Abstract
Aims/hypothesis
We investigated whether urinary markers of nucleic acid oxidation are associated with an increased risk of cancer in type 2 diabetes patients.
Methods
Urine samples from 1381 newly diagnosed diabetes patients were assayed for the oxidatively modified guanine nucleosides 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) and 8-oxo-7,8-dihydroguanosine (8-oxoGuo). Cox proportional hazards regression was used to examine the relationship between the urinary markers and cancer incidence.
Results
The crude analyses showed an association between overall cancer and urinary excretion of the RNA oxidation marker 8-oxoGuo (unadjusted hazard ratio for cancer per natural log increase in 8-oxoGuo 1.35 [95% CI, 1.01–1.81]), however, in the adjusted analyses, no significant associations between 8-oxodG or 8-oxoGuo and overall cancer were found. For site-specific cancers 8-oxodG was associated with breast cancer in the crude analyses (unadjusted hazard ratio for breast cancer per natural log increase in 8-oxodG was 2.37 [95% CI, 1.07–5.26]), although the association was attenuated in the adjusted analyses (sex- and age-adjusted hazard ratio 2.15 [95% CI, 0.92–5.02] and multivariate adjusted hazard ratio1.98 [95% CI, 0.95–4.10]).
Conclusions
Urinary excretion of the nucleic acid oxidation markers 8-oxodG and 8-oxoGuo at the time of diagnosis was not associated with cancer overall in type 2 diabetes patients. For site-specific cancers, risk elevations were seen for breast cancer (8-oxodG). These findings should be examined in future and larger studies.
Keywords: Type 2 diabetes; Cancer; RNA oxidation; DNA oxidation; 8‐oxo‐7,8‐dihydro‐2′‐deoxyguanosine; 8‐oxo‐7,8‐dihydroguanosine
Abbreviations: 8-oxodG, 8-oxo-7,8-dihydro-2'-deoxyguanosine; 8-oxoGuo, 8-oxo-7,8-dihydroguanosine; DCGP, Diabetes Care in General Practice; UPLC, Ultraperformance liquid chromatography
Introduction
Epidemiological studies suggest that type 2 diabetes is associated with increased risks of several cancer types, including cancers of the liver, pancreas, breast, endometrium, kidney, bladder, and colorectum [1–4]. Although diabetes may influence carcinogenesis by several mechanisms, e.g., hyperinsulinaemia, hyperglycaemia, or chronic inflammation, any exact mechanisms underlying the association between type 2 diabetes and cancer remain to be established. The mutagenic properties of oxidatively damaged DNA and the fact that diabetes is associated with increased urinary excretion of the DNA oxidation marker 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) [5] suggest that DNA oxidation could be one possible biological link between diabetes and cancer risk, and that urinary 8-oxodG may predict development of cancer in diabetes patients.
No previous studies have explored the relationship between 8-oxodG excretion and cancer incidence in type 2 diabetes patients. In general, the evidence on a potential association between urinary 8-oxodG excretion and cancer is limited, although some studies have demonstrated elevated levels of urinary 8-oxodG in patients with various malignancies [6–13]. We recently showed that urinary excretion of 8-oxo-7,8-dihydroguanosine (8-oxoGuo), the ribonucleoside counterpart to 8-oxodG, is an independent predictor of mortality in type 2 diabetes patients [14,15]. Whether 8-oxoGuo is also associated with cancer incidence is unknown.
In this study, we investigated whether urinary markers of oxidative stress 8-oxodG and 8-oxoGuo are associated with an increased risk of cancer in type 2 diabetes patients.
Methods
Study population
In the Diabetes Care in General Practice (DCGP) study [16], 474 general practitioners agreed to include all subjects on their practice list who fulfilled the following criteria: newly diagnosed diabetes based on hyperglycaemic symptoms and/or raised blood glucose values, diagnosed between 1 March 1989 and 28 February 1992, and aged 40 years or over. The diabetes diagnosis was subsequently confirmed with a single fasting whole blood/plasma glucose value of ≥7.0/8.0 mmol/l, measured in a major laboratory. The protocol-based exclusion criteria were life threatening somatic disease, severe mental illness, or unwillingness to participate. After exclusion of 162 patients, the study population consisted of 1381 newly diagnosed diabetes patients. Based on the onset of insulin treatment within 180 days of diagnosis, approximately 97.5% were considered to have type 2 diabetes [16]. Freshly voided morning urine samples were collected from all patients at the time of diagnosis.
