Abstract
Several pathological mutations have been identified in human POLG gene, encoding for the catalytic subunit of Pol γ, the solely mitochondrial replicase in animals and fungi. However, little is known regarding non-pathological polymorphisms found in this gene. Here we studied, in the yeast model Saccharomyces cerevisiae, eight human polymorphisms. We found that most of them are not neutral but enhanced both mtDNA extended mutability and the accumulation of mtDNA point mutations, either alone or in combination with a pathological mutation. In addition, we found that the presence of some SNPs increased the stavudine and/or zalcitabine-induced mtDNA mutability and instability.
Abbreviations: d4T, 3′-deoxy-2′,3′-didehydrothymidine or stavudine; ddC, 2′,3′-dideoxycytidine or zalcitabine; Ed4T, 2′,3′-didehydro-3′-deoxy-4′-ethynylthymidine; EryR, resistant to erythromycin; exo, exonuclease; FLT, 3′-fluoro-3′-deoxythymidine; HAART, highly active antiretroviral therapy; mtDNA, mitochondrial DNA; NRTI, nucleoside reverse transcriptase inhibitor; PEO, progressive external ophthalmoplegia; Pol γ, DNA polymerase γ; SNP, single nucleotide polymorphism; TP, triphosphate; wt, wild type
Keywords: POLG polymorphisms, MIP1, Yeast model, mtDNA point and extended mutability, NRTI, Pharmacogenetics
Highlights
-
•
We studied the effects of 8 human polymorphisms in Pol γ in the model system yeast.
-
•
Most polymorphisms increase mtDNA extended and point mutability.
-
•
Treatment with NRTIs determines mtDNA instability in wt and mutant strains.
-
•
Some polymorphisms make Mip1 more sensitive to NRTIs-induced mtDNA toxicity.
1. Introduction
Mitochondrial DNA (mtDNA) is replicated by the DNA polymerase γ, or Pol γ, which in animals is the only DNA polymerase present in mitochondria. The catalytic subunit of Pol γ belongs to a subfamily of the prokaryotic PolA family and its amino acid sequence is well conserved from yeast to humans. Structurally, human Pol γ is a heterotrimer composed of one catalytic subunit, which is encoded by POLG(1), and two accessory subunits, which are encoded by POLG2 (Yakubovskaya et al., 2006). The catalytic subunit contains an N-terminal exonuclease domain (residues 170–440), a C-terminal polymerase domain (residues 440–475 and 785–1239) and a spacer region encompassing residues 475–785 (Lee et al., 2009). To date, about 250 pathological mutations associated with severe mitochondrial disorders have been identified in POLG (http://tools.niehs.nih.gov/Polg/). Among them, a few mutations have been recently described to be involved in stavudine-induced toxicity (Chiappini et al., 2009; Yamanaka et al., 2007), in valproate-induced hepatotoxicity (Saneto et al., 2010; Stewart et al., 2010), in testicular cancer (Blomberg Jensen et al., 2008), in breast tumorigenesis (Azrak et al., 2012; Popanda et al., 2013; Singh et al., 2009) and in idiopathic sporadic parkinsonism (Luoma et al., 2007; Gui et al., 2012). Besides these pathological mutations, several single nucleotide polymorphisms (SNPs) have been identified in Pol γ, most of which appear to be, or are considered, neutral polymorphisms. Traditionally, a SNP is defined as a DNA sequence variation occurring within a population with an allelic frequency higher than 1%. Using next generation sequencing techniques applied to the exome, and to full genome sequencing, such as the 1000 Genomes Project (http://www.1000genomes.org/), a great number of new polymorphisms have been identified with a frequency much lower than 1%, so that now any nucleotide variation is generally considered as a SNP. A polymorphism differs from a pathological mutation since it is not associated with any pathology. However, several SNPs/polymorphisms behave as phenotypic modifiers of other mutations, or may alter the response to certain drugs or the susceptibility to environmental factors such as toxins, making them the subject of pharmacogenetic and toxicogenetic research.
