Abstract
Actin remodeling is a dynamic process associated with cell shape modification occurring during cell cycle and proliferation. Oxidative stress plays a role in actin reorganization via various systems including p38MAPK. Beside, the mitogenic response evoked by hydrogen peroxide (H2O2) in fibroblasts and smooth muscle cells (SMC) involves the metalloproteinase (MMPs)/sphingomyelinase 2 (nSMase2) signaling pathway. The aim of this work was to investigate whether this system plays a role in actin remodeling induced by H2O2.
Low H2O2 dose (5 µM) rapidly triggered a signaling cascade leading to nSMase2 activation, src and annexin 2 (AnxA2) phosphorylation, and actin remodeling, in fibroblasts and SMC. These events were blocked by pharmacological inhibitors of MMPs (Ro28-2653) and p38MAPK (SB203580), and were lacking in MMP2−/− and in nSMase2-mutant (fro) fibroblasts. Likewise, H2O2 was unable to induce actin remodeling in fro and MMP2−/− fibroblasts or in cells pretreated with p38MAPK, or MMP inhibitors. Finally we show that nSMase2 activation by H2O2, depends on MMP2 and p38MAPK, and is required for the src-dependent phosphorylation of AnxA2, and actin remodeling.
Taken together, these findings indicate for the first time that AnxA2 phosphorylation and actin remodeling evoked by oxidative stress depend on the sphingolipid pathway, via MMP2 and p38MAPK.
Abbreviations: nSMase, neutral sphingomyelinase; nSMase2, type 2 neutral sphingomyelinase; SMC, smooth muscle cell; mFbl, mouse fibroblast; wt, wild type; fro, fragilitas ossium; mef, mouse embryonic fibroblast; MMP, matrix metalloproteinases; AnxA2, annexin 2; H2O2, hydrogen peroxide; ROS, reactive oxygen species
Keywords: Oxidative stress, Fibroblasts, Smooth muscle cells, Sphingolipids, Actin, Vasculo-proliferative diseases
Graphical abstract
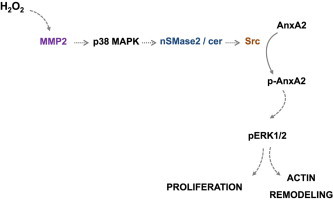
Schematic diagram of the signaling pathway triggered by low H2O2 concentration, leading to actin remodeling and cell proliferation. H2O2 (5 μM) activates MMP2 which leads to p38MAPK activation. P38MAPK activates nSMase2 which generates ceramide that activates src kinase. Src phosphorylates AnxA2 which leads to ERK1/2 phosphorylation involved in actin remodeling and cell proliferation.
Highlights
-
•
Low concentration of H2O2 activates matrix metalloproteinases MMP-2.
-
•
MMP-2 activates p38MAPK, type 2 neutral sphingomyelinase.
-
•
This signaling pathway induces annexin II phosphorylation via src.
-
•
This pathway is involved in actin remodeling due to H2O2 stimulation.
Introduction
Reactive oxygen species (ROS) regulate a huge number of cellular responses in mammalian cells, including migration, proliferation, contraction, growth arrest or apoptosis [1,2]. ROS include hydrogen peroxide (H2O2), which is a potent signaling agent [3], that exhibits proapoptotic and cytotoxic properties at high concentration [4], whereas low doses stimulate cell migration and proliferation of various cell types, such as fibroblasts or smooth muscle cells (SMC) [5]. H2O2 triggers the activation of mitogenic signaling pathways including the PDGFR-β receptor, PI–3K/Akt, src, or ERK1/2 [6]. We recently reported that low H2O2 concentration stimulate the proliferation of SMC and fibroblasts, via an activation of the sphingolipid (SL) pathway, represented by the neutral type 2 sphingomyelinase (nSMase2, the first step of the SL pathway), and by sphingosine kinase-1 (SK1) which generates the mitogenic and survival SL mediator sphingosine 1-phosphate (S1P). The signaling mechanism evoked by H2O2 involves a signaling cascade implicating src and the trans-activation of the PDGFR-β receptor [5]. In contrast, high H2O2 concentration inhibits SK1 (but not nSMase2) [5] and induces cell death [7].
Cell proliferation and migration involve early signaling events that affect cell movement, and require actin modification and polymerization. These events are coordinated by actin-binding proteins, and are regulated by signaling mechanisms implicating the PDGF-β receptor, PI3K, Ca2+ small G proteins, src and MAPK [8,9]. Annexin-II (AnxA2), a 36 kDa Ca2+-dependent phospholipid-binding protein, is a major regulator of actin remodeling, after undergoing phosphorylation by src on Tyr23 [10,11]. Membrane-bound AnxA2 is present at the inner surface of the plasma membrane, and acts as a platform regulating actin assembly and maintaining the dynamic of plasma membrane-associated actin cytoskeleton [12]. The expression of AnxA2 is associated with cell migration and proliferation, particularly in cancer, since poorly invasive tumor cells, such as MCF-7, express low level of AnxA2, whereas AnxA2 is highly expressed in very invasive cells. Moreover, increasing the expression of AnxA2 in MCF-7 stimulates their proliferation [13].
Cytoskeleton remodeling is one of the earliest targets of oxidative stress, via signaling implicating p38MAPK, as reported in endothelial cells [14] and in astrocytes [15], or NADPH oxidase and the translocation of phospho-PKC-δ, in SMC, as recently shown by Lv and coll [16]. A role for sphingolipid mediators, ceramide and sphingosine-1-phosphate (S1P) has been reported in actin remodeling [17], but the mechanisms are not yet identified. Since nSMase2 is a known target of reactive oxygen species (ROS) and since its activation involves p38MAPK [18], we aimed at investigating the role of nSMase2 in actin remodeling evoked by H2O2. We report that H2O2 activates nSMase2 in an MMP2 and p38MAPK-dependent manner, which results in the phosphorylation of AnxA2 by src, and subsequently ERK1/2 phosphorylation, actin remodeling and cell proliferation.
Materials and methods
Chemicals
[3H]Thymidine (5 Ci/mmol) was from PerkinElmer (Wellesley, US). Rabbit anti-AnxA2 and pTyr23 AnxA2 were from Santa Cruz Biotechnologies (Santa Cruz, CA) and rabbit anti-(activated-) phospho-ERK1/2, phospho-src, phospho-p38MAPKwere from cell Signaling. Ro28-2653 was given by H.-W. Krell (Roche Diagnostics, Penzberg, Germany). MMP2 substrate MCA-Pro-Leu-Ala-Nva-Dpa-ala-Arg-NH2 was from VWR. Other reagents were obtained from Sigma or Invitrogen (France).
