Abstract
“Oxidative stress” as a concept in redox biology and medicine has been formulated in 1985; at the beginning of 2015, approx. 138,000 PubMed entries show for this term. This concept has its merits and its pitfalls. Among the merits is the notion, elicited by the combined two terms of (i) aerobic metabolism as a steady-state redox balance and (ii) the associated potential strains in the balance as denoted by the term, stress, evoking biological stress responses. Current research on molecular redox switches governing oxidative stress responses is in full bloom. The fundamental importance of linking redox shifts to phosphorylation/dephosphorylation signaling is being more fully appreciated, thanks to major advances in methodology. Among the pitfalls is the fact that the underlying molecular details are to be worked out in each particular case, which is bvious for a global concept, but which is sometimes overlooked. This can lead to indiscriminate use of the term, oxidative stress, without clear relation to redox chemistry. The major role in antioxidant defense is fulfilled by antioxidant enzymes, not by small-molecule antioxidant compounds. The field of oxidative stress research embraces chemistry, biochemistry, cell biology, physiology and pathophysiology, all the way to medicine and health and disease research.
Keywords: Oxidative stress, Redox balance, Oxidants, Antioxidants, Redox signaling, Adaptive response
Graphical abstract
Highlights
-
•
Oxidative stress denotes deviation from redox steady state.
-
•
Oxidative stress is an attribute of aerobic metabolism.
-
•
Oxidative stress evokes stress responses.
-
•
Oxidative stress activates molecular redox switches.
Introduction
The concept of oxidative stress has been introduced for research in redox biology and medicine in 1985, now 30 years ago, in an introductory chapter 1 in a book entitled ‘Oxidative Stress’ [2]. A concurrent comprehensive review entitled ‘Biochemistry of Oxidative Stress’ [3] presented the knowledge on pro-oxidants and antioxidants and their endogenous and exogenous sources and metabolic sinks. Since then, Redox Biology as a research area has found fulminant development in a wide range of disciplines, starting from chemistry and radiation biology through biochemistry and cell physiology all the way into general biology and medicine.
A noteworthy insight, early on, was the perception that oxidation-reduction (redox) reactions in living cells are utilized in fundamental processes of redox regulation, collectively termed ‘redox signaling’ and ‘redox control’. A book ‘Antioxidant and Redox Regulation of Genes’ highlighted that development at an early stage [4]. Since then, an overwhelming and fascinating area of research has flourished, under the name of Redox Biology [5], [6]. The concept of oxidative stress was updated to include the role of redox signaling [7], and there were efforts of redefining oxidative stress [8], [9].
These developments were mirrored by the appearance of monographs, book series and the establishment of new research journals. Many volumes were published in Methods in Enzymology. An impressive number of new journals sprang up, Free Radical Research (initially Free Radical Research Communications), Free Radicals in Biology and Medicine, Redox Reports, Antioxidant Redox Signaling, and most recently Redox Biology.
Useful as the term ‘oxidative stress’ may be in research, there has been an inflationary development in research circles and more so in the medical field and, even more than that, in public usage outside scientific endeavors (I would call it ‘over-stressing’ the term). This led to a dilution of the meaning, to overuse and even misuse. Cautionary words were published [10] and even explicit criticism was voiced [11], [12]. “Over time, the mechanistic basis of the concept was largely forgotten and instead of the oxidative stress hypothesis becoming more precise in terms of molecular targets and mechanism, it became diffuse and nonspecific” [12]. In fact, an ‘oxidative stress hypothesis’ has not been formulated up to now. If anything, there were implicit deductions: for example, that because of the redox balance concept any single compound, e.g. a small-molecule redox-active vitamin, could alter the totality of the system. Such a view overlooks counterregulation and redundancies in the redox network. There is specificity inherent in the strategies of antioxidant defense [13]. Obviously, a general term describing a global condition cannot be meant to depict specific spatiotemporal chemical relationships in detail and in specific cells or organ conditions. Rather, it entails these, and directed effort is warranted to unravel the exact chemical and physical conditions and their significance in each case.
