
| RH60/1.5 | 311 (7) | 0.371 | 0.0 (3.2) | 401 (39) | 0.418 | −15.2 (0.3) | 4.56 (0.01) |
| RH60/2.3 | 499 (20) | 0.406 | 0.6 (0.4) | 405 (96) | 0.411 | −14.7 (0.7) | 4.20 (0.03) |
dv average hydrodynamic droplet diameter, PDI polydispersity index, ζ zeta potential
Effect of Surfactant Type and Ratio on Droplet Size, Zeta Potential, and Viscosity
In Table III are presented the results of droplet diameter and polydispersity index (PDI) and of ζ for the added to the MCC during pellet preparation and for the reconstituted emulsions, together with the viscosity of the added emulsions. Viscosity of the added emulsions was evaluated in order to be compared with the pellet properties. In Table IV are given the results of ANOVA for the main effects and interactions of the studied factors on the properties of added emulsions and self-emulsifying pellets.
Table IV.
ANOVA of the main effects and interactions of the type of drug (A), the type of surfactant (B), and the oil/surfactant ratio (C) on the properties of added emulsions and self-emulsifying pellets
| Property | Main and effects | Interactions | R a 2 | ||||
|---|---|---|---|---|---|---|---|
| A | B | C | A × B | B × C | A × C | ||
| Added emulsions | |||||||
| Droplet diam. (dv) | 0.333 | 0.135 | 0.070 | 0.164 | 0.413 | 0.568 | 0.742 |
| ζ-potential (ζ) | 0.006 | 0.213 | 0.202 | 0.481 | 0.486 | 0.562 | 0.943 |
| Viscosity (η) | 0.066 | 0.096 | 0.059 | 0.240 | 0.324 | 0.349 | 0.822 |
| Pellets | |||||||
| Median diameter | 0.025 | 0.016 | 0.614 | 0.049 | 0.104 | 0.191 | 0.951 |
| Fullness index (FI%) | 0.329 | 0.491 | 0.213 | 0.320 | 0.287 | 0.185 | 0.503 |
| Aspect ratio (AR) | 0.121 | 0.254 | 0.201 | 0.250 | 0.535 | 0.405 | 0.594 |
| Shape factor (eR) | 0.059 | 0.096 | 0.054 | 0.110 | 0.317 | 0.148 | 0.861 |
| Friability (%) | 0.021 | 0.028 | 0.012 | 0.046 | 0.169 | 0.044 | 0.959 |
| Tensile strength | 0.244 | 0.688 | 0.123 | 0.081 | 0.126 | 0.142 | 0.797 |
| Migration% | 0.132 | 0.473 | 0.204 | 0.822 | 0.972 | 0.620 | 0.248 |
| Disintegration time | 0.649 | 0.624 | 0.633 | 0.389 | 0.586 | 0.236 | 0.048 |
| Reconstituted emulsions | |||||||
| Droplet diameter (dv) | 0.025 | 0.259 | 0.095 | 0.084 | 0.777 | 0.641 | 0.858 |
| ζ-potential (ζ) | 0.013 | 0.112 | 0.177 | 0.212 | 0.373 | 0.511 | 0.901 |
Numbers in bold correspond to statistical significance p < 0.05 and numbers in italics to statistical significance p between 0.05 and 0.100
Droplet Size
Comparing the droplet diameters of added to the MCC emulsions with those of the corresponding reconstituted, it can be seen that they are significantly smaller, except in the case of propranolol with RH60 at oil/surfactant ratio 2.3. The range of PDI values increase from 0.205–0.406 for the added emulsions (the majority between 0.205 and 0.290) to 0.381–0.498 for the reconstituted, which is still under 0.500, thus, allowing comparison of the droplet diameters of the different formulations (30). The increase in reconstituted emulsions is by about a factor of 2, although still in the nano-size range (303–643 nm) which is desirable for absorption. The increase in the droplet size and the higher PDI values of the reconstituted emulsions, are attributed to the expected reduction in excess surface concentration of surfactant and drug in the oil/water interface due to the incomplete reconstitution of emulsions from pellets, as reported previously (12,22).