Cell culture
Mouse fibroblasts were isolated from nSMase2-deficient homozygous fro/fro mice [19] (fro/fro mFbl, smpd3fro/fro genotype) and from wild-type mice of the same genetic 129/SV strain background. MMP2−/− and wt mefs were from RIKEN BioResource Center (Ibaraki, Japan) [20]. Cells were grown in DMEM supplemented with 10% FCS, unless otherwise indicated. CRL 1999 human aortic SMC were from ATCC (Mölsheim, France), and were grown in RPMI-1640 supplemented with 10% fetal calf serum (FCS). Srck+ and Srckd mefs (a generous gift from Dr. S.J. Parsons, University of Virginia, Charlottesville, VA), derived from C3H10T1/2 transfected with a wild-type form of c-Src (Srck+) or with a mutated dominant-negative form of pp60c-Src deficient in kinase activity (Srckd cells, clone 430c-Src) [21]. The cells were grown in DMEM medium supplemented with 10% FCS and G418 (0.4 mg/ml). 24 h before the experiment, the medium was removed and replaced by serum-free RPMI. SiRNA directed against AnxA2 (SmartPool L061993) was from Dharmacon. The protocol used for transfecting fibroblasts with siRNA using oligofectamin reagent was similar to that previously reported in [5]. DNA synthesis was evaluated by [3H]thymidine incorporation as previously reported [22].
Atto-488 phalloidin labeling
Fibroblasts were seeded on glass coverslip. After stimulation by H2O2, cells were washed twice with pre-warmed PBS, pH 7.4, and fixed in 4% methanol free-formaldehyde solution in PBS for 10 min at room temperature. After washing twice with PBS, cells were incubated 5 min in PBS containing 0.1% Triton X-100, and stained with fluorescent atto-488 phalloidin (30 min at room temperature). Confocal analyses were done utilizing a Zeiss LSM 510 confocal microscope (Le Pecq, France) (fluorescein filter excitation 488 nm, emission 505 nm). The laser intensity was the same for all the picture capture. Fluorescence quantification was done with ImageJ software after subtraction of the background. Several cells were quantified for one condition experiment and each experimental condition was reproduced at least three times. Values from all independent experiments were averaged for a single data point. Results are presented as the normalization value of the mean value±S.E.M. of fluorescence emitted by cells treated with drugs±H2O2, vs. controls.
nSMase determination
Cells were homogenized by sonication in 0.1% Triton X-100, 10 mM MgCl2, 5 mM dithiothreitol, 0.1 mM Na3VO4, 10 mM glycerophosphate, 750 µM ATP, 1 mM PMSF, 2 mM EDTA, 10 µM leupeptin, and 10 µM pepstatin. The reaction mixture contained 100 µl of substrate [choline-methyl-14C]sphingomyelin (120,000 dpm/assay) in 0.1% Triton X-100, 20 mM HEPES buffer, pH 7.4, containing 1 mM MgCl2, and 100 µl of cell homogenate. After 2 h incubation at 37 °C, the liberated [methyl-14C]choline was partitioned under the previously used conditions [22] and quantified by liquid scintillation counting.
Metalloproteinase activity
Zymography experiments were done on a 10% acrylamide gel containing 0.1% gelatin (1 mg/ml). Cell culture supernatants (35 µl) were run at 20 mA, then incubated for 15 min in triton 2.5%, triton X100, and overnight in the reaction buffer (Tris–HCl 50 mM, NaCl 200 mM, CaCl2 5 mM, Brij-35 0.02% (m/v), pH 7.6). The gels were stained with Coomassie Blue R-250 for 30 min and the protease activity was identified as clear bands against a dark blue background after decoloration (acetic acid/methanol/water; 1/4/5).
MMP2 activity was determined on concentrated SMC media or cell pellet with the fluorogenic substrate MCA-Pro-Leu-Ala-Nva-Dpa-ala-Arg-NH2 (Calbiochem-WWR) as described [20]. The experiment was done in the presence and absence of EDTA (5 µM) and two controls were performed (without cell and without substrate). After 3 h incubation (37 °C), 1 ml Tris–HCl buffer, pH 7, was added, and the fluorescence was read (excitation and emission wavelengths, 325–395).
Western blots
Western blots were done as previously reported [22], and quantified using ImageJ. Protein concentration was determined using the Bradford reagent (Biorad).
Statistical analysis
Data are presented as mean±standard deviation. Statistical comparison of the data was performed using t-test to compare two groups, the one-way ANOVA (with Bonferroni correction) to compare more than 2 groups when only one factor was modified during the experiment and the two-way ANOVA test when two factors were changed (stimulation with or without H2O2 in the presence or absence of inhibitor) in the study (Prism 6 Software GraphPad Software, Inc., USA). Significance was set at P<0.05.
Results
Actin reorganization evoked by H2O2, requires ERK1/2 activation and AnxA2 phosphorylation
Wild type mouse fibroblasts (wt mFbl) incubated for 15 min, with H2O2 (5 µM) exhibited actin bundles corresponding to a reorganization of the actin network, when compared to actin fibers in quiescent cells (Fig. 1A–C). It is to note that the actin network remodeling is a temporary phenomenon which returned to the basal state after 2 h of treatment with low but not with high concentration of H2O2 (not shown). H2O2 stimulated ERK1/2 phosphorylation (Fig. 1D) that was required for actin remodeling and, as expected, for cell proliferation, since the MEK inhibitor PD98059, blocked thymidine uptake (Fig. 1E) and actin remodeling induced by H2O2 (Fig. 1F–H).
Fig. 1.
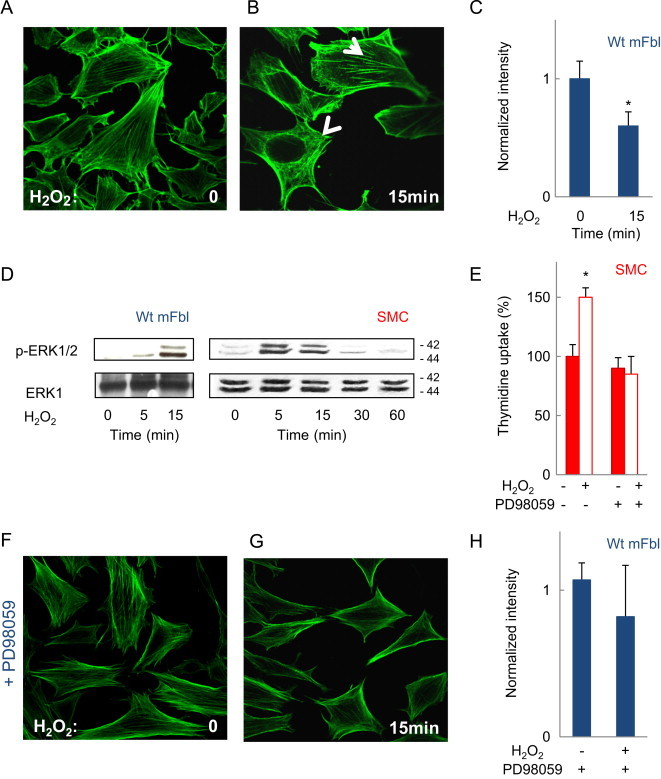
F-actin modifications evoked by H2O2 in wt mFbl depend on ERK1/2. (A, B) Representative F-actin pictures assessed by confocal microscopy of wt mFbl unstimulated (A), or stimulated by H2O2 (5 µM) for 15 min (B) and labeled with Alexa 488-phalloidin. (C) Intensity profiles of phalloidin staining for each cell were evaluated using ImageJ (expressed as normalized intensity) (D) time-course of ERK1/2 phosphorylation induced by H2O2 in fibroblasts and SMC stimulated by H2O2 (5 µM). (E) Thymidine uptake was quantified in SMC after treatment with H2O2w/wo the ERK1/2 inhibitor PD98059 (10 µM). (F–H) Representative F-actin confocal microscopy pictures and intensity quantification of phalloidin staining, evaluated as in 1A, w/wo PD98059 (10 µM). Data are mean±SEM from at least three independent experiments and are expressed relative to basal. ⁎, P<0.05 vs. basal.