In Pol γ, more than 1000 polymorphisms have been identified, most of which are non-coding or synonymous. Coding and non-synonymous SNPs have not yet been characterized so it is not known if they really are neutral polymorphisms. An exception is the E1143G mutation, which appears with a frequency of 3–4% in the European population (GeneSNps) and its effects on human Pol γ have been the subject of several studies. However, in vitro studies led to contradictory conclusions on the role of this mutation as a modulator of mutations or neutral polymorphism (Chan et al., 2006; Palin et al., 2010).
The Saccharomyces cerevisiae DNA polymerase γ, encoded by the MIP1 gene, shows 43% similarity with the human Pol γ catalytic subunit. Thanks to this similarity, yeast has been largely used to validate the role of human putative pathological mutations, to understand biochemical consequences associated with these mutations, to find molecules able to rescue their detrimental effects and to study the pharmacogenetics of drugs such as valproate and stavudine (Baile and Claypool, 2013; Baruffini and Lodi, 2010; Baruffini et al., 2006, 2007, 2011; Qian et al., 2014; Spinazzola et al., 2009; Stewart et al., 2010; Stricker et al., 2009; Stuart et al., 2006; Stumpf and Copeland, 2013; Stumpf et al., 2010; Szczepanowska and Foury, 2010). Yeast is a suitable model organism for the study of the effects of Pol γ mutations on mtDNA stability, thanks to its ability to survive in the absence of a functional mitochondrial genome. Yeast cells containing deletions-carrying mtDNA, called rho−, or cells which have completely lost mtDNA, called rho0, are respiratory-deficient and produce small-sized colonies, called petite. Petite mutants arise spontaneously with high frequency (approximately 10− 2) (Dujon, 1981), which is increased in the case of Mip1 mutant strains with reduced polymerase activity. Petite mutability can be easily measured and provides an estimate of the mtDNA extended mutability, i.e. loss or rearrangements in mtDNA. mtDNA point mutability can also be easily measured as the frequency of spontaneous mutants which are resistant to erythromycin (EryR mutants), an antibiotic that inhibits mitochondrial but not cytoplasmic translation. In fact, resistance to erythromycin is acquired through specific transversions or transitions in the mitochondrial gene encoding the 21 S rRNA (Cui and Mason, 1989; Sor and Fukuhara, 1982, 1984; Vanderstraeten et al., 1998). EryR mutability is increased by Mip1 mutations which reduce the fidelity of replication.
Yeast has also been used to evaluate the correlation between specific mutations in Mip1, corresponding to human mutations, and mtDNA mutability induced by treatment with stavudine (3′-deoxy-2′,3′-didehydrothymidine, or d4T) (Baruffini and Lodi, 2010), a nucleoside reverse transcriptase inhibitor (NRTI) which has been successfully used in the highly active antiretroviral therapy (HAART). Although HAART has significantly increased the life expectancy of HIV patients, prolonged treatment can induce side effects in some patients, most of which are due to the interference of the NRTIs with mitochondrial metabolism. Several observations suggest that NRTIs mitochondrial toxicity depends on their interference with Pol γ activity (Koczor and Lewis, 2010; Kohler and Lewis, 2007; Lee et al., 2003; Lewis et al., 2006), at least in part and especially for pyrimidine analogs such as stavudine and zalcitabine (2′,3′-dideoxycytidine, or ddC). It has been also demonstrated that several triphosphorylated NRTIs can inhibit the activity of human Pol γ in vitro (Johnson et al., 2001; Lim and Copeland, 2001; Martin et al., 1994). According to the “Pol γ hypothesis”, NRTI toxicity could be due to direct inhibition of polymerase catalytic activity, incorporation of NRTI in the nascent strand with subsequent chain termination or persistence of the analog in mitochondrial DNA because of inefficient excision (Lewis, 2007). Consequently, some mutations in Pol γ can result in different biochemical properties towards the NRTI-triphosphate (NRTI-TP), such as a greater Ki by the NRTI-TP, a lower NRTI-TP discrimination or a lower excision efficiency of the NRTI from the mtDNA and can increase the susceptibility to NRTIs toxicity, as was shown for patients carrying the mutation R964C (Bailey et al., 2009; Yamanaka et al., 2007). Thus, a pharmacogenetic approach in the NRTI treatment requires to establish whether polymorphisms are neutral or if they affect mtDNA polymerase activity.