Regarding the effect of drug type on the droplet diameter of reconstituted emulsions, the more lipophilic PR appears giving emulsions with lower overall diameter (ANOVA p = 0.025, Table IV), implying better stability of the reformed emulsion due to the easier incorporation and more firmly bound lipophilic drug to the oil/surfactant and to the greater surfactant availability. Regarding the effect of oil/surfactant ratio on droplet diameter, from Table III appears that for the added emulsions and for the same drug and surfactant, the diameters for the high oil/surfactant ratio are greater than those of the low ratio (p = 0.070, Table IV) which should be attributed to decreased interfacial film formation due to the lower surfactant content.
Zeta Potential (ζ)
The effects of formulation variables on the ζ of the added emulsions have been already presented and discussed during the comparison of inert and pharmaceutical emulsions. Now, comparing the ζ of the reconstituted emulsions with that of the added, it can be seen from Table III that ζ in the reconstituted emulsions changes considerably towards negative values, which can be ascribed to the effect of dilution on the composition of oil/water interface, and consequently, in the diffuse electrical layer around the droplets. In particular, this change shows the predominance of the free acids in the oil of the self-emulsifying mixture. ANOVA (Table IV) shows significant effect of the type of drug on ζ values for both added and reconstituted emulsions (p = 0.006 and p = 0.013, respectively) which means that drug charges still exist after reconstitution, resulting in significant differences in the droplet charges of the reconstituted emulsions of the two drugs. In fact, bivariate correlation analysis between the ζ values of added (1/10 dilution of oil/surfactant in water) and reconstituted emulsions gave linear relationship:
| 4 |
The higher dilution of droplets in the reconstituted emulsions is due to the volume of the reconstitution medium used in the test (200 mL in which 3 g pellets were suspended, corresponding to 0.6 g self-emulsifying mixture and 1/333 dilution instead of 1/10 in the prepared emulsions) and also due to the contact and adherence of the hydrophilic surfactants on the MCC fibrils of the dry pellets, resulting in incomplete reconstitution (12).
Viscosity
Viscosity of the added emulsions was determined as a quantitative measure of the difficulty to flow, which is expected to vary, since their appearance varied from clear to translucent or milky liquid, due to the different composition and relatively high content (25% w/w) of the drug/oil/surfactant mixture. More importantly, emulsion viscosity may affect the micromeritic and particularly the drug migration during pellet drying (19). The effect of drug addition on viscosity has already been discussed and now the surfactant type and oil/surfactant ratio will be considered.
From Table III, it appears that although the effect of surfactant on viscosity is of low statistical significance (p = 0.096, Table IV) RH40 contributes highest in viscosity, followed by RH60 and ELP. Taking into account the surfactant properties, Table II, we can see that their effect on emulsion viscosity should be a combination of molecular weight and HLB value, both of which (together with the surfactant content) affect their concentration in the oil/water interface. The increase of viscosity at lower oil/surfactant ratio is clear for all the emulsions studied and ANOVA confirmed this effect at significance p = 0.059 (Table IV) which can be attributed to the increased surfactant content at the oil/water interface and hence greater hydration and shearing resistance.
Pellet Properties
In Table V are summarized the micromeritic together with the mechanical properties and in Table VI the pharmaco-technological properties of the pellets respectively. From Table V it can be seen that the extra amount of water required, additionally to the emulsion (30 g) for the formation of pellets with narrowest size distribution and most spherical shape, increases slightly with oil/surfactant ratio for half of the batches of both drugs. Also, for a fixed ratio it increases in the order ELP < RH40 < RH60, which is the same as the order of their HLB value or hydrophilicity. Therefore, the effects of the surfactant content and type on the pellet properties should be ascribed to the hydration of MCC (12).
Table V.
