Abstract
Pressurized metered dose inhalers (pMDIs) are frequently used for the treatment of asthma and chronic obstructive pulmonary disease. The aerodynamic particle size distribution (APSD) of the residual particles delivered from a pMDI plays a key role in determining the amount and region of drug deposition in the lung and thereby the efficacy of the inhaler. In this study, a simulation model that predicts the APSD of residual particles from suspension pMDIs was utilized to identify the primary determinants for APSD. These findings were then applied to better understand the effect of changing drug concentration and micronized drug size on experimentally observed APSDs determined through Andersen Cascade Impactor testing. The experimental formulations evaluated had micronized drug mass median aerodynamic diameters (MMAD) between 1.2 and 2.6 μm and drug concentrations ranging from 0.01 to 1% (w/w) with 8.5% (w/w) ethanol in 1,1,1,2-tetrafluoroethane (HFA-134a). It was determined that the drug concentration, micronized drug size, and initially atomized droplet distribution have a significant impact in modulating the proportion of atomized droplets that contain multiple suspended drug particles, which in turn increases the residual APSD. These factors were found to be predictive of the residual particle MMAD for experimental suspension HFA-134a formulations containing ethanol. The empirical algebraic model allows predicting the residual particle size for a variety of suspension formulations with an average error of 0.096 μm (standard deviation of 0.1 μm).
KEY WORDS: aerodynamic particle size distribution (APSD), formulation, pressurized metered dose inhaler (pMDI), suspension
INTRODUCTION
Inhalation drug therapy is the primary modality of drug delivery for patients that suffer from asthma or chronic obstructive pulmonary disease (COPD). It allows for topical treatment of the lungs, while minimizing systemic exposure of the drug. One of the widely utilized methods for delivering drugs to the lungs is through pressurized metered dose inhalers (pMDIs), which use pressurized propellant as the energy source to atomize formulation into droplets containing medications. In addition to pMDI formulations containing propellants and drug(s), they may also contain cosolvents (e.g., ethanol) and/or dissolved excipients. The drug may be dissolved in the formulation, rendering a solution, or the micronized drug particles may be dispersed in the formulation, rendering a suspension.
The clinical efficacy from any pMDI depends on a variety of clinical and delivery system factors. While many clinical factors are independent of the device, developing a delivery system that can produce residual particles that can efficiently deposit in the patient’s lung is paramount to the success of the inhaler. One of the primary performance metrics evaluated for pharmaceutical aerosols is the residual aerodynamic particle size distribution (APSD) of the aerosolized drug product. The residual APSD is characterized by the residual mass median aerodynamic diameter (MMAD) and the geometric standard deviation (GSD). The MMAD signifies the aerodynamic diameter at which half of the aerosolized drug mass lies below the stated diameter. Particles with an aerodynamic diameter of approximately 0.5 to 5 μm have the highest probability of depositing in the lung, with the smaller particles having a greater probability to penetrate into the deep lung. Particles with aerodynamic particles much larger than 5 μm tend to impact in the oropharyngeal cavity (1). The larger the GSD value, the greater the spread of the aerodynamic diameters of the residual particles.
When a pMDI is actuated, the formulation exits a spray orifice and is broken up into many initial atomized droplets that contain a representative proportion of each excipient that is dissolved in the formulation. If the drug is in solution, the atomized droplets will also contain an identical proportion of drug as that found in the formulation. However, if the drug is suspended in the formulation, varying number of drug particles are found in a given droplet, with the concentration and size of the solid suspended drug and the size of the initial droplet influencing the number of drug particles in a given droplet (2). Given the volatile nature of the propellant, the propellant rapidly evaporates rendering an intermediate droplet that contains cosolvent, non-volatile excipient and the drug. The cosolvent also evaporates over the due course of droplet evolution leaving residual particles (if complete evaporation occurs) that constitute of only non-volatile mass, which can deposit in the human lungs. These residual particles are significantly smaller than their corresponding initial droplets.
For formulations where the drug is in solution in the propellant-cosolvent system, each atomized droplet contains a proportional amount of drug as that found in the bulk formulation. Throughout the process of droplet evolution from initial droplets into residual particles, each residual particle will contain the same amount of drug as that in its corresponding initial droplet. This amount of drug will contribute to the residual APSD. Thus, the residual particle size for solution pMDI formulations is dependent on the size distribution of the initial droplets and the concentration of the drug. The residual APSD is readily estimated by Eq. 1. Formulations with larger initial droplet sizes result in a larger residual APSD compared to those with smaller initial droplet sizes.
| 1 |
where MMADR (μm) is the mass median aerodynamic diameter of the residual particles, MMDI (μm) is the mass median diameter (MMD) of the initial droplets, CNV (weight fraction) is the concentration of the non-volatile components (e.g., dissolved drug and excipients) in the formulation, and ρI and ρR are the densities (g/cm3) of the formulation and the residual particles, respectively.
The residual APSD from suspension pMDI formulations cannot be adequately described by a simple equation. Atomized droplets may contain some number (i.e., 0, 1, 2, 3, etc.) of different sized suspended drug particles. The probability that a given initial droplet will contain a certain number of drug particles can be described by a Poisson distribution (3,4). The Poisson distribution is a function of the volume of the initial droplet and the number of suspended drug particles per unit volume (PPUV) in the formulation. The PPUV increases with decreasing drug size and increasing drug concentration. Thus, it is expected that the residual APSD for suspension pMDI formulations is largely dependent on the size of the drug particles and the proportion of droplets that contain multiple drug particles. However, a simple and empirical algebraic expression to describe this relationship for suspension pMDIs does not exist.