Given the enormous variety and range of pro-oxidant and antioxidant enzymes and compounds, attempts were made to classify subforms of oxidative stress [7] and to conceptually introduce intensity scales ranging from physiological oxidative stress to excessive and toxic oxidative burden [14], as indicated in Table 1. There is ample evidence for the role of oxidation products of DNA, RNA, carbohydrates, proteins and lipids.
Table 1.
Oxidative stress: definition, specific forms, classification according to intensity.
| Category | Term | Reference |
|---|---|---|
| Definition, original | “A disturbance in the prooxidant-antioxidant balance in favor of the former” | [1] |
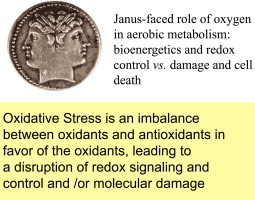
| Definition, updated | “An imbalance between oxidants and antioxidants in favor of the oxidants, leading to a disruption of redox signaling and control and/or molecular damage” | [7] |
| Specific form | Nutritional oxidative stress Dietary oxidative stress Postprandial oxidative stress Physiological oxidative stress Photooxidative stress Ultraviolet (UV-A, UV-B) Infrared-A Radiation-induced oxidative stress Nitrosative stress Reductive stress |
[7] |
| Related terms | Oxidant stress, Pro-oxidant stress Oxidative stress status (OSS) |
|
| Classification | Basal oxidative stress Low intensity oxidative stress Intermediate intensity oxidative stress High intensity oxidative stress |
[14] |
What are the merits and pitfalls of ‘oxidative stress’ today?
A comprehensive treatment of this question is to be deferred to an in-depth treatment (in preparation). However, for the purpose of the present Commentary it may suffice to collect a few thoughts: from its very nature, it is a challenge to combine the basic chemical notion of oxidation-reduction, including electron transfer, free radicals, oxygen metabolites (such as the superoxide anion radical, hydrogen peroxide, hydroxyl radical, electronically excited states such as singlet molecular oxygen, as well as the nitric oxide radical and peroxynitrite) with a biological concept, that of stress, first introduced by Selye in his research of adaptive responses [15], [16]. The two words ‘oxidative’ and ‘stress’ elicit a notion which, in a nutshell, focuses on an important sector of fundamental processes in biology. This is a merit.
Pitfalls are close-by: in research, simply to talk of ‘exposing cells or organisms to oxidative stress’ should clearly be discouraged. Instead, the exact molecular condition employed to change the redox balance of a given system is what is important; for example, in an experimental study cells were exposed to hydrogen peroxide, not to oxidative stress. Such considerations are even more appropriate in applications in the medical world. Quite often, redox components which are thought to be centrally important in disease processes are flatly denoted as oxidative stress; this can still be found in numerous schemes in the current biomedical literature. The underlying biochemically rigorous foundation may often be missing. Constructive criticism in this sense has been voiced repeatedly [11], [12], [17]. A related pitfall in this sense is the use of the term ROS, which stands for reactive oxygen species (the individual chemical reactants which were named in the preceding paragraph); whenever the specific chemical entity of the oxidant is known, that oxidant should be mentioned and discussed, not the generic ‘ROS’.
This ‘one-size-fits-all’ mentality pervades also into the analytics: measuring so-called ‘total antioxidant capacity (TAC)’ in a blood plasma sample will not give useful information on the state of the organism, and should be discouraged [18]. Rather, individual antioxidant enzyme activities and patterns of antioxidant molecules need to be assessed.
In view of the knowledge that the major burden of antioxidant defense is shouldered by antioxidant enzymes [13], it seems puzzling—in hindsight—that large human clinical studies based on one or two low-molecular-weight antioxidant compounds were undertaken.
3. What is attractive about ‘oxidative stress’?
3.1. Molecular redox switches
What seems to be attractive about the term is the implicit notion of adaptation, coming from the general association of stress with stress response. This goes back to Selye's concept of stress as the ‘general adaptation syndrome’ [19]. The enormously productive field of molecular switches was opened by the discovery of phosphorylation/dephosphorylation, serving a mechanism in molecular signaling [20]. The role of redox switches came into focus more recently, foremost the dynamic role of cysteines in proteins, opening the field of the redox proteome, currently flourishing because of advances in mass spectrometric and imaging methodology [21], [22], [23], [24]. A bridge between phosphorylation/dephosphorylation and protein cysteine reduction/oxidation is given by the redox sensitivity of critical cysteinyl residues in protein phosphatases, opening the molecular pathway for signaling cascades as fundamental processes throughout biology.