Extra water required during MCC wet massing, micromeritic (size, shape) and mechanical (friability and tensile strength) properties of self-emulsifying pellets [mean, (SD), n = 3 and for tensile strength n = 10]
| Cremophor/ratio | Extra water (g) | Modala fraction% | Median diameter (μm) | Shape parametersb | Friabilityb (%) | Tensileb strength (MPa) | ||
|---|---|---|---|---|---|---|---|---|
| FI (%) | AR | eR | ||||||
| Furosemide | ||||||||
| ELP/1.5 | 2 | 79 | 1,071 | 3.34 | 1.09 | 0.53 | 3.6 | 0.81 |
| ELP/2.3 | 3 | 69 | 1,096 | 3.49 | 1.09 | 0.54 | 2.2 | 0.67 |
| RH40/1.5 | 2 | 70 | 1,083 | 3.45 | 1.11 | 0.47 | 3.6 | 0.88 |
| RH40/2.3 | 3 | 80 | 1,062 | 3.84 | 1.10 | 0.50 | 2.5 | 0.67 |
| RH60/1.5 | 5 | 87 | 1,000 | 3.47 | 1.09 | 0.52 | 2.3 | 1.10 |
| RH60/2.3 | 5 | 87 | 1,012 | 3.11 | 1.08 | 0.55 | 1.7 | 0.78 |
| Propranolol | ||||||||
| ELP/1.5 | 3 | 79 | 1,043 | 3.47 | 1.10 | 0.51 | 2.8 | 0.99 |
| ELP/2.3 | 3 | 84 | 1,037 | 3.34 | 1.08 | 0.54 | 2.4 | 0.72 |
| RH40/1.5 | 3 | 78 | 1,048 | 3.90 | 1.12 | 0.44 | 2.1 | 0.98 |
| RH40/2.3 | 4 | 86 | 1,018 | 3.18 | 1.11 | 0.50 | 1.6 | 0.65 |
| RH60/1.5 | 5 | 90 | 1,013 | 6.11 | 1.18 | 0.37 | 2.0 | 0.60 |
| RH60/2.3 | 5 | 89 | 1,019 | 3.22 | 1.10 | 0.49 | 1.9 | 0.52 |
FI% fullness index, AR aspect ratio, e R shape factor
aModal size fraction: 850–1,200 μm
bStandard deviation for FI% 0.37–0.94, for AR 0.020–0.058, for eR 0.051–0.090, for friability 0.0–0.2, and for tensile strength 0.05–0.10
Table VI.
Pharmaco-technological properties of pellets (disintegration time, drug content in outer and inner part of pellets, migration%) and drug solubility in oil/surfactant mixtures [mean, (SD), n = 3]
| Cremophor/ratio | Disin/tion time (min) | Drug (μg) in | Migration (%) | Drug solubilitya in oil/surfactant (mg/mL) | |
|---|---|---|---|---|---|
| Outer part | Inner part | ||||
| Furosemide | |||||
| ELP/1.5 | 6.2 (0.4) | 210 (6) | 193 (4) | 9.0 | 26.0 (0.5) |
| ELP/2.3 | 4.5 (0.7) | 235 (8) | 215 (2) | 9.8 | 24.7 (0.9) |
| RH40/1.5 | 4.5 (0.2) | 214 (9) | 209 (2) | 2.5 | 40.7 (0.9) |
| RH40/2.3 | 4.4 (0.4) | 241 (5) | 212 (3) | 14.5 | 28.0 (2.0) |
| RH60/1.5 | 4.8 (0.3) | 206 (1) | 197 (2) | 4.3 | 29.9 (0.7) |
| RH60/2.3 | 4.5 (0.1) | 233 (1) | 195 (1) | 18.5 | 29.8 (2.5) |
| Propranolol | |||||
| ELP/1.5 | 4.2 (0.1) | 194 (2) | 192 (1) | 0.8 | 63.2 (2.7) |
| ELP/2.3 | 3.7 (0.1) | 210 (4) | 189 (6) | 11.0 | 47.6 (2.3) |
| RH40/1.5 | 5.7 (0.1) | 218 (1) | 214 (2) | 1.8 | 61.5 (1.3) |
| RH40/2.3 | 4.9 (0.1) | 220 (5) | 211 (1) | 4.3 | 49.6 (2.8) |
| RH60/1.5 | 10.8 (0.3) | 202 (1) | 199 (1) | 1.3 | 57.0 (2.5) |
| RH60/2.3 | 4.3 (0.1) | 220 (1) | 217 (2) | 1.8 | 49.9 (2.6) |
aData from Nikolakakis and Malamataris 2014 (22)
Pellet Size
The results in Table V show that most pellet batches have a high percentage (69–89%) in the modal size fraction 850–1,200 μm and median diameters in a narrow size range (1,000–1,096 μm). ANOVA (Table IV) showed interaction of the effects of drug type and surfactant type on the median diameter (p = 0.049). This is depicted in Fig. 3a by comparing the mean values of pellet diameters for the two drugs and the three surfactants. It can be seen that the difference between drugs are pronounced for pellets with ELP and RH40 but small for those with RH60. Also, for FS, the difference between ELP and RH60 or between RH40 and RH60 is large; whereas for PR, it is small, indicating that the effect of drug depends on the surfactant. The decreasing size of the pellets with increasing surfactant hydrophilicity (Fig. 3a) can be explained due to the greater extra water required for their preparation (Table V) and hence greater water loss and decrease in diameter during drying. As can be seen in Table V the range of required extra water is smaller for propranolol compared to furosemide (2 to 5 compared with 3 to 5) which accounts for the different effect of surfactant type for the two drugs.