A useful model that does not require an arsenal of technical calculations and experimentation would greatly benefit the formulation development and quality control process for suspension pMDI manufacturing. It would allow for improved high-throughput screening of plausible suspension pMDI products, decreased time and resource investment in current trial-and-error approach to evaluating test pMDIs, and better understanding of the link between pMDI device and formulation variables to product performance. The objective of this study is to thoroughly evaluate the dependency of various parameters (i.e., micronized drug particle size distribution, drug concentration, and initially atomized droplet size) that affect the residual APSD of suspension 1,1,1,2-tetrafluoroethane (HFA-134a) pMDIs through a previously published simulation model (5,6) along with experimental in vitro cascade impactor data. These factors are analyzed as they relate to intermediate factors such as the proportion of drug-containing droplets that contain multiple suspended drug particles and PPUV. The results presented herein will provide the understanding necessary to facilitate suspension pMDI formulation and device design.
EXPERIMENTAL METHODS
Computational Algorithm
A Monte Carlo simulation model has been developed in Microsoft Visual Basic® 6.5 and embedded into Microsoft Excel® 2007 (Redmond, WA, USA) to predict residual APSD from hydrofluoroalkane pMDI formulations containing a single drug in suspension with varying cosolvent concentration, actuator orifice diameter, and metering valve size (5,6). The algorithm for this model is presented in Fig. 1. This model assumes that the formulation is an ideal suspension without preferential interactions of any component of the system. To simulate the residual particle size of any given pMDI formulation, the user provides formulation details, and the size distributions of the initial atomized droplets and micronized drug. For the simulations presented in this paper, Eq. 2 was used to calculate the effective initial droplet MMD for aerosol particles that would be sized by the cascade impactor (7), based on knowledge of the formulation and device parameters. Each atomized droplet is then simulated individually until sufficient drug-containing residual particles (at least 10,000 particles) are obtained to provide a representative size distribution estimate (8). For any given droplet, a physical initial droplet diameter is randomly selected from the lognormal distribution determined from Eq. 2 with a GSD of 1.8 (7). Once the volume of the droplet is calculated, a cumulative Poisson distribution is utilized to randomly determine the number of drug particles in the droplet based on the droplet volume and PPUV (see Eq. 3). The size of each drug particle within that droplet is then randomly selected based on the lognormal size distribution of the micronized drug. Thereafter, the aerodynamic diameter of the residual particle is calculated as a factor of the number, volume, and density of drug particles contained within the residual particle. The size distribution of all the residual particles simulated is then determined using statistical software as presented in the Statistical Analysis section.
| 2 |
Fig. 1.
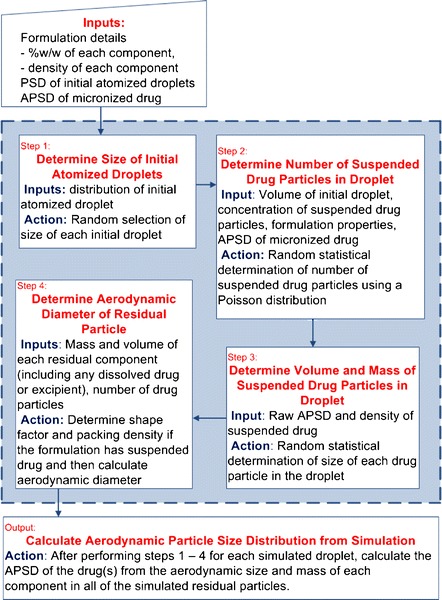
Depiction of the Monte Carlo simulation algorithm utilized to model suspension pMDI formulations. Steps 1 to 4 are repeated until sufficient initial droplets are simulated. Particle size distribution is abbreviated as PSD. All other abbreviations can be found in text
where VS (μL) is the metering valve size, CEtOH (weight fraction) is the concentration of the ethanol in the formulation, and OD (mm) is the actuator orifice diameter.
| 3 |
where PPUV (#/cm3) is the number of suspended drug particles per unit volume, Cdrug (weight fraction) is the concentration of the drug, ρdrug (g/cm3) is the drug particle density, and GSDdrug and MMDdrug (μm) are the geometric standard deviation and mass median diameter, respectively, of the micronized drug used in the formulation.
The Poisson distribution is a discrete distribution that describes the probability that a given droplet, of a defined size, will contain some number of drug particles. It accounts for the size of the simulated initial droplet and the PPUV, to determine the probability that the droplet will have a given number of drug particles. Using a uniform 0 to 1 random number generator (R) and the droplet-specific cumulative Poisson distribution, a specific number of drug particles are found to be contained in the droplet being simulated based on the R value, which denotes the probability of the fraction less than within the Poisson distribution. If the R value is close to one, the droplet will contain a relatively larger number of drug particles; if the value is close to zero, the droplet will contain very few or no drug particles.
One way of characterizing the residual particle distribution, other than using the MMAD and GSD, is to evaluate the proportion of drug-containing particles that contain one or multiple drug particles. Atomized particles that do not contain drug do not contribute mass or size to the APSD of the drug and can therefore be ignored in this analysis. Residual drug-containing particles that contain only one drug particle are termed as “singlets,” while those that contain more than one drug particle are termed as “multiplets.” Since the sum of the percentage of singlets and multiplets always equals 100, generally only one of values will be provided for a given simulation.
Simulated APSD—Description of Configurations Evaluated
To better understand the sensitivity of the residual MMAD on the effect of changing micronized drug MMAD, drug concentration, and initial droplet MMD, several theoretical formulations were simulated with a variety of HFA-134a suspension pMDI configurations. For all simulations, unless otherwise noted, the initial droplet MMD was 10.7 μm (which corresponds with the initial MMD predicted from Eq. 2 using a 50 μL valve size and 0.3 mm actuator orifice diameter), with a GSD of 1.8 (7). The ethanol concentration was fixed at 8% (w/w). The model drug concentrations ranged from 0.002 to 4% (w/w) with micronized drug size varying from 0.5 to 3 μm MMAD (ρdrug = 1.25 g/cm3).
