Abstract
The aim of this study was to prepare candesartan cilexetil-loaded niosomes and mixed niosomes to enhance the aqueous solubility of the drug, thus improving its oral bioavailability. The formulations were prepared using various types and combinations of surfactants, copolymers, and charge-inducing agents. The candesartan cilexetil entrapment efficiency, particle size, and zeta potential of these niosomes varied within the range of 99.06 ± 1.74 to 36.26 ± 2.78, 157.3 ± 3.3 to 658.3 ± 12.7 nm, and −14.7 ± 2.8 to −44.5 ± 1.5 mV, respectively. The in vitro drug release from niosomes was improved after niosomal entrapment compared to pure candesartan cilexetil. The sedimentation behavior study and formulation stability tests against bile salt revealed that mixed niosomes prepared by combining Span 60 and Pluronic P85 demonstrated better stability. The differential scanning calorimetry analysis showed the conversion of crystal structure of candesartan cilexetil to the soluble amorphous form after niosomal encapsulation which induced the drug release. Consequently, oral drug delivery by Span 60/Pluronic P85-mixed niosomes seems feasible due to enhanced drug release and stability.
KEY WORDS: in vitro drug release, niosomes, oral drug delivery, stability, surfactants
INTRODUCTION
The bioavailability of the drugs is influenced by a number of factors. The route of drug administration is the most important of all factors (1). It is well known that oral administration of drugs is preferred over alternatives due to its convenience and lower costs. The oral administration of several drugs is hampered due to their unstable and water-insoluble nature that leads to low bioavailability. Presystemic metabolism, low intestinal permeability, and lack of drug release are some other factors that lower the oral bioavailability. In recent years, there has been an increasing interest on the usage of drug delivery systems to improve the oral bioavailability of active agents, and particularly, nanosized drug delivery systems (NDDSs) are emerging in this field (2).
The efficacy of NDDS for bioavailability enhancement is a challenge. The success of the NDDS is highly influenced by its ability to carry the drug through the biological membranes without degradation and specificity of its biodistribution (3). Several transportation mechanisms have been proposed for NDDS passage through gastrointestinal barriers. Paracellular passage, endocytosis uptake, and lymphatic uptake via M cells in Peyer’s patches are the main pathways.
Candesartan cilexetil (CC), an angiotensin II receptor blocker, is widely indicated for the treatment of hypertension and heart failure. Among the other angiotensin receptor agonists, CC is more potent and exhibits fewer side effects. After oral administration, CC is hydrolyzed to its active form, candesartan, during absorption in the intestinal wall. However, its bioavailability is very low (about 40%). Poor aqueous solubility (0.0003 mg/mL) of CC is the major problem in formulation development step (4–6). There are several approaches to improve the oral bioavailability of CC including the formulation of NDDS (5,7–9).
This work was designed to develop CC-loaded niosomes and characterize these carriers for oral CC administration. CC was chosen as a model drug. Niosomes are well-known NDDS formed from nonionic amphiphiles in vesicular form. A number of nonionic amphiphiles have been used to prepare niosomes. The idea of preparing mixed niosomes by using more than one amphiphiles in niosome formulation to improve the niosome properties such as stability and drug-loading capacity has not been adequately explored. Most of the studies focus on Span and Tween surfactants. One of the goals of this study was to evaluate the potential of Pluronics to form niosomes by themselves or combined with Span 60. Pluronics are widely used triblock copolymers consisting of hydrophilic poly(ethylene oxide) (PEO) and hydrophobic poly(propylene oxide) (PPO) groups. These polymers were chosen due to their excellent ability to interact with body membranes and hydrophobic surfaces, thus enhancing the drug transport across cellular barriers such as intestinal epithelial cells (10). Three different Pluronic polymers—Pluronic F127 (EO100-PO65-EO100), Pluronic P85 (EO26-PO40-EO26), and Pluronic L64 (EO13-PO30-EO13)—and Span 60 were used to prepare niosomes and mixed niosomes. Due to their colloidal properties, prepared formulations were characterized in terms of particle size, zeta potential, drug encapsulation efficiency, sedimentation behaviors, and in vitro drug release. The formulations were compared for their stability against bile salts. The thermal analysis and microscopic visualization were further performed for characterization.
MATERIALS AND METHODS
Materials
Span 60, cholesterol, dicetyl phosphate (DCP), stearylamine (SA), sodium chloride, and sodium deoxycholate were purchased from Sigma-Aldrich (Milwaukee, WI, USA). CC and Pluronics were gifts from Abdi Ibrahim (Istanbul, Turkey) and BASF (Ludwigshafen, Germany). Monobasic potassium phosphate was acquired from Fluka (Buchs, Switzerland). Tween 80, isopropyl alcohol, and methanol were purchased from Merck (Darmstadt, Germany). Sodium hydroxide was bought from Riedel-de Haën (Seelze, Germany). Ultrapure water purified by Milli-Q Plus system (Millipore Corp., Molsheim, France) was used in all experiments.
Preparation of CC-Bearing Niosomes
Drug-loaded niosomes were formed by film hydration method combined with sonication. Niosome formulations were prepared by using various surfactant (Span 60, Pluronic L64, and Pluronic F127) combinations and different charge-inducing agents [dicetyl phosphate (DCP) or stearyl amine (SA)]. The mixture of surfactant, cholesterol, DCP or SA, and CC was dissolved with chloroform in a rounded bottom flask at the molarities shown in Table I. Chloroform was removed by Rotavapor (Buchi R200, BUCHI Labortechnik AG, Flawil, Switzerland) at 55°C with a rotation speed of 68 rpm. In order to remove the traces of chloroform, vacuum was applied to the flask overnight. Subsequently, the thin film was hydrated with 10-mL ultrapure water at 60°C by 15-min vortexing and 15-min bath sonication. Further probe sonication was applied at 42 W for 15 min. The obtained niosome dispersions were stored in a refrigerator at 5 ± 3°C. Formulations were characterized after a 2-day incubation period.
Table I.