Fig. 3.

Interaction plots of the effects of drug and surfactant on a pellet diameter and b friability, and c interaction plots of the effects of drug and oil/surfactant ratio on friability (solid symbols with solid lines for furosemide and open symbols with dotted lines for propranolol)
Pellet Shape
From Table V, it can be seen that, except for formulation of PR with RH60 at oil/surfactant ratio 1.5, the values of the fullness index (FI% <3.90), aspect ratio (AR <1.12) and shape factor (eR >0.44) indicate production of spherical pellets. However, the values of eR for the different formulations vary considerably (0.37–0.55), meaning that eR is more discriminative. Comparing pellet batches of the two drugs, it can be seen that eR is lower for PR batches (drug effect significant at p = 0.059, Table V). For the different surfactants, the results in Table V do not show a certain trend, whereas for different oil/surfactant ratios eR increases clearly with the increase of oil/surfactant ratio or lower surfactant content (p = 0.054, Table V). Comparing the effects of the three studied factors on pellet shape and emulsion properties, we can see that viscosity and eR are both affected at similar significant level by the type of drug and the oil/surfactant ratio. Therefore, the differences in eR values should be related to the viscosity of emulsions used for the preparation of the pellets.
More specifically, considering the data in Tables III and V, it can be seen that emulsions of furosemide with RH40 at oil/surfactant ratio 1.5 and 2.3 (denoted as FS/RH40/1.5 and FS/RH40/2.3) and emulsions of propranolol with RH40 and RH60 at ratio 1.5 (denoted as PR/RH40/1.5 and PR/RH60/1.5) with higher viscosity, correspond to more irregular pellets with higher FI% and AR and lower eR. Accordingly, the relationship between pellet shape and emulsion viscosity was examined by plotting data of the more established shape parameters aspect ratio (AR) and shape factor (eR) against emulsion viscosity. The plots in Fig. 4a, b show that except for formulation PR/RH60/1.5, AR increases linearly with viscosity (R2 = 0.711 and 0.893 for FS and PR, respectively, Fig. 4a), whereas eR decreases (R2 = 0.740 and 0.977, Fig. 4b).
Fig. 4.

Plots of a aspect ratio and b shape factor (eR) with the viscosity of emulsions used for the preparation of pellets (round symbols for furosemide and square for propranolol)
The increased sphericity with decreasing viscosity should be due to even spreading of emulsions in MCC powder resulting in more homogeneous wet mass and improved distribution of the self-emulsifying oil/surfactant/drug mixture in the pellets. The deviation of formulation PR/RH60/1.5 representing high drug lipophilicity, high surfactant hydrophilicity and content, may be ascribed to the difficulty of spreading of the respective emulsion in MCC, resulting in high FI% and low eR values (Table V).
