Abstract
Peroxisomes are ubiquitous organelles present in nearly all eukaryotic cells. Conserved functions of peroxisomes encompass beta-oxidation of fatty acids and scavenging of reactive oxygen species generated from diverse peroxisomal metabolic pathways. Peroxisome content, number, and size can change quickly in response to environmental and/or developmental cues. To achieve efficient peroxisome homeostasis, peroxisome biogenesis and degradation must be orchestrated. We review the current knowledge on redox regulated peroxisome biogenesis and degradation with an emphasis on yeasts and plants.
Abbreviations: APX, ascorbate peroxidase; DHAR, dehydroascorbate reductase; GR, glutathione reductase; Grx, glutaredoxins; GSSG, oxidized glutathione; MAPK, mitogen-activated protein kinase; MDAR, monodehydroascorbate peroxidase; PAS, phagophore assembly site; PexAD, peroxisome-associated protein degradation; RADAR, receptor accumulation and degradation pathway; ROS, reactive oxygen species; UPS, ubiquitin-proteasome system.
Keywords: Peroxisome, Pexophagy, Redox, Catalase, Oxidative stress
Graphical abstract
Highlights
-
•
Conserved functions of peroxisomes include β-oxidation of fatty acids and scavenging of ROS.
-
•
Peroxisome homeostasis is achieved by coordinating biogenesis and degradation.
-
•
Repression of peroxisome biogenesis under oxidative stress.
-
•
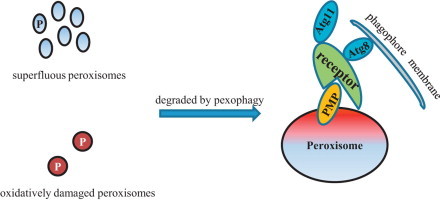
Superfluous and oxidative damaged peroxisomes are degraded by pexophagy.
Introduction
Peroxisomes are multifunctional organelles harboring at least two conserved metabolic pathways: fatty acid beta-oxidation and detoxification of hydrogen peroxide [1,2]. Moreover, peroxisomal metabolism varies tremendously within different organisms, encompassing glycolysis in Trypanosome, the glyoxylate cycle in seedlings, and photorespiration in leaves [1,3]. Peroxisome number and size adapt rapidly to environmental and developmental cues. Recent data suggest that redox plays an important role in peroxisome homeostasis by coordinating peroxisome biogenesis and degradation [4,5]. Here, we discuss how redox regulates peroxisome homeostasis, focusing on yeasts and plants.
Antioxidant system in peroxisomes
In addition to fatty acid beta-oxidation, in lower eukaryotic cells, peroxisomes are crucial compartments for secondary metabolism, including the catabolism of oleic acid, methanol, polyamines, purine bases, n-alkanes, and d-amino acids [2,6]. These reactions result in the production of high levels of hydrogen peroxide, which needs to be scavenged to maintain functional peroxisomes. Catalase, peroxidase, and small molecule thiol such as glutathione are major players of the peroxisomal antioxidant system [7–9]. Hydrogen peroxide produced in the peroxisome lumen is mainly scavenged by peroxisomal catalase and glutathione peroxidase, encoded by PMP20 in Candida boidini and GPX1 in Saccharomyces cerevisiae[7,10]. Removal of hydrogen peroxide by catalases, but not glutathione peroxidases, is independent of cellular reducing cofactors, such as glutathione or thioredoxin, as they catalyze a dismutation reaction converting H2O2 to water and O2. However, peroxisomal glutathione peroxidase requires glutathione as a cellular reductant to reduce H2O2 to water [10]. Glutathione has been found to be present in yeast peroxisomes [7]. However, how glutathione is imported into peroxisomes is not clear. It has been suggested that the peroxisomal membrane is freely permeable to small metabolites; therefore, cytosolic glutathione is presumably delivered to the peroxisome lumen by diffusing across the peroxisomal membrane [11]. Oxidized glutathione (GSSG) is thought to be exported to the cytosol through Opt2, a peroxisomal glutathione transporter, wherein it is reduced to GSH by cytosolic glutathione reductase in an NADPH-dependent manner [12].
Besides glutathione peroxidase, glutaredoxins (Grx) also utilize glutathione as a cofactor to reduce disulfide bridges of oxidized proteins. A small family of glutaredoxins exists in S. cerevisiae[13]. However, it remains unclear whether any of them reside within the peroxisome lumen. It has been shown recently that S. cerevisiae Gto1, one of the three omega-class glutathione transferases whose function is related to the dithiol glutaredoxins, Grx1 and Grx2, is targeted to the peroxisome lumen through the PTS1 pathway [14].