What was particularly exciting to many researchers was the discovery of master switch systems [25], prominent examples being OxyR in bacteria [26] and NFkB [27] and Nrf2/Keap1 [28] in higher organisms. That batteries of enzyme activities are mustered by activation of gene transcription through a ‘simple’ redox signal is still an exciting strategy. Much of current effort in redox biology is addressed towards these response systems. Obviously, medical and pharmacological intervention attempts are a consequence.
Outlook
Current interest into the linkage of oxidative stress to inflammation and inflammatory responses is adding a new perspective. For example, inflammatory macrophages release glutathionylated peroxiredoxin-2, which then acts as a ‘danger signal’ to trigger the production of tumor necrosis factor-alpha [29]. The orchestrated responses to danger signals related to damage-associated molecular patterns (DAMPs) include relations to oxidative stress [30]. Under oxidative stress conditions, a protein targeting factor, Get3 in yeast (mammalian TRC40) functions as an ATP-independent chaperone [31]. More detailed molecular understanding will also deepen the translational impact into biology and medicine; as mentioned above, these aspects are beyond this Commentary and will be treated elsewhere. However, it might be mentioned, for example, that viral and bacterial infections are often associated with deficiencies in micronutrients, including the essential trace element, selenium, the redox-active moiety in selenoproteins. Selenium status may affect the function of cells in both adaptive and innate immunity [32]. Major diseases, now even diabetes Type 2, are being considered as ‘redox disease’ [33].
Molecular insight will enhance the thrust of the concept of oxidative stress, which is intimately linked to cellular energy balance. Thus, the subcellular compartmentation of redox processes and redox components is being studied at a new level, in mammalian cells [34] as well as in phototrophic organisms [35]. New insight from spatiotemporal organization of hydrogen peroxide metabolism [36] complements the longstanding interest in hydroperoxide metabolism in mammalian organs and its relationship to bioenergetics [37].
The following quote attributed to Hans Selye [38] might well apply to the concept of oxidative stress: “If only stress could be seen, isolated and measured, I am sure we could enormously lengthen the average human life span”.
Acknowledgments
I gratefully acknowledge the input and friendship of many colleagues in shaping ideas in this multidisciplinary field; of many close associates, Enrique Cadenas and Wilhelm Stahl in particular, and close colleagues Dean Jones, Bruce Ames, Lester Packer, Alberto Boveris. Also to Masayasu Inoue for the translation of the book ‘Oxidative Stress’ into Japanese. Last, but not least, to the many active scientists in this research field, gathered under the umbrella of the Society for Free Radical Research International (SFRRI) and related organizations such as the Oxygen Club of California (OCC). I would like to express thanks to Victor Darley-Usmar for fruitful discussion during the writing of this manuscript.
I also am thankful for the research support by the National Foundation for Cancer Research (NFCR), Bethesda, MD, USA, and the Deutsche Forschungsgemeinschaft and the Alexander von Humboldt-Stiftung.
References
- 1.Sies H. In: Oxidative Stress. Sies H., editor. Academic Press; London: 1985. Oxidative stress: introductory remarks; pp. 1–8. [Google Scholar]
- 2.H. Sies (Ed.), Oxidative Stress, Academic Press, London, 1985, pp. 1–507.
- 3.Sies H. Biochemistry of oxidative stress. Angewandte Chemie International Edition. 1986;25(12):1058–1071. (in German) [Google Scholar]; Sies H. Biochemie des oxidativen Stress. Angewandte Chemie. 1986;98(12):1061–1075. doi: 10.1002/ange.19860981203. [DOI] [Google Scholar]
- 4.C.K. Sen, H. Sies, P.A. Baeuerle (Eds.), Antioxidant and Redox Regulation of Genes, Academic Press, London, 2000, pp. 1–562.
