Abstract
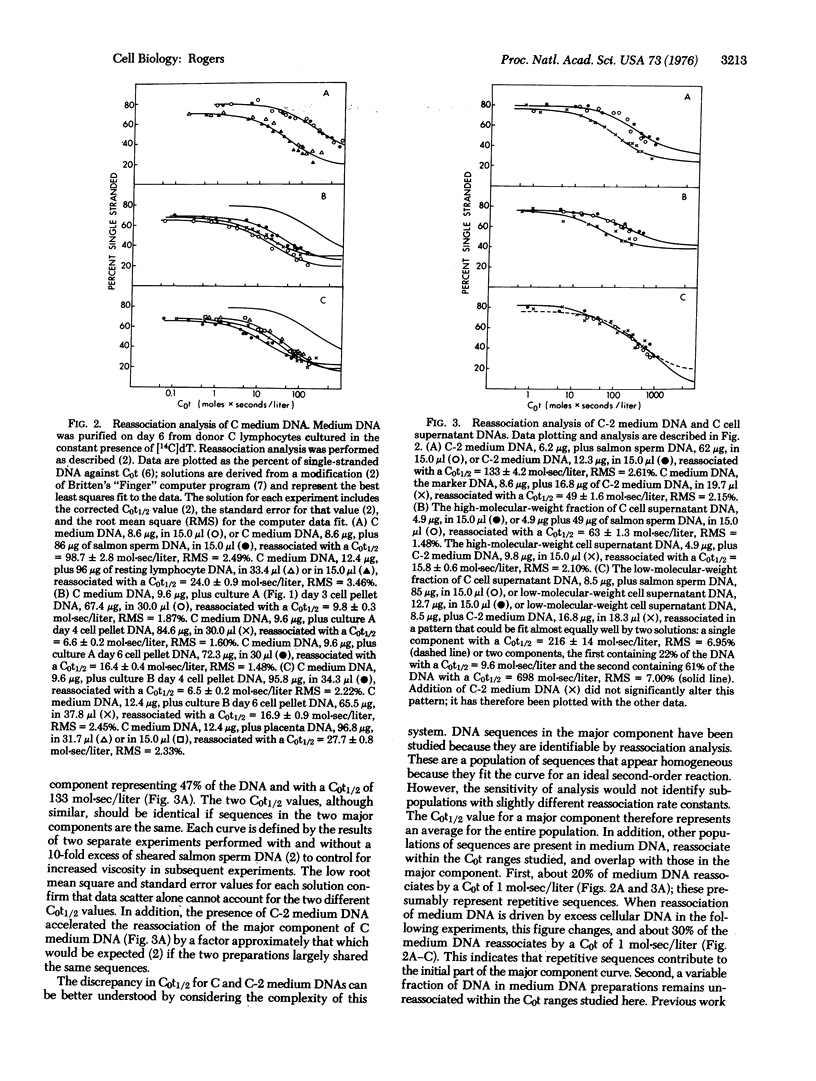
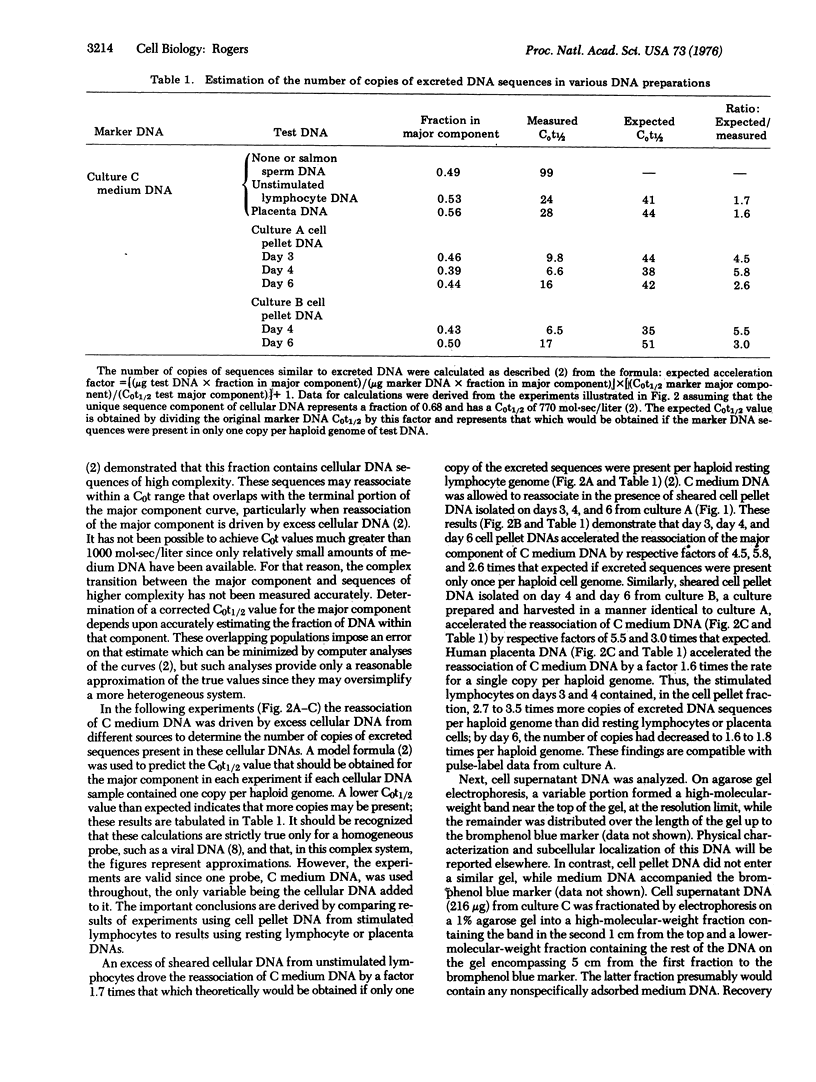
Phytohemagglutinin-stimulated human peripheral blood lymphocytes in vitro synthesize DNA that is excreted into the culture medium. When such cells are pulse-labeled with [3H]thymidine during the peak of DNA synthesis on day 3 of culture, then cultured for 3 more days in the absence of isotope, labeled DNA moves slowly into the Hirt supernatant cell fraction from the pellet fraction containing chromosomal DNA,and then into the culture medium. The number of copies of excreted DNA sequences in the Hirt pellet fraction was determined for lymphocytes harvested on days 3,4, and 6 after stimulation and compared to the number found in resting lymphocyte DNA and in placenta DNA. While resting lymphocyte and placenta DNAs contain one to two copies of sequences similar to excreted DNA per haploid genome, stimulated lymphocytes on days 3 and 4 of culture contain 3- to 4-fold more copies; by day 6 of culture, stimulated lymphocytes contain only 1- to 2-fold more copies than resting lymphocytes. Thus, phytohemagglutinin induces lymphocytes to selectively replicate several copies of a limited portion of their genome, copies which are then excreted into the culture medium. As determined by reassociation kinetics analysis, a high-molecular-weight DNA fraction from the Hirt supernatant contains sequences found in excreted DNA. This DNA may represent an intermediate formed prior to release of excreted sequences from the cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Dawid I. B. Specific gene amplification in oocytes. Oocyte nuclei contain extrachromosomal replicas of the genes for ribosomal RNA. Science. 1968 Apr 19;160(3825):272–280. doi: 10.1126/science.160.3825.272. [DOI] [PubMed] [Google Scholar]
- Gall J. G. Differential synthesis of the genes for ribosomal RNA during amphibian oögenesis. Proc Natl Acad Sci U S A. 1968 Jun;60(2):553–560. doi: 10.1073/pnas.60.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelb L. D., Kohne D. E., Martin M. A. Quantitation of Simian virus 40 sequences in African green monkey, mouse and virus-transformed cell genomes. J Mol Biol. 1971 Apr 14;57(1):129–145. doi: 10.1016/0022-2836(71)90123-9. [DOI] [PubMed] [Google Scholar]
