Abstract
Supramolecular hydrogels formed by cyclodextrins and polymers have been widely investigated as a biocompatible, biodegradable and controllable drug delivery system. In this study, a supramolecular hydrogel based on biodegradable poly(caprolactone)–poly(ethylene glycol)–poly(caprolactone) (PCL-PEG-PCL) triblock copolymers and γ-cyclodextrin (γ-CD) was prepared through inclusion complexation as an injectable, sustained-release vehicle for insulin. The triblock copolymer PCL-PEG-PCL was synthesised by the ring-opening polymerisation method, using microwave irradiation. The polymerisation reaction and the copolymer structures were evaluated by nuclear magnetic resonance (NMR) and gel permeation chromatography (GPC). The supramolecular hydrogel was prepared in aqueous solution by blending an aqueous γ-CD solution with an aqueous solution of PCL-PEG-PCL triblock copolymer at room temperature. In vitro insulin release through the hydrogel system was studied. The relative surface hydrophobicity of standard and released insulin from the SMGel was estimated using 8-anilino-1-naphthalene sulfonic acid (ANS). Results of 1HNMR and gel permeation chromatography revealed that microwave irradiation is a simple and reliable method for synthesis of PCL-PEG-PCL copolymer. Gelation occurred within a minute. The supramolecular hydrogel obtained by mixing 10.54% (w/v) γ-CD and 2.5% (w/v) copolymer had an excellent syringeability. Insulin was released up to 80% over a period of 20 days. Insulin kept its initial folding after formulating and releasing from SMGel. A supramolecular hydrogel based on complexation of triblock PCL-PEG-PCL copolymer with γ-cyclodextrin is a suitable system for providing sustained release of therapeutic proteins, with desirable flow behaviour.
Key words: insulin, PCL-PEG-PCL, supramolecular hydrogel, triblock copolymer, γ-CD
INTRODUCTION
Supramolecular hydrogels (SMGels) are a class of physical hydrogels in which the network formation is achieved through the noncovalent interaction of polymer segments that absorb a large amount of water (1). As one of the most potential classes of biomaterials, supramolecular hydrogels formed by cyclodextrins and polymers have been widely investigated for use as controllable drug delivery systems (2).
Cyclodextrins are a series of cyclic oligosaccharides composed of six (α-CD), seven (β-CD) and eight (γ-CD) d(+)-glucose units linked by α-1,4-linkage (3), which can thread onto some linear polymer chains; the resulting supramolecular complex tends to aggregate to form hydrogels (4). Li et al. (5) reported, for the first time, a supramolecular hydrogel based on inclusion complexation between α-cyclodextrin and high-molecular-weight poly(ethylene glycol). Later, it was found that the mentioned SMGel is thixotropic and has exceptional properties, paving the way for potential biomedical applications in view of the excellent biocompatibility of both PEG and α-CD (6).
The formation of such supramolecular hydrogels generally consists of two stages. First, the hydrophobic interactions between the guest polymer and the CD cavity form a cross-linked network, and then the network is stabilised by hydrogen bonding between neighbouring CDs (7). During the sol-to-gel transition, the polymer chains noncovalently aggregate at their CD-rich segments, and these domains continue to assemble into larger particles, leading to turbid sol and subsequent viscose gel as a function of temperature and/or shearing (8). Due to the dynamic nature and weak noncovalent interaction of their networks, SMGels exhibit reversible sol–gel transitions upon exposure to shear forces. These interesting thixotropic effects have led to numerous studies on SMGels for the controlled and sustained release of drugs (9).
Such supramolecular hydrogels can encapsulate drugs physically in situ during gel formation. These systems increase drug loading efficacy and prevent the structural changes in the drug categories, such as proteins, peptides and nucleic acids (10–12).
The reversible sol–gel transition properties of SMGels make them injectable and syringeable biomaterials capable of establishing versatile drug delivery systems (13–16).
Protein drugs have emerged as potent medicines for various types of widespread human diseases (17–19), and insulin is one of the most important and commonly used drugs to treat diabetes mellitus patients in clinics (20). For over 80 years, parenteral administration of insulin formulations has been the major route of insulin delivery for the treatment of insulin-dependent diabetes mellitus (IDDM) in clinical practice (21). It is obvious that the development of a controlled insulin release system would increase patient compliance by reducing the frequency of administration, thereby improving its therapeutic effectiveness.
In order to achieve this purpose, encapsulation, release manner and the therapeutic effects of insulin within various biodegradable polymers have been investigated extensively over the past decade (22–24). In particular, hydrogels and supramolecular hydrogels have been evaluated as capable protein carriers, due to their high water content, consistency and biocompatibility (25–27).
In the current study, γ-CD was selected to prepare an insulin-loaded SMGel, due to its low haemolytic activity and superior biodegradability compared to those of α-CD (10,28). We aimed to develop a sustained insulin release system and study the details of in vitro evaluations of γ-CD and poly(caprolactone)–poly(ethylene glycol)–poly(caprolactone) (PCL-PEG-PCL) hydrogels for efficient insulin encapsulation and delivery as an injectable formulation.
MATERIALS AND METHODS
Materials
Poly(ethylene glycol) with a molecular weight of 20,000 was purchased from Merck (Darmstadt, Germany). ε-Caprolactone, stannous octanoate (tin(II) ethyl hexanoate) and 8-anilino-1-naphthalene sulfonic acid were procured from Sigma Aldrich (St. Louis, MO, USA). Insulin was kindly donated by the Novo Nordisk representative in Tehran, Iran. All other analytical grade chemical reagents and solvents were obtained from Merck (Darmstadt, Germany) and used as received.