The protocol was approved by the ethics committee of Copenhagen and Frederiksberg and informed consent was obtained from all patients.
Assessment of urinary markers and covariates
The urine samples were assayed between 2009 and 2010 for the oxidatively modified guanine nucleosides 8-oxodG and 8-oxoGuo using a validated method of ultraperformance liquid chromatography (UPLC) and tandem mass spectrometry [17]. 8-oxodG and 8-oxoGuo were normalized against urinary creatinine concentration. The assessment of the remaining patient characteristics at baseline has been described elsewhere [16].
Ascertainment of cancer
Information on cancer incidence was obtained from the Danish Cancer Registry [18], which contains accurate and virtually complete records of cancer cases in Denmark. The patients were followed for cancer occurrence from date of diabetes diagnosis until 1 January 2009.
Statistical analysis
The patients were grouped according to the quartiles of their urinary 8-oxodG and 8-oxoGuo levels in order to examine the associations between patient characteristics at diagnosis and the corresponding levels of oxidative stress. The medians and interquartile ranges (for continuous characteristics) or percentages (for categorical characteristics) were reported for each quartile, and associations were assessed by Kruskal–Wallis or chi-square tests, respectively.
Associations between oxidative stress and cancer risk were estimated by Cox proportional hazards regression models based on time from diagnosis to cancer event or censoring. Oxidative stress was represented by the natural logarithm of 8-oxodG and 8-oxoGuo, and by a four-class ordinal variable corresponding to the quartiles of the distribution. Three models were estimated for each of the oxidative stress variables and each outcome: an unadjusted model, a model adjusted for age and sex, and a third model adjusting for sex, age, smoking status, physical activity, education, BMI, alcohol consumption and cohabitation status.
We restricted cancer site-specific analyses to the most common cancer types in the cohort (more than 20 events). In these analyses, the above-mentioned models were used, and oxidative stress was represented by the natural logarithm of 8-oxodG and 8-oxoGuo.
Cancer incidence was plotted against follow-up time using the Kaplan–Meier method.
Reported p values were two-sided and p<0.05 was considered to be significant. Analyses were performed with SAS version 9.2.
Results
Patient characteristics
The median age at diagnosis of diabetes was 65.4 years (interquartile range, 55.7–73.6 years), with a slight male preponderance (53%). Tables 1 and 2 show the baseline characteristics according to quartiles of 8-oxodG and 8-oxoGuo, respectively. For both 8-oxodG and 8-oxoGuo, patients in the highest quartiles were older, more often women, more often living alone, and had higher levels of glycated haemoglobin. In addition, patients in the highest quartiles of 8-oxodG had lower BMI, total cholesterol and serum creatinine, and patients in the highest quartiles of 8-oxoGuo were more often highly educated, less often smokers, and less physically active.
Table 1.
Baseline characteristics according to quartiles of 8-oxodG.