Moreover, fatty acid beta-oxidation in germinating seeds and photorespiration in leaves are important sources of hydrogen peroxide generation in plant peroxisomes [1]. The antioxidant defense system in plant peroxisomes is much more sophisticated than what has been found in lower eukaryotic cells (Table 1). In addition to catalases and glutathione peroxidases, an ascorbate–glutathione cycle is involved in decomposing hydrogen peroxide [15–17]. In Arabidopsis thaliana, the ascorbate–glutathione cycle is composed of four types of peroxisomal enzymes: ascorbate peroxidase 3 (APX3), monodehydroascorbate peroxidase 1 and 4 (MDAR1/4), dehydroascorbate reductase 1 (DHAR1), and glutathione reductase 1 (GR1) as well as two reductants, ascorbate and glutathione [18–21]. Other antioxidative enzymes, including Cu/Zn SOD and glutathione S-transferases, participate in removing superoxide radicals and hydroperoxides, respectively [20,22,23].
Table 1.
Peroxisomal antioxidatant enzymes in Saccharomyces cerevisiae and Arabidopsis thaliana.
| Gene locus | Acronym | Annotation | Localization | Reference |
|---|---|---|---|---|
| YDR256C | ScCta1 | Catalase | Matrix | [9] |
| YKL026C | ScGpx1 | Glutathione peroxidase | Matrix | [10] |
| YPR194C | ScOpt2 | Glutathione transporter | Peroxisomal membrane | [12] |
| AT4G35000 | AtAPX3 | Ascorbate peroxidase | Peroxisomal membrane | [18] |
| AT1G20630 | AtCAT1 | Catalase | Matrix | [20] |
| AT4G35090 | AtCAT2 | Catalase | Matrix | [20] |
| AT1G20620 | AtCAT3 | Catalase | Matrix | [20] |
| AT5G18100 | AtCSD3 | Copper/Zinc superoxide dismutase | Matrix | [20,22] |
| AT1G19570 | AtDHAR1 | Dehydroascorbate reductase | Matrix | [21] |
| AT3G24170 | AtGR1 | Glutathione reductase | Matrix | [20] |
| AT5G41210 | AtGSTT1 | Glutathione transferase | Matrix | [20,23] |
| AT5G41240 | AtGSTT2 | Glutathione transferase | Matrix | [23] |
| AT5G41220 | AtGSTT3 | Glutathione transferase | Matrix | [23] |
| AT3G52880 | AtMDAR1 | Monodehydroascorbate reductase | Matrix | [19] |
| AT3G27820 | AtMDAR4 | Monodehydroascorbate reductase | Peroxisomal membrane | [19] |
Reactive oxygen species (ROS) are not solely by-products of peroxisomal metabolism. As signaling molecules, peroxisomal ROS can affect peroxisome homeostasis, e.g. the biogenesis and degradation of peroxisomes [4,5]. The latter is also named pexophagy, the selective degradation of peroxisomes in the vacuole [24,25].
Repression of peroxisome biogenesis under oxidative stress
Besides oxidative damage of peroxisomal proteins, peroxisomal matrix protein import and peroxisome proliferation are impaired when the peroxisomal antioxidant system breaks down, such as in the absence of antioxidative enzymes or severe abiotic stresses [4,5]. Therefore, maintenance of the peroxisomal redox balance is crucial for preventing peroxisomal proteins from oxidative damage and sustaining functional peroxisomes.
The subcellular localization and activities of several peroxisomal matrix proteins are known to be regulated by redox. Upon exposure to osmotic stress, S. cerevisiae Gpd1, a NAD+-dependent glycerol 3-phosphate dehydrogenase, changes its subcellular localization from the peroxisome lumen to the cytosol and nucleus [26]. Moreover, it has been shown recently that redox switches confer the alternative targeting of Arabidopsis plastidic glucose-6-phosphate dehydrogenase to peroxisomes [27]. Furthermore, the activity of Arabidopsis 3-ketoacyl-CoA thiolase, an essential enzyme in the beta-oxidation pathway, is controlled by a redox sensitive switch in the peroxisome within a physiological range [28,29].
Although it is quite clear that peroxisome biogenesis could be damaged under oxidative stress, the underlying mechanism is still elusive. It is speculated that the peroxisomal protein import machinery is damaged under oxidative stress. This hypothesis is supported by evidence that few peroxins, proteins involved in peroxisome biogenesis, are known to be modified or impaired by an imbalance of cellular ROS [30–32].
The minimal peroxisomal translocon is composed of Pex5 and Pex14 [33,34]. Pex5 is the receptor of PTS1 proteins and shuttles between the cytosol and peroxisome lumen. This process relies on a conserved cysteine at the N-terminus of Pex5. After unloading peroxisomal cargo in the matrix, the recycling of Pex5 is initiated by monoubiquitination on this conserved N-terminal cysteine [35,36]. Cys 10 of Pichia pastoris Pex5 plays a critical role in cargo binding and release since disulfide bond-linked and reduced Pex5 show differential cargo binding affinity [30]. Dissipation of the redox balance between the cytosol and the peroxisome matrix activates the receptor accumulation and degradation pathway (RADAR), resulting in an import defect. Cys 11 in human Pex5 functions as a redox sensitive residue as well, although a different model of regulation of PTS1 import was proposed [37]. Also, one of the two major components of the peroxisomal translocon, Pex14, is degraded in the absence of PMP20, probably by the ubiquitin-proteasome system (UPS) [31]. Therefore, under oxidative stress, the import of peroxisomal proteins would be shut down immediately as the peroxisomal translocon is disassembled. A third redox sensitive peroxin is Pex11, whose homodimerization via intermolecular disulfide bonds, along with the increasing oxidative metabolism within old peroxisomes, inhibits peroxisomal division [32].