The Composition of Niosome Formulations
| Organic phase | Aqueous phase | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CC | Span 60 | Pluronic L64 | Pluronic F127 | Pluronic P85 | Cholesterol | DCP | SA | Chloroform (mL) | Ultrapure water (mL) | ||
| F1 | mM | 11.4 | 11.4 | − | − | − | 11.4 | 1.20 | − | 10 | q.s. 10 |
| mg | 69.6 | 49.3 | − | − | − | 44.2 | 6.49 | − | |||
| F2 | mM | 11.4 | 5.70 | 5.70 | − | − | 11.4 | 1.20 | − | 10 | q.s. 10 |
| mg | 69.6 | 24.6 | 165 | − | − | 44.2 | 6.49 | − | |||
| F3 | mM | 11.4 | − | 11.4 | − | − | 11.4 | 1.20 | − | 10 | q.s. 10 |
| mg | 69.6 | − | 330 | − | − | 44.2 | 6.49 | − | |||
| F4 | mM | 11.4 | 5.70 | − | 5.70 | − | 11.4 | 1.20 | − | 10 | q.s. 10 |
| mg | 69.6 | 24.6 | − | 718 | − | 44.2 | 6.49 | − | |||
| F5 | mM | 11.4 | − | − | 11.4 | − | 11.4 | 1.20 | − | 10 | q.s. 10 |
| mg | 69.6 | − | − | 1436 | − | 44.2 | 6.49 | − | |||
| F6 | mM | 11.4 | − | − | 11.4 | 11.4 | 1.20 | − | 10 | q.s. 10 | |
| mg | 69.6 | − | − | 524 | 44.2 | 6.49 | − | ||||
| F7 | mM | 11.4 | 5.70 | − | 5.70 | 11.4 | 1.20 | − | 10 | q.s. 10 | |
| mg | 69.6 | 24.6 | − | 262 | 44.2 | 6.49 | − | ||||
| F8 | mM | 11.4 | 5.70 | − | 5.70 | 11.4 | 6.00 | − | 10 | q.s. 10 | |
| mg | 69.6 | 24.6 | − | 262 | 44.2 | 32.5 | − | ||||
| F9 | mM | 11.4 | 5.70 | − | 5.70 | 11.4 | − | 1.20 | 10 | q.s. 10 | |
| mg | 69.6 | 24.6 | − | 262 | 44.2 | − | 3.23 | ||||
| F10 | mM | 11.4 | 5.70 | − | 5.70 | 11.4 | − | 6.00 | 10 | q.s. 10 | |
| mg | 69.6 | 24.6 | − | 262 | 44.2 | − | 16.2 | ||||
CC candesartan cilexetil, DCP dicetyl phosphate, SA stearylamine, q.s. quantity sufficient
Determination of CC Encapsulation Efficiency
The encapsulation efficiency was determined after removing unloaded CC from drug-loaded niosomes by ultracentrifugation at 150,000g for 1.5 h. Obtained precipitate was diluted with water to obtain drug-loaded niosomal dispersion. Subsequently, 10–50 μL of niosome dispersion was taken and 50–200 μL of isopropyl alcohol was added to disrupt niosomes. Samples were diluted to 3 mL with methanol for drug assay. CC content was estimated by UV spectrophotometer (Schimadzu 1202 UV Visible, Japan) at λmax 254.5 nm. The assay method was validated, and the analytical validation parameters (accuracy, precision, limit of detection, limit of quantification) were calculated. The linearity range of the method was 0.0025–0.0300 mg/mL, and determination coefficient value (r2) was 0.999. The relative standard deviations for the types of precision and accuracy were always within the acceptable limits (less than 5%). Detection and quantification limits of the method were 3.73 × 10−4 and 1.13 × 10−3 mg/mL, respectively.
The results of CC encapsulation were reported as the encapsulation efficiency percentage which is calculated as the percentage of drug loaded with respect to the total amount added in the preparation and weight percentage of drug entrapped in the total niosome weight.
Size and Size Distribution Measurements
The mean particle size and size distributions of niosomes were determined by photon correlation spectroscopy (PCS) using a Zetasizer Nano ZS (Malvern Instruments, Worcestershire, UK) at 25°C. Formulations were incubated for 2 days before performing the analysis; formulations were diluted with water and filtered through Whatman no. 42 filter. The particle size distribution was measured as the polydispersity index (PDI). All measurements were conducted in triplicate, and results were presented as the mean ± standard error (SE).
Surface Charge
The surface charge of niosomes was obtained by measuring the zeta potential of niosomes. The measurements were performed by using Zetasizer Nano ZS (Malvern Instruments, Worcestershire, UK) utilizing laser Doppler anemometry. The results were reported as the average of six measurements.
Sedimentation Behavior of the Formulations in Storage Conditions
In order to evaluate the stability of niosomes, their sedimentation behavior was observed. Three milliliters of each formulation was introduced into capped vials and incubated in refrigerator at 5 ± 3°C and climatic chamber at 25 ± 2°C to evaluate visible sedimentation or flocculation. The photographic images of the formulations were captured at the 6th and 24th hours using a Nikon D3100 (Japan) digital camera, and the height of the sediment layer was measured for the precipitated formulations. The value of sedimentation volume (F) was calculated by using Eq. 1:
| 1 |
- Vu
The volume of the sedimented solid within a certain time
- Vo
The total volume of the formulation
In Vitro Release of CC from Niosomes
In in vitro drug release experiments, simulated intestinal fluid (SIF) (pH 6.8), containing 0.1% (v/w) Tween 80, was employed as the dissolution media and dialysis method was used. Niosomal vesicles containing 1 mg CC were placed into dialysis bag and sunk in 50 mL of release medium at 37°C. The samples were placed in a water bath and shaken at 60 rpm 37°C. At fixed time intervals, 3 mL of samples were taken from the release medium and the same amount of fresh medium was added back to the vessel for replacement. CC concentrations were determined spectrophotometrically at 256 nm (n = 3). The drug assay method for release studies was validated, and the analytical validation parameters were calculated. The linearity range of the method was 0.0025–0.030 mg/mL, and determination coefficient value (r2) was 0.999. The relative standard deviations for the types of precision and accuracy were always within the acceptable limits (less than 5%). Detection and quantification limits of the method were 3.55 × 10−4 and 1.08 × 10−3 mg/mL, respectively.