Materials
Micronized albuterol sulfate were provided by 3M Drug Delivery Systems (St. Paul, MN, USA) and Micron Technologies Limited (Dartfort, Kent, UK). Metering valves, actuators, and Pluronic L10 (BASF Corp., Florham Park, NJ, USA) were provided by 3M Drug Delivery Systems. Pressure resistant glass vials were purchased from Research Products International Corporation (Mt. Prospect, IL, USA). 200 proof ethanol was purchased from Decon Labs (King of Prussia, PA, USA) and HFA-134a, from Atofina Chemicals Incorporated (Philadelphia, PA, USA). HPLC-grade methanol and phosphoric acid were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Evaluated Experimental Formulations
Three different lots of micronized albuterol sulfate were used. The particle size distribution of two lots were determined using the Model 3321 Aerodynamic Particle Sizer Spectrometer™ (APS) with the Model 3433 Small Scale Powder Disperser (both from TSI Inc., Shoreview, MN, USA). The two lots evaluated had a micronized MMAD of 2.62 μm (GSD of 1.81) and 1.77 μm (GSD of 1.57). A third drug lot was obtained by high shear homogenization of the 2.62 μm lot in 200 proof ethanol using a technique described by Jinks (9) and James et al. (10). The particle size of this lot was determined to have a size distribution of 1.22 μm MMAD (GSD of 1.57) using the Malvern Mastersizer 2000 (Malvern Instruments Ltd., Malvern, Worcestershire, UK)
A total of 12 suspension pMDI formulations we prepared containing one of three different sizes of the micronized albuterol sulfate at one of four concentrations between 0.01 to 1% (w/w), with a nominal 8.5% (w/w) 200 proof ethanol in HFA-134a were prepared in pressure-resistant glass vials fitted with 50 μL Spraymiser™ metering valves. Once the vials contained the desired weight of drug and ethanol, HFA-134a was filled using a cold-transfer technique. Each of the vials was crimped with a valve using a small-scale bottle crimper. Immediately, prior to using the vials, the vials were sonicated for approximately 1 min to disperse the suspension.
Andersen Cascade Impactor Testing
Before beginning each Andersen Cascade Impactor (ACI) test, the stages of the ACI were rinsed with 50% methanol in water, followed by 100% methanol and air-dried. Once dry, the stages and throat were coated with 50:50 methanol/Pluronic L10 and the methanol was allowed to evaporate in order to prevent particle bounce. Actuators (orifice diameter of 0.3 mm and jet length of 0.7 mm with round mouthpiece of 20 mm) were used for all tests. The sequence of testing aerosol vials was randomized with respect to drug concentration. For each vial, the vial was actuated three times to prime the valve, and the stem of the valve was subsequently rinsed with the diluent, 77:23 water/methanol. Once the valve stem was dry, it was fitted with a clean actuator. The air flow through the ACI was adjusted to 28.3 L/min and was verified using a TSI Series 4000 flow meter (TSI Inc., Shoreview, MN, USA). Each vial was tested in triplicate, and the number of actuations for each test varied between 2 and 25, based on the drug concentration of the vial used. The vial was hand-shaken in a slow, rolling manner for 20 s between actuations to aid dispersing the suspension formulation and prevent cooling effects on delivered dose. The valve stem, actuator, USP throat, stages 0 to 7, and the filter were rinsed with appropriate volumes of the diluent. The amount of drug deposited on each part was determined by high-performance liquid chromatography (HPLC).
Analytical Assay
The HPLC system consisted of a Waters 2690 Separations Module coupled to a Waters 996 Photodiode Array Detector (both from Waters, Milford, MA, USA). An Apollo C18 5 μm 150 mm × 4.6 mm column was used. Forty microliters of the sample was injected onto the column, and the mobile phase used for the separation was 0.1% phosphoric acid/methanol (77:23 (v/v)) at a flow rate of 0.75 mL/min. The data was collected and processed using Millennium Version 3.20 with UV detection at λmax of albuterol sulfate (225 nm). Quantitation was conducted based on peak area using a linear standard curve between 0.250 and 250 μg/mL albuterol sulfate. The retention time for the drug was 3.3 min. No leachable or extractable compounds from vials or bags used to rinse the ACI stages were detected upon analysis of the HPLC data.
Statistical Analysis
Residual APSDs were determined using Chimera Technologies’ DistFit™ (Forest Lake, MN, USA), a data fitting program. Data from simulations and experimental tests were assumed to have a unimodal lognormal distribution. For simulated data, the aerodynamic diameters and corresponding mass for the 10,000 droplets simulated were grouped into 20 bins. These data were then inputted into DistFit to determine the MMAD and GSD of the aerosol. For each ACI run, the residual APSD of the albuterol sulfate was determined based off of HPLC results. The cumulative drug mass percentage recovered in the ACI on each stage was included in the particle size analysis. A χ2 goodness-of-fit test with a p value <0.02 was assumed to yield a reasonable lognormal fit for the data.
Multiple linear regressions presented in this paper were performed using Stata Corporation’s Stata® 10.0 (College Station, TX, USA). Analysis of variance presented in this paper was performed using Minitab® 17 (Minitab Inc., State College, PA, USA). An a priori p value of 0.05 was used to delineate statistical significance. Adjusted R2 values have been reported for any multiple linear regression models with more than one independent variable.
RESULTS
Factors Influencing the Fraction of Atomized Droplets That Contain Drug Particles
For a single actuation of a pMDI, 700,000 to 300,000,000 droplets can be atomized depending on the formulation and hardware configuration (11). However, for a suspension pMDI formulation, only some of the atomized droplets contain drug particles. The size and concentration of the micronized drug in a suspension pMDI formulation are two important factors that influence the number of atomized droplets that contain drug particles. Figures 2 and 3 present simulated data for representative HFA-134a suspension formulations with varying concentrations of drug and 8% (w/w) ethanol; the data in Fig. 2 is for a micronized suspended drug size of 1.5 μm MMAD (GSD of 2.0), while Fig. 3 represents a 2.5 μm MMAD (GSD of 2.0) distribution.
Fig. 2.