| Characteristic |
1st quartile (N=338) |
2nd quartile (N=338) |
3rd quartile (N=338) |
4th quartile (N=338) |
p Value⁎ |
|---|---|---|---|---|---|
| 8-oxodG (nmol/mmol creatinine) | <1.60 | 1.60–2.09 | 2.10–2.77 | >2.77 | |
| Male sex (%) | 62.4 | 53.0 | 53.6 | 44.1 | <0.001 |
| Age (yr) | 62.1 (54.2–70.1) | 65.6 (54.5–73.5) | 64.7 (54.8–72.3) | 69.4 (59.4–75.7) | <0.001 |
| Smoking status (%) | 0.06 | ||||
| Never | 26.0 | 30.2 | 28.6 | 37.0 | |
| Previous | 36.4 | 37.5 | 34.7 | 30.7 | |
| Current | 37.6 | 32.3 | 36.8 | 32.2 | |
| Physical activity (%) | 0.09 | ||||
| Low | 27.1 | 25.4 | 26.3 | 32.3 | |
| Moderate | 66.9 | 66.7 | 65.9 | 64.4 | |
| High | 6.1 | 8.0 | 7.9 | 3.3 | |
| Cohabitation status (living alone) (%) | 27.4 | 30.3 | 29.6 | 41.0 | <0.001 |
| Education (higher) (%) | 76.2 | 79.6 | 79.8 | 80.6 | 0.52 |
| Glycated haemoglobin (%) | 9.9 (8.3–11.2) | 9.8 (8.4–11.7) | 10.3 (8.9–11.9) | 10.7 (9.1–12.2) | <0.001 |
| Total cholesterol (mmol/l) | 6.5 (5.6–7.4) | 6.2 (5.4–7.0) | 6.2 (5.4–7.1) | 6.0 (5.2–6.9) | 0.001 |
| Fasting triglycerides (mmol/l) | 2.28 (1.44–3.38) | 1.89 (1.41–2.66) | 2.05 (1.38–2.93) | 1.87 (1.37–2.65) | 0.05 |
| Serum creatinine (µmol/l) | 90.0 (83.0–103.0) | 90.0 (80.0–101.0) | 89.0 (79.0–99.0) | 87.0 (78.5–99.0) | 0.03 |
| Urinary albumin (mg/l) | 10.4 (5.3–25.5) | 10.8 (5.5–23.9) | 11.2 (6.2–29.1) | 15.5 (6.9–35.9) | 0.63 |
| Hypertension (%)† | 76.0 | 74.3 | 74.0 | 73.4 | 0.87 |
| BMI (kg/m2) | 29.7 (26.7–33.2) | 29.7 (26.4–33.6) | 29.0 (26.0–32.4) | 28.3 (24.9–31.2) | <0.001 |
| Retinopathy (%) | 3.3 | 4.7 | 5.0 | 4.9 | 0.75 |
| Peripheral neuropathy (%) | 17.2 | 20.5 | 18.9 | 20.4 | 0.66 |
| History of acute myocardial infarction (%) | 10.4 | 8.6 | 7.7 | 7.1 | 0.45 |
| History of stroke (%) | 5.3 | 3.0 | 4.1 | 5.0 | 0.44 |
Medians (interquartile ranges) are shown.
p Values are from chi-square tests (for categorical data) or from analysis of variance (for continuous data).
Hypertension was defined as systolic/diastolic blood pressure ≥160/90 mmHg or the use of antihypertensive drugs.
Table 2.
Baseline characteristics according to quartiles of 8-oxoGuo.
| Characteristic |
1st quartile (N=336) |
2nd quartile (N=337) |
3rd quartile (N=337) |
4th quartile (N=337) |
p Value⁎ |
|---|---|---|---|---|---|
| 8-oxoGuo (nmol/mmol creatinine) | <2.86 | 2.86–3.63 | 3.64–4.77 | >4.77 | |
| Male sex (%) | 64.9 | 59.6 | 46.6 | 41.5 | <0.001 |
| Age (yr) | 60.8 (51.3–69.1) | 62.0 (53.6–70.1) | 66.8 (58.6–74.5) | 70.2 (61.9–76.6) | <0.001 |
| Smoking status (%) | 0.02 | ||||
| Never | 25.2 | 29.7 | 28.8 | 38.3 | |
| Previous | 36.5 | 33.6 | 37.7 | 31.3 | |