Stability of Niosomes in Bile Salt Solution
The stability of niosomes against bile salt solution formulations was determined by following the previously reported method (11). A series of solutions containing different concentrations (4, 8, 12, 16, and 20 mM) of sodium deoxycholate were prepared in phosphate buffered saline (pH 7.4). Forty microliters of niosomes was added to 760 μL of diluted bile salt solutions and incubated at 37°C. After 1 h, the turbidity of the samples was measured spectrophotometrically at 400 nm using bile salt solutions without niosomes as blank for each salt concentration. The turbidity percentage calculated via Eq. 2 was graphed versus sodium deoxycholate concentration.
| 2 |
- a
Absorbance of niosome formulations after incubation in bile salt solution
- b
Absorbance of niosome formulations after incubation in pH 7.4 phosphate buffered saline
Transmission Electron Microscope Observation of Niosomes
The morphological appearance of niosomes was observed by transmission electron microscopy (TEM). In order to prepare the samples for the microscopy, niosome dispersion was dropped onto carbon-coated 200-mesh copper grids and held horizontally to allow the penetration. The excess sample was removed by filter paper, and one drop of 2% uranyl acetate was added to the grid for staining. The negatively stained samples were then imaged on a FEI Tecnai G2 Spirit Bio (TWIN) electron microscope (FEI, Eindhoven, Netherlands) at 120 kV.
Differential Scanning Calorimetry Analysis
Thermal properties of CC, ingredients, CC-loaded F9 niosomes, and physical mixtures of niosome components were investigated with differential scanning calorimetry using Shimadzu DSC-60 (Tokyo, Japan). F9 niosomes were lyophilized before the analysis by using Christ Gamma 2-16 LSC freeze dryer (Harz, Germany). The physical mixture of niosome formulation containing the same ingredients at the same concentrations was prepared by mixing the powders in a glass mortar. Samples were placed in aluminum pans and sealed. The thermograms were obtained at a scan rate of 20°C/min by heating the samples from 20 to 300°C under nitrogen atmosphere. Indium was used as a reference for calibration during the analysis.
RESULTS AND DISCUSSION
Determination of CC Encapsulation Efficiency
CC-loaded niosomes were prepared by using different surfactants and/or amphiphilic copolymers and charge-inducing agents at various concentrations. The encapsulation efficiency (% EE) of CC in F1–F9 formulations varied between 36.26 ± 2.78 and 99.06 ± 1.74 (Table II). F10 formulation prepared by combining Span 60 and Pluronic P85 did not form niosomes because of high SA concentration leading to enhanced electrostatic interaction forming tight aggregates after ultracentrifugation step for removing the unloaded CC.
Table II.
Candesartan Cilexetil (CC) Encapsulation Efficiency and Weight Fraction in Niosomes (Mean ± Standard Error)
| Formulation code | Surfactant | HLB | Charging agent | Encapsulation efficiency (%) | Weight of loaded drug (%) |
|---|---|---|---|---|---|
| F1 | Span 60 | 4.7 | DCP | 94.99 ± 0.76 | 39.55 ± 0.19 |
| F2 | Span 60, Pluronic L64 | 13.7 | DCP | 73.21 ± 2.93 | 19.65 ± 1.60 |
| F3 | Pluronic L64 | 15 | DCP | 36.26 ± 2.78 | 11.79 ± 0.42 |
| F4 | Span 60, Pluronic F127 | 21.4 | DCP | 66.48 ± 3.34 | 4.56 ± 1.25 |
| F5 | Pluronic F127 | 22 | DCP | 92.32 ± 1.07 | 1.67 ± 0.13 |
| F6 | Pluronic P85 | 16 | DCP | 80.11 ± 2.42 | 6.27 ± 1.20 |
| F7 | Span 60, Pluronic P85 | 15 | DCP | 99.06 ± 1.74 | 13.42 ± 1.40 |
| F8 | Span 60, Pluronic P85 | 15 | DCP | 62.73 ± 4.58 | 13.62 ± 1.42 |
| F9 | Span 60, Pluronic P85 | 15 | SA | 59.82 ± 1.89 | 15.75 ± 0.52 |
| F10 | Span 60, Pluronic P85 | 15 | SA | ND | ND |
HLB hydrophilic-lipophilic balance, DCP dicetyl phosphate, SA stearylamine, ND not determined
The encapsulation efficiency percentage showed a tendency to decrease when the amount of DCP was increased or SA was incorporated. This decrease may be due to decreased niosome stability aroused from enhanced repulsion within the bilayers of niosomes (12). As the hydrophilic-lipophilic balance (HLB) of Pluronics increased, the entrapment efficiency of CC also increased in the niosomes prepared from plain Pluronics (F3, F5, and F6). The highest encapsulation was obtained for Pluronic F127 (EO100-PO65-EO100) niosomes followed by Pluronic P85 (EO26-PO40-EO26) and Pluronic L64 (EO13-PO30-EO13) niosomes. This result could be attributed to the increased capacity of the lipophilic environment in the niosome bilayer resulting from the increasing PPO chain length and the increasing molecular weight. This relationship weakened upon combined usage of Pluronics with Span 60 in mixed niosomes. The molecular weights of Pluronic F127, Pluronic P85, Pluronic L64, Span 60, and CC are 12,600, 4600, 2900, 430.62, and 610.67 respectively. It is reported that combination of a low molecular weight amphiphile to Pluronics significantly affects the association or disassociation of these polymers to form micelles. Furthermore, the high interactions between water soluble polymers and amphiphile molecules have been reported (13). Span 60 is a hydrophobic amphiphile with a HLB value of 4.7, and CC is also hydrophobic. Therefore, when Pluronic F127 which has the highest HLB and longest PPO chain length is combined with Span 60, the entrapment site inside the bilayer has to be shared between CC and the hydrophobic amphiphile. This can explain the decrease of drug loading from 92 to 66%. On the other hand, combination of Span 60 with Pluronic P85 and Pluronic L64, which have lower molecular weight and own longer hydrophilic PEO chain than PPO chain, increases the hydrophobicity level, thus contributing to drug loading (14).
Size and Size Distribution Measurements
The size of NDDS plays a critical role on their absorption behaviors through intestine. It is reported that the extent of particle uptake is negatively correlated with the particle size but has an optimum size range around 100–200 nm (15). The particle size of F2, F3, and F6 is within this range, which has been encouraging in terms of absorption enhancement of poorly water-soluble drug, CC (Table III).
Table III.