Pellet Friability and Tensile Strength
From the results in Table V, it appears that all batches of self-emulsifying pellets exhibit friability >1.6%. This is expected considering the relatively high proportion of self-emulsifying mixture (20% w/w), resulting in weaker MCC interparticle bonding and less coherent pellets. More specifically, from Table V, it appears that overall, furosemide pellets show higher friability than propranolol and that for the same drug and surfactant, the friability of pellets with higher oil/surfactant ratio is always lower. This should be related to the greater deformability of pellets due to the higher oil content and hence their greater ability to absorb shocks during tumbling in the friabilator and also to lubricating action of the oil. From the results of ANOVA (Table IV), it is seen that all studied formulation factors affect friability and there are significant interactions between the effect of drug and surfactant type (p = 0.046) as well as between the effect of drug type and oil/surfactant ratio (p = 0.044).
The former of the two interactions is depicted in Fig. 3b, where the differences in friability for the two drugs are seen to be greater for Cremophor RH40, with FS showing highest and PR lowest friability, whereas there are no differences for Cremophor RH60. The latter of the two interactions is depicted in the plots of Fig. 3b, where the decrease in friability with oil/surfactant ratio is seen to be steeper for FS. The greater friability of FS pellets compared to PR and, in particular, those made with Cremophor RH 40 (Fig. 3b) can be ascribed to the higher viscosity of the added FS/RH40 emulsions (7.75 and 4.98 cP at oil/surfactant ratios 1.5 and 2.3 for FS compared with 4.94 and 3.97 cP for PR, Table III). The higher viscosity impairs the movement of emulsions and distribution into MCC powder, as manifested by high extrusion forces (18), and consequently the efficiency of incorporation of oil/surfactant in the dry pellet, which adversely affects pellet deformability or the ability to absorb stresses during friability testing. The low and similar friability of the pellets of both drugs made with RH60 could be ascribed to the increased presence of the highly hydrophilic surfactant (HLB = 15.7) on the pellet surface, migrating along with the water during drying and also, to its high molecular weight and solid nature (Table II), resulting in harder pellet surface. The different effect of oil/surfactant ratio for the two drugs on friability (interaction shown in Fig. 3c) can be ascribed to the greater difference in the viscosities of the emulsions of the two drugs at ratio 1.5 (1.39 cP, Table III) compared with ratio 2.3 (1.33 cP) and consequently greater difference in pellet deformability as explained above.
From the data in Table V, it appears that the type of drug and the surfactant do not have an effect on tensile strength. However, for the same drug and surfactant type, tensile strength clearly decreases with the increase in oil/surfactant ratio which should be attributed to the weakening of the interparticle MCC bonds due to the masking action of the oil (12).
Pellet Disintegration
From Table III, the disintegration time does not seem to be influenced by the type of drug or surfactant. However, for the same drug and surfactant type, there is a general decrease with the increase in oil/surfactant ratio. This is in agreement with the weaker MCC interparticle bonding which is responsible for the tensile strength reduction due to the increased oil content and, additionally, it can be ascribed to lesser gel formation due to the lower surfactant content (11,12). The nearly double disintegration time of formulation PR/RH60/1.5 can be ascribed to the inefficient spreading and uneven distribution of oil/surfactant in the pellets which probably limits the presence of surfactant and wetting at certain parts of the pellet surface only, resulting in less efficient liquid penetration and longer disintegration time. This is also indicated by the lowest sphericity of PR/RH60/1.5 pellets (highest FI% and AR and lowest eR, Table V).
Drug Migration in Pellets
The results of drug content in outer and inner part of pellets together with the calculated migration (%) and solubility of drug in the corresponding oil/surfactant mixture are given in Table VI. Migration appears greater for the less lipophilic furosemide and particularly for higher oil/surfactant ratio.