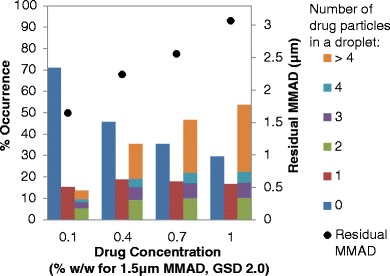
The approximate occurrence of residual particles containing 0, 1, or more than 1 drug particle (left y-axis) and residual MMAD (right y-axis) with respect to drug concentration for micronized suspended drug with a MMAD of 1.5 μm (GSD of 2.0) for a HFA-134a formulation containing 8% (w/w) ethanol and no other excipients
Fig. 3.
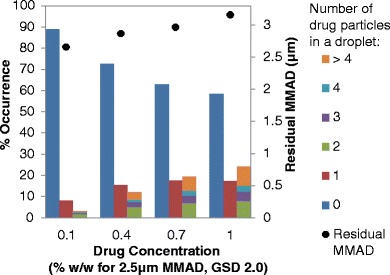
The approximate occurrence of residual particles containing 0, 1, or more than 1 drug particle (left y-axis) and residual MMAD (right y-axis) with respect to drug concentration for micronized suspended drug with a MMAD of 2.5 μm (GSD of 2.0) for a HFA-134a formulation containing 8% (w/w) ethanol and no other excipients
For all of the micronized drug sizes or concentrations examined, a significant portion of atomized droplets do not contain any drug particles, as represented by the column on the left for each drug concentration (i.e., the dark blue columns) on the bar charts (see Figs. 2 and 3). For example, for formulations with 2.5 μm MMAD drug, the percentage of atomized droplets that did not contain drug particles ranged from 58% (at a drug concentration of 1% (w/w)) to 89% (at a drug concentration of 0.1% (w/w)). Formulations with 1.5 μm MMAD micronized drug had fewer “empty” droplets (i.e., droplets without suspended drug particles) compared to formulations with 2.5 μm MMAD micronized drug, although 30 to 71% of the simulated droplets did not contain drug for the 1.5 μm MMAD micronized drug depending on drug concentration of the formulations examined. In addition, of the droplets that contain drug particles, many have only one drug particle, but some droplets contain multiple drug particles.
The number of atomized droplets that contain drug particles is directly related to the number of drug PPUV in the formulation. Increasing the drug concentration or decreasing the size of the micronized drug results in an increased PPUV, which correspondingly increases the number of atomized droplets containing drug. The percentage of atomized droplets that contain suspended drug particles for the formulations presented in Figs. 2 and 3 can be determined by summing the middle and right bars for each drug concentration series or simply subtracting the percentage of atomized droplets that do not contain drug particles from 100%. Increasing the suspended drug concentration from 0.1 to 1% (w/w) increased the percentage of drug-containing droplets from 29 to 70% for 1.5 μm MMAD (see Fig. 2) and 11 to 42% for 2.5 μm MMAD drug formulation (see Fig. 3).
In addition to the drug concentration and micronized drug size, the initial droplet size also influences the proportion of atomized droplets that contain suspended drug particles. Figure 4 presents the percentage of aerosolized droplets that contain micronized drug for three different initial droplet sizes using a 2.5 μm MMAD model micronized drug (GSD of 2.0) and a range of drug concentrations (0.1 to 1% w/w) from a HFA-134a system with 8% (w/w) ethanol. Atomized droplets with larger initial droplet MMDs and higher drug concentrations tend to have more drug-containing droplets than droplets with smaller initial droplet MMDs and lower drug concentrations as presented by Fig. 4 and further evaluated by Sheth et al. (2). Increasing the simulated initial droplet MMD from 9.1 to 12.3 μm results in increasing the proportion of drug-containing droplets in a sample of atomized droplets from 11.2 to 15.3% and from 41.6 to 50.8% for formulations with 0.1 and 1% (w/w) 2.5 μm MMAD drug, respectively.
Fig. 4.
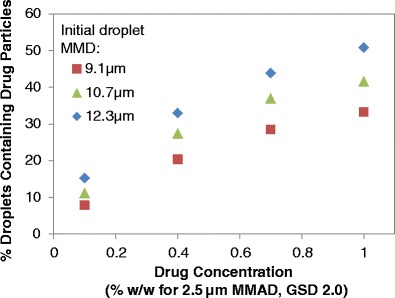
The proportion of atomized droplets containing suspended drug particles for formulations with 0.1 to 1% (w/w) drug for a HFA-134a formulation containing 8% (w/w) ethanol. Three different sizes of initial droplet sizes were evaluated: 9.1, 10.7, and 12.3 μm (GSD of 1.8). The MMAD of the suspended micronized drug is 2.5 μm (GSD of 2.0). No other excipients were included in the simulated formulation
Factors Influencing the Fraction of Multiplets
Factors that influence the proportion of atomized droplets from a pMDI suspension formulation that contain suspended drug particles also influence the proportion of drug-containing atomized droplets that contain single or multiple drug particles. Thus, the micronized drug size and concentration, and the initial droplet size distribution influence percentage of singlets and multiplets in samples of drug-containing droplets.
The Influence of Micronized Drug Size and Concentration on the Proportion of Multiplets
Increasing the micronized drug size for a given drug concentration results in an increase in the occurrence of singlets and a significant decrease in multiplets among a sample of drug-containing droplets. For instance, for formulations with 0.1% (w/w) suspended 1.5 μm MMAD micronized drug, 15.3% of atomized droplets contain a single drug particle and 13.7% contain more than one drug particle, as determined from simulations (see Fig. 2). This can also be expressed as 53% singlets and 47% multiplets among a sample of drug-containing droplets for this formulation. Similarly, for formulations containing 0.1% (w/w) suspended 2.5 μm MMAD micronized drug, 8.2% of atomized droplets contain a single drug particle and 3.1% of atomized droplets contain more than one drug particle, which equals to 73% singlets and 27% multiplets (see Fig. 3). Thus, increasing the micronized drug size from 1.5 to 2.5 μm MMAD results in far fewer multiplets being present which, as will be discussed later, has a significant influence on the MMAD of the residual aerosol. Similarly, the proportion of droplets containing multiple drug particles starkly increases with increasing drug concentration. In particular, Figs. 2 and 3 show that the number of droplets containing greater than four drug particles dramatically increases with increasing drug concentration or decreasing micronized drug particle size. For example, with 0.1% (w/w) 2.5 μm MMAD micronized drug, there are only 0.5% of drug-containing particles that contain more than four drug particles; increasing the concentration to 1% (w/w) leads to increasing the percentage of drug-containing particles with more than four drug particles to 9.0% (an 18.2-fold increase).