Synthesis of Triblock Copolymer
PCL-PEG-PCL copolymers were synthesised with a PCL/PEG ratio of 1:10 by using the ring-opening polymerisation technique with microwave irradiation, as described previously (29). Briefly, 20 g poly(ethylene glycol), MW 20,000, was introduced into a round-bottom flask equipped with a condenser and placed in a microwave. The PEG was irradiated for 10 min at 800 W and 100°C to dry by increasing its viscosity. Then, 2 g ε-caprolactone and catalyst Sn(Oct)2 was added to the dried viscous PEG, and the mixture was further irradiated and stirred at 800 W and 130°C for 15 min. The synthesised copolymer was purified by dissolving it in 25 mL dichloromethane and precipitated by adding 800 mL diethyl ether. The purification process was repeated three times, and the precipitates were freeze-dried and kept at −20°C until use.
The yield of the reaction was calculated by dividing the amount of the obtained product by the amount of the starting materials used in the copolymer synthesis process.
Characterisation of PCL-PEG-PCL Triblock Copolymer
The proton nuclear magnetic resonance (1H NMR) spectrum of the synthesised PCL-PEG-PCL triblock copolymer was recorded on a Bruker Ac-80 spectrophotometer (Germany) in CDCl3 at 25°C. The number average molecular weight (Mn) and PCL-to-PEG ratio were estimated by integrating the signals pertaining to each monomer according to the method established by Jeong et al. (30). The molecular weight (Mw) and polydispersity of the obtained copolymer were determined by gel permeation chromatography, using an Agilent GPC-Addon system and RID-A refractive index signal detector coupled to the PLgel columns. Tetrahydrofuran was used as an eluent (flow 1 mL/min); the sample injection volume was 10 μL.
Formation and Characterisations of Supramolecular Hydrogels
An aqueous solution of 2.5% (w/v) PCL-PEG-PCL was mixed with an aqueous solution of 10.54% (w/v) of γ-CD under vigorous stirring at room temperature, until hydrogels formed. To prepare the insulin-loaded supramolecular hydrogel, 3 mg insulin in 150 μL of HCl 0.1 N was added to the 0.5-mL aqueous copolymer solution (100 mg/mL). Then, 232 mg γ-CD was dissolved in 2.2 mL of deionised water and gradually added to the drug and copolymer solution under vigorous stirring. The three formulations were prepared according to the above procedure and evaluated in terms of gelling times (31).
Investigation of Supramolecular Hydrogel and Physical Mixture Morphology
Scanning electron microscopy images (LEO-1350 VP, Germany) were used to study the pore structure of the insulin-loaded hydrogel. The freeze-dried samples were mounted on an aluminium base with adhesive carbon tape and coated with 2–5 nm gold under vacuum for 5 min to prevent charging and distortion prior to obtaining the SEM images. The structural shapes of the insulin-loaded supramolecular hydrogel and those of the physical mixture of γ-CD/copolymer/insulin were compared.
Swelling Ratio Measurement
Swelling behaviour was monitored gravimetrically. For this purpose, 100 mg of dried hydrogel was immersed in 250 mL of deionised water at room temperature. The swelling ratio of the hydrogel was measured every 15 min for three replicas using Eq. 1 (32):
| 1 |
X-ray Powder Diffraction
The crystalline phases of the insulin-loaded supramolecular hydrogel and the physical mixture of γ-CD/copolymer/insulin were determined using a Phillips analytical X-ray B.V. (USA), consisting of a PW3710 diffractometer and an X-ray tube (30 mA and 40 kV) with a copper anode. The experiment was performed at a scanning rate of 0.033°/s at an angle of 2θ and a range of 5°–35°.
Differential Scanning Calorimetry
The thermal transitions of SMGel, insulin-loaded SMGel, insulin, triblock copolymer and γ-CD were determined using a differential scanning calorimeter (Mettler Toledo DSC 822, Switzerland). The sample sizes were approximately 3–5 mg, and each sample was subjected to thermal cycles of 25–175°C, at a rate of 20°C/min.
Evaluation of Formulation Injectability
A syringe with a 25- and 28-gauge micro-fine insulin needle was filled with the insulin-loaded supramolecular hydrogel, and the injectability of the formulations was determined by passing them through the syringe at 25°C.
In vitro Release Study
The formulation (2 mL) was immersed in 20-mL tubes, with a cross-sectional area of 2 cm2, containing 10 mL phosphate buffer solution (PBS, pH = 7.4). The tubes were transferred to a shaker incubator water bath (Faraz Teb, Iran) set at 40 rpm and 37°C. Samples (300 μL) were withdrawn at specific times and replaced by adding 300 μL of fresh release medium.
The samples were assayed for insulin content by Bradford assay (31,33). A blend of each sample with Bradford reagent (n = 3) was analysed in a 96-well plate by spectrophotometry measurement at A = 600 nm using an ELISA Microplate Reader (Stat Fax 2100, USA).
Investigation of Insulin Folding
The relative surface hydrophobicity of standard insulin and released insulin from the SMGel was estimated using 8-anilino-1-naphthalene sulfonic acid (ANS). ANS solutions (200 μM) were added to the insulin solution (1 μM), and the mixture was incubated for 30 min at room temperature. Then, the samples were excited at 370 nm, and the emission spectra were recorded between 400 and 600 nm, using a spectrofluorometer (Shimadzu, Japan).