In contrast to peroxisomal membrane protein quality control by the UPS, three modes of clearance of damaged peroxisomal matrix proteins have been proposed: (I) Degradation in the peroxisome lumen by the peroxisomal Lon protease [38,39]; (II) export from the peroxisomal matrix and degraded by the proteasome, a process called peroxisome-associated protein degradation (PexAD) in plants [40,41]; and (III) sequestering in daughter cells and removal through coordinated fission and degradation by autophagy [42]. The first mode has been found in both yeasts and plants, while modes 2 and 3 were observed in Arabidopsis seedlings and Hansenula polymorpha, respectively. Pexophagy may be triggered when oxidative damage is unmanageable by peroxisomal quality control mechanisms resulting in complete loss of the whole organelle.
Nutrient adaption triggered pexophagy
Pexophagy has been best studied in methylotrophic yeasts, especially P. pastoris and H. polymorpha[24,25]. Two types of pexophagy, macropexophagy and micropexophagy, have been characterized extensively in P. pastoris. Macropexophagy is activated by moving P. pastoris cells from methanol to ethanol or from oleate to glucose. Thus, proliferated peroxisomes produced in methanol or oleate become superfluous organelles under peroxisome biogenesis repressed conditions and are engulfed individually by pexophagosomes (Fig. 1A). The process of macropexophagy is completed by fusing pexophagosomes with the vacuole to deliver peroxisomes into the vacuole lumen for degradation. A remarkable feature of methanol-induced peroxisomes is the formation of peroxisome clusters. How individual peroxisomes are separated from a cluster and engulfed by pexophagosomes is not known, although macropexophagy in P. pastoris has been firmly proven by fluorescence microscopy. Mao et al. showed that Dnm1 and Vps1 mediated peroxisomal fission is critical for the efficient degradation of the organelle [43]. Whether this is also true for macropexophagy needs further investigation. In contrast, micropexophagy is triggered by transferring cells from methanol to glucose, wherein a cluster of peroxisomes is directly delivered to the vacuole through fusion of the vacuolar sequestering membranes with the micropexophagy specific apparatus. Regarding the signaling events, the Subramani laboratory screened the S. cerevisiae kinase/phosphatase knockout strain collection for pexophagy defects and found that a mitogen-activated protein kinase (MAPK), Slt2, and its upstream components are essential for the pexophagy pathway [44]. Furthermore, the Klionsky group found that S. cerevisiae Slt2 is required not only for pexophagy, but also for mitophagy [45].
Fig. 1.
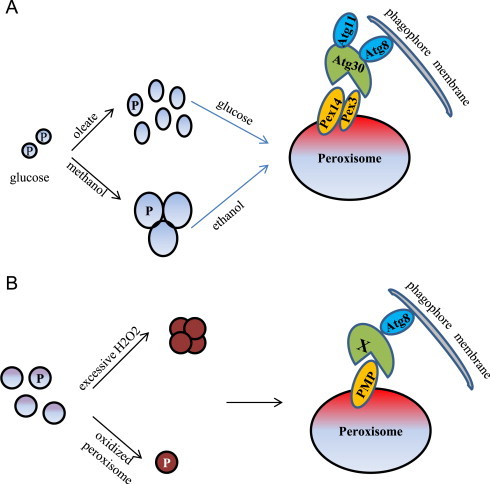
Mechanistic views of pexophagy in yeasts and plants. (A) Peroxisome proliferation is induced when P. pastoris cells are grown in methanol or oleate medium. Macropexophagy is triggered by shifting cells from methanol to ethanol or from oleate to glucose. Atg30 is the pexophagy receptor and interacts with Pex14 and Pex3 on the peroxisomal membrane. Moreover, it binds the autophagy adaptor protein Atg11 and the ubiquitin-like protein Atg8 at the pexophagy-specific PAS. Phosphorylation of Atg30 coordinates its interaction with Atg8 and Atg11. (B) In plants, peroxisomes become oxidatively damaged when the redox status in the peroxisomal lumen is unmanageable. Oxidized peroxisomes are degraded by pexophagy. However, the plant pexophagy-specific receptor (designated as X), a homolog of Atg30 or Atg36, has not been characterized. And it is also not known how the plant pexophagy-specific receptor is associated with the damaged peroxisomes, but it may interact with a PMP.