Tyrosine phosphorylation (Tyr23) of AnxA2 is associated with cell proliferation and ERK1/2 activation [23]. H2O2 treatment stimulated the phosphorylation of AnxA2 on Tyr23 in SMC and in wt mFbl (Fig. 2A, B). AnxA2 was required for ERK1/2 activation and cell proliferation induced by H2O2, as supported by the inhibitory effect of AnxA2 siRNA on ERK1/2 phosphorylation (Fig. 2B) and thymidine uptake (Fig. 2C). Since Tyr23-phosphorylated AnxA2 is involved in the dynamic restructuring of the actin cytoskeleton [10,11], we investigated the effect of AnxA2 silencing on actin remodeling. In fibroblasts siRNA-silenced for AnxA2, the cells exhibited a ‘splinter-like’ bundle aspect which was not modified upon H2O2 stimulation (Fig. 2D, E).
Fig. 2.
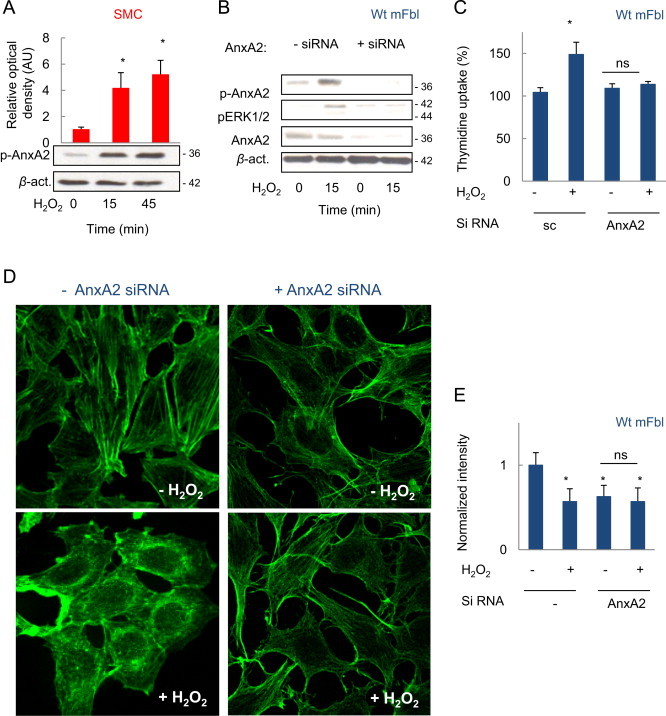
AnxA2 phosphorylation evoked by H2O2 is involved in ERK1/2 activation and actin remodeling. (A) Western-blot experiments showing the phosphorylation of AnxA2 (on Tyr23) induced by H2O2 (5 µM) in SMC. (B, C) AnxA2 silencing by siRNA in wt mFbl, suppressed the phosphorylation of ERK1/2 (B), and thymidine uptake (C) evoked by H2O2. (D, E) Confocal microscopy pictures of F-actin labeled with Alexa 488-phalloidin in wt mFbl siRNA silenced for AnxA2 (D), and intensity quantification of phalloidin staining using ImageJ experiments were performed at least 3 times: ⁎p<0.05 vs. basal.
As H2O2 triggers the phosphorylation and activation of src in fibroblasts [5] and SMC (Fig. 3A) we checked whether src is involved in AnxA2 phosphorylation. The src inhibitor PP2 inhibited AnxA2 phosphorylation induced by H2O2 (Fig. 3B). Likewise, H2O2 was unable to trigger AnxA2 phosphorylation in Srckd fibroblasts (Fig. 3C), indicating that src is necessary for AnxA2 phosphorylation by H2O2. In agreement with these findings, ERK1/2 phosphorylation (Fig. 3D) and actin remodeling induced by H2O2, were inhibited by PP2 (Fig. 3E, F).
Fig. 3.
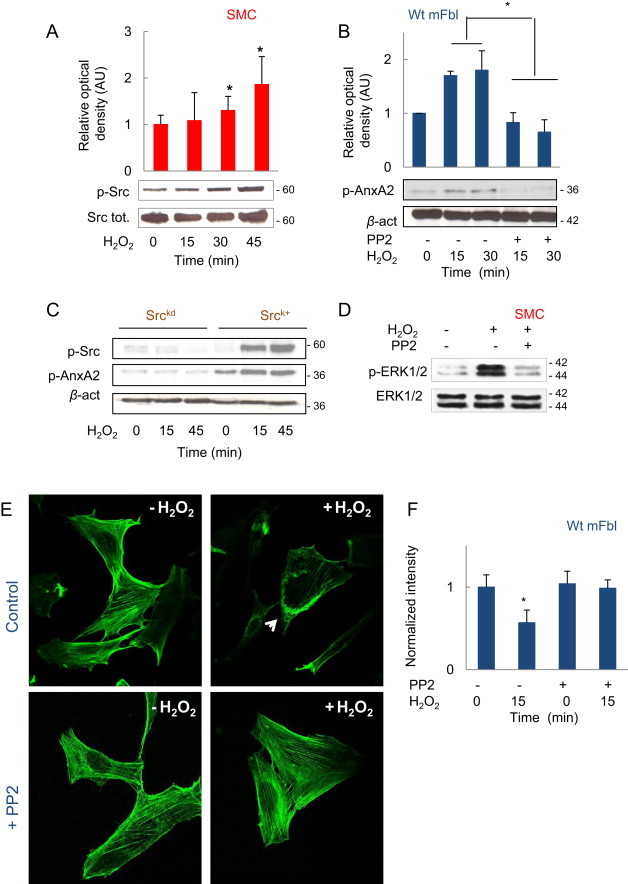
Src phosphorylation leads to AnxA2 and ERK1/2 phosphorylations. (A) Time-course of src phosphorylation in SMC stimulated by H2O2 (5 µM). (B) Effect of the src inhibitor PP2 (10 µM) on AnxA2 phosphorylation induced by H2O2 (5 µM). (C) Time-course of AnxA2 phosphorylation by H2O2 in srcK+ and srckd mefs. (D) Effect of PP2 (10 µM) on ERK1/2 phosphorylation in SMC stimulated by H2O2 (5 µM). (E, F) Representative confocal microscopy pictures of F-actin and intensity quantification of phalloidin staining showing the effect of PP2 (10 µM) in wt mFbl stimulated by H2O2 stimulation. Experiments were performed at least 3 times: * p<0.05 vs. basal.
AnxA2 phosphorylation depends on nSMase2 activation
We recently reported that src activation by H2O2 in SMC and fibroblasts, depends on the activation of nSMase2 [5], which suggests that nSMase2 may be involved in the phosphorylation of AnxA2 by src. Fibroblasts isolated from fragilitas ossium (fro) mice [19], are mutant for nSMase2, which cannot be activated by H2O2 as reported [5] and (Fig. 4A). No AnxA2 phosphorylation was observed in these cells upon H2O2 stimulation by comparison to wt mFbl (Fig. 4B, C).
Fig. 4.