Similar trends are seen with a wide variety of formulations with a fixed initial droplet size distribution. Figure 5 presents a contour plot for percentage of multiplets as a factor of micronized drug size and drug concentration for HFA-134a formulations with 8% (w/w) ethanol. A total of 60 simulations were conducted to define the design space presented in Fig. 5. As previously described, changes in the drug concentration and MMAD of the micronized drug have a systematic effect on the percent of multiplets. For instance, for simulations at 0.1% (w/w) drug concentration, there are approximately 81 and 16% multiplets, for 0.5 and 3 μm MMAD micronized drug, respectively. The percent of multiplets is also impacted by the drug concentration to a lesser extent than micronized drug size. This is due to the fact that the number concentration of suspended drug particles in the formulation (PPUV, see Eq. 3) is proportional to the drug concentration to the first power, but inversely proportional to the MMAD of the drug to the third power.
Fig. 5.
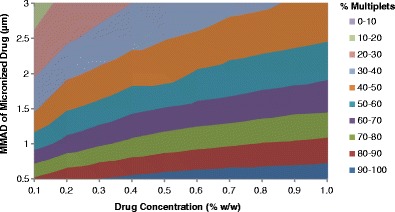
The percentage of simulated aerosolized droplets that contain two or more drug particles in a sample of only drug-containing particles as a function of the drug concentration and the MMAD of the micronized drug. All simulations contained micronized drug with a GSD of 1.8, 8% (w/w) ethanol in HFA-134a; no other excipients were included in the simulations
Effect of Initial Droplet Size Distribution on Percentage of Multiplets
The initially atomized droplets from a pMDI are not entirely uniform in physical size; actually, these droplets follow a lognormal distribution with a given mass median diameter (MMD) and GSD (7). For the simulated data presented in this paper, the MMD of the initial droplet was empirically estimated using Eq. 2 and the GSD was assumed to be 1.8 for the USP inlet based on the research conducted by Stein and Myrdal (7). Increasing the initial droplet MMD results in a greater propensity of having larger initial droplets within the initial droplet distribution compared to smaller initial droplet MMD.
Simulations were done in order to assess how the initial droplet size influences the proportion of multiplets in the aerosol. The initial droplet MMD can be varied based on device parameters (i.e., metering valve size and actuator orifice diameter) and formulation (i.e., concentration of cosolvent), as presented in Eq. 2. For the simulations presented in Fig. 6, the valve size and orifice diameters were varied while the ethanol concentration was fixed at 8% (w/w) to modulate the initial droplet MMD while the formulation was fixed with 0.4% (w/w) suspended drug (having either 1.0 or 2.5 μm MMAD). With the increase in initial droplet MMD, there is a significant decrease in percent singlets and increase in percent multiplets. For instance, for a formulation containing 1.0 μm MMAD micronized drug, the percentage of drug-containing droplets that contained only one drug particle decreased from 37.8 to 20.7% as the initial droplet MMD is increased from 8.0 to 13.0 μm. Moreover, with the increase in initial droplet size, the percentage of droplets that contain a large number of drug particles greatly increased. This is true for both formulations, but is especially evident for formulations with smaller micronized drug particle size. For example, 29.4% of drug-containing atomized droplets from a 0.4% suspended formulation containing 1.0 μm MMAD micronized drug with 13.0 μm initial droplet MMD have more than nine drug particles; whereas only 11.9% for the same drug size with 8.0 μm initial droplet have more than nine drug particles. These percentages decrease starkly when the micronized drug is changed to 2.5 μm MMAD: 4.2% for the 13.0 μm and 1.0% for the 8.0 μm initial droplet MMD.
Fig. 6.
The percentage of drug-containing atomized droplets that contain some number of drug particles for 0.4% (w/w) suspended drug, with 8% ethanol in HFA-134a. The purple bars represent 2.5 μm MMAD (GSD of 1.8) micronized drug with an initial droplet MMD of 8.0 or 13.0 μm (GSD of 1.8). The green bars represent 1.0 μm MMAD (GSD of 1.8) micronized drug with an initial droplet MMD of 8.0 or 13.0 μm (GSD of 1.8)
The Relationship Between Proportion of Multiplets and the Residual MMAD
The residual particle MMAD is influenced both by the size of the micronized drug as well as the relative proportion of multiplets in the aerosol. The residual MMAD for any pMDI suspension formulation, containing no additional non-volatile excipient, is a property of the distribution of drug-containing particles. Many of the atomized droplets do not contain suspended drug particles; as a result, these droplets do not contribute to the residual size of the suspended drug. For dilute formulations, most of the droplets containing suspended drug particles are singlets. Thus, the residual MMAD mimics the MMAD of the micronized drug. However, with the increase in concentration or decrease in micronized drug size, more drug-containing droplets will have multiple drug particles, which effectively results in the residual MMAD deviating more significantly from the MMAD of the micronized drug. For a given input drug size and initial droplet MMD, the ratio of residual MMAD to the micronized drug MMAD as a function of drug concentration increases in a predictable manner, as presented in Fig. 7. At low concentrations, the ratio is approximately 1 for all three drug sizes modeled. This indicates that in relatively dilute formulations, the residual MMAD is nearly identical to that of the micronized drug MMAD. However, as the drug concentration increases, the residual MMAD also increases since more multiplets are obtained. This increase is much more dramatic for formulations with smaller micronized drug sizes since many more micronized drug particles are present in the formulation, thus increasing the likelihood of having more droplets containing multiple drug particles.