Stability of Insulin
The structural stability of released insulin from hydrogel was detected by sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS–PAGE) and compared with native insulin. Protein samples were diluted with Tris-buffer (pH 6.8) with 2% SDS and electrophoresis of samples was performed by using a Bio-RadMiNi-Protein II electrophoresis system at a constant voltage of 200 V in a Tris/glycine/SDS buffer. After migration, the staining procedure was carried out with Coomassie bright blue in methanol–acetic–water (2.5:1:6.4).
Circular Dichroism Spectroscopy
This test was performed on the samples to evaluate the conformational stability of insulin released from hydrogel. The samples were first filtered through a 0.2-μm syringe filter to separate the protein from the polymer. As control, circular dichroism (CD) spectra of dissolved insulin in the extracting medium were collected. The analysis was performed using a Jasco J-810 spectropolarimeter (Tokyo, Japan) with a 1-mm light path quartz cell (Hellma, Müllheim, Germany). Data were acquired at a bandwidth of 0.1 nm with a scan speed of 50 nm/min and a response time of 8 s. The samples and standard insulin solution were prepared in 25 mM Tris–HCl solution (pH = 7.8) and scanned over the wavelength range of 190–250 nm.
RESULTS
PCL-PEG-PCL Synthesis and Characterisations
The copolymer was successfully synthesised by ring-opening polymerisation with a yield of 88%. The 1H NMR spectrum of the PCL-PEG-PCL copolymer is shown in Fig. 1. The signal at 3.6 ppm is attributed to the CH2 of the PEG block in the copolymer chains. Its integral serves as an internal standard to calculate the average chain length of the PEG. The signals at 4.1, 2.2 and 1.5 ppm are related to the CH2 of PCL block, and their integrals can be used to estimate the average chain length of the PCL. The results of these analyses of the copolymer are summarised in Table I.
Fig. 1.
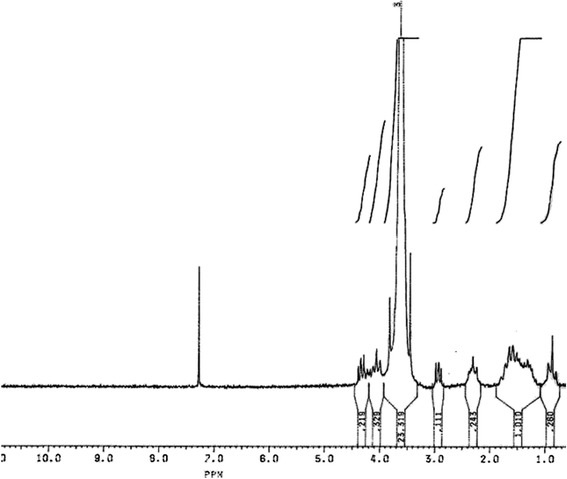
1H NMR of PCL1,000-PEG20,000-PCL1,000 triblock copolymer in CDCl3. The signals relate to CH2 blocks of PEG and PCL as shown in the above structure
Table I.
Copolymer Characteristics Determined by 1H NMR and GPC
| 1HNMR | GPC | ||||
|---|---|---|---|---|---|
| M n a (PCL-PEG-PCL) | PCL/PEGb | M n c | M n d | M w e | M w/M n f |
| 981.54-20,000-981.54 | 0.098 | 21,960 | 23,851 | 25,965 | 1.08 |
1 H NMR proton nuclear magnetic resonance, GPC gel permeation chromatography, PCL-PEG-PCL poly(caprolactone)–poly(ethylene glycol)–poly(caprolactone)
aAverage molecular weight determined by 1H NMR
bPCL/PEG ratio determined by 1H NMR
cNumber average molecular weight determined by 1H NMR
dNumber average molecular weight determined by GPC
eAverage molecular weight determined by GPC
fPolydispersity determined by GPC
Formation of the Supramolecular Hydrogel
In this study, the SMGel was formed successfully using a PCL1,000-PEG20,000-PCL1,000 copolymer with a PCL/PEG ratio of 1:10 at concentrations of about 10.54% (w/v) of γ-CD and 2.5% (w/v) of copolymer. The mixtures of γ-CD, insulin and triblock copolymer transformed into a gel in only 40 s (n = 3).
Swelling Ratio
The swelling profile of the hydrogel is shown in Fig. 2. The hydrogel showed good water-swelling ability in distilled water; the weight of the supramolecular hydrogel increased 1.2 times in 30 min.
Fig. 2.
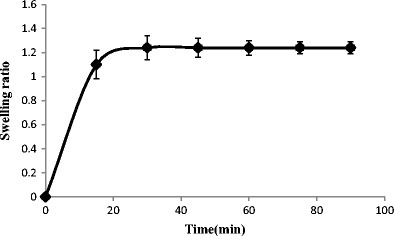
Swelling ratio of supramolecular hydrogel over 90 min
Syringeability of Supramolecular Hydrogels
The prepared insulin formulation passed easily through a 25- and 28-gauge syringe at 25°C and was injectable. Eventually, it regained its gel network after passing through a needle in less than a minute. This is attributable to the thixotropic behaviour of the SMGel (34).
SEM Imaging
The structural shape of the freeze-dried supramolecular hydrogel and the physical mixture of the insulin/γ-CD/copolymer were analysed by SEM. As shown in Fig. 3, in contrast to the physical mixture of the components, the supramolecular hydrogel exhibited a 3D porous foam-like morphology.
Fig. 3.