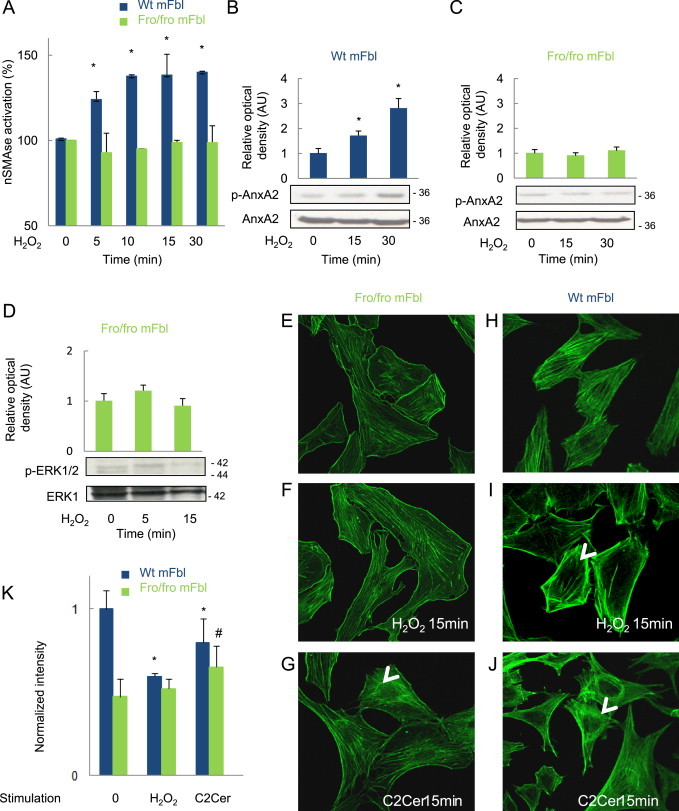
Role of nSMase in AnxA2 phosphorylation and ERK1/2 activation induced by H2O2. (A) Time-course of nSMase activation by H2O2 (5 µM), in wt (black bars) and fro/fro mFbl (white bars). (B, C) AnxA2 phosphorylation induced by H2O2 in wt (B) and in fro/fro mFbl (C). (D) Lack of ERK1/2 phosphorylation in fro/fro mFbl upon H2O2 stimulation. (E–K) Representative confocal microscopy pictures of F-actin of fro/fro (E–G) or wt mFbl (H, I) or stimulated or not (E, H) with H2O2 (F, I) or with C2 ceramide (5 µM, G, J) for 15 min (K) intensity quantification of phalloidin staining (expressed as normalized intensity) was evaluated using ImageJ and is indicated in the corresponding picture. Data are mean±SEM from at least three independent experiments and are expressed relative to basal. *, P<0.05 vs. basal.
We previously demonstrated that fro/fro mFbl do not proliferate upon H2O2 stimulation [5]. As expected, neither ERK1/2 phosphorylation (Fig. 4D), nor actin remodeling were observed in nSMase2-mutant (fro) cells (Fig. 4E–K). The actin network in fro/fro mFbl was different from that observed in wt mFbl (short fibers in fro/fro mFbl vs. long fiber running across the cells in control cells; Fig. 4E, H), no effect of H2O2 on the network observed in fro/fro mFbl (Fig. 4F, I). However, when fro/fro mFbl were treated with 5 µM exogenous C2 ceramide, the organization of stress actin fibers was comparable to that observed in wt mFbl treated with H2O2 or (Fig. 4G, J). Altogether, these data suggest that nSMase2 and ceramide contribute to actin remodeling evoked by H2O2.
MMP2 and p38MAPK are required for nSMase2 activation by H2O2
We previously reported that the activation of nSMase2 by stress-inducing agents such as TNF-α or oxidized LDL, requires MMP2 [20,22]. Since MMP2 is activated by H2O2 (Fig. 5), we checked whether it is implicated in actin remodeling via nSMase2 activation.
Fig. 5.
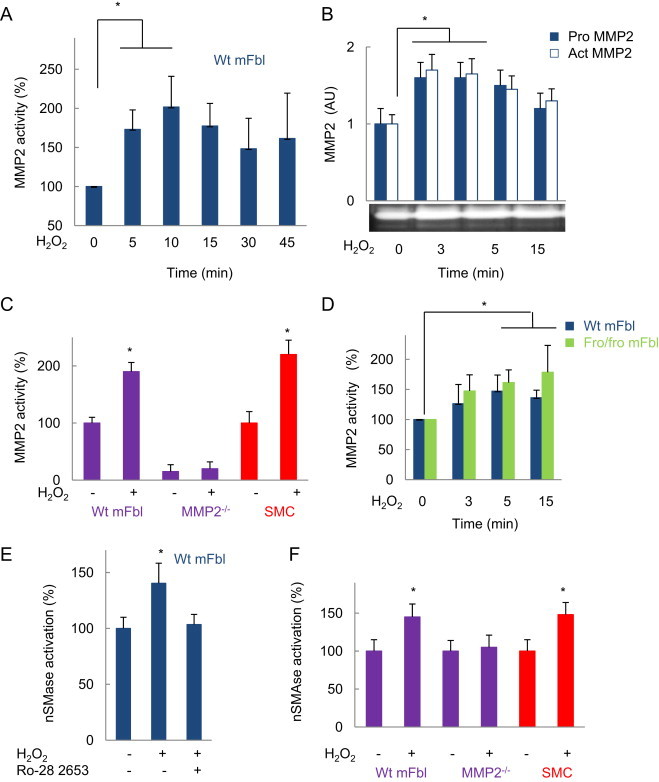
MMP2 activation by H2O2 precedes nSMase2. (A, B) Time-course of MMP2 activation by H2O2 using (A) the specific fluorogenic substrate (MCA-Pro-Leu-Ala-Nva-Dpa-ala-Arg-NH2) or (B) zymography experiments, as described in Exerimental section. (C, D) MMP2 enzymatic activity determined with the fluorogenic substrate, in wt mFbl, MMP2−/− fibroblasts, SMC (C) simulated 15 min, and in fro/fro or wt mFbl (D), after stimulation by H2O2 (5 µM). (E) Effect of the MMP inhibitor Ro-26 2853 (10 nM) on nSMase activation evoked by H2O2 in wt mFbl. (F) nSMase activation by H2O2. In wt mFbl and MMP2−/− fibroblasts. Data are mean±SEM from at least three independent experiments and are expressed relative to basal. ⁎, P<0.05 vs. basal.
H2O2 was unable to trigger nSMase2 activation in MMP2−/− fibroblasts (Fig. 5), while Ro-28 2653, an MMP inhibitor of large specificity, inhibited nSMase2 activation, in agreement with our previous reports [22] and (Fig. 5). In contrast, MMP2 was activated by H2O2 in fro/fro fibroblasts (Fig. 5), indicating that nSMase2 activation is downstream MMP2.
Among the mechanisms possibly involved in the activation of nSMase2 by MMP2, we investigated the role of p38MAPK, which is early activated in response to agents such as TNF-α [18] or endothelin-1 [24]. Results presented in Fig. 6 indicate that p38MAPK activation by H2O2, needs MMP2, since no phosphorylation of p38MAPK was observed in MMP2−/− fibroblasts (A), whereas in nSMase2 mutant fro/fro mFbl, p38MAPK was phosphorylated (B). In addition, the p38MAPK pharmacological inhibitor SB203580, blocked the activation of nSMase in wt fibroblasts (Fig. 6C), thereby indicating that p38MAPK activation by H2O2 did not implicate the sphingolipid pathway, but was necessary to the activation of nSMase.