The Particle Size, Polydispersity Index, and Zeta Potential Measurements of the Niosome Formulations (Mean ± Standard Error)
| Formulation code | Particle size (nm) | PDI | Zeta potential (mV) |
|---|---|---|---|
| F1 | 242.0 ± 6.6 | 0.32 ± 0.02 | −39.8 ± 1.8 |
| F2 | 157.3 ± 3.3 | 0.23 ± 0.02 | −35.7 ± 0.7 |
| F3 | 160.2 ± 0.8 | 0.19 ± 0.01 | −31.0 ± 1.5 |
| F4 | 350.7 ± 7.0 | 0.28 ± 0.01 | −26.5 ± 0.4 |
| F5 | 658.3 ± 12.7 | 0.30 ± 0.01 | −27.6 ± 0.5 |
| F6 | 159.0 ± 1.1 | 0.09 ± 0.01 | −23.8 ± 1.2 |
| F7 | 220.9 ± 2.1 | 0.48 ± 0.02 | −23.1 ± 1.5 |
| F8 | 283.7 ± 15.7 | 0.27 ± 0.02 | −44.5 ± 1.5 |
| F9 | 480.7 ± 15.2 | 0.49 ± 0.16 | −14.7 ± 2.8 |
| F10 | ND | ND | ND |
PDI polydispersity index, ND not determined
The average niosome size was varied between 157 and 658 nm and increased as the HLB value of surfactants increased. This result is compatible with the literature findings (16). As expected, an increase in the length of PEO and PPO moieties in the structure of Pluronics, thus the molecular weight of the polymers, leads to the formation of larger niosomes. The higher hydrophilic chain lengths lead to the formation of less rigid and looser bilayers (14). The average size of niosomes prepared with Pluronic F127 (F5) was 658 nm, whereas after addition of Span 60 (F4), the size decreased down to 350 nm. The addition of an amphiphile, which has a dominant hydrophobic character, to this formulation leads to the formation of a more intensive and rigid lipid layer resulting in reduced particle size. This can be considered as an advantage for the stability of the formulation so that the amount of the encapsulated drug can be preserved during storage.
The charge-inducing agents such as DCP and SA are known to stabilize niosomes against aggregation and precipitation by increasing their zeta potential. Furthermore, these agents enhance the permeability of the niosome membrane to water and lead to the formation of large niosomes (12). This relation was observed in F8 formulation in which niosome size increased from 220.9 ± 2.1 to 283.7 ± 15.7 nm as DCP concentration was increased. The niosome size became 480.7 ± 15.2 nm when SA was used as a charge inducer (F9 formulation).
The quality and uniformity of the dispersed systems is expressed with the polydispersity index values. The values less than 0.7 are considered as suitable measurements (17). In the niosome formulations, PDI values ranged between 0.09 ± 0.01 and 0.49 ± 0.16. The low PDI values demonstrated the narrow size distribution and uniformity of the niosomal suspension (15).
Surface Charge
One of the parameters for interpreting the stability of colloidal systems is their zeta potential. As the zeta potential increases, the charged particles repel one another, and this stabilizes the system against aggregation. Systems with the zeta potential value of ±30 mV or higher are considered to be stable (4). The zeta potential of the niosomes was changed within a range of −44.5 ± 1.5 and −14.7 ± 2.8 mV. In this respect, some of the formulations did not have adequate stability due to low electrostatic stabilization. The composition of the niosomal bilayer and the amount and type of the charge-inducing agents affected the zeta potential of niosomes. As the amount of DCP increased, the zeta potential was also increased negatively from −23.1 ± 1.5 mV (F7 niosomes) to −44.5 ± 1.5 mV (F8 niosomes) (Table III). The addition of SA which is known as positive charge inducer increased the zeta potential positively from −23.1 ± 1.5 mV (F7 niosomes) to −14.7 ± 2.8 mV (F9 niosomes) as expected.
Sedimentation Behavior of the Formulations in Storage Conditions
The storage temperature was found to be effective on the sedimentation behaviors of niosomes. Prior studies indicated the provocative role of increasing storage temperature on the extent of coalescence of nanosized particles (18). As seen in the photographic images in Fig. 1, sedimentation and flocculation were observed in several niosome formulations which increased by temperature and incubation time. However, all of the formulations were easily redispersed in the aqueous medium by hand shaking, with the exception of the F10 formulation, where caking was present. Due to cake formation, the zeta potential and size of the vesicles were not measurable. The cause of the aggregation in vesicular systems is mainly due to van der Waals attractions (19). The aggregation of niosomes can be attributed to several causes including (a) the possibly reduced electrostatic repulsion between vesicles due to the ion density of the dispersion medium that shield the surface charge of the vesicle (20), (b) the broad particle size distribution of the formulations (21), (c) the extensive energy input (sonication, etc.) required during the preparation process which creates thermodynamically unstable systems (22), and (d) the decreasing number of water-binding sites on membranes (19). The sedimentation in F7 niosomes was inhibited as DCP concentration was increased by preparing F8 niosomes. This is compatible with the general agreement stating the enhanced steric stabilization due to DCP inclusion (20). The zeta potential of F7 and F8 niosomes were −23.1 ± 1.5 and −44.5 ± 1.5 mV. The repulsion between F8 niosomes is higher due to high zeta potential; as a result of this, precipitation is retarded for F8 niosomes. F1 and F4–F8 niosomes were deflocculating system that precipitate according to stokes law and present cloudy supernatants. F9 niosomes formed a flocculating system in which a distinct boundary between the sediment and the clear supernatant is observed. The attraction forces between vesicles lead them to form fluffy conglomerates called flocs. Flocculation is considered as a reversible event. Depending on the flocs size and porosity of the network between the flocs, the sedimentation rate is determined. Precipitation can be inhibited upon a decrease on the floc density depending on their structure. In F2 and F3 formulation, a creaming instability was observed in which the niosomes were raised to the surface of the dispersions and considered as unstable. The average vesicle size of F2 and F3 formulations were 157.3 ± 3.3 and 160.2 ± 0.8 nm which are the smallest among all formulations. The instability of these formulations might be related with their small size. Suspensions with an F value of 1 are considered as ideal systems demonstrating flocculation without sedimentation or caking (23). Among all of the formulations, F values for F9 niosomes are closest to 1 at both 5 ± 3 and 25 ± 2°C, and it can be considered as the most stable formulation (Fig. 2). Furthermore, storing niosomes in refrigerator at 5 ± 3°C would be more appropriate to prevent the sedimentation of the system. F1, F4, and F5 are at equilibrium at 5 ± 3°C and can be considered as stable.
Fig. 1.