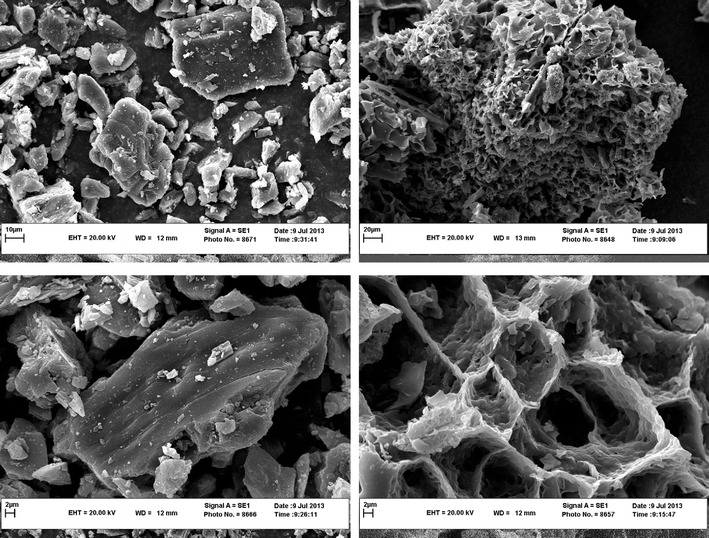
SEM image of freeze-dried hydrogel (left) and physical mixture of pure components (right)
X-ray Powder Diffraction
The X-ray powder patterns of the copolymer, γ-cyclodextrin and insulin, as well as their physical mixture and the SMGel formulations, are shown in Fig. 4.
Fig. 4.
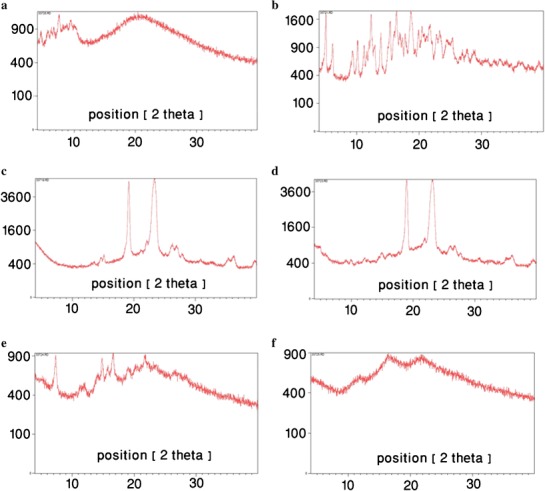
X-ray powder pattern of insulin (a), γ-CD (b), copolymer (c), physical mixture of insulin/copolymer/γ-CD (d), SMGel (e) and insulin-loaded SMGel (f)
Differential Scanning Calorimetry
To confirm the formation of SMGel, a differential scanning calorimetry (DSC) thermogram of the copolymer, CD, insulin, their physical mixture and the insulin-loaded SMGel are presented in Fig. 5.
Fig. 5.
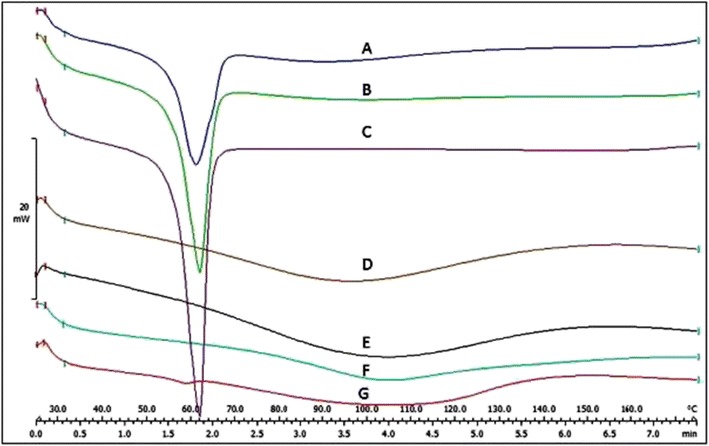
DSC thermogram of physical mixture of insulin/copolymer/γ-CD (A), physical mixture of polymer/γ-CD (B), polymer (C), insulin (D), hydrogel of insulin/polymer/γ-CD (E), γ-CD (F) and hydrogel of polymer/γ-CD (G)
In vitro Release
Figure 6 shows the release profile of insulin through the SMGel in PBS at pH 7.4 at 37°C to mimic the physiological condition.
Fig. 6.
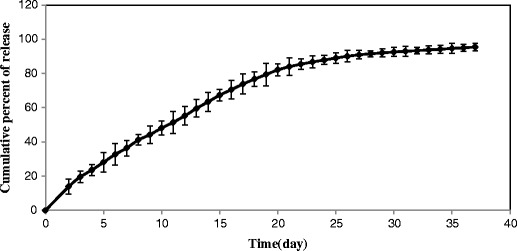
Release profile of insulin-loaded SMGel
The release kinetics of insulin proved the results and showed the best fit for the Higuchi model (R2 = 0.967) and zero-order release kinetics (R2 = 0.983) (35–37).
Insulin Folding
The ANS fluorescence emission spectra of both the standard and released insulin after 35 days were similar, suggesting unaltered surface hydrophobicity due to this formulation (Fig. 7).
Fig. 7.