| Current | 38.3 | 36.7 | 33.4 | 30.4 | |
| Physical activity (%) | <0.001 | ||||
| Low | 21.3 | 24.8 | 26.3 | 38.9 | |
| Moderate | 68.7 | 67.2 | 70.0 | 58.1 | |
| High | 10.0 | 8.0 | 3.7 | 3.0 | |
| Cohabitation status (living alone) (%) | 25.8 | 20.3 | 33.0 | 42.8 | <0.001 |
| Education (higher) (%) | 70.9 | 79.7 | 79.3 | 86.4 | <0.001 |
| Glycated haemoglobin (%) | 9.7 (8.3–11.0) | 9.8 (8.5–11.4) | 10.4 (8.8–12.0) | 11.0 (9.1–12.4) | <0.001 |
| Total cholesterol (mmol/l) | 6.2 (5.4–7.1) | 6.2 (5.5–7.1) | 6.2 (5.4–7.1) | 6.2 (5.3–7.2) | 0.99 |
| Fasting triglycerides (mmol/l) | 1.96 (1.30–2.86) | 1.94 (1.34–2.89) | 1.99 (1.47–2.83) | 2.02 (1.49–2.93) | 0.32 |
| Serum creatinine (µmol/l) | 90.0 (82.0–101.0) | 90.0 (80.0–100.0) | 88.0 (79.0–101.0) | 89.0 (79.0–102.0) | 0.21 |
| Urinary albumin (mg/l) | 10.0 (5.4–21.2) | 11.6 (5.5–23.7) | 11.5 (6.3–29.5) | 15.5 (7.0–42.2) | 0.21 |
| Hypertension (%)† | 73.5 | 69.7 | 77.4 | 77.4 | 0.06 |
| BMI (kg/m2) | 29.1 (26.2–32.2) | 29.4 (26.6–32.9) | 29.4 (26.4–33.1) | 28.7 (25.1–32.6) | 0.24 |
| Retinopathy (%) | 4.1 | 4.7 | 2.6 | 6.5 | 0.14 |
| Peripheral neuropathy (%) | 15.0 | 16.3 | 21.3 | 24.0 | 0.009 |
| History of acute myocardial infarction (%) | 9.0 | 7.1 | 7.5 | 10.4 | 0.40 |
| History of stroke (%) | 3.6 | 4.5 | 4.5 | 5.0 | 0.83 |
Medians (interquartile ranges) are shown.
p Values are from chi-square tests (for categorical data) or from analysis of variance (for continuous data).
Hypertension was defined as systolic/diastolic blood pressure ≥160/90 mmHg or the use of antihypertensive drugs.
Nucleic acid oxidation and overall cancer risk
We identified a total of 264 incident cancers during follow-up. Kaplan–Meier estimates of cancer incidence for all subjects according to quartiles of urinary 8-oxodG and 8-oxoGuo are shown in Fig. 1.
Fig. 1.
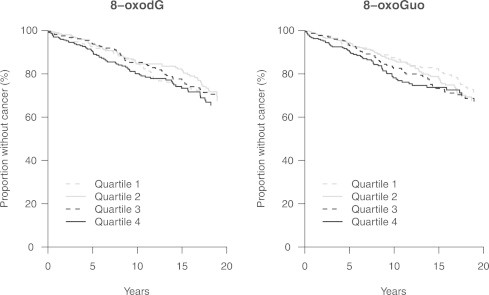
Kaplan–Meier estimates of cancer incidence for all subjects according to quartiles of urinary 8-oxodG and 8-oxoGuo.
In the unadjusted Cox regression analyses, log8-oxoGuo was significantly associated with cancer (Table 3). The unadjusted hazard ratio for cancer per natural log increase in 8-oxoGuo was 1.35 (95% confidence interval [CI], 1.01–1.81; p=0.04). In the adjusted analyses, no significant associations between 8-oxodG or 8-oxoGuo and overall cancer were found. Both analyses using the quartiles of distribution and log8-oxodG and log8-oxoGuo as continuous covariates showed no association between urinary excretion of the two markers and risk of cancer.
Table 3.
Association of urinary 8-oxodG and 8-oxoGuo with overall cancer risk.