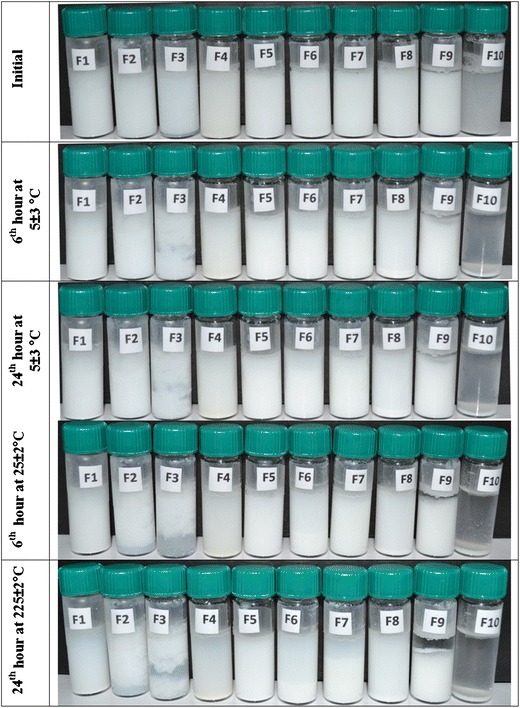
The sedimentation behaviors of the formulations stored at 5 ± 3 and 25 ± 2°C for 6 and 24 h
Fig. 2.
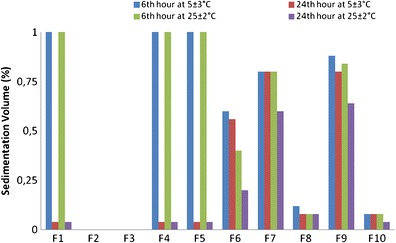
The sedimentation volumes of the formulations stored at 5 ± 3 and 25 ± 2°C for 6 and 24 h
In Vitro Release of CC from Niosome Formulations
The CC release profiles from pure CC and F1–F9 niosomes in SIF (pH 6.8), containing 0.1% (v/w) Tween 80, are given in Fig. 3. The in vitro drug release profiles demonstrated that the release rate of free CC is significantly lower than CC released from niosomes except F6 niosomes (p > 0.05). This demonstrated the solubilizing effect of niosomes leading to enhanced drug release. The release profile of free CC is similar to the findings of previous studies (4,7). In Fig. 3, the amounts released at the end of 4 h are also shown. The release profiles indicate the sustained drug release of CC from niosomes which is a common drug release manner (17). The cumulative release of CC was significantly highest in the F5 and F9 niosomes with 66.84 ± 2.11 and 67.12 ± 0.88% at the end of the 4th hour (p > 0.05). The release rate was ordered as F5 > F6 > F3 in niosomes prepared with plain Pluronics, in which the PEO chain length was also increased. This was attributed to the hydrophilic PEO chains surrounding the niosome surface and leading to a decrease in surface tension which provokes the drug release. Besides, as the length of the hydrophilic chain increases, this forms a looser bilayer in the niosomal structure and improves the drug release. This explains the high drug release rate from F5 niosomes prepared from Pluronic F127. Higher density of PEO chains on the surface layer might have caused increased water loading on the surface thus enhancing the drug release as it was stated in previous researches (17). The drug release rate and drug loading were increased when Span 60 was combined with Pluronic P85 (F7 niosomes). Increased DCP amount contributed to the drug release rate due to its hydrophilic nature (F8 niosomes).
Fig. 3.
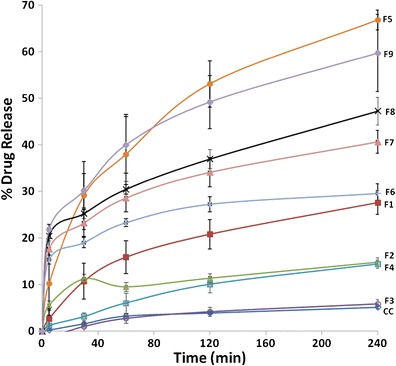
The candesartan cilexetil (CC) release profiles from pure candesartan cilexetil (CC) and F1–F9 niosomes in simulated intestinal fluid (SIF) (pH 6.8), containing 0.1% (v/w) Tween 80
Stability of Niosomes in Bile Salt Solution
In order to prepare effective vesicular systems, it is important to preserve their integrity in gastrointestinal system. An orally administered vesicular system should carry the encapsulated drug to its absorption site in the gastrointestinal system by protecting it against degradation. Besides, in order to enhance the oral bioavailability of drugs via the absorption-enhancing effect of the vesicular systems, the integrity of the carrier should be preserved during gastrointestinal transit. In our previous study, we demonstrated the protective effect of niosomes against enzymatic degradation (trypsin, a-chymotrypsin, and pepsin) (24). Rowland and Woodley have investigated the effect of bile salts, pancreatic lipase, and different pH conditions on the stability of conventional multilamellar liposomes. Among these parameters especially bile salts were found to be the most critical factor that threats the liposome integrity. Bile salts caused the liposomes to release more than 80% of the encapsulated marker (25). They found that the lipid composition of liposomes and the type of the encapsulated agent are two dominant parameters that affect the liposome’s membrane destabilization. The findings of Chiang and Weiner also confirm this relationship (26). Similar researches emphasize the importance of vesicle stability in bile salts on their fate upon oral application. Bile salts are involved in lipid digestion after being synthesized in the liver, and they are released to the gastrointestinal tract. The interaction of bile salts with vesicular systems results in the disintegration of bilayer membrane to mixed micelles and thus leads to the leaking of the active agent (27,28). The steps of this transformation is described as follows: (i) penetration of the bile salts between water and lipid phase and formation of mixed bilayer vesicles with altered permeability, (ii) coexistence of mixed bilayer vesicles with mixed bilayer sheets, (iii) saturation of the bilayers with bile molecules and mixed micelle formation, (iv) disappearance of the bilayer structure. This disruption mechanism highly depends on the bile salt (detergent)/lipid interactions (29). In order to overcome the low bile salt stability problems, researchers investigate on the formulation of more stable vesicles. Several attempts including the usage of different lipids (highly fluorinated phospholipids (29)), usage of nonionic surfactants (polysorbate 20 (30)), incorporation of polymers to the bilayer structure (chitosan (27)), and vesicle coating (polysaccharides (31)) have increased the vesicle stability.
In this study, all of the niosome formulations were subjected to stability tests in sodium deoxycholate solutions with different concentrations for an hour. Sodium deoxycholate is one of the bile salts and has a similar structure with synthetic surfactants. It is known that they form micellar formations in aqueous solutions (32). The graph of turbidity measurements as the indicator of the stability versus salt concentration is given in Fig. 4.