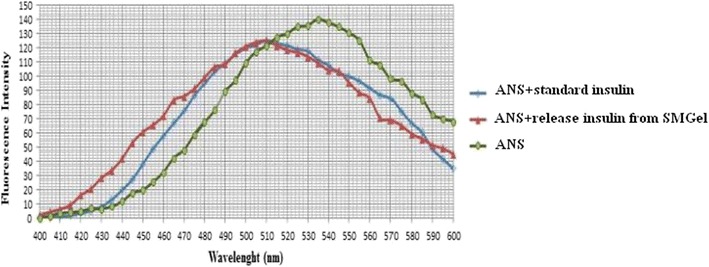
ANS fluorescence spectra of standard insulin, released insulin from SMGel and ANS probe
Stability of Insulin
The SDS–PAGE was performed and the results were illustrated in Fig. 8.
Fig. 8.

SDS-PAGE result for intact insulin (1) and releases of insulin from SMGel after 35 days (2)
Circular Dichroism Spectroscopy
The CD spectrum of released insulin from hydrogel after 35 days was illustrated in Fig. 9. As can be seen, the profile of released insulin from hydrogel was superimposed to that of the control group.
Fig. 9.
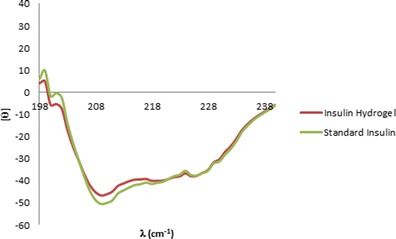
The CD spectrum of released insulin from SMGel after 35 days (red line) and standard insulin (green line)
DISCUSSION
The results of polymer characterisation tests verify that microwave irradiation is a reliable method for the synthesis of PCL-PEG-PCL triblock copolymer. By using a microwave-assisted polymerisation, the high-speed, reproducible and scalable production of materials such as polymers with limited side reactions and by-products and also a higher yield of reaction are possible. Moreover, it facilitates scale-up procedure and technology transfer from bench to industry (38,39). In our previous study, we compared the conventional and the microwave-assisted synthesis of PCL-PEG-PCL that affirmed the superiority of this new method. Microwave irradiation reduced the time of reaction from 6 h to only 15 min. Furthermore, the yield of reaction increased up to 95% which was approximately 65% in conventional methods (29).
The PEG segment in the PCL1,000-PEG20,000-PCL1,000 backbones was found to form inclusion complexes with γ-CD molecules, resulting in the formation of the supramolecular hydrogel in less than a minute. The stoichiometric necklace-like inclusion complex is formed by α-CD threading onto PEG blocks (15). It was previously proved that gelation property was affected by various factors, including the PEG content of the polymers, the solution concentration, the mixing ratio of cyclodextrin and polymer, and temperature (9). For extremely hydrophobic polymers, such as PCL, slow CD threading kinetics prevents complex formation. Therefore, block copolymers composed of hydrophobic and hydrophilic blocks were used to obtain complex stability with moderate threading kinetics. In polymers such as PCL-PEG-PCL, hydrophobic interaction between the PCL segments act as a barrier against γ-CD to thread onto the PCL blocks. In some cases, aggregations of the unthreaded hydrophobic polymer blocks strengthen the hydrogel structures to form more stable cross-linked networks (40).
In this system, the small quantity of hydrophobic PCL blocks and high quantity of hydrophilic blocks enhanced aggregations of γ-CD threading onto PEG blocks. In this regard, one of the critical parameters that need to be considered is the proper balance between hydrophobic and hydrophilic segments of the copolymer (41). For copolymers with a large PCL/PEG ratio and a low concentration of α-CD, the copolymer could not turn into a gel because of the long hydrophobic PCL blocks and high level of hydrophobic aggregations that reduces the chance of α-CD to thread onto the PEG blocks.
Although the PCL-PEG-PCL is among the thermo-responsive copolymers and shows the irreversible sol–gel transition, the results proved that in the prepared SMGel, the hydrophobic interactions compared with the hydrophilic interactions were much less than the threshold condition necessary for gel formation at the applicable temperature. We supposed that this phenomenon is due the very large hydrophilic PEG segments of copolymer in comparison with its very short hydrophobic PCL blocks. In fact, the running force of gel formation, which is hydrophobic interactions, is extremely low before adding γ-CD. Thus, when the γ-CD solution was added to the insulin/triblock copolymer solution, since it covered the PEG blocks and enhanced the opportunity of PCL block interactions, the solution became opaque within several seconds and changed to a gel in less than a minute. Indisputably, it is one of the major advantages of such systems which provide the condition of having a gel even with low concentration of copolymer; as a result, it adds to the biocompatibility of formulations and reduces the probable risk of inflammation at the injection site due to the copolymers.
The prepared supramolecular hydrogel swelled in the presence of water. Supramolecular hydrogels have a porous and hydrophilic network structure, so they can absorb water into the matrix in an aqueous medium. High quantity of water makes the hydrogels biocompatible and less irritant than other implantable systems (42).
As it was shown, the prepared supramolecular hydrogel is injectable and shows the thixotropic behaviour. Such materials, called shear-thinning or pseudo-plastic, lost their high viscosity as a function of shear rate and reclaim it when there is no more agitation, over a period of time (14).
The time of recovery is also crucial for suitable drug delivery systems. If it takes a long time to gel shapes, the detrimental burst release of drug and dose dumping phenomenon which may cause severe adverse reactions will emerges. Fortunately, this supramolecular hydrogel recovered its gel structure rapidly, and as we indicated in the section of in vitro release results, the burst release was less than 5%.
SEM confirmed the porous configuration of the system. As we mentioned above, such highly porous structure gerents the supramolecular hydrogels’ high capacity of water absorption. The spongy structure only formed after threading of γ-CD onto the PEG blocks.