- 5.C. Gitler, A. Danon (Eds.), Cellular Implications of Redox Signaling, Imperial College Press, London, 2003, pp. 1–427.
- 6.Herrmann J.M., Dick T.P. Redox biology on the rise. Biological Chemistry. 2012;393(9):999–1004. doi: 10.1515/hsz-2012-0111. 22944698 [DOI] [PubMed] [Google Scholar]
- 7.Sies H., Jones D. In: 2nd ed. Fink G., editor. Vol. 3. Elsevier; Amsterdam: 2007. Oxidative stress; pp. 45–48. (Encyclopedia of Stress). [Google Scholar]
- 8.Jones D.P. Redefining oxidative stress. Antioxidants & Redox Signaling. 2006;8(9–10):1865–1879. doi: 10.1089/ars.2006.8.1865. 16987039 [DOI] [PubMed] [Google Scholar]
- 9.Jones D.P. Radical-free biology of oxidative stress. American Journal of Physiology—Cell Physiology. 2008;295(4):C849–C868. doi: 10.1152/ajpcell.00283.2008. 18684987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sies H. In: Oxidative Stress: Oxidants and Antioxidants. Sies H., editor. Academic Press; London: 1991. Oxidative stress: introduction; pp. xv–xxii. [Google Scholar]
- 11.Azzi A., Davies K.J., Kelly F. Free radical biology—terminology and critical thinking. FEBS Letters. 2004;558(1–3):3–6. doi: 10.1016/S0014-5793(03)01526-6. 14982062 [DOI] [PubMed] [Google Scholar]
- 12.Levonen A.-L., Hill B.G., Kansanen E., Zhang J., Darley-Usmar V.M. Redox regulation of antioxidants, autophagy, and the response to stress: implications for electrophile therapeutics. Free Radical Biology and Medicine. 2014;71:196–207. doi: 10.1016/j.freeradbiomed.2014.03.025. 24681256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sies H. Strategies of antioxidant defense. European Journal of Biochemistry. 1993;215(2):213–219. doi: 10.1111/j.1432-1033.1993.tb18025.x. 7688300 [DOI] [PubMed] [Google Scholar]
- 14.Lushchak V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chemico-Biological Interactions. 2014;224C:164–175. doi: 10.1016/j.cbi.2014.10.016. 25452175 [DOI] [PubMed] [Google Scholar]
- 15.Selye H. A syndrome produced by diverse nocuous agents. Nature. 1936;138(3479):32. doi: 10.1038/138032a0. [DOI] [PubMed] [Google Scholar]
- 16.Selye H. Stress and disease. Science. 1955;122(3171):625–631. doi: 10.1126/science.122.3171.625. [DOI] [PubMed] [Google Scholar]
- 17.Nathan C., Cunningham-Bussel A. Beyond oxidative stress: an immunologist's guide to reactive oxygen species. Nature Reviews Immunology. 2013;13(5):349–361. doi: 10.1038/nri3423. 23618831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pompella A., Sies H., Wacker R., Brouns F., Grune T., Biesalski H.K., Frank J. The use of total antioxidant capacity as surrogate marker for food quality and its effect on health is to be discouraged. Nutrition. 2014;30(7–8):791–793. doi: 10.1016/j.nut.2013.12.002. 24984994 [DOI] [PubMed] [Google Scholar]
- 19.Selye H. The general-adaptation-syndrome. Annual Review of Medicine. 1951;2:327–342. doi: 10.1146/annurev.me.02.020151.001551. 14847556 [DOI] [PubMed] [Google Scholar]
- 20.Fischer E.H., Krebs E.G. Conversion of phosphorylase-b to phosphorylase-a in muscle extracts. Journal of Biological Chemistry. 1955;216(1):121–132. 13252012 [PubMed] [Google Scholar]
- 21.Go Y.M., Jones D.P. The redox proteome. Journal of Biological Chemistry. 2013;288(37):26512–26520. doi: 10.1074/jbc.R113.464131. 23861437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cremers C.M., Jakob U. Oxidant sensing by reversible disulfide bond formation. Journal of Biological Chemistry. 2013;288(37):26489–26496. doi: 10.1074/jbc.R113.462929. 23861395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindemann C., Leichert L.I. Quantitative redox proteomics: the NOxICAT method. Methods in Molecular Biology. 2012;893:387–403. doi: 10.1007/978-1-61779-885-6_24. 22665313 [DOI] [PubMed] [Google Scholar]