Pexophagy shares core autophagic machinery with general autophagy, including the Atg1 kinase complex (a basic scaffold for assembling of the phagophore assembly site, PAS), the Atg9 cycling system (essential for phagophore elongation), the PI3K complexes for PtdIns3P generation, and the ubiqutin-like conjugation systems for autophagosome formation [24,25]. But in addition, pexophagy recruits unique components for its selectivity, such as Atg11, Atg26, Atg30/Atg36, and Atg37 [46–49]. Atg30 and Atg36 are the pexophagy-specific receptors identified in P. pastoris and S. cerevisiae, respectively [46,48]. P. pastoris Atg30 serves as a bridge between the peroxisomes to be degraded and the phagophore assembly site. Atg30 interacts with two peroxisomal membrane proteins, Pex14 and Pex3, under pexophagy conditions, a process regulated by phosphorylation [46]. Furthermore, in mammalian systems, Pex14 and Pex3 also have a role in peroxisome degradation, although the underlying molecular details differ. When starved Chinese Hamster Ovary cells are transferred to nutrient-rich medium, Pex14 binds to the processed and lipidated form of LC3, an animal ortholog of yeast Atg8 [50,51]. Moreover, mammalian pexophagy may be triggered by monoubiquitination of Pex3 or PMP34 [52]. On the other hand, Atg30 or Atg36 interacts with the ubiquitin-like protein Atg8 and the scaffold protein Atg11, which requires phosphoregulation as well [53]. In S. cerevisiae, it has been recently shown that Hrr25-mediated phosphorylation of Atg36 enhances its interactions with the common adaptor Atg11 [54].
Activation of pexophagy by oxidative stress
In the past decade, efforts have been mainly focused on how superfluous peroxisomes are degraded by pexophagy. However, in contrast to lower eukaryotic cells, peroxisome number in plants is constant during the entire lifecycle. Therefore, the major role of pexophagy in plants is to remove oxidatively damaged peroxisomes, rather than the clear superfluous peroxisomes. Only recently large progress has been achieved on how oxidized peroxisomes are removed by pexophagy in plants (Fig. 1B) [55,56]. Shibata et al. showed that in the leaves of Arabidopsis atg2 , atg7, and atg18a mutants, peroxisomes form large aggregates and separate from chloroplasts and mitochondria, organelles generally clustered together with peroxisomes to facilitate the shuttling of metabolites generated during photorespiration [55]. The aggregated peroxisomes in these atg mutants accumulate inactive catalases, suggesting that the peroxisome aggregates result from peroxisomes that were oxidized and damaged by excessive hydrogen peroxide. This hypothesis is supported by the observation of high concentrations of hydrogen peroxide in the atg2 mutant and a more oxidized intraperoxisomal state in atg2 and atg5 mutants compared to wild-type. Furthermore, the cat2 mutant also contained peroxisome aggregates. In addition, Atg8 was found to frequently colocalize with aggregated peroxisomes, suggesting oxidatively damaged, dysfunctional peroxisomes are selectively degraded by autophagy. Interestingly, Yoshimoto et al. found that in the atg5 mutant a proportion of peroxisome-containing protein aggregates, mainly composed of inactive catalase, is segregated or “torn off” from the whole organelle by unknown mechanisms and degraded through pexophagy [56]. A similar phenomenon has been observed in H. polymorpha. The Van der Klei group demonstrated that peroxisomal matrix protein aggregates were removed by concerted peroxisomal fission and autophagy [42]. Whether this is also true in plants requires further investigation.
In addition to pexophagy found in Arabidopsis leaves, peroxisomes are selectively degraded during the functional transition of seedling glyoxysomes to leaf peroxisomes [40]. Seedling glyoxysomes play a pivotal role in lipid metabolism, producing acetyl-CoA used by the glyoxylate cycle to form organic acids, which are converted into sugars by gluconeogenesis [1]. Glyoxysomes can transform into leaf glyoxysomes by specifically removing malate synthase and isocitrate lyase, two peroxisomal enzymes of the glyoxylate cycle, through PexAD, concomitant with the import of enzymes required for photorespiration [40]. Furthermore, several groups have recently shown that this organelle remodeling process can be accelerated by selectively degrading oxidized glyoxysomes when the LON2 protease is disabled [39,57]. However, it is still unknown whether LON2 regulates pexophagy directly or indirectly. It is estimated that in mice liver 20–30% of excess peroxisomes are destroyed by peroxisomal Lon protease mediated degradation [58]. Kim et al. showed that pexophagy preferably happens in hypocotyls, while it is less obvious at the whole-seedling level [59]. Therefore, it seems that pexophagy plays a crucial role in preventing the accumulation of damaged peroxisomes in plants at different developmental stages.
Although in plants, oxidized peroxisomes have been demonstrated to be degraded by pexophagy, neither the plant pexophagy-specific receptor, a functional homolog of Atg30, nor the signaling events of the plant pexophagy pathway have been characterized. ATG genes required for general autophagy are conserved among different organisms [60]. However, it seems that genes needed for the selective autophagy pathways are organism-specific. For example, Atg30 and Atg36 are conserved only among a few yeast species [46,48]. Therefore, identifying these plant specific ATG proteins will shed more light on the molecular mechanisms of pexophagy in higher eukaryotic cells.