The aim of this study is to characterize, by the use of specific ad hoc yeast models, some polymorphisms in Pol γ, in order to assess whether they are neutral nucleotide variations or not. Specifically, we will try to answer the following questions: (i) Is the mutation neutral, i.e. does the mutant Mip1 behave in vivo like the wt Mip1 concerning mtDNA extended and point mutability? (ii) Is the mutation a phenotypic modifier or mutation modulator, i.e. can the mutation worsen the phenotype of an in cis pathological mutation? (iii) Does the mutation increase the toxicity induced by stavudine or zalcitabine treatment? An answer to the last question is of particular relevance in view of possible pharmacogenetic applications.
We found that most of the considered polymorphisms are not neutral but rather behave as phenotypic modifiers, and that three polymorphisms, besides the R964C and E1143G mutations previously studied (Baruffini and Lodi, 2010), showed an altered sensitivity to stavudine and/or zalcitabine toxicity.
2. Materials and methods
2.1. Strains, plasmids and media
The yeast strains used in this work are reported in Table 1. The primers used are reported in Supplementary Table 1. Yeast media are YP (1% yeast extract (Formedium), 2% peptone (Formedium)) or SC (0.69% yeast nitrogen base without amino acids (Formedium), 0.1% drop out mix according to Kaiser et al., 1994). Appropriate carbon sources were added to the medium at a final concentration of 2%. 5-FOA plates contained 0.69% yeast nitrogen base without amino acids, 1% fluoroorotic acid (Formedium), 40 mg/l of each amino acid or base necessary to complement the auxotrophy, 40 mg/l uracil and 2% glucose. YPGen and YPHyg were YP supplemented with 200 mg/l G418 sulfate (Gibco) or 250 mg/l hygromycin B (Formedium). YPAEG-Ery medium contained 1% yeast extract (Difco), 2% peptone (Difco), 100 mg/l adenine, 3% [v/v] glycerol, 3% [v/v] ethanol, 3 g/l erythromycin (Sigma). If necessary, the medium was solidified by adding 2% agar (Formedium).
Table 1.
Strains used in this work.
| Strain | Genotype | Origin |
|---|---|---|
| DWM-5A | Matα ade2-1 leu2-3,112 ura3-1 trp1-1 his3-11,15 can1-100 mip1::KanR | Baruffini et al. (2006) |
| YLV3t3m3 | Mata ade2-1 leu2-3,112::hENT1-LEU2 ura3-1 trp1-1::HSV-TK-trp1::KanR his3-11,15 can1-100 mip1::HphR | Baruffini and Lodi (2010) |
| W303-1B | Matα ade2-1 leu2-3,112 ura3-1 trp1-1 his3-11,15 can1-100 | Thomas and Rothstein (1989) |
| YLV3t3m3ΔH | Mata ade2-1 leu2-3,112::hENT1::SpHIS5 ura3-1 trp1-1::HSV-TK-trp1::KanR his3-11,15 can1-100 mip1::HphR | This study, disruption of hENT1 with SpHIS5 in YLV3t3m3 |
| W1BCK1 | Matα ade2-1 leu2-3,112::hDCK1-LEU2 ura3-1 trp1-1 his3-11,15 can1-100 | This study, integration of human DCK1 at the leu2 locus of W303-1B |
| W1BCK1-10 | Matα ade2-1 leu2-3,112::hDCK1-LEU2 ura3-1 trp1-1 his3-11,15 can1-100 mip1::HphR | This study, disruption of MIP1 with HphMX4 cassette in W1BCK1 |
Human ENT1 was disrupted by using the one step gene disruption technique, with the HIS5 gene from Schizosaccharomyces pombe cassette. The SpHIS5 cassette, flanked by 40–45 bp of hENT1, was amplified by using pUG27 (Güldener et al., 2002) as a template and oligonucleotides hENT1DFw and hENT1D2Rv as primers. The cassette was introduced in strain YLV3t3m3 and the disruption was confirmed by PCR, thus obtaining the YLV3t3m3∆H strain.