The X-ray diffraction patterns of pure insulin, γ-CD and the copolymer were shown in Fig. 3. In the diffraction pattern of the copolymer, two peaks in 2θ = 19 and 23 were observed. These two peaks were preserved in the pattern of physical mixture, but they disappeared in that of supramolecular hydrogel. This means that in the hydrogel, the copolymer was interacted with CD molecules and because of such interaction, the X-ray diffraction pattern of insulin/SMGel exhibited less crystallinity than the physical mixture. It was previously shown that the reflection at d = 4.46 Å and 2θ = 19.8 is a characteristic peak, which is the identity of necklace-like structures. In contrast to α-CD inclusion complexes, γ-CD inclusion complexes exhibit less crystallinity and, consequently, do not exhibit sharp peaks at d = 4.46 Å and 2θ = 19.8. Because two polymer chains can be included within the large cavity of γ-CD, the crystallinity degree of this SMGel is low, and it shows more elasticity (31).
DSC findings confirmed the formation of SMGel. The endothermic peak related to the copolymer was observed in the physical mixtures of copolymer/CD and copolymer/CD/insulin, as well as the pure copolymer. However, the endothermic peak in the SMGel system completely disappeared. The results indicate that the SMGel was appropriately prepared.
The SMGel controlled the release of insulin at 37°C for about 1 month. The insulin release from the SMGel exhibited an initial burst release of less than 5%, followed by a sustained release for up to 37 days. The initial burst release, which is an unwanted occurrence in a drug release profile, was low here. This can be considered a noticeable advantage of such SMGel drug delivery systems compared to other sustained delivery systems.
The insulin-loaded SMGel demonstrated a suitable continuous release profile of more than 80% of the drug up to 20 days. After 20 days, the release profile was followed by a plateau, and ∼15% of the remainder of the drug was released over 17 days. After 20 days, some cracks appeared in the hydrogel matrixes, which are indicative of copolymer degradation. Therefore, the remaining drug was released more by a copolymer degradation mechanism, which had a slower rate compared to the first section of the release profile, in which diffusion was dominant.
As it was previously reported, the large molecules are mostly released by polymer degradation rather than diffusion, especially the ones that incorporated into the depth (43). However, because of a large porosity of the SMGel, the insulin molecules that had been closer to the surface of hydrogel could diffuse through the holes of networks. Yet, concentration gradient was decreased over a release procedure due to the elimination of drug quantity and mounting of a distance between drug molecules and surface of hydrogel. It can reduce the diffusion rates; thus, the release kinetics shifts to the zero-order release because of polymer erosion.
Because insulin can lose its activity during formulation, it is important for the controlled delivery system to release insulin in its active form.
It is understood that ANS is a hydrophobic site-responsive probe that has negligible fluorescence in aqueous media. Binding of this probe to hydrophobic sites in proteins results in a several-fold enhancement in its fluorescence, with a shift in the emission maximum to a lower wavelength (10).
The similar fluorescence emission of standard and released insulin affirmed that insulin molecules retain their activity in the formulation.
SDS–PAGE results showed that during preparation and release processes, insulin was intact (Fig. 8). These results were in agreement with earlier findings.
CD spectra showed that the conformation of released insulin from hydrogel after 35 days was unchanged and insulin was released in its native state (Fig. 9). CD analysis demonstrated that secondary structural integrity of insulin was maintained during preparation and release processes.
CONCLUSION
SMGel loaded with insulin was successfully prepared by mixing an aqueous solution of insulin and copolymer with an aqueous γ-CD solution. The results indicated that insulin encapsulates efficiently under mild conditions in an aqueous environment and in the absence of organic solvents. The unique advantage of preparing drug-loaded SMGel is that the drug can be easily encapsulated during sol-to-gel transformation.
In this study, we demonstrated the formation of an insulin/γ-CD SMGel of PCL-PEG-PCL, based on the self-assembly of PEG threaded with γ-CD without any covalent interactions. The release rate of insulin from the SMGel occurred in a sustained and controlled manner. These results suggest the potential use of the prepared hydrogel as an injectable sustained-release system for insulin.
Acknowledgments
This project was supported by a grant from Vice Chancellor of Research, Mashhad University of Medical Sciences (MUMS), Mashhad, Iran. The results described in this paper were part of a Pharm D student thesis proposal related to Zinat Heidari.
Conflict of Interest
The authors declare that they have no conflict of interest.
Contributor Information
Sayyed A. Sajadi Tabassi, Phone: +98 511 38823255, Email: sajadia@mums.ac.ir.
Farzin Hadizadeh, Phone: +98 513 7112420, Email: hadizadehf@mums.ac.ir.