| Outcome |
Model 1 HR (95% CI) |
p Value |
Model 2 HR (95% CI) |
p Value |
Model 3 HR (95% CI) |
p Value |
|---|---|---|---|---|---|---|
| Any cancer (n=264) | ||||||
| 8-oxodG | 0.25⁎ | 0.74⁎ | 0.98⁎ | |||
| 1st quartile | 1.00 | 1.00 | 1.00 | |||
| 2nd quartile | 0.92 (0.63–1.32) | 0.63 | 0.85 (0.59–1.24) | 0.40 | 0.85 (0.58–1.24) | 0.40 |
| 3rd quartile | 0.97 (0.69–1.36) | 0.87 | 0.88 (0.62–1.25) | 0.48 | 0.88 (0.62–1.25) | 0.46 |
| 4th quartile | 1.22 (0.85–1.74) | 0.27 | 1.05 (0.73–1.52) | 0.79 | 1.00 (0.69–1.46) | 0.99 |
| Log 8-oxodG | 1.13 (0.82–1.54) | 0.46 | 1.00 (0.72–1.38) | 0.99 | 0.94 (0.68–1.29) | 0.68 |
| 8-oxoGuo | 0.06⁎ | 0.64⁎ | 0.91⁎ | |||
| 1st quartile | 1.00 | 1.00 | 1.00 | |||
| 2nd quartile | 1.15 (0.82–1.61) | 0.41 | 1.07 (0.76–1.50) | 0.72 | 0.97 (0.68–1.37) | 0.85 |
| 3rd quartile | 1.25 (0.90–1.74) | 0.18 | 1.00 (0.70–1.43) | 0.99 | 0.96 (0.67–1.37) | 0.82 |
| 4th quartile | 1.39 (0.98–1.98) | 0.07 | 1.13 (0.77–1.64) | 0.53 | 0.98 (0.67–1.44) | 0.92 |
| Log 8-oxoGuo | 1.35 (1.01–1.81) | 0.04 | 1.08 (0.79–1.49) | 0.63 | 0.98 (0.71–1.37) | 0.93 |
Model 1: unadjusted model. Model 2: adjusted for age and sex. Model 3: adjusted for sex, age, smoking status, physical activity, education, BMI, alcohol consumption and cohabitation status.
p Value for linear trend across quartiles.
Risk of site-specific cancers
For both 8-oxodG and 8-oxoGuo, the site-specific hazard ratios varied in direction and magnitude (Table 4), probably due to the small numbers of cancer cases. Elevated risk estimates were observed for breast cancer. The unadjusted hazard ratio for breast cancer per natural log increase in 8-oxodG was 2.37 (95% CI, 1.07–5.26; p=0.03). When adjusting for covariates in models 2 and 3, the corresponding hazard ratios were 2.15 (95% CI, 0.92–5.02; p=0.08) and 1.98 (95% CI, 0.95–4.10; p=0.07), respectively.
Table 4.
Association of urinary 8-oxodG and 8-oxoGuo with cancer, by cancer type.
| Outcome |
Model 1 HR (95% CI) |
p Value |
Model 2 HR (95% CI) |
p Value |
Model 3 HR (95% CI) |
p Value |
|---|---|---|---|---|---|---|
| Log 8-oxodG | ||||||
| Colorectal cancer (n=49) | 0.84 (0.43–1.65) | 0.62 | 0.72 (0.37–1.38) | 0.32 | 0.61 (0.31–1.22) | 0.16 |
| Lung cancer (n=25) | 0.79 (0.33–1.86) | 0.58 | 0.74 (0.31–1.80) | 0.51 | 0.67 (0.26–1.70) | 0.40 |
| Breast cancer (n=31) | 2.37 (1.07–5.26) | 0.03 | 2.15 (0.92–5.02) | 0.08 | 1.98 (0.95–4.10) | 0.07 |
| Prostate cancer (n=35) | 0.86 (0.45–1.65) | 0.65 | 0.74 (0.40–1.36) | 0.33 | 0.66 (0.32–1.37) | 0.26 |
| Urinary tract cancer (n=29) | 0.68 (0.33–1.44) | 0.31 | 0.68 (0.30–1.53) | 0.35 | 0.69 (0.28–1.68) | 0.42 |
| Log 8-oxoGuo | ||||||
| Colorectal cancer (n=49) | 0.70 (0.33–1.49) | 0.36 | 0.49 (0.22–1.11) | 0.09 | 0.46 (0.20–1.03) | 0.06 |
| Lung cancer (n=25) | 2.03 (0.94–4.39) | 0.07 | 1.95 (0.87–4.38) | 0.11 | 1.84 (0.63–5.36) | 0.26 |
| Breast cancer (n=31) | 1.47 (0.74–2.94) | 0.27 | 1.22 (0.56–2.67) | 0.62 | 1.01 (0.51–2.02) | 0.98 |
| Prostate cancer (n=35) | 1.22 (0.63–2.36) | 0.55 | 0.74 (0.38–1.46) | 0.38 | 0.74 (0.38–1.47) | 0.39 |
| Urinary tract cancer (n=29) | 0.80 (0.28–2.28) | 0.68 | 0.76 (0.21–2.70) | 0.67 | 0.59 (0.16–2.13) | 0.42 |
Model 1: unadjusted model. Model 2: adjusted for age and sex. Model 3: adjusted for sex, age, smoking status, physical activity, education, BMI, alcohol consumption and cohabitation status.