Fig. 7.
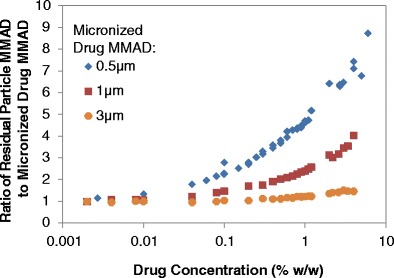
A comparison of the ratio of the residual particle MMAD to the micronized drug MMAD for various sized micronized drug at different concentrations. All simulations were conducted with 8% ethanol in HFA-134a, with no additional excipients
In addition to the concentration and size of the micronized drug, the initial droplet size distribution also has an impact on the resulting residual MMAD. Figure 6 presents data for two different micronized suspended drugs at a concentration of 0.4% (w/w) with two different initial droplet MMDs. The residual particle sizes for the four formulations depicted in Fig. 6 increased with increasing initial droplet MMD. For instance, for the 1.0 μm MMAD micronized drug, the residual MMAD was 1.6 μm for a configuration with initial droplet MMD of 8.0 μm; the residual MMAD increased to 2.4 μm when the initial droplet MMD was increased to 13.0 μm. Similar trend was seen for the 2.5 μm MMAD micronized drug: increasing the initial droplet MMD from 8.0 to 13.0 μm, resulted in increasing the residual MMAD from 2.8 to 3.2 μm. The increase in residual MMAD with increasing initial droplet MMD can be primarily attributed to the corresponding increase in percentage of multiplets. For instance, increasing the initial droplet MMD by 5.0 μm increased the percentage of multiplets from 26.7% for 8.0 μm to 44.6% for 13.0 μm initial droplet MMD for the formulations with 2.5 μm micronized drug. This led to an overall increase in residual MMAD by 0.42 μm. For a larger variety of suspended pMDI formulations (with micronized drug MMAD of 0.5 to 3 μm and drug concentrations ranging from 0.004 to 1% (w/w), data not shown), increasing the initial droplet MMD from 8.6 to 13.2 μm resulted in increasing the residual MMAD for all micronized drug sizes and drug concentrations.
Relationship Between Particles per Unit Volume and Percentage of Multiplets on Residual MMAD
Interestingly, when the percentage of multiplets among drug-containing droplets or the ratio of the MMADs of the residual particle to that of the micronized drug is compared to the PPUV, the data from simulations with varying micronized drug size (0.5 to 3 μm MMAD) and varying drug concentration (0.1 to 1.2% (w/w)) all lie on the same curve, as seen in Fig. 8. The unshaded symbols in Fig. 8 correspond to the percentage of multiplets (on the left y-axis), and the shaded symbols correspond to the relative change in residual MMAD as a factor of the MMAD of the micronized drug (on the right y-axis). For a 10-fold change in drug concentration, from 0.1 to 1% (w/w), with a fixed initial droplet MMD, the PPUV for all micronized drug sizes increase by a factor of 10 (see Eq. 3). The PPUV for the 2.5 μm MMAD micronized drug ranged from 7.6 × 108 to 7.6 × 109/cm3 for 0.1 and 1% drug concentration, respectively. For the same range of drug concentrations, the PPUV for the 1.0 μm drug was much larger and ranged from 1.2 × 1010 to 1.2 × 1011/cm3. With a 10-fold increase in the PPUV, the percent multiplets for the 1.0 μm MMAD micronized drug increased by 26.9% (from 55.7 to 82.5%), and the relative MMAD increased by 60% (from ratio of 1.5 to 2.4). For the 2.5 μm MMAD drug, the same increase in the PPUV yielded a similar increase in absolute percentage of multiplets (from 21.2 to 49.1%, for 7.6 × 108 and 7.6 × 109/cm3 suspended drug particles, respectively) and a significantly lower change in the relative MMAD (21% increase, from ratio of 1.1 to 1.3) compared to that of the 1.0 μm drug.
Fig. 8.
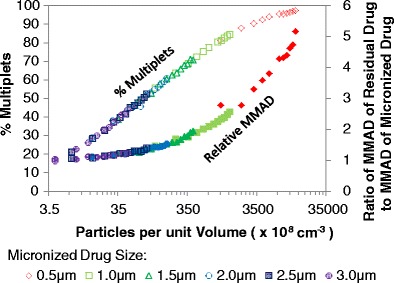
The percentage of multiplets (on the left y-axis) with the ratio of residual drug MMAD to the MMAD of the micronized drug (on the right y-axis) for a given number of particles per unit volume (PPUV). The various symbols refer to different micronized drug size (all with GSD of 1.8). Unshaded symbols correspond to the percentage of multiplets axis. Shaded symbols correspond to the MMAD ratio. All simulated formulations contained drug concentration between 0.1 and 1.2% (w/w), 8% ethanol in HFA-134a, with no additional excipients
Increasing the drug size leads to decreasing the PPUV, decreasing the percentage of multiplets, and decreasing the relative MMAD, as seen by the short segments of each series for a given curve in Fig. 8. When the PPUV is low for a formulation, the percentage of multiplets is also low. The percent of multiplets for the given initial size distribution can be modeled by Eq. 4. In addition, as evidenced with the data presented in Fig. 7, the ratio of the MMADs of the residual particle to the micronized drug is nearly 1 for formulations with low percentage of multiplets. However, with the increase in percentage of multiplets, the PPUV and the MMAD ratio also increase. In fact, the relative change in residual MMAD as a factor of the size of the micronized drug, for an initial droplet MMD of 10.7 μm with GSD of 1.8, can be modeled by Eq. 5. Increasing the initial droplet MMD from 10.7 μm would yield a greater proportion of multiplets than what is modeled by Eq. 4 and a greater ratio of residual MMAD to micronized drug MMAD than what is modeled by Eq. 5.
| 4 |
where Multiplets is the percentage of multiplets for formulations with an initial droplet MMD of 10.7 μm, and PPUV (#/cm3) is the number of drug particles per unit volume in the formulation, which can be calculated from Eq. 3.
| 5 |
where MMADR is the residual MMAD of the formulation, and MMADDrug is the micronized drug MMAD, which collectively provide the relative increase in residual MMAD as a factor of the micronized drug size. The PPUV (#/cm3) can be calculated from Eq. 3.