Acknowledgments
This study was funded by the Foundation for Taishan Scholar (Grant no. tshw20130962) from the People's Government of Shandong Province to C.M.
References
- 1.Hu J., Baker A., Bartel B., Linka N., Mullen R.T., Reumann S., Zolman B.K. Plant peroxisomes: biogenesis and function. Plant Cell. 2012;24(6):2279–2303. doi: 10.1105/tpc.112.096586. 22669882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hiltunen J.K., Mursula A.M., Rottensteiner H., Wierenga R.K., Kastaniotis A.J., Gurvitz A. The biochemistry of peroxisomal beta-oxidation in the yeast Saccharomyces cerevisiae. FEMS Microbiology Reviews. 2003;27(1):35–64. doi: 10.1016/S0168-6445(03)00017-2. 12697341 [DOI] [PubMed] [Google Scholar]
- 3.Bauer S., Morris J.C., Morris M.T. Environmentally regulated glycosome protein composition in the African trypanosome. Eukaryotic Cell. 2013;12(8):1072–1079. doi: 10.1128/EC.00086-13. 23709182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar S., Kawałek A., van der Klei I.J. Peroxisomal quality control mechanisms. Current Opinion in Microbiology. 2014;22C:30–37. doi: 10.1016/j.mib.2014.09.009. 25305535 [DOI] [PubMed] [Google Scholar]
- 5.Nordgren M., Fransen M. Peroxisomal metabolism and oxidative stress. Biochimie. 2014;98:56–62. doi: 10.1016/j.biochi.2013.07.026. 23933092 [DOI] [PubMed] [Google Scholar]
- 6.Platta H.W., Hagen S., Reidick C., Erdmann R. The peroxisomal receptor dislocation pathway: to the exportomer and beyond. Biochimie. 2014;98:16–28. doi: 10.1016/j.biochi.2013.12.009. 24345375 [DOI] [PubMed] [Google Scholar]
- 7.Horiguchi H., Yurimoto H., Kato N., Sakai Y. Antioxidant system within yeast peroxisome. Biochemical and physiological characterization of CbPmp20 in the methylotrophic yeast Candidae boidinii. Journal of Biological Chemistry. 2001;276(17):14279–14288. doi: 10.1074/jbc.M011661200. 11278957 [DOI] [PubMed] [Google Scholar]
- 8.Horiguchi H., Yurimoto H., Goh T., Nakagawa T., Kato N., Sakai Y. Peroxisomal catalase in the methylotrophic yeast Candidae boidinii: transport efficiency and metabolic significance. Journal of Bacteriology. 2001;183(21):6372–6383. doi: 10.1128/JB.183.21.6372-6383.2001. 11591682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrova V.Y., Drescher D., Kujumdzieva A.V., Schmitt M.J. Dual targeting of yeast catalase A to peroxisomes and mitochondria. Biochemical Journal. 2004;380(2):393–400. doi: 10.1042/BJ20040042. 14998369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohdate T., Inoue Y. Involvement of glutathione peroxidase 1 in growth and peroxisome formation in Saccharomyces cerevisiae in oleic acid medium. Biochimica et Biophysica Acta. 2012;1821(9):1295–1305. doi: 10.1016/j.bbalip.2012.05.004. 22659048 [DOI] [PubMed] [Google Scholar]
- 11.Antonenkov V.D., Hiltunen J.K. Transfer of metabolites across the peroxisomal membrane. Biochimica et Biophysica Acta. 2012;1822(9):1374–1386. doi: 10.1016/j.bbadis.2011.12.011. 22206997 [DOI] [PubMed] [Google Scholar]
- 12.Elbaz-Alon Y., Morgan B., Clancy A., Amoako T.N., Zalckvar E., Dick T.P., Schwappach B., Schuldiner M. The yeast oligopeptide transporter Opt2 is localized to peroxisomes and affects glutathione redox homeostasis. FEMS Yeast Research. 2014;14:1055–1067. doi: 10.1111/1567-1364.12196. 25130273 [DOI] [PubMed] [Google Scholar]
- 13.Collinson E.J., Wheeler G.L., Garrido E.O., Avery A.M., Avery S.V., Grant C.M. The yeast glutaredoxins are active as glutathione peroxidases. Journal of Biological Chemistry. 2002;277(19):16712–16717. doi: 10.1074/jbc.M111686200. 11875065 [DOI] [PubMed] [Google Scholar]
- 14.Barreto L., Garcerá A., Jansson K., Sunnerhagen P., Herrero E. A peroxisomal glutathione transferase of Saccharomyces cerevisiae is functionally related to sulfur amino acid metabolism. Eukaryotic Cell. 2006;5(10):1748–1759. doi: 10.1128/EC.00216-06. 16936141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corpas F.J., Barroso J.B., del Río L.A. Peroxisomes as a source of reactive oxygen species and nitric oxide signal molecules in plant cells. Trends in Plant Sciences. 2001;6(4):145–150. doi: 10.1016/s1360-1385(01)01898-2. 11286918 [DOI] [PubMed] [Google Scholar]
- 16.Kamigaki A., Mano S., Terauchi K., Nishi Y., Tachibe-Kinoshita Y., Nito K., Kondo M., Hayashi M., Nishimura M., Esaka M. Identification of peroxisomal targeting signal of pumpkin catalase and the binding analysis with PTS1 receptor. Plant Journal. 2003;33(1):161–175. doi: 10.1046/j.0960-7412.2003.001605.x. 12943550 [DOI] [PubMed] [Google Scholar]