Fig. 6.
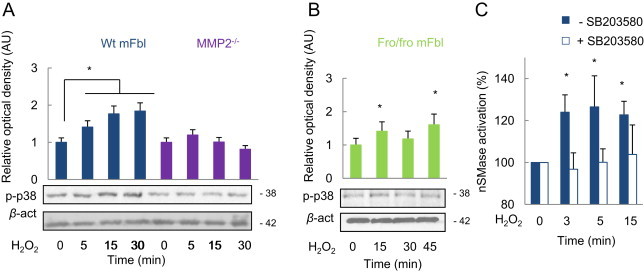
MMP2-dependent nSMase2 activation by H2O2 involves p38MAPK. Wt mFbl, MMP2−/− (A) and fro/fro fibroblasts (B) were stimulated with H2O2 at the indicated times. The phosphorylation of p38MAPK was evaluated by western-blot. Alternatively, nSMase activity was measured in wt mFbl incubated with H2O2w/wo the SB203580 (10 µM) (C). These experiments were performed in triplicate. ⁎, P <0.05 vs. basal.
MMP2 and p38MAPK are required for src, AnxA2 phosphorylation and actin remodeling.
We tested the effect of MMP2 and p38MAPK inhibitors on src activation, that is necessary for AnxA2 phosphorylation (Fig. 7). H2O2 did not stimulate the phosphorylation of src in MMP2−/− fibroblasts and in fro/fro mFbl (Fig. 7A, B) and in cells treated with the p38MAPK inhibitor (SB203580) (C). Likewise, AnxA2 phosphorylation was absent in cells treated with SB203580 in MMP2−/− fibroblasts upon stimulation by H2O2 (Fig. 7E, F), as well as actin remodeling, ERK1/2 phosphorylation and cell proliferation (Fig. 8).
Fig. 7.
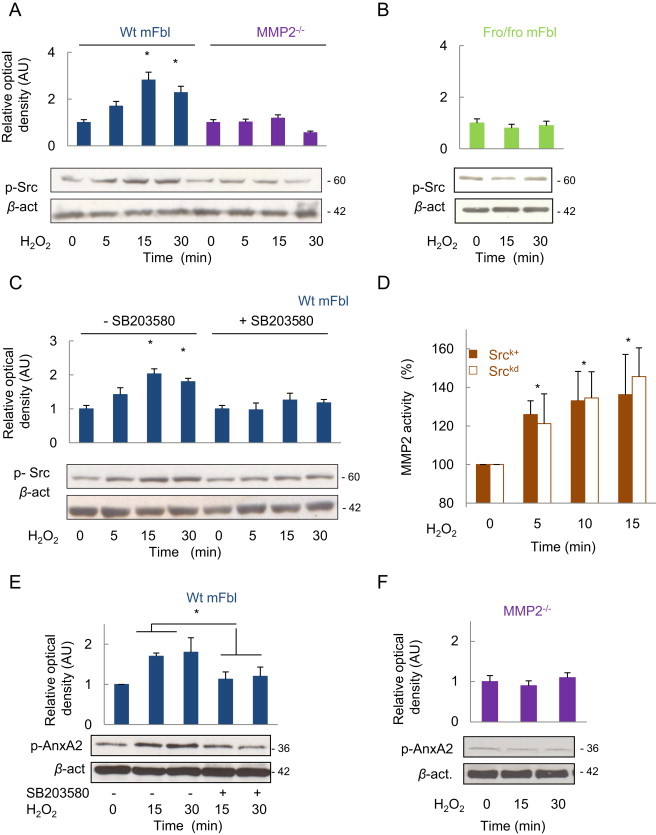
The phosphorylation of Src and AnxA2 depends on the activation of nSMase2, via MMP2 and p38MAPK. Cells were stimulated by H2O2 at the indicated times, and src phosphorylation was determined on wt and MMP2−/− (A), and on fro/fro mFbl (B). In C, effect of the p38MAPK inhibition by H2O2 in srck+ and srckd fibroblasts, determined using the specific fluorogenic assay as reported in the experimental section. (E) Effect of SB203580 on AnxA2 phosphorylation in wt mFbl. (F) AnxA2 phosphorylation by H2O2 in MMP2−/− fibroblasts. Data are means±SEM from at least three independent experiments and are expressed relative to basal. ⁎, P<0.05 vs. basal.
Fig. 8.
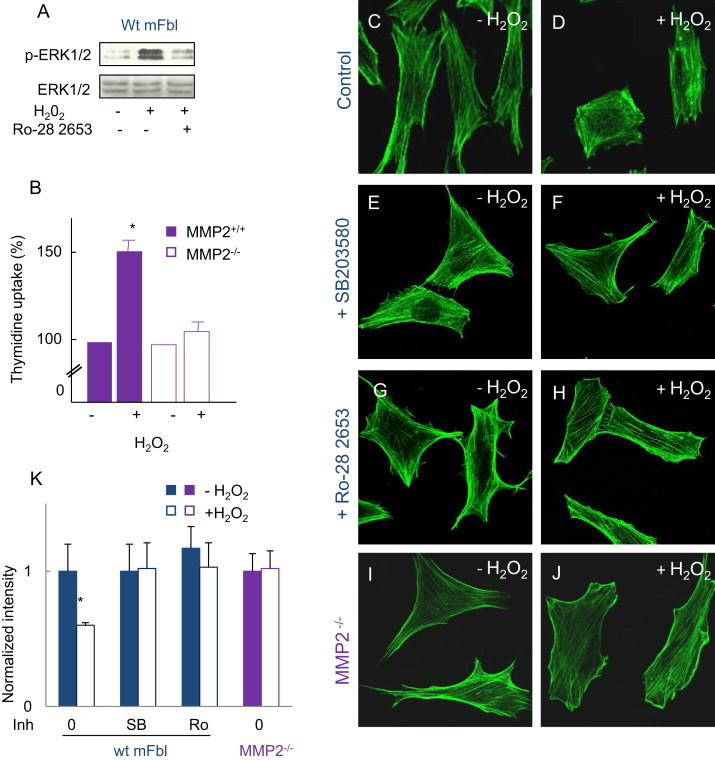
MMPs and p38MAPK are involved in actin remodeling, ERK1/2 activation and cell proliferation, (A) effect of the MMP inhibitor Ro-28 2653 (10 nM), on ERK1/2 phosphorylation induced by H2O2 in wt mFbl. (B) Thymidine uptake in MMP2−/− and wt mFbl stimulated by H2O2 (5 µM). (C–J) Representative confocal microscopy pictures of F-actin in wt (C–H) or MMP2-KO (I, J) fibroblasts and effect of SB203580 (10 µM, E, F), Ro-28 2653 (10 nM, G, H), was added 30 min before H2O2 (5 µM). (K) Intensity quantification of phalloidin (expressed as normalized intensity). All the experiments were done in triplicate. ⁎p<0.05 vs. basal.