Discussion
This cohort study that included 1381 newly diagnosed type 2 diabetes patients observed for up to 20 years is the first prospective study to explore the association between urinary excretion of markers of oxidative stress and cancer in diabetes patients.
We found an apparent association with urinary excretion of the RNA oxidation marker 8-oxoGuo in the crude analyses, however, this disappeared in the adjusted analyses. In none of the analyses, excretion of 8-oxodG was associated with cancer overall. For site-specific cancer, elevated risk estimates were seen for some sites, however, a consistent pattern over the varying levels of confounder adjustment was only seen for breast cancer and excretion of 8-oxodG. However, the statistical precision of notably the site specific analyses was limited and small to moderate associations between the nucleic acid markers and cancer could have been missed. Hence, the analyses should be repeated in other larger studies.
In two nested case–control/case–cohort studies within a large population-based prospective cohort study, Loft et al. examined associations between urinary excretion of 8-oxodG and risk of lung cancer [19] and breast cancer [20], respectively. In the first study 260 cases with lung cancer and a sub-cohort of 263 individuals matched on sex, age and smoking duration were included. In accordance with our findings Loft et al. found that overall the excretion of 8-oxodG was not significantly associated with the risk of lung cancer [19]. However, among never-smokers a high excretion of 8-oxodG was significantly associated with an increased risk of developing lung cancer [19]. Interestingly, the results from the other study by Loft et al. [20] are also in accordance with our findings regarding breast cancer incidence in type 2 diabetes patients. Loft et al. included 336 matched case–control pairs and found a borderline significant positive association between 8-oxodG and risk of all breast cancer (IRR: 1.08; 95% CI 1.00–1.17 per unit increase in nmol/mmol creatinine), which was significant with respect to the risk of oestrogen receptor-positive cancers (IRR: 1.11; 95% CI 1.01–1.23) and among women with low dietary iron intake (IRR: 1.10; 95% CI 1.06–1.37) [20]. Future studies are needed to confirm this apparent association between urinary 8-oxodG excretion and breast cancer.
The mechanistic evidence for the mutagenic effects of DNA oxidation by modification has been well established [21–24]. However, our data combined with the other epidemiological evidence indicate that from a clinical point of view the contributing effect of oxidative DNA modifications to human carcinogenesis is modest.
The main strengths of our study include the long follow-up period, the low attrition rate, the measurement of 8-oxodG and 8-oxoGuo with a validated method of UPLC and tandem mass spectrometry, the high quality in cancer diagnoses from the Danish Cancer Registry, and the availability of important potential clinical confounders.
The main limitation of our study was the sample size. Despite a total number of 264 cancer events, the statistical precision was low, limiting our ability to detect small and moderate associations between the oxidative stress markers and cancer risk, notably for site-specific cancers. This means that if the nucleic acid oxidation markers actually are associated with increased risk of cancer, this risk is too small to be detected in this study. Another limitation of this study was the reliance on a single measurement of the nucleic acid oxidation markers in a morning spot urine sample at the time of diagnosis. Thus the markers only reflect the oxidative burden at this specific time point. The possibility exists that the risk of cancer is associated with the accumulated oxidative burden during the follow-up period, which again could depend on the glycaemic regulation in the patients in this period.
In summary, urinary excretion of the nucleic acid oxidation markers 8-oxodG and 8-oxoGuo at the time of diagnosis was not associated with cancer overall in our large cohort of type 2 diabetes patients. For site-specific cancers, risk elevations were seen for breast cancer (8-oxodG). However, these findings should be examined in future and larger studies.
Duality of interest
The authors declare that there is no duality of interest associated with their involvement in this manuscript.
Contribution statement
KB researched data, contributed to discussion, wrote the manuscript, and reviewed and edited the manuscript. VS, TH, AW, MP, JTA, EJ-S, LJH, JEH, SJB, NdFO, SF and HEP researched data, contributed to discussion, and reviewed and edited the manuscript.