References
- 1.Li J. Self-assembled supramolecular hydrogels based on polymer–cyclodextrin inclusion complexes for drug delivery. NPG Asia Mater. 2010;2(3):112–8. doi: 10.1038/asiamat.2010.84. [DOI] [Google Scholar]
- 2.Bromberg LE, Ron ES. Temperature-responsive gels and thermogelling polymer matrices for protein and peptide delivery. Adv Drug Deliv Rev. 1998;31(3):197–221. doi: 10.1016/S0169-409X(97)00121-X. [DOI] [PubMed] [Google Scholar]
- 3.Bender ML, Komiyama M. Cyclodextrin chemistry. Berlin: Springer; 1978. [Google Scholar]
- 4.Harada A, Li J, Kamachi M. Double-stranded inclusion complexes of cyclodextrin threaded on poly (ethylene glycol) Nature. 1994;370(6485):126–8. doi: 10.1038/370126a0. [DOI] [Google Scholar]
- 5.Li J, Harada A, Kamachi M. Sol–gel transition during inclusion complex formation between α-cyclodextrin and high molecular weight poly (ethylene glycol) s in aqueous solution. Polym J. 1994;26(9):1019–26. doi: 10.1295/polymj.26.1019. [DOI] [Google Scholar]
- 6.Li J, Ni X, Leong KW. Injectable drug-delivery systems based on supramolecular hydrogels formed by poly (ethylene oxide) s and α-cyclodextrin. J Biomed Mater Res A. 2003;65(2):196–202. doi: 10.1002/jbm.a.10444. [DOI] [PubMed] [Google Scholar]
- 7.Harada A, Li J, Kamachi M. The molecular necklace: a rotaxane containing many threaded α-cyclodextrins. Nature. 1992;356(6367):325–7. doi: 10.1038/356325a0. [DOI] [Google Scholar]
- 8.Li J, Li X, Toh KC, Ni X, Zhou Z, Leong KW. Inclusion complexation and formation of polypseudorotaxanes between poly [(ethylene oxide)-r an-(propylene oxide)] and cyclodextrins. Macromolecules. 2001;34(26):8829–31. doi: 10.1021/ma011129b. [DOI] [Google Scholar]
- 9.Huh KM, Cho YW, Chung H, Kwon IC, Jeong SY, Ooya T, et al. Supramolecular hydrogel formation based on inclusion complexation between poly (ethylene glycol)-modified chitosan and α-cyclodextrin. Macromol Biosci. 2004;4(2):92–9. doi: 10.1002/mabi.200300037. [DOI] [PubMed] [Google Scholar]
- 10.Higashi T, Hirayama F, Misumi S, Arima H, Uekama K. Design and evaluation of polypseudorotaxanes of pegylated insulin with cyclodextrins as sustained release system. Biomaterials. 2008;29(28):3866–71. doi: 10.1016/j.biomaterials.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 11.Ma D, Zhang H-B, Chen D-H, Zhang L-M. Novel supramolecular gelation route to in situ entrapment and sustained delivery of plasmid DNA. J Colloid Interface Sci. 2011;364(2):566–73. doi: 10.1016/j.jcis.2011.08.051. [DOI] [PubMed] [Google Scholar]
- 12.Prabaharan M, Mano J. Chitosan derivatives bearing cyclodextrin cavitiesas novel adsorbent matrices. Carbohydr Polym. 2006;63(2):153–66. doi: 10.1016/j.carbpol.2005.08.051. [DOI] [Google Scholar]
- 13.Ganta S, Devalapally H, Shahiwala A, Amiji M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J Control Release. 2008;126(3):187–204. doi: 10.1016/j.jconrel.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Li X, Ni X, Wang X, Li H, Leong KW. Self-assembled supramolecular hydrogels formed by biodegradable PEO–PHB–PEO triblock copolymers and α-cyclodextrin for controlled drug delivery. Biomaterials. 2006;27(22):4132–40. doi: 10.1016/j.biomaterials.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 15.Vyas A, Saraf S, Saraf S. Cyclodextrin based novel drug delivery systems. J Incl Phenom Macrocycl Chem. 2008;62(1–2):23–42. doi: 10.1007/s10847-008-9456-y. [DOI] [Google Scholar]
- 16.Yu J, Fan H, Huang J, Chen J. Fabrication and evaluation of reduction-sensitive supramolecular hydrogel based on cyclodextrin/polymer inclusion for injectable drug-carrier application. Soft Matter. 2011;7(16):7386–94. doi: 10.1039/c1sm05426k. [DOI] [Google Scholar]
- 17.Harries M, Smith I. The development and clinical use of trastuzumab (Herceptin) Endocr-Relat Cancer. 2002;9(2):75–85. doi: 10.1677/erc.0.0090075. [DOI] [PubMed] [Google Scholar]
- 18.Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer. 2002;2(10):727–39. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- 19.Marshall H. Anti-CD20 antibody therapy is highly effective in the treatment of follicular lymphoma. Trends Immunol. 2001;22(4):183–4. doi: 10.1016/s1471-4906(01)01909-3. [DOI] [PubMed] [Google Scholar]
- 20.McCarthy AA. New approaches to diabetes disease control, insulin delivery, and monitoring. Chem Biol. 2004;11(12):1597–8. doi: 10.1016/j.chembiol.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Banting F, Best CH, Collip JB, Campbell WR, Fletcher AA. Pancreatic extracts in the treatment of diabetes mellitus. Can Med Assoc J. 1922;12(3):141. [PMC free article] [PubMed] [Google Scholar]
- 22.Onuki Y, Morishita M, Takayama K. Formulation optimization of water-in-oil-water multiple emulsion for intestinal insulin delivery. J Control Release. 2004;97(1):91–9. doi: 10.1016/j.jconrel.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Takenaga M, Yamaguchi Y, Kitagawa A, Ogawa Y, Kawai S, Mizushima Y, et al. Optimum formulation for sustained-release insulin. Int J Pharm. 2004;271(1):85–94. doi: 10.1016/j.ijpharm.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 24.Takenaga M, Yamaguchi Y, Kitagawa A, Ogawa Y, Mizushima Y, Igarashi R. A novel sustained-release formulation of insulin with dramatic reduction in initial rapid release. J Control Release. 2002;79(1):81–91. doi: 10.1016/S0168-3659(01)00518-1. [DOI] [PubMed] [Google Scholar]