- 17.Oshima Y., Kamigaki A., Nakamori C., Mano S., Hayashi M., Nishimura M., Esaka M. Plant catalase is imported into peroxisomes by Pex5p but is distinct from typical PTS1 import. Plant & Cell Physiology. 2008;49(4):671–677. doi: 10.1093/pcp/pcn038. 18308759 [DOI] [PubMed] [Google Scholar]
- 18.Shen G., Kuppu S., Venkataramani S., Wang J., Yan J., Qiu X., Zhang H. Ankyrin repeat-CONTAINING PROTEIN 2A is an essential molecular chaperone for peroxisomal membrane-bound ascorbate PEROXIDASE3 in Arabidopsis. Plant Cell. 2010;22(3):811–831. doi: 10.1105/tpc.109.065979. 20215589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lisenbee C.S., Lingard M.J., Trelease R.N. Arabidopsis peroxisomes possess functionally redundant membrane and matrix isoforms of monodehydroascorbate reductase. Plant Journal. 2005;43(6):900–914. doi: 10.1111/j.1365-313X.2005.02503.x. 16146528 [DOI] [PubMed] [Google Scholar]
- 20.Reumann S., Babujee L., Ma C., Wienkoop S., Siemsen T., Antonicelli G.E., Rasche N., Lüder F., Weckwerth W., Jahn O. Proteome analysis of Arabidopsis leaf peroxisomes reveals novel targeting peptides, metabolic pathways, and defense mechanisms. Plant Cell. 2007;19(10):3170–3193. doi: 10.1105/tpc.107.050989. 17951448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reumann S., Quan S., Aung K., Yang P., Manandhar-Shrestha K., Holbrook D., Linka N., Switzenberg R., Wilkerson C.G., Weber A.P. In-depth proteome analysis of Arabidopsis leaf peroxisomes combined with in vivo subcellular targeting verification indicates novel metabolic and regulatory functions of peroxisomes. Plant Physiology. 2009;150(1):125–143. doi: 10.1104/pp.109.137703. 19329564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang C.H., Kuo W.Y., Weiss C., Jinn T.L. Copper chaperone-dependent and -independent activation of three copper–zinc superoxide dismutase homologs localized in different cellular compartments in Arabidopsis. Plant Physiology. 2012;158(2):737–746. doi: 10.1104/pp.111.190223. 22186608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dixon D.P., Hawkins T., Hussey P.J., Edwards R. Enzyme activities and subcellular localization of members of the Arabidopsis glutathione transferase superfamily. Journal of Experimental Botany. 2009;60(4):1207–1218. doi: 10.1093/jxb/ern365. 19174456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farré J.C., Krick R., Subramani S., Thumm M. Turnover of organelles by autophagy in yeast. Current Opinion in Cell Biology. 2009;21(4):522–530. doi: 10.1016/j.ceb.2009.04.015. 19515549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manjithaya R., Nazarko T.Y., Farré J.C., Subramani S. Molecular mechanism and physiological role of pexophagy. FEBS Letters. 2010;584(7):1367–1373. doi: 10.1016/j.febslet.2010.01.019. 20083110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung S., Marelli M., Rachubinski R.A., Goodlett D.R., Aitchison J.D. Dynamic changes in the subcellular distribution of Gpd1p in response to cell stress. Journal of Biological Chemistry. 2010;285(9):6739–6749. doi: 10.1074/jbc.M109.058552. 20026609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyer T., Hölscher C., Schwöppe C., von Schaewen A. Alternative targeting of Arabidopsis plastidic glucose-6-phosphate dehydrogenase G6PD1 involves cysteine-dependent interaction with G6PD4 in the cytosol. Plant Journal. 2011;66(5):745–758. doi: 10.1111/j.1365-313X.2011.04535.x. 21309870 [DOI] [PubMed] [Google Scholar]
- 28.Pye V.E., Christensen C.E., Dyer J.H., Arent S., Henriksen A. Peroxisomal plant 3-ketoacyl-CoA thiolase structure and activity are regulated by a sensitive redox switch. Journal of Biological Chemistry. 2010;285(31):24078–24088. doi: 10.1074/jbc.M110.106013. 20463027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sundaramoorthy R., Micossi E., Alphey M.S., Germain V., Bryce J.H., Smith S.M., Leonard G.A., Hunter W.N. The crystal structure of a plant 3-ketoacyl-CoA thiolase reveals the potential for redox control of peroxisomal fatty acid beta-oxidation. Journal of Molecular Biology. 2006;359(2):347–357. doi: 10.1016/j.jmb.2006.03.032. 16630629 [DOI] [PubMed] [Google Scholar]