Comparison of Simulated Data with Experimental Data
While the above results and conclusions were determined through a simulation model developed by Stein et al. (5,6), similar trends are seen with experimental cascade impactor testing using the USP inlet. The experimental formulations evaluated had nominal concentrations by weight of 0.01% (average dose of 6.9 μg) to 1% (average dose of 480 μg) with one of three micronized drug sizes. With dilute drug concentrations, the residual MMAD closely mimics that of the micronized drug. For instance, the average residual MMAD (for the lowest concentration, (w/w)) for each of the micronized drug sizes experimentally evaluated are 1.47 ± 0.04 μm (0.010%), 1.73 ± 0.005 μm (0.033%), and 2.55 ± 0.03 μm (0.033%) for the 1.22, 1.77, and 2.62 μm MMAD micronized drug, respectively. As the drug concentration increased, the ratio of the MMAD of the residual particle to that of the micronized drug also substantially increased, indicating that the residual particle MMAD increased. When compared to simulated data, the residual MMADs of these formulations varied by a maximum of 8.8%, which corresponds to an absolute difference of 0.22 μm; the average (±standard deviation) difference is 4.03% (±4.66%), which corresponds to 0.096 μm (±0.088 μm). When comparing the experimental residual MMAD to the percentage of multiplets (determined through simulations), it is evident that the increase in the residual MMAD is closely associated with the increase in the percentage of multiplets.
Furthermore, the calculated PPUV was used to calculate the relative increase in residual MMAD as a function of the micronized drug size for formulations containing nominal 8% (w/w) ethanol in HFA-134a (see Eq. 5, above) and was compared to the experimental values. This theoretical ratio predicted the experimental ratio of residual MMAD to micronized drug MMAD with modest accuracy, with an absolute error being between 0.002 and 0.27 (average of 0.085). Equation 5 was further utilized to predict the residual MMAD for twelve experimental formulations. The experimental and predicted residual MMADs were well correlated with an R2 of 0.907, with an absolute difference of 0.010 and 0.39 μm between the two MMAD values. Of note, Eq. 5 was developed based on simulated formulations containing 8% (w/w) ethanol; however, the experimental formulations contained an average of 8.5 ± 0.3% ethanol. Not surprisingly, Eq. 5 under-predicts the residual MMAD for most of these formulations, since it assumes that the initial droplet size is smaller than if it was calculated using Eq. 2 with the experimental ethanol concentration.
Through analysis of variance on the MMAD of the residual aerosol as a function of the input drug size and drug concentration in the formulation, all terms including the interaction term and second-order terms were statistically significant (p value <0.025 for all terms) indicating strong and substantially non-linear influence of the drug size and concentration on the residual MMAD. Since Eq. 5 does not effectively predict residual MMAD from formulations with varying calculated initial droplet MMDs, a multiple linear regression model was developed using Stata 10 to further expand the range of formulations that may be evaluated. Equation 6 was found to be a reasonable empirical model with each variable having a p value <0.05 and an overall p value <0.001 with an adjusted R2 of 0.94. The residual MMAD from suspension pMDI formulations is highly dependent on the micronized drug size and concentration, and initial droplet size, which all play a key role in determining the number of multiplets among a sample of drug-containing droplets. The resulting residual MMAD from Eq. 6 was compared to experimental residual MMAD values derived from Andersen Cascade measurements, as depicted in Fig. 9. Experimental residual MMADs were well correlated to Eq. 6, with an R2 of 0.95 about the line of unity, suggesting that with the knowledge of the values for the parameters in Eq. 6, one can effectively correlate the residual MMAD from HFA-134a suspension pMDI formulations with only one drug and no other excipients.
| 6 |
Fig. 9.
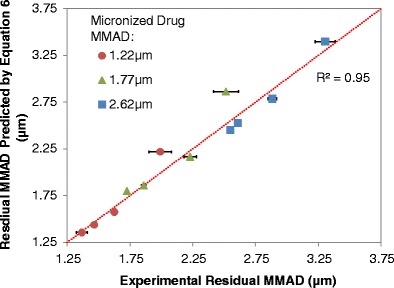
A comparison of experimental residual MMAD values derived by Andersen Cascade Impactor measurements to those values derived from Eq. 6, with 8.5% (w/w) used as the ethanol concentration for calculating the initial droplet MMD. The red dotted diagonal represents the line of unity. The model micronized drug MMAD varied from 1.22 to 2.62 μm (with varying micronized drug GSD). All formulations contained varying concentrations of drug with a nominal 8.5% (w/w) ethanol and HFA-134a. All of the aerosol vials were fitted with 50 μL Spraymiser™ valves with 0.3 mm actuator orifice diameters
where MMADR (μm) is the residual MMAD from a suspension HFA-134a pMDI formulation, MMADDrug (μm) is the micronized drug MMAD, MMDI (μm) is the initial droplet MMD (calculated from Eq. 2), and CDrug (weight fraction) is the concentration of the suspended drug.
DISCUSSION
The relationships evaluated above can be explained by considering the determinants of residual particle size for suspension pMDI formulations. Many of the atomized droplets do not contain drug; hence, they do not have an impact on the residual particle size. The residual particle MMAD is only dependent on atomized droplets that contained at least one drug particle. The primary determinants of the aerodynamic diameter of a given simulated residual particle are the size and the number of the drug particles within that droplet and the size of the droplet. As more atomized drug-containing droplets for a given formulation contain multiple drug particles, the larger the residual MMAD will be.