Funding
This study was supported by the Research Committee at Copenhagen University Hospital – Rigshospitalet (Rigshospitalets Forskningsudvalg), the Research Committee at Bispebjerg Hospital (Bispebjerg Hospitals Forskningsudvalg), the Capital Region of Denmark (Region Hovedstaden), the Danish Medical Research Council, Aase and Ejnar Danielsen Foundation, P. Carl Petersen Foundation, the Augustinus Foundation, the Lundbeck Foundation, the Danish Research Foundation for General Practice, the Health Insurance Foundation, the Danish Ministry of Health, Novo Nordisk Farmaka Denmark Ltd., the A.P. Møller Foundation for the Advancement of Medical Science, and the Pharmacy Foundation.
Acknowledgments
The authors gratefully acknowledge the technical assistance of laboratory technician Katja Luntang Christensen (Rigshospitalet).
References
- 1.Giovannucci E., Harlan D.M., Archer M.C. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33(7):1674–1685. doi: 10.2337/dc10-0666. 20587728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coughlin S.S., Calle E.E., Teras L.R., Petrelli J., Thun M.J. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. American Journal of Epidemiology. 2004;159(12):1160–1167. doi: 10.1093/aje/kwh161. 15191933 [DOI] [PubMed] [Google Scholar]
- 3.Vigneri P., Frasca F., Sciacca L., Pandini G., Vigneri R. Diabetes and cancer. Endocrine Related Cancer. 2009;16(4):1103–1123. doi: 10.1677/ERC-09-0087. 19620249 [DOI] [PubMed] [Google Scholar]
- 4.Hemminki K., Li X., Sundquist J., Sundquist K. Risk of cancer following hospitalization for type 2 diabetes. Oncologist. 2010;15(6):548–555. doi: 10.1634/theoncologist.2009-0300. 20479278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broedbaek K., Weimann A., Stovgaard E.S., Poulsen H.E. Urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine as a biomarker in type 2 diabetes. Free Radical Biology and Medicine. 2011;51(8):1473–1479. doi: 10.1016/j.freeradbiomed.2011.07.007. 21820047 [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto T., Hosokawa K., Tamura T., Kanno H., Urabe M., Honjo H. Urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG) levels in women with or without gynecologic cancer. Journal of Obstetrics and Gynaecology Research. 1996;22(4):359–363. doi: 10.1111/j.1447-0756.1996.tb00989.x. 8870419 [DOI] [PubMed] [Google Scholar]
- 7.Dziaman T., Huzarski T., Gackowski D. Elevated level of 8-oxo-7,8-dihydro-2′-deoxyguanosine in leukocytes of BRCA1 mutation carriers compared to healthy controls. International Journal of Cancer. 2009;125(9):2209–2213. doi: 10.1002/ijc.24600. 19623658 [DOI] [PubMed] [Google Scholar]
- 8.Kuo H.W., Chou S.Y., Hu T.W., Wu F.Y., Chen D.J. Urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG) and genetic polymorphisms in breast cancer patients. Mutation Research. 2007;631(1):62–68. doi: 10.1016/j.mrgentox.2007.04.009. 17512776 [DOI] [PubMed] [Google Scholar]
- 9.Erhola M., Toyokuni S., Okada K. Biomarker evidence of DNA oxidation in lung cancer patients: association of urinary 8-hydroxy-2′-deoxyguanosine excretion with radiotherapy, chemotherapy, and response to treatment. FEBS Letters. 1997;409(2):287–291. doi: 10.1016/s0014-5793(97)00523-1. 9202163 [DOI] [PubMed] [Google Scholar]
- 10.Thanan R., Murata M., Pinlaor S. Urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine in patients with parasite infection and effect of antiparasitic drug in relation to cholangiocarcinogenesis. Cancer Epidemiology, Biomarkers & Prevention. 2008;17(3):518–524. doi: 10.1158/1055-9965.EPI-07-2717. 18349269 [DOI] [PubMed] [Google Scholar]