- 25.Hoare T, Pelton R. Charge-switching, amphoteric glucose-responsive microgels with physiological swelling activity. Biomacromolecules. 2008;9(2):733–40. doi: 10.1021/bm701203r. [DOI] [PubMed] [Google Scholar]
- 26.Lin C-C, Metters AT. Hydrogels in controlled release formulations: network design and mathematical modeling. Adv Drug Deliv Rev. 2006;58(12):1379–408. doi: 10.1016/j.addr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 27.van Dijk‐Wolthuis W, Van Steenbergen M, Underberg W, Hennink W. Degradation kinetics of methacrylated dextrans in aqueous solution. J Pharm Sci. 1997;86(4):413–7. doi: 10.1021/js9604220. [DOI] [PubMed] [Google Scholar]
- 28.Irie T, Uekama K. Pharmaceutical applications of cyclodextrins. III. Toxicological issues and safety evaluation. J Pharm Sci. 1997;86(2):147–62. doi: 10.1021/js960213f. [DOI] [PubMed] [Google Scholar]
- 29.Khodaverdi A, Akbari A, Tekie FSM, Mohajeri SA, Zohuri G, Hadizadeh F. Sustained delivery of amphotericin B and vancomycin hydrochloride by an injectable thermogelling tri-block copolymer. PDA J Pharm Sci Technol. 2013 doi: 10.5731/pdajpst.2013.00908. [DOI] [PubMed] [Google Scholar]
- 30.Jeong B, Choi Y, Bae Y, Zentner G, Kim S. New biodegradable polymers for injectable drug delivery systems. J Control Release. 1999;62(1):109–14. doi: 10.1016/S0168-3659(99)00061-9. [DOI] [PubMed] [Google Scholar]
- 31.Abu Hashim II, Higashi T, Anno T, Motoyama K, Abd-ElGawad A-EH, El-Shabouri MH, et al. Potential use of γ-cyclodextrin polypseudorotaxane hydrogels as an injectable sustained release system for insulin. Int J Pharm. 2010;392(1):83–91. doi: 10.1016/j.ijpharm.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 32.Khodaverdi E, Rajabi O, Farhadi F, Jalali A, Mirzazadeh TF. Preparation and investigation of poly (N-isopropylacrylamide-acrylamide) membranes in temperature responsive drug delivery. Iran J Basic Med Sci. 2010;13(3):102–10. [Google Scholar]
- 33.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1):248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 34.Li X, Li J. Supramolecular hydrogels based on inclusion complexation between poly (ethylene oxide)‐b‐poly (ε‐caprolactone) diblock copolymer and α-cyclodextrin and their controlled release property. J Biomed Mater Res A. 2008;86(4):1055–61. doi: 10.1002/jbm.a.31710. [DOI] [PubMed] [Google Scholar]
- 35.Masaro L, Zhu X. Physical models of diffusion for polymer solutions, gels and solids. Prog Polym Sci. 1999;24(5):731–75. doi: 10.1016/S0079-6700(99)00016-7. [DOI] [Google Scholar]
- 36.Möckel JE, Lippold BC. Zero-order drug release from hydrocolloid matrices. Pharm Res. 1993;10(7):1066–70. doi: 10.1023/A:1018931210396. [DOI] [PubMed] [Google Scholar]
- 37.Sarkar P, Bharill S, Gryczynski I, Gryczynski Z, Nair MP, Lacko AG. Binding of 8-anilino-1-naphthalenesulfonate to lecithin: cholesterol acyltransferase studied by fluorescence techniques. J Photochem Photobiol B Biol. 2008;92(1):19–23. doi: 10.1016/j.jphotobiol.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 38.Hoogenboom R, Schubert US. Microwave-assisted polymer synthesis: recent developments in a rapidly expanding field of research. Macromol Rapid Commun. 2007;28(4):368–86. doi: 10.1002/marc.200600749. [DOI] [Google Scholar]
- 39.Sosnik A, Gotelli G, Abraham GA. Microwave-assisted polymer synthesis (MAPS) as a tool in biomaterials science: how new and how powerful. Prog Polym Sci. 2011;36(8):1050–78. doi: 10.1016/j.progpolymsci.2010.12.001. [DOI] [Google Scholar]
- 40.Zhao S-P, Zhang L-M, Ma D. Supramolecular hydrogels induced rapidly by inclusion complexation of poly(epsilon-caprolactone)-poly(ethylene glycol)-poly(epsilon-caprolactone) block copolymers with alpha-cyclodextrin in aqueous solutions. J Phys Chem B. 2006;110(25):12225–9. doi: 10.1021/jp057506u. [DOI] [PubMed] [Google Scholar]
- 41.Ni X, Cheng A, Li J. Supramolecular hydrogels based on self-assembly between PEO-PPO-PEO triblock copolymers and α-cyclodextrin. J Biomed Mater Res A. 2009;88(4):1031–6. doi: 10.1002/jbm.a.31906. [DOI] [PubMed] [Google Scholar]
- 42.Kopecek J. Hydrogels: from soft contact lenses and implants to self-assembled nanomaterials. J Polym Sci A Polym Chem. 2009;47(22):5929–46. doi: 10.1002/pola.23607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khodaverdi E, Tekie FSM, Mohajeri SA, Ganji F, Zohuri G, Hadizadeh F. Preparation and investigation of sustained drug delivery systems using an injectable, thermosensitive, in situ forming hydrogel composed of PLGA–PEG–PLGA. AAPS PharmSciTech. 2012;13(2):590–600. doi: 10.1208/s12249-012-9781-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