For construction of the strain W1BCK1, a fragment of pFL61 containing the PGK promoter and the PGK terminator was digested with BamHI and BglII from plasmid pFL61, and subcloned in BamHI-digested pFL26 integrative plasmid (Bonneaud et al., 1991; Minet et al., 1992). Human DCK1 cDNA was amplified with primers DCKcFw and DCKcRv, digested with NotI and subcloned in pFL26PGK under the control of the PGK promoter. The plasmid was introduced into the strain W303-1B and transformants in which the plasmid was integrated at the leu2 locus were selected on SC medium without leucine. Integration was verified by PCR.
For construction of the strain W1BCK1-10B, the mip1::HphMX4 cassette was amplified from DWM-9A genomic DNA (Baruffini et al., 2009) with primers MIP1DFw and MIP1Drv, and introduced into the W1BCK strain. Disruption was verified by phenotypic analysis and PCR.
Human CNT3 cDNA was amplified with primers hCNT3Fw and hCNT3Rv, and cloned in the centromeric plasmid pUSG-E12 (a personal gift of A. Inga) after digestion with XhoI and NotI, and in the episomal plasmid pYES2 (Life Technologies) after digestion with HindIII and NotI. In both plasmids, the hCNT3 ORF was cloned under the GAL1-10 promoter.
2.2. Construction of mutant mip1 strains
mip1 mutant alleles were constructed through mutagenic overlap PCR (Ho et al., 1989). After overlap PCR, the MIP1 fragments containing mutations E166Q, E166R, P207L or L340V were digested with NotI and AvrII and subcloned in pFL39MIP1 (Baruffini et al., 2006), whereas MIP1 fragments containing mutations Y753F, S889R, S899W, K903R or K903C were digested with AvrII and BsrGI and subcloned in the same plasmid. mip1 mutant alleles harboring G224A, A692T, E698G, Q766R, Q766C and E900G were constructed previously (Baruffini et al., 2006, 2007; Spinazzola et al., 2009; Stricker et al., 2009). mip1 double mutant alleles were constructed by overlap PCR using mip1A692T as template instead of MIP1 wt allele.
Strains DWM-5A/pFL38MIP1, YLV3t3m3/pFL38MIP1 and W1BCK1-10/pFL38MIP1 were transformed by the LiAc/ssDNA/PEG method (Gietz and Schiestl, 2007a,b) with pFL39 plasmid harboring mip1 wt and mutant alleles, thus obtaining heteroallelic strains. Subsequently, pFL38MIP1 was counter-selected in the presence of 5-fluoroorotic acid as previously reported (Baruffini et al., 2010), thus obtaining mip1 mutant haploid strains.
2.3. Northern blot, RT-PCR and RT-qPCR
For Northern blot on strain YLV3t3m3, total RNA was prepared from cells grown to OD600 = 1 in YP medium supplemented with 2% glucose, which inhibits expression by GAL promoter, 0.3% galactose or 2% galactose, which induces expression by GAL promoter, with or without d4T, by extraction with hot acidic phenol (Ausubel et al., 1994). Northern analysis was carried out as previously reported (Sherman et al., 1986). ENT1, HSV-TK and ACT1 probes were obtained by PCR-amplification using primers hENT1Fw and hENT1Rv, TK1Fw and TK1Rv, ACT1Fw and ACT1Rv, respectively. The probes were labeled with Easytides [α-32P]dCTP 3000 Ci/mmol (PerkinElmer) by the rediprime DNA-labeling system (Amersham). Signals were quantified using Multi-Analyst software (Bio-Rad).