- 11.Miyake H., Hara I., Kamidono S., Eto H. Oxidative DNA damage in patients with prostate cancer and its response to treatment. Journal of Urology. 2004;171(4):1533–1536. doi: 10.1097/01.ju.0000116617.32728.ca. 15017214 [DOI] [PubMed] [Google Scholar]
- 12.Tagesson C., Källberg M., Klintenberg C., Starkhammar H. Determination of urinary 8-hydroxydeoxyguanosine by automated coupled-column high performance liquid chromatography: a powerful technique for assaying in vivo oxidative DNA damage in cancer patients. European Journal of Cancer. 1995;31A(6):934–940. doi: 10.1016/0959-8049(94)00490-0. 7646926 [DOI] [PubMed] [Google Scholar]
- 13.Evans M.D., Dizdaroglu M., Cooke M.S. Oxidative DNA damage and disease: induction, repair and significance. Mutation Research. 2004;567(1):1–61. doi: 10.1016/j.mrrev.2003.11.001. 15341901 [DOI] [PubMed] [Google Scholar]
- 14.Broedbaek K., Siersma V., Henriksen T. Association between urinary markers of nucleic acid oxidation and mortality in type 2 diabetes: a population-based cohort study. Diabetes Care. 2013;36(3):669–676. doi: 10.2337/dc12-0998. 23150279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broedbaek K., Siersma V., Henriksen T. Urinary markers of nucleic acid oxidation and long-term mortality of newly diagnosed type 2 diabetic patients. Diabetes Care. 2011;34(12):2594–2596. doi: 10.2337/dc11-1620. 21994431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olivarius N.F., Beck-Nielsen H., Andreasen A.H., Hørder M., Pedersen P.A. Randomised controlled trial of structured personal care of type 2 diabetes mellitus. British Medical Journal. 2001;323(7319):970–975. doi: 10.1136/bmj.323.7319.970. 11679387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henriksen T., Hillestrøm P.R., Poulsen H.E., Weimann A. Automated method for the direct analysis of 8-oxo-guanosine and 8-oxo-2′-deoxyguanosine in human urine using ultraperformance liquid chromatography and tandem mass spectrometry. Free Radical Biology and Medicine. 2009;47(5):629–635. doi: 10.1016/j.freeradbiomed.2009.06.002. 19501155 [DOI] [PubMed] [Google Scholar]
- 18.Gjerstorff M.L. The Danish cancer registry. Scandinavian Journal of Public Health. 2011;39(7 Suppl.):42–45. doi: 10.1177/1403494810393562. 21775350 [DOI] [PubMed] [Google Scholar]
- 19.Loft S., Svoboda P., Kasai H. Prospective study of 8-oxo-7,8-dihydro-2′-deoxyguanosine excretion and the risk of lung cancer. Carcinogenesis. 2006;27(6):1245–1250. doi: 10.1093/carcin/bgi313. 16364924 [DOI] [PubMed] [Google Scholar]
- 20.Loft S., Olsen A., Møller P., Poulsen H.E., Tjønneland A. Association between 8-oxo-7,8-dihydro-2′-deoxyguanosine excretion and risk of postmenopausal breast cancer: nested case-control study. Cancer Epidemiology, Biomarkers & Prevention. 2013;22(7):1289–1296. doi: 10.1158/1055-9965.EPI-13-0229. 23658396 [DOI] [PubMed] [Google Scholar]
- 21.David S.S., O’Shea V.L., Kundu S. Base-excision repair of oxidative DNA damage. Nature. 2007;447(7147):941–950. doi: 10.1038/nature05978. 17581577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melis J.P., van Steeg H., Luijten M. Oxidative DNA damage and nucleotide excision repair. Antioxidants and Redox Signaling. 2013;18(18):2409–2419. doi: 10.1089/ars.2012.5036. 23216312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dizdaroglu M., Kirkali G., Jaruga P. Formamidopyrimidines in DNA: mechanisms of formation, repair, and biological effects. Free Radical Biology and Medicine. 2008;45(12):1610–1621. doi: 10.1016/j.freeradbiomed.2008.07.004. 18692130 [DOI] [PubMed] [Google Scholar]
- 24.Nohmi T., Kim S.R., Yamada M. Modulation of oxidative mutagenesis and carcinogenesis by polymorphic forms of human DNA repair enzymes. Mutation Research. 2005;591(1–2):60–73. doi: 10.1016/j.mrfmmm.2005.03.033. 16081110 [DOI] [PubMed] [Google Scholar]