The drug lipophilicity and oil/surfactant ratio should affect migration due to their combined influences on the solubilization of drug in the oil of droplets and on capillary flow (viscosity) of the emulsion used. In pellets, before drying, drug may exist as solubilized in the oil droplets and as dissolved or suspended in the continuous aqueous emulsion phase, Fig. 1. Since the solubilities of the two studied drugs in water at the ambient temperature of pellet preparation, are similar (0.063 mg/mL for FS and 0.051 mg/mL for PR) and remarkably lower (about 3 orders of magnitude) than those in oil/surfactant mixtures (24.7–63.2 mg/mL), the drug lipophilicity should determine partitioning in the two emulsion phases and the concentration in the droplets (13,15). During drying, instability of the emulsion and deposition of drug dissolved in the aqueous phase may occur. Therefore, in dried pellets the drug exists as solubilized in the oil/surfactant mixture dispersed in the MCC particles, and/or as solid particles, mainly in the outer part of the pellets, carried there by the migrating aqueous phase of the emulsion during drying.
In addition, viscosity affects capillary flow of liquid phase towards the pellet surface and drug deposition due to water evaporation during drying together with surface tension as has been already reported for wet granulations dried in conventional oven (19). The contribution of surface tension on migration is not expected to be significant for the examined formulations, since it is similar for the aqueous solutions of the two drugs (64.0 and 66.0 mN/m) and the three surfactants (41.3–44.8 mN/m, Table II).
Comparing the effects of drug lipophilicity and oil/surfactant ratio on emulsion viscosity and drug migration (%) (Tables III and VI), there is apparently some controversy. As expected, decreased emulsion viscosity due to increase of oil/surfactant ratio (for the same drug) corresponds to increased migration (%) but decreased viscosity due to increased drug lipophilicity corresponds to decreased migration (%). This controversy reveals predominance of drug lipophilicity and solubility in oil droplets on migration (%) over that of capillary flow.
From the above discussion, it is clear that both the viscosity of the emulsion (η) and drug solubility in oil/surfactant mixture (S) should control drug migration towards pellet surface. Accordingly, from the drug migration (M%) plots against the product [η·S], Fig. 5, it can be seen that M% decreases exponentially with the product [η·S], following the simple equation (R2 = 0.856):
| 5 |
that could be useful during formulation work.
Fig. 5.

Plot of drug migration with the product of viscosity (η) and solubility of drug (S) in oil/surfactant mixtures
CONCLUSIONS
Regarding the emulsion properties, viscosity is affected by the type of drug and oil/surfactant ratio, the droplet size by the oil/surfactant ratio, while zeta potential is affected by the drug.
For the micromeritic properties and mechanical strength of pellets, pellet shape is affected by the studied formulation variables and there was significant interaction between the effects of the drug and surfactant type on pellet diameter. There were also significant interactions between the effects of drug and surfactant type and between the effects of drug and oil/surfactant ratio on pellet friability.
Regarding the pharmaco-technological properties of pellets, drug migration is higher for furosemide of lower lipophilicity and for higher oil/surfactant ratio. The droplet size of the reconstituted emulsions and the zeta potential of both added to the MCC (for the preparation of pellets) and reconstituted emulsions are affected by the type of drug. Linear relationships between the emulsion viscosity and shape parameters of pellets (aspect ratio and shape factor) and exponential relationship between drug migration (%) and the product of viscosity and solubility of drug in oil/surfactant were established, which may be useful during formulation work of self-emulsifying pellets.
Acknowledgments
The work is partly from the Master’s Thesis of Andrea Salis under Erasmus Program between the School of Pharmacy, Thessaloniki, Greece and Sassari, Italy. The authors are grateful to Help Hellas (Athens, Greece) for the generous donation of furosemide and to BASF (Ludwigshafen, Germany) for the surfactants.