Altogether these results emphasize the role of nSMase2 in AnxA2 phosphorylation (via src) and actin remodeling evoked by H2O2, via a signaling mechanisms implicating MMP2 and p38MAPK.
Discussion
Several mechanisms have been proposed to explain the effect of ROS on actin cytoskeleton including ATP depletion, oxidative modification or activation of Ca2+ dependent proteins and protein kinase pathways [25]. In this article we show that nSMase2 is involved in the phosphorylation by src of AnxA2 and actin remodeling evoked by H2O2, via a signaling mechanism implicating an upstream activation of MMP2 and p38MAPK. Note that the remodeling of actin is reversible in the presence of low H2O2 concentration, while it leads to a full break of the actin network when higher toxic H2O2 dose is used ([5] and unpublished observations).
AnxA2 belongs to a large family of highly conserved proteins characterized by their ability to bind and order membrane phospholipids, particularly membranes enriched in cholesterol. It can be phosphorylated by growth factors receptors [26] PKCs [27], and src [11]. Moreover AnxA2 is involved in membrane trafficking and cell polarity [28]. The phosphorylation of AnxA2 by src on Tyr23, mediates several cellular events such as cell scattering and branching morphogenesis [10], Rho-mediated actin rearrangement and cell adhesion and cancer cell proliferation [10,23]. Our data indicate that the phosphorylation of AnxA2 depends on src that is activated subsequently to nSMase2 activation. We previously reported the role of nSMase2 in src activation by H2O2, the trans-activation of the PDGFβ receptor and subsequently of sphingosine kinase-1 (SK1) [5,29]. Other reports indicate that src may upregulate nSMase2 activity and ceramide generation, via p38 MAPK in HAE cells exposed to oxidative stress [30]. AnxA2 phosphorylation is linked to actin bundling in fibroblasts and is necessary to transduce ERK1/2 activation and the mitogenic signaling of H2O2, since fibroblasts siRNA-silenced for AnxA2, did not phosphorylate ERK1/2 upon H2O2 treatment, nor proliferate, and exhibited a disorganized actin network.
Our data confirm that nSMase2 activation by H2O2 requires an activation of MMP2 as previously reported for various stress-inducing agents [20,22,29]. The role of MMP2 in cell proliferation and migration could involve the proteolytic degradation of basement membranes and extracellular matrix components [32]. Previous studies reported that MMP2 can be activated and secreted by actin remodeling or upon treatment by cytoskeletal disrupting agents [33,34]. Here we show that MMP2 is necessary for the activation of nSMase2 and actin remodeling evoked by H2O2 via p38MAPK, which is activated upstream nSMase2, in agreement with previous reports [35,36]. Of note, the role of p38MAPK in actin remodeling, has been reported in astrocytes [15] and endothelial cells [14].
Ceramide and S1P are known to rearrange cytoskeleton [17,31], mainly through the regulation of Rho GTPases [37], or ezrin phosphorylation, as reported for cisplatin on actin cytoskeleton, which involves acidic SMase activation and ceramide generation [38]. Interestingly close interactions exist between ezrin and AnxA2, as AnxA2 could regulate the level of ezrin expression [39], and the association of these two proteins could constitute an interface between endosome, plasma membrane and cytoskeleton [40]. AnxA2 contributes to the actin regulatory machinery that regulates the endosomal trafficking and activation of Src [41] to induce ERK1/2 activation [42].
Cellular regulation of actin polymerization and organization is a highly complex process that involves a number of actin-binding proteins, including severing, sequestering, cross-linking, and membrane-anchoring proteins, all of which being under the regulation of various signal transduction pathways. The mechanisms linking actin organization/disorganization and the mitogenic signaling cascade are not well clarified. Yue's group proposed that the inhibition of stress fiber actin formation may contribute to cell proliferation [43], while Triesman and coll. proposed that actin remodeling is involved in cell proliferation via the activation of the serum response transcription factor [44]. In addition, actin regulation could participate to MT1-MMP or MMP2 secretion and activation [33,34]. Our data show that MMP2 inhibition blocks actin remodeling, suggesting a possible positive feedback loop between these two events. Moreover, it is possible that src targeted to raft domains could turn off an actin-assembly activity mediated by AnxA2, which may contribute to modify actin dynamics, characteristic of proliferating cells [12]. This is in agreement with reports showing that growth factors promote the reorganization of actin filaments [45], which can be inhibited by antioxidants and p38MAPK or ERK1/2 inhibitors [21].
In summary, our data support the conclusion that nSMase2 activation by H2O2 regulates actin remodeling and proliferation in SMC and fibroblasts, by triggering src activation and the subsequent phosphorylation of AnxA2, leading to ERK1/2 phosphorylation. This pathway involves an early signaling mechanism implicating MMP2 and p38MAPK, which are required for nSMase2 activation. The implication of phosphorylated AnxA2 as a signal transducer in cell proliferation and actin remodeling remains to be determined.
Acknowledgments
The authors acknowledge INSERM, Université Toulouse-3, Agence de Biomedicine and COST CM1001 for logistic and financial support. The authors wish to thank MH. Grazide, E. Mucher, and Plateforme Imagerie for excellent technical assistances.
References
- 1.Blanc A., Pandey N.R., Srivastava A.K. Synchronous activation of ERK 1/2, p38MAPK and PKB/Akt signaling by H2O2 in vascular smooth muscle cells: potential involvement in vascular disease. International Journal of Molecular Medicine. 2003;11(2):229–234. 12525883 [PubMed] [Google Scholar]
- 2.Mehdi M.Z., Azar Z.M., Srivastava A.K. Role of receptor and nonreceptor protein tyrosine kinases in H2O2-induced PKB and ERK1/2 signaling. Cell Biochemistry and Biophysics. 2007;47(1):1–10. doi: 10.1385/cbb:47:1:1. 17406055 [DOI] [PubMed] [Google Scholar]
- 3.Stone J.R., Yang S. Hydrogen peroxide: a signaling messenger. Antioxidants and Redox Signaling. 2006;8(3–4):243–270. doi: 10.1089/ars.2006.8.243. 16677071 [DOI] [PubMed] [Google Scholar]