Fig. 4.
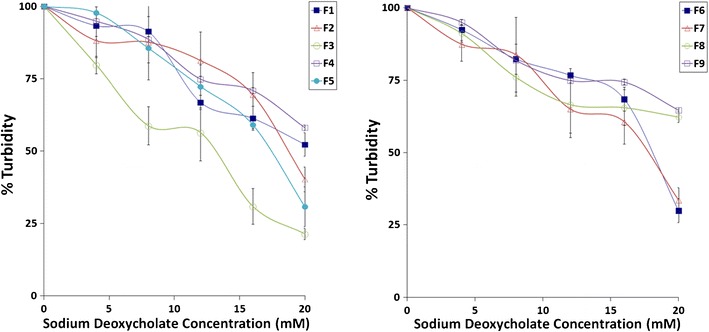
The stability of formulations in sodium deoxycholate solutions
As the bile salt concentration increased, the turbidity decreased. A possible interaction between bilayer composition and bile salts might have led to the formation of mixed vesicles at elevated bile salt concentrations. The decrease on turbidity was highest for F3, F5, F6, and F7 demonstrating their low stability against bile salts. Absence of Span 60 in bilayer composition (F3, F5, F6 formulations) decreased the stability of niosomes against bile salts. Especially the F1, F4, F8 and F9 niosomes were more stable and partly lysed demonstrating a more rigid bilayer structure in terms of preserving the encapsulated drug in gastrointestinal conditions. If the results of in vitro drug release test were also considered, F8 and F9 niosomes seem more ideal among these four formulations. Combined usage of Pluronic P85 and Span 60 seems feasible to improve niosome stability.
Transmission Electron Microscope Observation of Niosomes
The TEM micrographs of niosomes are given in Fig. 5. The TEM images confirmed the formation of niosomes. The shapes of the vesicles were spherical, and they were similar with the typical niosome micrographs obtained in prior studies (33,34). The size of the niosomes was around the average particle size (480.7 ± 15.2 nm) measured by Zetasizer. The particle size distribution histogram revealed the bimodal size distribution of F9 formulation. This was also confirmed by the TEM analysis demonstrating several niosomes around 5 μm. But the percentage of larger niosomes was observed to be very low compared to the general size distribution.
Fig. 5.
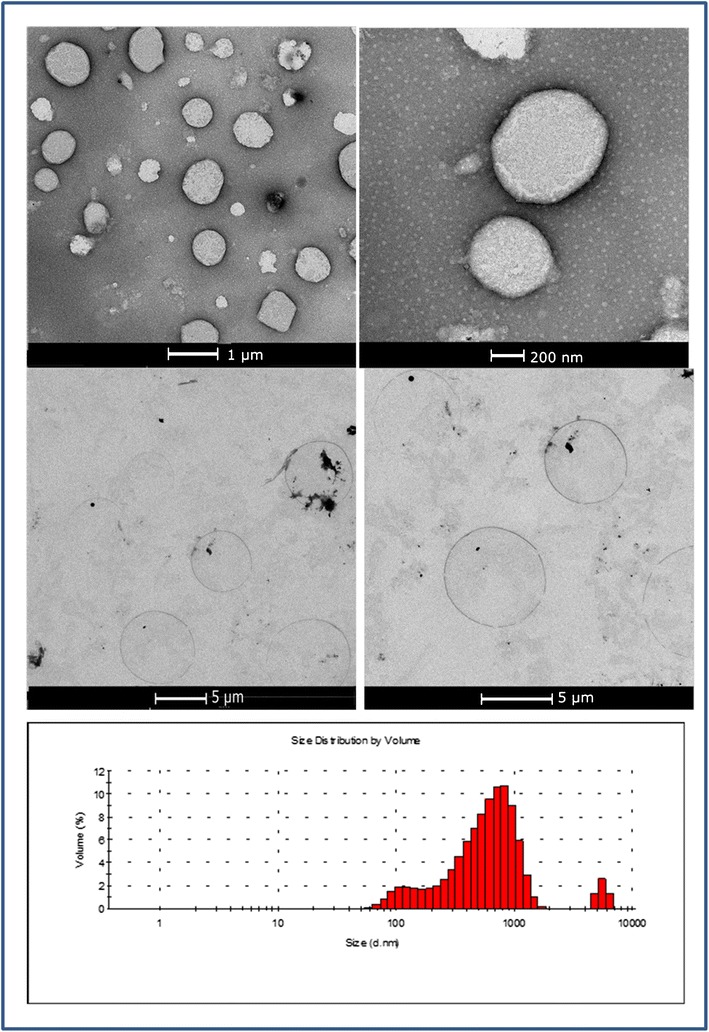
Transmission electron microscopy (TEM) micrographs of candesartan cilexetil (CC)-loaded F9 niosomes and their particle size distribution histograms
Differential Scanning Calorimetry Analysis
Thermal analysis was performed for niosomes to evaluate the drug-excipient interactions in niosomes. The thermogram of CC revealed the melting point of the active agent at 168°C as an endothermic peak indicating that CC is in the crystalline form (Fig. 6(c)). This data is comparable with the literature findings (4,6,35). The later exothermic peaks demonstrated the decomposition of CC. Differential scanning calorimetry (DSC) results has demonstrated that cholesterol, Span 60, and SA were in crystal structure exhibiting sharp melting peaks at 148.7, 55.7, and 55.0°C (Fig. 6(d–f)). Two different peaks were observed for Span 60 (Fig. 6(f)). The first peak at 55.7°C was the melting point of the molecule in crystal structure, and the second peak at 132.7°C showed the flash point. The melting points of each sample were compatible with literature data (36). The DSC analysis carried out with Pluronic F127 revealed the melting point of the copolymer at 42.9°C.
Fig. 6.