References
- 1.Bechgaard H, Hegermann NG. Controlled-release multiple-units and single-unit doses. Drug Dev Ind Pharm. 1978;4:53–67. doi: 10.3109/03639047809055639. [DOI] [Google Scholar]
- 2.Schulz P, Kleinebudde P. A new multiparticulate delayed release system. Part I: dissolution properties and release mechanism. J Control Release. 1997;47:181–9. doi: 10.1016/S0168-3659(97)01634-9. [DOI] [Google Scholar]
- 3.Constantinides PP. Lipid microemulsions for improving drug dissolution and oral absorption: physical and biopharmaceutical aspects. Pharm Res. 1995;12(11):1561–72. doi: 10.1023/A:1016268311867. [DOI] [PubMed] [Google Scholar]
- 4.Pouton CW. Formulation of self-emulsifying drug delivery systems. Adv Drug Deliv Rev. 1997;25:47–58. doi: 10.1016/S0169-409X(96)00490-5. [DOI] [Google Scholar]
- 5.Newton M, Petersson J, Podczeck F, Clarke A, Booth S. The influence of formulation variables on the properties of pellets containing a self-emulsifying mixture. J Pharm Sci. 2001;90(8):987–95. doi: 10.1002/jps.1051. [DOI] [PubMed] [Google Scholar]
- 6.Serratoni M, Newton JM, Booth S, Clarke A. Controlled drug release from pellets containing water-insoluble drugs dissolved in a self-emulsifying system. Eur J Pharm Biopharm. 2007;65:94–8. doi: 10.1016/j.ejpb.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Newton JM. Gastric emptying of multi-particulate dosage forms. Int J Pharm. 2010;395:2–8. doi: 10.1016/j.ijpharm.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 8.Tuleu K, Newton M, Rose J, Euler D, Saklatvala R, Clarke A, et al. Comparative bioavailability study in dogs of a self-emulsifying formulation of progesterone presented in a pellet and liquid form compared with an aqueous suspension of progesterone. J Pharm Sci. 2004;93(6):1495–502. doi: 10.1002/jps.20068. [DOI] [PubMed] [Google Scholar]
- 9.Iosio T, Voinovich D, Grassi M, Pinto JF, Perissutti B, Zacchina M, et al. Bi-layered self-emulsifying pellets prepared by co-extrusion and spheronization: Influence of formulation variables and preliminary study on the in vivo absorption. Eur J Pharm Biopharm. 2008;69:686–97. doi: 10.1016/j.ejpb.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Sun J, Wang Y, Liu X, Liu Y, Fu Q, et al. Solid self-emulsifying nitrendipine pellets: Preparation and in vitro/in vivo evaluation. Int J Pharm. 2010;383:1–6. doi: 10.1016/j.ijpharm.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 11.Nazzal S, Smalyukh II, Lavrentovich OD, Khan MA. Preparation and in vitro characterization of a eutectic based semisolid self-nanoemulsified drug delivery system (SNEDDS) of ubiquinone: mechanism and progress of emulsion formation. Int J Pharm. 2002;235:247–65. doi: 10.1016/S0378-5173(02)00003-0. [DOI] [PubMed] [Google Scholar]
- 12.Matsaridou I, Barmpalexis P, Salis A, Nikolakakis I. The Influence of Surfactant HLB and Oil/Surfactant Ratio on the Formation and Properties of Self-emulsifying Pellets and Microemulsion Reconstitution. AAPS PharmSciTech. 2012;13(4):1319–30. doi: 10.1208/s12249-012-9855-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah NH, Carvajal MT, Patel CI, Infeld MH, Malick AW. Self-emulsifying drug delivery systems (SEDDS) with polyglycolyzed glycerides for improving in vitro dissolution and oral absorption of lipophilic drugs. Int J Pharm. 1994;106(1):15–23. doi: 10.1016/0378-5173(94)90271-2. [DOI] [Google Scholar]
- 14.Nielsen FS, Gibault E, Ljusberg-Wahren H, Arleth L, Pedersen JS, Mullertz A. Characterization of prototype self-nanoemulsifying formulations of lipophilic compounds. J Pharm Sci. 2007;96(4):876–92. doi: 10.1002/jps.20673. [DOI] [PubMed] [Google Scholar]