- 4.Goldkorn T., Balaban N., Shannon M., Chea V., Matsukuma K., Gilchrist D., Wang H., Chan C. H2O2 acts on cellular membranes to generate ceramide signaling and initiate apoptosis in tracheobronchial epithelial cells. Journal of Cell Science. 1998;111(21):3209–3220. doi: 10.1242/jcs.111.21.3209. 9763515 [DOI] [PubMed] [Google Scholar]
- 5.Cinq-Frais C., Coatrieux C., Grazide M.H., Hannun Y.A., Nègre-Salvayre A., Salvayre R., Augé N. A signaling cascade mediated by ceramide, src and PDGFRbeta coordinates the activation of the redox-sensitive neutral sphingomyelinase-2 and sphingosine kinase-1. Biochimica et Biophysica Acta. 2013;1831:1344–1356. doi: 10.1016/j.bbalip.2013.04.014. 23651497 [DOI] [PubMed] [Google Scholar]
- 6.González-Rubio M., Voit S., Rodríguez-Puyol D., Weber M., Marx M. Oxidative stress induces tyrosine phosphorylation of PDGF alpha-and beta-receptors and pp60c-src in mesangial cells. Kidney International. 1996;50(1):164–173. doi: 10.1038/ki.1996.299. 8807585 [DOI] [PubMed] [Google Scholar]
- 7.Pchejetski D., Kunduzova O., Dayon A., Calise D., Seguelas M.H., Leducq N., Seif I., Parini A., Cuvillier O. Oxidative stress-dependent sphingosine kinase-1 inhibition mediates monoamine oxidase A-associated cardiac cell apoptosis. Circulation Research. 2007;100(1):41–49. doi: 10.1161/01.RES.0000253900.66640.34. 17158340 [DOI] [PubMed] [Google Scholar]
- 8.Gerthoffer W.T. Mechanisms of vascular smooth muscle cell migration. Circulation Research. 2007;100(5):607–621. doi: 10.1161/01.RES.0000258492.96097.47. 17363707 [DOI] [PubMed] [Google Scholar]
- 9.Maness P.F., Walsh R.C., Jr. Dihydrocytochalasin B disorganizes actin cytoarchitecture and inhibits initiation of DNA synthesis in 3T3 cells. Cell. 1982;30(1):253–262. doi: 10.1016/0092-8674(82)90031-9. 6215122 [DOI] [PubMed] [Google Scholar]
- 10.De Graauw M., Tijdens I., Smeets M.B., Hensbergen P.J., Deelder A.M., van de Water B. Annexin A2 phosphorylation mediates cell scattering and branching morphogenesis via cofilin activation. Molecular and Cellular Biology. 2008;28(3):1029–1040. doi: 10.1128/MCB.01247-07. 18070928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rescher U., Ludwig C., Konietzko V., Kharitonenkov A., Gerke V. Tyrosine phosphorylation of annexin A2 regulates Rho-mediated actin rearrangement and cell adhesion. Journal of Cell Science. 2008;121(13):2177–2185. doi: 10.1242/jcs.028415. 18565825 [DOI] [PubMed] [Google Scholar]
- 12.Hayes M.J., Shao D., Bailly M., Moss S.E. Regulation of actin dynamics by annexin 2. EMBO Journal. 2006;25(9):1816–1826. doi: 10.1038/sj.emboj.7601078. 16601677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu B., Zhang F., Yu M., Zhao P., Ji W., Zhang H., Han J., Niu R. Up-regulation of Anxa2 gene promotes proliferation and invasion of breast cancer MCF-7 cells. Cell Proliferation. 2012;45(3):189–198. doi: 10.1111/j.1365-2184.2012.00820.x. 22452352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huot J., Houle F., Marceau F., Landry J. Oxidative stress-induced actin reorganization mediated by the p38 mitogen-activated protein kinase/heat shock protein 27 pathway in vascular endothelial cells. Circulation Research. 1997;80(3):383–392. doi: 10.1161/01.res.80.3.383. 9048659 [DOI] [PubMed] [Google Scholar]
- 15.Zhu D., Tan K.S., Zhang X., Sun A.Y., Sun G.Y., Lee J.C. Hydrogen peroxide alters membrane and cytoskeleton properties and increases intercellular connections in astrocytes. Journal of Cell Science. 2005;118(16):3695–3703. doi: 10.1242/jcs.02507. 16046474 [DOI] [PubMed] [Google Scholar]
- 16.Lv P., Miao S.B., Shu Y.N., Dong L.H., Liu G., Xie X.L., Gao M., Wang Y.C., Yin Y.J., Wang X.J., Han M. Phosphorylation of smooth muscle 22alpha facilitates angiotensin II-induced ROS production via activation of the PKCdelta-P47phox axis through release of PKCdelta and actin dynamics and is associated with hypertrophy and hyperplasia of vascular smooth muscle cells in vitro and in vivo. Circulation Research. 2012;111(6):697–707. doi: 10.1161/CIRCRESAHA.112.272013. 22798525 [DOI] [PubMed] [Google Scholar]
- 17.Canals D., Jenkins R.W., Roddy P., Hernández-Corbacho M.J., Obeid L.M., Hannun Y.A. Differential effects of ceramide and sphingosine 1-phosphate on ERM phosphorylation: probing sphingolipid signaling at the outer plasma membrane. Journal of Biological Chemistry. 2010;285(42):32476–32485. doi: 10.1074/jbc.M110.141028. 20679347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clarke C.J., Guthrie J.M., Hannun Y.A. Regulation of neutral sphingomyelinase-2 (nSMase2) by tumor necrosis factor-alpha involves protein kinase C-delta in lung epithelial cells. Molecular Pharmacology. 2008;74(4):1022–1032. doi: 10.1124/mol.108.046250. 18653803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aubin I., Adams C.P., Opsahl S., Septier D., Bishop C.E., Auge N., Salvayre R., Negre-Salvayre A., Goldberg M., Guénet J.L., Poirier C. A deletion in the gene encoding sphingomyelin phosphodiesterase 3 (Smpd3) results in osteogenesis and dentinogenesis imperfecta in the mouse. Nature Genetics. 2005;37(8):803–805. doi: 10.1038/ng1603. 16025116 [DOI] [PubMed] [Google Scholar]
- 20.Tellier E., Nègre-Salvayre A., Bocquet B., Itohara S., Hannun Y.A., Salvayre R., Augé N. Role for furin in tumor necrosis factor alpha-induced activation of the matrix metalloproteinase/sphingolipid mitogenic pathway. Molecular and Cellular Biology. 2007;27(8):2997–3007. doi: 10.1128/MCB.01485-06. 17283058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng C.X., Chen Z.H., Zeng C.H., Qin W.S., Li L.S., Liu Z.H. Triptolide protects podocytes from puromycin aminonucleoside induced injury in vivo and in vitro. Kidney International. 2008;74(5):596–612. doi: 10.1038/ki.2008.203. 18509322 [DOI] [PubMed] [Google Scholar]
- 22.Augé N., Maupas-Schwalm F., Elbaz M., Thiers J.C., Waysbort A., Itohara S., Krell H.W., Salvayre R., Nègre-Salvayre A. Role for matrix metalloproteinase-2 in oxidized low-density lipoprotein-induced activation of the sphingomyelin/ceramide pathway and smooth muscle cell proliferation. Circulation. 2004;110(5):571–578. doi: 10.1161/01.CIR.0000136995.83451.1D. 15277330 [DOI] [PubMed] [Google Scholar]
- 23.Wang Y.Q., Zhang F., Tian R., Ji W., Zhou Y., Sun X.M., Liu Y., Wang Z.Y., Niu R.F. Tyrosine 23 phosphorylation of annexin A2 Promotes proliferation, invasion, and Stat3 phosphorylation in the nucleus of human breast cancer SK-BR-3 cells. Cancer Biology & Medicine. 2012;9(4):248–253. doi: 10.7497/j.issn.2095-3941.2012.04.005. 23691485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohanian J., Forman S.P., Katzenberg G., Ohanian V. Endothelin-1 stimulates small artery VCAM-1 expression through p38MAPK-dependent neutral sphingomyelinase. Journal of Vascular Research. 2012;49(4):353–362. doi: 10.1159/000336649. 22627111 [DOI] [PubMed] [Google Scholar]