- 30.Ma C., Hagstrom D., Polley S.G., Subramani S. Redox-regulated cargo binding and release by the peroxisomal targeting signal receptor, Pex5. Journal of Biological Chemistry. 2013;288(38):27220–27231. doi: 10.1074/jbc.M113.492694. 23902771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bener Aksam E., Jungwirth H., Kohlwein S.D., Ring J., Madeo F., Veenhuis M., van der Klei I.J. Absence of the peroxiredoxin Pmp20 causes peroxisomal protein leakage and necrotic cell death. Free Radical Biology and Medicine. 2008;45(8):1115–1124. doi: 10.1016/j.freeradbiomed.2008.07.010. 18694816 [DOI] [PubMed] [Google Scholar]
- 32.Marshall P.A., Dyer J.M., Quick M.E., Goodman J.M. Redox-sensitive homodimerization of Pex11p: a proposed mechanism to regulate peroxisomal division. Journal of Cell Biology. 1996;135(1):123–137. doi: 10.1083/jcb.135.1.123. 8858168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma C., Schumann U., Rayapuram N., Subramani S. The peroxisomal matrix import of Pex8p requires only PTS receptors and Pex14p. Molecular Biology of the Cell. 2009;20(16):3680–3689. doi: 10.1091/mbc.E09-01-0037. 19570913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meinecke M., Cizmowski C., Schliebs W., Krüger V., Beck S., Wagner R., Erdmann R. The peroxisomal importomer constitutes a large and highly dynamic pore. Nature Cell Biology. 2010;12(3):273–277. doi: 10.1038/ncb2027. 20154681 [DOI] [PubMed] [Google Scholar]
- 35.Platta H.W., El Magraoui F., Schlee D., Grunau S., Girzalsky W., Erdmann R. Ubiquitination of the peroxisomal import receptor Pex5p is required for its recycling. Journal of Cell Biology. 2007;177(2):197–204. doi: 10.1083/jcb.200611012. 17452527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Platta H.W., El Magraoui F., Bäumer B.E., Schlee D., Girzalsky W., Erdmann R. Pex2 and pex12 function as protein-ubiquitin ligases in peroxisomal protein import. Molecular and Cellular Biology. 2009;29(20):5505–5516. doi: 10.1128/MCB.00388-09. 19687296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Apanasets O., Grou C.P., Van Veldhoven P.P., Brees C., Wang B., Nordgren M., Dodt G., Azevedo J.E., Fransen M. PEX5, the shuttling import receptor for peroxisomal matrix proteins, is a redox-sensitive protein. Traffic. 2014;15(1):94–103. doi: 10.1111/tra.12129. 24118911 [DOI] [PubMed] [Google Scholar]
- 38.Bartoszewska M., Williams C., Kikhney A., Opaliński Ł., van Roermund C.W., de Boer R., Veenhuis M., van der Klei I.J. Peroxisomal proteostasis involves a Lon family protein that functions as protease and chaperone. Journal of Biological Chemistry. 2012;287(33):27380–27395. doi: 10.1074/jbc.M112.381566. 22733816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goto-Yamada S., Mano S., Nakamori C., Kondo M., Yamawaki R., Kato A., Nishimura M. Chaperone and protease functions of LON protease 2 modulate the peroxisomal transition and degradation with autophagy. Plant & Cell Physiology. 2014;55(3):482–496. doi: 10.1093/pcp/pcu017. 24492254 [DOI] [PubMed] [Google Scholar]
- 40.Lingard M.J., Monroe-Augustus M., Bartel B. Peroxisome-associated matrix protein degradation in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(11):4561–4566. doi: 10.1073/pnas.0811329106. 19246395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burkhart S.E., Lingard M.J., Bartel B. Genetic dissection of peroxisome-associated matrix protein degradation in Arabidopsis thaliana. Genetics. 2013;193(1):125–141. doi: 10.1534/genetics.112.146100. 23150599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manivannan S., de Boer R., Veenhuis M., van der Klei I.J. Lumenal peroxisomal protein aggregates are removed by concerted fission and autophagy events. Autophagy. 2013;9(7):1044–1056. doi: 10.4161/auto.24543. 23614977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mao K., Liu X., Feng Y., Klionsky D.J. The progression of peroxisomal degradation through autophagy requires peroxisomal division. Autophagy. 2014;10(4):652–661. doi: 10.4161/auto.27852. 24451165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manjithaya R., Jain S., Farré J.C., Subramani S. A yeast MAPK cascade regulates pexophagy but not other autophagy pathways. Journal of Cell Biology. 2010;189(2):303–310. doi: 10.1083/jcb.200909154. 20385774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mao K., Wang K., Zhao M., Xu T., Klionsky D.J. Two MAPK-signaling pathways are required for mitophagy in Saccharomyces cerevisiae. Journal of Cell Biology. 2011;193(4):755–767. doi: 10.1083/jcb.201102092. 