- 24.Groitl B., Jakob U. Thiol-based redox switches. Biochimica et Biophysica Acta. 2014;1844(8):1335–1343. doi: 10.1016/j.bbapap.2014.03.007. 24657586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’Autréaux B., Toledano M.B. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nature Reviews Molecular Cell Biology. 2007;8(10):813–824. doi: 10.1038/nrm2256. 17848967 [DOI] [PubMed] [Google Scholar]
- 26.Zheng M., Aslund F., Storz G. Science. 1998;279(5357):1718–1721. doi: 10.1126/science.279.5357.1718. 9497290 [DOI] [PubMed] [Google Scholar]
- 27.Schreck R., Rieber P., Baeuerle P.A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO Journal. 1991;10(8):2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. 2065663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Itoh K., Chiba T., Takahashi S., Ishii T., Igarashi K., Katoh Y., Oyake T., Hayashi N., Satoh K., Hatayama I., Yamamoto M., Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochemical and Biophysical Research Communications. 1997;236(2):313–322. doi: 10.1006/bbrc.1997.6943. 9240432 [DOI] [PubMed] [Google Scholar]
- 29.Salzano S., Checconi P., Hanschmann E.-, Lillig C.H., Bowler L.D., Chan P., Vaudry D., Mengozzi M., Coppo L., Sacre S., Atkuri K.R., Sahaf B., Herzenberg L.A., Herzenberg L.A., Mullen L., Ghezzi P. Linkage of inflammation and oxidative stress via release of glutathionylated peroxiredoxin-2, which acts as a danger signal. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:12157–12162. doi: 10.1073/pnas.1401712111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaefer L. Complexity of danger: the diverse nature of damage-associated molecular patterns. Journal of Biological Chemistry. 2014;289(51):35237–35245. doi: 10.1074/jbc.R114.619304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voth W., Schick M., Gates S., Li S., Vilardi F., Gostimskaya I., Southworth D.R., Schwappach B., Jakob U. The protein targeting factor Get3 functions as ATP-independent chaperone under oxidative stress conditions. Molecular Cell. 2014;56(1):116–127. doi: 10.1016/j.molcel.2014.08.017. 25242142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steinbrenner H., Al-Quraishy S., Dkhil M.A., Wunderlich F., Sies H. Dietary selenium in adjuvant therapy of viral and bacterial infections. Advances in Nutrition. 2015;6:73–82. doi: 10.3945/an.114.007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watson J.D. Type 2 diabetes as a redox disease. Lancet. 2014;383(9919):841–843. doi: 10.1016/S0140-6736(13)62365-X. 24581668 [DOI] [PubMed] [Google Scholar]
- 34.Kaludercic N., Deshwal S., Di Lisa F. Reactive oxygen species and redox compartmentalization. Frontiers in Physiology. 2014;5:285. doi: 10.3389/fphys.2014.00285. [Pubmed: 25161621] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmitt F.J., Renger G., Friedrich T., Kreslavski V.D., Zharmukhamedov S.K., Los D.A., Kuznetsov V.V., Allakhverdiev S.I. Reactive oxygen species: re-evaluation of generation, monitoring and role in stress-signaling in phototrophic organisms. Biochimica et Biophysica Acta. 2014;1837(6):835–848. doi: 10.1016/j.bbabio.2014.02.005. 24530357 [DOI] [PubMed] [Google Scholar]
- 36.Sies H. Role of metabolic H2O2 generation: redox signaling and oxidative stress. Journal of Biological Chemistry. 2014;289(13):8735–8741. doi: 10.1074/jbc.R113.544635. 24515117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiological Reviews. 1979;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. 37532 [DOI] [PubMed] [Google Scholar]
- 38.Jasmin G., Bois P., Selye H. In: Fink G., editor. Vol. 3. Academic Press; London: 2000. pp. 417–418. (Encyclopedia of Stress). [Google Scholar]