- Gerber P. Activation of Epstein-Barr virus by 5-bromodeoxyuridine in "virus-free" human cells (complement-fixing antigen-immunofluorescence-leukocytes). Proc Natl Acad Sci U S A. 1972 Jan;69(1):83–85. doi: 10.1073/pnas.69.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampar B., Derge J. G., Martos L. M., Walker J. L. Synthesis of Epstein-Barr virus after activation of the viral genome in a "virus-negative" human lymphoblastoid cell (Raji) made resistant to 5-bromodeoxyuridine (thymidine kinase-virus antigen-immunofluorescence-herpesvirus fingerprints). Proc Natl Acad Sci U S A. 1972 Jan;69(1):78–82. doi: 10.1073/pnas.69.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helling R. B., Goodman H. M., Boyer H. W. Analysis of endonuclease R-EcoRI fragments of DNA from lambdoid bacteriophages and other viruses by agarose-gel electrophoresis. J Virol. 1974 Nov;14(5):1235–1244. doi: 10.1128/jvi.14.5.1235-1244.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Lerner R. A., Meinke W., Goldstein D. A. Membrane-associated DNA in the cytoplasm of diploid human lymphocytes. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1212–1216. doi: 10.1073/pnas.68.6.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinke W., Goldstein D. A. Reassociation and dissociation of cytoplasmic membrane-associated DNA. J Mol Biol. 1974 Jul 15;86(4):757–773. doi: 10.1016/0022-2836(74)90352-0. [DOI] [PubMed] [Google Scholar]
- Rogers J. C., Boldt D., Kornfeld S., Skinner A., Valeri C. R. Excretion of deoxyribonucleic acid by lymphocytes stimulated with phytohemagglutinin or antigen. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1685–1689. doi: 10.1073/pnas.69.7.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J. C. Characterization of DNA excreted from phytohemagglutinin-stimulated lymphocytes. J Exp Med. 1976 May 1;143(5):1249–1264. doi: 10.1084/jem.143.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T., Nordman C. T., Gräsbeck R. Separation of lymphocyte-stimulating and agglutinating activities in phytohaemagglutinin (PHA) from Phaseolus vulgaris. Scand J Haematol. 1967;4(1):77–80. doi: 10.1111/j.1600-0609.1967.tb01601.x. [DOI] [PubMed] [Google Scholar]