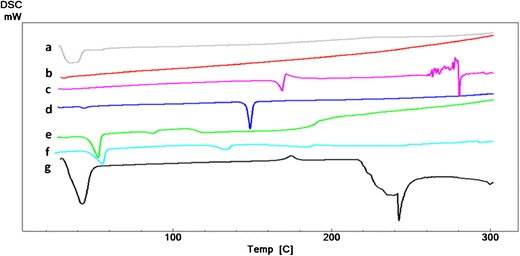
Differential scanning calorimetry (DSC) thermograms of a physical mixture of candesartan cilexetil (CC) + cholesterol + Span 60 + stearylamine (SA) + Pluronic P85, b F9 niosomes, c candesartan cilexetil (CC), d cholesterol, e Span 60, f stearylamine (SA), and g Pluronic P85
Within the studied temperature range, the DSC thermal curve of lyophilized niosomes did not show any thermal effect (Fig. 6(b)). The characteristic peak of CC at 168°C was not present in the thermogram of lyophilized F9 niosome (Fig. 6(b)) demonstrating the incorporation of CC in niosomes and formation of its amorphous form after incorporation (6,35,37). The molecular distribution of amorphous CC in niosomes can explain this situation as stated by Dong and Feng (38). The formation of amorphous structure of CC after niosomal entrapment can be considered as an advantage in terms of enhancing the drug release. Disappearance of the CC peak can be also attributed to the high interactions between CC and niosome components that leads to high encapsulation efficiency (39).
The peaks of Pluronic P85 and Span 60 were preserved in the physical mixture, but they became smaller compared to their single thermograms demonstrating the partial conversion to amorphous structure or their lower amounts in the aluminum pan (Fig. 6(a)).
CONCLUSION
In this study, niosomes and mixed niosomes encapsulating a poorly water-soluble drug (CC) were successfully prepared by using different compositions. Among the mixed niosome formulations prepared by combining Span 60 and Pluronic P85, F9-mixed niosomes containing low level of SA as charge-inducing agent represented better sedimentation stability in storage conditions and improved dissolution rate of CC. Besides, they demonstrated remarkable stability against bile salt disruption conditions. Our findings suggest F9-mixed niosomes as worthy of further exploration because they might have great potential in improving the oral bioavailability of poorly soluble drugs and getting into the drug market.
Acknowledgments
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Meringer P, Winter M. Clinical pharmacokinetics and pharmacodynamics. In: Troy D, editor. Remington: the science and practice of pharmacy. 59. 21. Philadelphia: Lippincott Williams & Wilkins; 2006. p. 1193. [Google Scholar]
- 2.Ensign LM, Cone R, Hanes J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv Drug Deliv Rev. 2012;64(6):557–70. doi: 10.1016/j.addr.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vauthier C. Formulating nanoparticles to achieve oral and intravenous delivery of challenging drugs. In: Tiddy G, Tan R, editors. NanoFormulation. Cambridge: RSC Publishing; 2012. [Google Scholar]
- 4.Detroja C, Chavhan S, Sawant K. Enhanced antihypertensive activity of candesartan cilexetil nanosuspension: formulation, characterization and pharmacodynamic study. Sci Pharm. 2011;79(3):635–51. doi: 10.3797/scipharm.1103-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nekkanti V, Pillai R, Venkateshwarlu V, Harisudhan T. Development and characterization of solid oral dosage form incorporating candesartan nanoparticles. Pharm Dev Technol. 2009;14(3):290–8. doi: 10.1080/10837450802585278. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Z, Gao F, Bu H, Xiao J, Li Y. Solid lipid nanoparticles loading candesartan cilexetil enhance oral bioavailability: in vitro characteristics and absorption mechanism in rats. Nanomedicine. 2012;8(5):740–7. doi: 10.1016/j.nano.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Gao F, Zhang Z, Bu H, Huang Y, Gao Z, Shen J, et al. Nanoemulsion improves the oral absorption of candesartan cilexetil in rats: performance and mechanism. J Control Release. 2011;149(2):168–74. doi: 10.1016/j.jconrel.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Vaculikova E, Grunwaldova V, Kral V, Dohnal J, Jampilek J. Preparation of candesartan and atorvastatin nanoparticles by solvent evaporation. Molecules. 2012;17(11):13221–34. doi: 10.3390/molecules171113221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Z, Gao F, Bu H, Xiao J, Li Y. Solid lipid nanoparticles loading candesartan cilexetil enhance oral bioavailability: in vitro characteristics and absorption mechanism in rats. Nanomedicine. 2012;8(5):740–7. doi: 10.1016/j.nano.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 10.Tavano L, Vivacqua M, Carito V, Muzzalupo R, Caroleo MC, Nicoletta F. Doxorubicin loaded magneto-niosomes for targeted drug delivery. Colloids Surf B: Biointerfaces. 2013;102:803–7. doi: 10.1016/j.colsurfb.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 11.Sezgin-Bayindir Z, Onay-Besikci A, Vural N, Yuksel N. Niosomes encapsulating paclitaxel for oral bioavailability enhancement: preparation, characterization, pharmacokinetics and biodistribution. J Microencapsul. 2013;30:796–804. doi: 10.3109/02652048.2013.788088. [DOI] [PubMed] [Google Scholar]
- 12.Carafa M, Santucci E, Alhaique F, Coviello T, Murtas E, Riccieri FM, et al. Preparation and properties of new unilamellar non-ionic:ionic surfactant vesicles. Int J Pharm. 1998;160:51–9. doi: 10.1016/S0378-5173(97)00294-9. [DOI] [Google Scholar]
- 13.Kurumada K, Robinson BH. Viscosity studies of Pluronic F127 in aqueous solution. Prog Colloid Polym Sci. 2004;123:12–5. [Google Scholar]
- 14.Liu T, Guo R. Preparation of a highly stable niosome and its hydrotrope-solubilization action to drugs. Langmuir ACS J Surf Colloids. 2005;21(24):11034–9. doi: 10.1021/la051868b. [DOI] [PubMed] [Google Scholar]
- 15.Win KY, Feng SS. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials. 2005;26(15):2713–22. doi: 10.1016/j.biomaterials.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 16.Pardakhty A, Moazeni E, Varshosaz J, Hajhashemi V, Rouholamini NA. Pharmacokinetic study of niosome-loaded insulin in diabetic rats. Daru. 2011;19(6):404–11. [PMC free article] [PubMed] [Google Scholar]