The Poisson distribution is utilized to determine the number of drug particles within each simulated atomized droplet, thus it also determines the percentage of multiplets among drug-containing droplets. The Poisson distribution changes for each initially atomized droplet, based on the average value of having a given number of drug particles within a droplet. This average value is defined as the product of the initial droplet volume and the particles per unit volume (PPUV) for the formulation. The initial droplet volume is a function of the initial droplet MMD. The PPUV is a calculated value and increases with increasing drug concentration and decreasing micronized drug volume. The micronized drug volume is a function of the micronized drug MMAD. This leads to a high PPUV for high concentration formulations with small micronized drug.
Equation 6 was developed to predict the residual MMAD from suspension pMDIs with ethanol in HFA-134a. It was found that the micronized drug MMAD and concentration, and initial droplet MMD (which is modulated by metering valve size, actuator orifice diameter, and cosolvent concentration and calculated from Eq. 2) are significant predictors for the residual MMAD. Interestingly, all of these variables are determinants for the percentage of multiplets in a sample of drug-containing droplets. With the increase in PPUV of the formulation (a function of micronized drug concentration and size) and initial droplet volume, the residual particle MMAD increases. Furthermore, at dilute concentrations, when the PPUV is relatively low, the residual particle size mimics that of the micronized size of the drug. At high drug concentrations, the micronized size of the drug continues to play a critical role in determining the residual MMAD, but the relationship between micronized drug size and residual particle size is not direct. At high concentrations of smaller micronized drug, the resulting residual particle MMAD is primarily determined by the number of multiplets and the size of the drug particle aggregates. For high concentration formulations with larger micronized drug size, the deviation of the resulting residual particle MMAD from the MMAD of the micronized drug is not substantial. For such formulations, it is physically less possible to fit multiple drug particles into a given atomized droplet, thus the resulting residual particle MMAD is primarily determined by the number and size of singlets, which can be thought of as the lack of substantial percentage of multiplets.
CONCLUSION
Overall, the study presented herein shows that residual particle MMAD from single drug-containing suspension HFA-134a formulations can be empirically predicted for a variety of micronized drug size using basic arithmetic operations. The residual particle MMAD is highly dependent on the micronized drug size and concentration, and the initial droplet MMD. The initial droplet MMD for such formulations can be calculated with knowledge of the metering valve size, actuator orifice diameter, and ethanol concentration. Increases in drug concentration, micronized drug size, and initial droplet MMD lead to an increase in residual particle MMAD. Furthermore, increase in drug concentration and initial droplet MMD leads to a substantial increase in the ratio of the residual particle MMAD to the micronized drug MMAD. This effect is especially pronounced for high concentration formulations with relatively small micronized drug MMAD.
Acknowledgments
The authors would like to acknowledge the Pharmaceutical Research and Manufacturers of America (PhRMA) Foundation for a pharmaceutics predoctoral fellowship that supported this work.
Conflict of Interest
Stephen W. Stein is currently employed by 3M Drug Delivery Systems. Poonam Sheth and Paul B. Myrdal declared that no conflict of interest exists.
Contributor Information
Poonam Sheth, Phone: (520) 626-3847, sheth@email.arizona.edu.
Stephen W. Stein, Email: swstein@mmm.com
Paul B. Myrdal, Email: myrdal@pharmacy.arizona.edu
REFERENCES
- 1.Heyder J, Gebhart J, Rudolf G, Schiller CF, Stahlhofen W. Deposition of particles in the human respiratory tract in the size range 0.005–15 μm. J Aerosol Sci. 1986;17(5):811–25. doi: 10.1016/0021-8502(86)90035-2. [DOI] [Google Scholar]
- 2.Sheth P, Stein SW, Myrdal PB. The influence of initial atomized droplet size on residual particle size from pressurized metered dose inhalers. Int J Pharm. 2013;455:57–65. doi: 10.1016/j.ijpharm.2013.07.061. [DOI] [PubMed] [Google Scholar]
- 3.Gonda I. Development of a systematic theory of suspension inhalation aerosols. I. A framework to study the effects of aggregation on the aerodynamic behaviour of drug particles. Int J Pharm. 1985;27:99–116. doi: 10.1016/0378-5173(85)90189-9. [DOI] [Google Scholar]
- 4.Raabe OG. The dilution of monodispersed suspensions for aerosolization. Am Ind Hyg Assoc J. 1968;29:439–43. doi: 10.1080/00028896809343031. [DOI] [PubMed] [Google Scholar]
- 5.Stein SW, Sheth P, Karayiannis C, Chiou H, Myrdal PB. Modeling MDI delivery: a priori predictions, empirical models and experiments. Resp Drug Deliv. 2010;1:353–64. [Google Scholar]
- 6.Stein SW, Sheth P, Myrdal PB. A model for predicting size distributions delivered from pMDIs with suspended drug. Int J Pharm. 2012;422(1):101–15. doi: 10.1016/j.ijpharm.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 7.Stein SW, Myrdal PB. A theoretical and experimental analysis of formulation and device parameters affecting solution MDI size distributions. J Pharm Sci. 2004;93(8):2158–75. doi: 10.1002/jps.20116. [DOI] [PubMed] [Google Scholar]
- 8.Stein SW. The influence of sample size on the accuracy of particle size distribution measurements. Resp Drug Deliv. 2008;333–336.
- 9.Jinks PA. Preparation and utility of sub-micron lactose, a novel excipient for HFA MDI suspension formulations. Proceedings of Drug Delivery to the Lungs, 14; 2003.
- 10.James J, Crean B, Davies M, Toon R, Jinks P, Roberts CJ. The surface characterisation and comparison of two potential sub-micron, sugar bulking excipients for use in low-dose, suspension formulations in metered dose inhalers. Int J Pharm. 2008;361(1):209–21. doi: 10.1016/j.ijpharm.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 11.Stein SW. Estimating the number of droplets and drug particles emitted from MDIs. AAPS PharmSciTech. 2008;9(1):112–5. doi: 10.1208/s12249-007-9006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