- 15.Chidambaram N, Burgess DJ. Effect of nonionic surfactant on transport of surface-active and non-surface-active model drugs and emulsion stability in triphasic systems. AAPS Pharm Sci. 2000;2(3):1–11. doi: 10.1208/ps020319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sznitowska M, Janicki S, Dabrowska E, Zurowska-Pryczkowska K. Submicron emulsions as drug carriers. Studies on destabilization potential of various drugs. Eur J Pharm Sci. 2001;12(3):175–9. doi: 10.1016/S0928-0987(00)00115-9. [DOI] [PubMed] [Google Scholar]
- 17.Ktistis G. A viscosity study on oil-in-water microemulsions. Int J Pharm. 1990;61:213–8. doi: 10.1016/0378-5173(90)90211-L. [DOI] [Google Scholar]
- 18.Podczeck F, Maghetti A, Newton JM. The influence of non-ionic surfactants on the rheological properties of drug/microcrystalline cellulose/water mixtures and their use in the preparation and drug release performance of pellets prepared by extrusion/spheronization. Eur J Pharm Sci. 2009;37:334–40. doi: 10.1016/j.ejps.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Kapsidou T, Nikolakakis I, Malamataris S. Agglomeration state and migration of drugs in wet granulations during drying. Int J Pharm. 2001;227:97–112. doi: 10.1016/S0378-5173(01)00788-8. [DOI] [PubMed] [Google Scholar]
- 20.Poutiainen S, Honkanen M, Becker J, Nachtweide D, Järvinen K, Ketolainen J. X-ray microtomography analysis of intragranular drug migration during fluidized bed and oven tray drying. J Pharm Sci. 2012 doi: 10.1002/jps.23051. [DOI] [PubMed] [Google Scholar]
- 21.Schrank S, Hodzic A, Zimmer A, Glasser JB, Khinast J, Roblegg E. Ibuprofen-loaded calcium stearate pellets: drying-induced variations in dosage form properties. AAPS PharmSciTech. 2012;13(2):686–98. doi: 10.1208/s12249-012-9791-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nikolakakis I, Malamataris S. Self-emulsifying pellets: relations between kinetic parameters of drug release and emulsion reconstitution, influence of formulation variables. J Pharm Sci. 2014;103(5):1453–65. doi: 10.1002/jps.23919. [DOI] [PubMed] [Google Scholar]
- 23.Ktistis G, Niopas I. A study on the in-vitro percutaneous absorption of propranolol from disperse systems. J Pharm Pharmacol. 1998;50(4):413–8. doi: 10.1111/j.2042-7158.1998.tb06881.x. [DOI] [PubMed] [Google Scholar]
- 24.Podczeck F, Newton JM. A shape factor to characterize the quality of spheroids. J Pharm Pharmacol. 1994;46:82–5. doi: 10.1111/j.2042-7158.1994.tb03745.x. [DOI] [PubMed] [Google Scholar]
- 25.Shipway PH, Hutchings IM. Attrition of brittle spheres by fracture under compression and impact loading. Powder Technol. 1993;76:23–30. doi: 10.1016/0032-5910(93)80037-B. [DOI] [Google Scholar]
- 26.Montgomery DC. Design and analysis of experiments. 4. New York: Wiley; 1987. pp. 108–10. [Google Scholar]
- 27.Thi TD, Van Speybroeck M, Barillaro V, Martens J, Annaert P, Augustijns P, et al. Formulate-ability of ten compounds with different physicochemical profiles in SMEDDS. Eur J Pharm Sci. 2009;38:479–88. doi: 10.1016/j.ejps.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 28.Avdeef A, Box KJ, Comer JEA, Hibbert C, Tam KY. pH-Metric logP 10. Determination of Liposomal membrane-water partition coefficients of ionizable drugs. Pharm Res. 1998;15(2):209–15. doi: 10.1023/A:1011954332221. [DOI] [PubMed] [Google Scholar]
- 29.Florence AT, Attwood D. Physicochemical principles of pharmacy. 2. New York: The Macmillan Press; 1988. pp. 239–40. [Google Scholar]
- 30.Zetasizer Nanoseries User Mannual MANO 317 Issue 1.1 Feb. 2004 page 5.6. Malvern Instruments Ltd, Worcestershire.
- 31.Alvarez-Nunez FA, Yalkowsky SH. Relationship between polysorbate 80 solubilization descriptors and octanol-water partition coefficients of drugs. Int J Pharm. 2000;200:217–22. doi: 10.1016/S0378-5173(00)00386-0. [DOI] [PubMed] [Google Scholar]