- 17.Yang DB, Zhu JB, Huang ZJ, Ren HX, Zheng ZJ. Synthesis and application of poly(ethylene glycol)-cholesterol (Chol-PEGm) conjugates in physicochemical characterization of nonionic surfactant vesicles. Colloids Surf B: Biointerfaces. 2008;63(2):192–9. doi: 10.1016/j.colsurfb.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 18.De S, Robinson DH. Particle size and temperature effect on the physical stability of PLGA nanospheres and microspheres containing Bodipy. AAPS PharmSciTech. 2004;5(4):e53. doi: 10.1208/pt050453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cevc G, Richardsen H. Lipid vesicles and membrane fusion. Adv Drug Deliv Rev. 1999;38(3):207–32. doi: 10.1016/S0169-409X(99)00030-7. [DOI] [PubMed] [Google Scholar]
- 20.Uchegbu IF, Florence AT. Non-ionic surfactant vesicles (niosomes): physical and pharmaceutical chemistry. Adv Colloid Interf Sci. 1995;58:1–55. doi: 10.1016/0001-8686(95)00242-I. [DOI] [Google Scholar]
- 21.An L, Pan Y, Shen X, Lu H, Yang Y. Rod-like attapulgite/polyimide nanocomposites with simultaneously improved strength, toughness, thermal stability and related mechanisms. J Mater Chem. 2008;18:4928–41. doi: 10.1039/b805849k. [DOI] [Google Scholar]
- 22.Lentz BR, Carpenter TJ, Alford DR. Spontaneous fusion of phosphatidylcholine small unilamellar vesicles in the fluid phase. Biochemistry. 1987;26(17):5389–97. doi: 10.1021/bi00391a026. [DOI] [PubMed] [Google Scholar]
- 23.Nutan MTH, Reddy IK. General principles of suspensions. In: Kulshreshtha AK, Singh ON, Wall GM, editors. Pharmaceutical suspensions: from formulation development to manufacturing. New York: Springer; 2010. pp. 52–5. [Google Scholar]
- 24.Bayindir ZS, Yuksel N. Characterization of niosomes prepared with various nonionic surfactants for paclitaxel oral delivery. J Pharm Sci. 2010;99(4):2049–60. doi: 10.1002/jps.21944. [DOI] [PubMed] [Google Scholar]
- 25.Rowland RN, Woodley JF. The stability of liposomes in vitro to pH, bile salts and pancreatic lipase. Biochim Biophys Acta. 1980;620(3):400–9. doi: 10.1016/0005-2760(80)90131-9. [DOI] [PubMed] [Google Scholar]
- 26.Chiang CM, Weiner N. Gastrointestinal uptake of liposomes. 1. In vitro and in situ studies. Int J Pharm. 1987;37(1–2):75–85. doi: 10.1016/0378-5173(87)90011-1. [DOI] [Google Scholar]
- 27.Li H, Yu Y, Faraji Dana S, Li B, Lee CY, Kang L. Novel engineered systems for oral, mucosal and transdermal drug delivery. J Drug Target. 2013;21(7):611–29. doi: 10.3109/1061186X.2013.805335. [DOI] [PubMed] [Google Scholar]
- 28.Freund O, Amedee J, Roux D, Laversanne R. In vitro and in vivo stability of new multilamellar vesicles. Life Sci. 2000;67(4):411–9. doi: 10.1016/S0024-3205(00)00640-8. [DOI] [PubMed] [Google Scholar]
- 29.Gadras C, Santaella C, Vierling P. Improved stability of highly fluorinated phospholipid-based vesicles in the presence of bile salts. J Control Release. 1999;57(1):29–34. doi: 10.1016/S0168-3659(98)00109-6. [DOI] [PubMed] [Google Scholar]
- 30.Di Marzio L, Esposito S, Rinaldi F, Marianecci C, Carafa M. Polysorbate 20 vesicles as oral delivery system: in vitro characterization. Colloids Surf B Biointerfaces. 2013;104:200–6. doi: 10.1016/j.colsurfb.2012.10.036. [DOI] [PubMed] [Google Scholar]
- 31.Sihorkar V, Vyas SP. Polysaccharide coated niosomes for oral drug delivery: formulation and in vitro stability studies. Pharmazie. 2000;55(2):107–13. [PubMed] [Google Scholar]
- 32.Kumar P, Bohidar HB. Aqueous dispersion stability of multi-carbon nanoparticles in anionic, cationic, neutral, bile salt and pulmonary surfactant solutions. Colloids Surf A Physicochem Eng Asp. 2010;361:13–24. doi: 10.1016/j.colsurfa.2010.03.009. [DOI] [Google Scholar]
- 33.Aboelwafa AA, El-Setouhy DA, Elmeshad AN. Comparative study on the effects of some polyoxyethylene alkyl ether and sorbitan fatty acid ester surfactants on the performance of transdermal carvedilol proniosomal gel using experimental design. AAPS PharmSciTech. 2010;11(4):1591–602. doi: 10.1208/s12249-010-9539-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guinedi AS, Mortada ND, Mansour S, Hathout RM. Preparation and evaluation of reverse-phase evaporation and multilamellar niosomes as ophthalmic carriers of acetazolamide. Int J Pharm. 2005;306(1–2):71–82. doi: 10.1016/j.ijpharm.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 35.Satturwar P, Eddine MN, Ravenelle F, Leroux JC. pH-responsive polymeric micelles of poly(ethylene glycol)-b-poly(alkyl(meth)acrylate-co-methacrylic acid): influence of the copolymer composition on self-assembling properties and release of candesartan cilexetil. Eur J Pharm Biopharm. 2007;65(3):379–87. doi: 10.1016/j.ejpb.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 36.Villasmil-Sanchez S, Rabasco AM, Gonzalez-Rodriguez ML. Thermal and 31P-NMR studies to elucidate sumatriptan succinate entrapment behavior in phosphatidylcholine/cholesterol liposomes. Comparative 31P-NMR analysis on negatively and positively-charged liposomes. Colloids Surf B: Biointerfaces. 2013;105:14–23. doi: 10.1016/j.colsurfb.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 37.Wang M, Yuan Y, Gao Y, Ma HM, Xu HT, Zhang XN, et al. Preparation and characterization of 5-fluorouracil pH-sensitive niosome and its tumor-targeted evaluation: in vitro and in vivo. Drug Dev Ind Pharm. 2012;38(9):1134–41. doi: 10.3109/03639045.2011.641565. [DOI] [PubMed] [Google Scholar]
- 38.Dong Y, Feng SS. Poly(d, l-lactide-co-glycolide)/montmorillonite nanoparticles for oral delivery of anticancer drugs. Biomaterials. 2005;26(30):6068–76. doi: 10.1016/j.biomaterials.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 39.Nasr M, Mansour S, Mortada ND, Elshamy AA. Vesicular aceclofenac systems: a comparative study between liposomes and niosomes. J Microencapsul. 2008;25(7):499–512. doi: 10.1080/02652040802055411. [DOI] [PubMed] [Google Scholar]