21576396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farré J.C., Manjithaya R., Mathewson R.D., Subramani S. PpAtg30 tags peroxisomes for turnover by selective autophagy. Developmental Cell. 2008;14(3):365–376. doi: 10.1016/j.devcel.2007.12.011. 18331717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nazarko T.Y., Ozeki K., Till A., Ramakrishnan G., Lotfi P., Yan M., Subramani S. Peroxisomal Atg37 binds Atg30 or palmitoyl-CoA to regulate phagophore formation during pexophagy. Journal of Cell Biology. 2014;204(4):541–557. doi: 10.1083/jcb.201307050. 24535825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Motley A.M., Nuttall J.M., Hettema E.H. Pex3-anchored Atg36 tags peroxisomes for degradation in Saccharomyces cerevisiae. EMBO Journal. 2012;31(13):2852–2868. doi: 10.1038/emboj.2012.151. 22643220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nazarko T.Y., Polupanov A.S., Manjithaya R.R., Subramani S., Sibirny A.A. The requirement of sterol glucoside for pexophagy in yeast is dependent on the species and nature of peroxisome inducers. Molecular Biology of the Cell. 2007;18(1):106–118. doi: 10.1091/mbc.E06-06-0554. 17079731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hara-Kuge S., Fujiki Y. The peroxin Pex14p is involved in LC3-dependent degradation of mammalian peroxisomes. Experimental Cell Research. 2008;314(19):3531–3541. doi: 10.1016/j.yexcr.2008.09.015. 18848543 [DOI] [PubMed] [Google Scholar]
- 51.Jiang L., Hara-Kuge S., Yamashita S.I., Fujiki Y. Peroxin Pex14p is the key component for coordinated autophagic degradation of mammalian peroxisomes by direct binding to LC3-II. Genes to Cells. 2014 doi: 10.1111/gtc.12198. 25358256 [DOI] [PubMed] [Google Scholar]
- 52.Kim P.K., Hailey D.W., Mullen R.T., Lippincott-Schwartz J. Ubiquitin signals autophagic degradation of cytosolic proteins and peroxisomes. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(52):20567–20574. doi: 10.1073/pnas.0810611105. 19074260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Farré J.C., Burkenroad A., Burnett S.F., Subramani S. Phosphorylation of mitophagy and pexophagy receptors coordinates their interaction with Atg8 and Atg11. EMBO Reports. 2013;14(5):441–449. doi: 10.1038/embor.2013.40. 23559066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanaka C., Tan L.J., Mochida K., Kirisako H., Koizumi M., Asai E., Sakoh-Nakatogawa M., Ohsumi Y., Nakatogawa H. Hrr25 triggers selective autophagy-related pathways by phosphorylating receptor proteins. Journal of Cell Biology. 2014;207(1):91–105. doi: 10.1083/jcb.201402128. 25287303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shibata M., Oikawa K., Yoshimoto K., Kondo M., Mano S., Yamada K., Hayashi M., Sakamoto W., Ohsumi Y., Nishimura M. Highly oxidized peroxisomes are selectively degraded via autophagy in Arabidopsis. Plant Cell. 2013;25(12):4967–4983. doi: 10.1105/tpc.113.116947. 24368788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoshimoto K., Shibata M., Kondo M., Oikawa K., Sato M., Toyooka K., Shirasu K., Nishimura M., Ohsumi Y. Organ-specific quality control of plant peroxisomes is mediated by autophagy. Journal of Cell Science. 2014;127(6):1161–1168. doi: 10.1242/jcs.139709. 24463818 [DOI] [PubMed] [Google Scholar]
- 57.Farmer L.M., Rinaldi M.A., Young P.G., Danan C.H., Burkhart S.E., Bartel B. Disrupting autophagy restores peroxisome function to an Arabidopsis lon2 mutant and reveals a role for the LON2 protease in peroxisomal matrix protein degradation. Plant Cell. 2013;25(10):4085–4100. doi: 10.1105/tpc.113.113407. 24179123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yokota S., Dariush Fahimi H. Degradation of excess peroxisomes in mammalian liver cells by autophagy and other mechanisms. Histochemistry and Cell Biology. 2009;131(4):455–458. doi: 10.1007/s00418-009-0564-6. 19229553 [DOI] [PubMed] [Google Scholar]
- 59.Kim J., Lee H., Lee H.N., Kim S.H., Shin K.D., Chung T. Autophagy-related proteins are required for degradation of peroxisomes in Arabidopsis hypocotyls during seedling growth. Plant Cell. 2013;25(12):4956–4966. doi: 10.1105/tpc.113.117960. 24368791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hanaoka H., Noda T., Shirano Y., Kato T., Hayashi H., Shibata D., Tabata S., Ohsumi Y. Leaf senescence and starvation-induced chlorosis are accelerated by the disruption of an Arabidopsis autophagy gene. Plant Physiology. 2002;129(3):1181–1193. doi: 10.1104/pp.011024. 12114572 [DOI] [PMC free article] [PubMed] [Google Scholar]