- 25.Bellomo G., Mirabelli F. Oxidative stress and cytoskeletal alterations. Annals of the New York Academy of Sciences. 1992;663:97–109. doi: 10.1111/j.1749-6632.1992.tb38653.x. 1482106 [DOI] [PubMed] [Google Scholar]
- 26.Brambilla R., Zippel R., Sturani E., Morello L., Peres A., Alberghina L. Characterization of the tyrosine phosphorylation of calpactin I (annexin II) induced by platelet-derived growth factor. Biochemical Journal. 1991;278(2):447–452. doi: 10.1042/bj2780447. 1654883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He K.L., Sui G., Xiong H., Broekman M.J., Huang B., Marcus A.J., Hajjar K.A. Feedback regulation of endothelial cell surface plasmin generation by PKC-dependent phosphorylation of annexin A2. Journal of Biological Chemistry. 2011;286(17):15428–15439. doi: 10.1074/jbc.M110.185058. 21115493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayes M.J., Rescher U., Gerke V., Moss S.E. Annexin–actin interactions. Traffic. 2004;5(8):571–576. doi: 10.1111/j.1600-0854.2004.00210.x. 15260827 [DOI] [PubMed] [Google Scholar]
- 29.Coatrieux C., Sanson M., Negre-Salvayre A., Parini A., Hannun Y., Itohara S., Salvayre R., Auge N. MAO-A-induced mitogenic signaling is mediated by reactive oxygen species, MMP-2, and the sphingolipid pathway. Free Radical Biology and Medicine. 2007;43(1):80–89. doi: 10.1016/j.freeradbiomed.2007.03.036. 17561096 [DOI] [PubMed] [Google Scholar]
- 30.Goldkorn T., Filosto S., Chung S. Lung injury and Lung cancer caused by cigarette smoke-induced oxidative stress: molecular mechanisms and therapeutic opportunities involving the ceramide-generating machinery and epidermal growth factor receptor. Antioxidants & Redox Signaling. 2014;21:2149–2174. doi: 10.1089/ars.2013.5469. 24684526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shikata Y., Birukov K.G., Garcia J.G. S1P induces FA remodeling in human pulmonary endothelial cells: role of Rac, GIT1, FAK, and paxillin. Journal of Applied Physiology. 2003;94(3):1193–1203. doi: 10.1152/japplphysiol.00690.2002. 12482769 [DOI] [PubMed] [Google Scholar]
- 32.Bauvois B. New facets of matrix metalloproteinases MMP-2 and MMP-9 as cell surface transducers: outside-in signaling and relationship to tumor progression. Biochimica et Biophysica Acta. 2012;1825(1):29–36. doi: 10.1016/j.bbcan.2011.10.001. 22020293 [DOI] [PubMed] [Google Scholar]
- 33.Tomasek J.J., Halliday N.L., Updike D.L., Ahern-Moore J.S., Vu T.K., Liu R.W., Howard E.W. Gelatinase A activation is regulated by the organization of the polymerized actin cytoskeleton. Journal of Biological Chemistry. 1997;272(11):7482–7487. doi: 10.1074/jbc.272.11.7482. 9054450 [DOI] [PubMed] [Google Scholar]
- 34.Schram K., Ganguly R., No E.K., Fang X., Thong F.S., Sweeney G. Regulation of MT1-MMP and MMP-2 by leptin in cardiac fibroblasts involves Rho/ROCK-dependent actin cytoskeletal reorganization and leads to enhanced cell migration. Endocrinology. 2011;152(5):2037–2047. doi: 10.1210/en.2010-1166. 21385940 [DOI] [PubMed] [Google Scholar]
- 35.Clarke C.J., Truong T.G., Hannun Y.A. Role for neutral sphingomyelinase-2 in tumor necrosis factor alpha-stimulated expression of vascular cell adhesion molecule-1 (VCAM) and intercellular adhesion molecule-1 (ICAM) in lung epithelial cells: p38 MAPK is an upstream regulator of nSMase2. Journal of Biological Chemistry. 2007;282(2):1384–1396. doi: 10.1074/jbc.M609216200. 17085432 [DOI] [PubMed] [Google Scholar]
- 36.Shapiro S., Khodalev O., Bitterman H., Auslender R., Lahat N. Different activation forms of MMP-2 oppositely affect the fate of endothelial cells. American Journal of Physiology – Cell Physiology. 2010;298(4):C942–C951. doi: 10.1152/ajpcell.00305.2009. 20071690 [DOI] [PubMed] [Google Scholar]
- 37.Donati C., Bruni P. Sphingosine 1-phosphate regulates cytoskeleton dynamics: implications in its biological response. Biochimica et Biophysica Acta. 2006;1758(12):2037–2048. doi: 10.1016/j.bbamem.2006.06.015. 16890187 [DOI] [PubMed] [Google Scholar]
- 38.Zeidan Y.H., Jenkins R.W., Hannun Y.A. Remodeling of cellular cytoskeleton by the acid sphingomyelinase/ceramide pathway. Journal of Cell Biology. 2008;181(2):335–350. doi: 10.1083/jcb.200705060. 18426979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hein Z., Schmidt S., Zimmer K.P., Naim H.Y. The dual role of annexin II in targeting of brush border proteins and in intestinal cell polarity. Differentiation. 2011;81(4):243–252. doi: 10.1016/j.diff.2011.01.009. 21330046 [DOI] [PubMed] [Google Scholar]
- 40.Harder T., Kellner R., Parton R.G., Gruenberg J. Specific release of membrane-bound annexin II and cortical cytoskeletal elements by sequestration of membrane cholesterol. Molecular Biology of the Cell. 1997;8(3):533–545. doi: 10.1091/mbc.8.3.533. 9188103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayes M.J., Moss S.E. Annexin 2 has a dual role as regulator and effector of v-Src in cell transformation. Journal of Biological Chemistry. 2009;284(15):10202–10210. doi: 10.1074/jbc.M807043200. 19193640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Diesbach P., Medts T., Carpentier S., D’Auria L., Van Der Smissen P., Platek A., Mettlen M., Caplanusi A., van den Hove M.F., Tyteca D., Courtoy P.J. Differential subcellular membrane recruitment of Src may specify its downstream signalling. Experimental Cell Research. 2008;314(7):1465–1479. doi: 10.1016/j.yexcr.2008.01.015. 18316074 [DOI] [PubMed] [Google Scholar]
- 43.Yue J., Shukla R., Accardi R., Zanella-Cleon I., Siouda M., Cros M.P., Krutovskikh V., Hussain I., Niu Y., Hu S., Becchi M., Jurdic P., Tommasino M., Sylla B.S. Cutaneous human papillomavirus type 38 E7 regulates actin cytoskeleton structure for increasing cell proliferation through CK2 and the eukaryotic elongation factor 1A. Journal of Virology. 2011;85(17):8477–8494. doi: 10.1128/JVI.02561-10. 21697493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gineitis D., Treisman R. Differential usage of signal transduction pathways defines two types of serum response factor target gene. Journal of Biological Chemistry. 2001;276(27):24531–24539. doi: 10.1074/jbc.M102678200. 11342553 [DOI] [PubMed] [Google Scholar]
- 45.Sharma V.M., Litersky J.M., Bhaskar K., Lee G. Tau impacts on growth-factor-stimulated actin remodeling. Journal of Cell Science. 2007;120(5):748–757. doi: 10.1242/jcs.03378. 17284520 [DOI] [PubMed] [Google Scholar]