For the reverse transcription, total RNA prepared from YLV3t3m3 or YLV3t3m3ΔH was treated with DNase I (New England Biolabs), retro-transcribed with M-MuLV Reverse Transcriptase (New England Biolabs) with oligo (dT)20primer (Euroclone) and murine RNase inhibitor (New England Biolabs). PCR on retro-transcribed hCNT3 and, as reference, ACT1, was performed using hCNT3cFw and hCNT3cRv or ACT1Fw and ACT1Rv primers. qPCR on retro-transcribed hDCK1, MIP1 and, as reference, ACT1 was performed by using Power Sybr Green mix with ROX (Life Technologies), supplemented with primers DCK1qFw and DCK1qRv, MIP1qFw and MIP1qFw, or ACT1qFw and ACT1qRv, at a final concentration of 120 nM in the AB 7300 (Life Technologies) instrument at default settings: 50 °C 2 min, 95 °C 10 min, 41 cycles at 95 °C for 15 s and 60 °C for 1 min, and one cycle at 95 °C for 15 s and at 60 °C for 15 s. Statistical analysis was performed through an unpaired two-tailed t-test.
2.4. Measurement of petite frequency and EryR mutant frequency
Petite frequency was measured in DWM-5A mip1 strains and in W1BCK1 mip1 strains as previously reported (Baruffini et al., 2010), and on YLV3t3m3 as previously reported (Baruffini and Lodi, 2010) in at least four independent clones for each strain. Statistical analysis was performed through a paired two-tailed t-test.
EryR mutant frequency was measured using the method of the median (Lea and Coulson, 1949) as previously described (Baruffini et al., 2010), except for YLV3t3m3 mip1 strains, which were not grown on SC medium but on YP medium supplemented with 2% galactose. Each value is the mean of three independent fluctuation test experiments with 16–20 samples each. Statistical analysis of EryR mutant frequency was performed through an unpaired two-tailed t-test.
2.5. Measurement of mtDNA levels
W1BCK1 and YLV3t3m3 wild type and mutant strains were grown in the same condition used to determine petite frequency. Total DNA was extracted as previously reported (Hoffman and Winston, 1987). qPCR was performed on mtDNA gene COX1 and, as reference, on nDNA gene ACT1 using primers COX1-forward and COX1-reverse (Taylor et al., 2005), and ACT1Fw and ACT1Rv as reported in Section 2.3.
3. Results
3.1. Polymorphism selection and in silico analysis
A large number of polymorphisms have been found in the POLG(1) gene and their descriptions are collected in databases of the NHLBI exome sequencing project, in the SNP database from NCBI, and in the Human DNA Polymerase γ Mutation Database. We examined these databases to select suitable polymorphisms for our study, in particular those i) which have been found with a frequency higher than 0.1% and ii) which involve substitutions of an amino acid which is conserved between yeast and human or which is located in a region conserved between the two organisms. The latter is a prerequisite to be able to introduce the corresponding mutation in the ortholog position of the yeast Mip1 protein.
The NHLBI GO Exome Sequencing Project database (http://evs.gs.washington.edu/EVS/) contains data from the exome sequencing of more than 6200 people, of which approximately 2000 are African–American and 4300 are European–American unrelated individuals. Among the 283 POLG SNPs found in the database, 81 are coding and missense, of which 17 have a frequency higher than 0.1% in either the African–American (AA) population or in the European–American (EA) population (Supplementary Table 2).
The NCBI SNP database (http://www.ncbi.nlm.nih.gov/snp) contains 842 human SNPs in POLG, most of which derive from the 1000 Genomes Project. Four of these SNPs have been verified and have a MAF (Minimal Allele Frequency) higher than 0.001 or 0.1% (Supplementary Table 3).
The Human DNA Polymerase γ Mutation Database (http://tools.niehs.nih.gov/Polg/) describes approximately 250 mutations and polymorphisms obtained from literature. Of these mutations, 15 are reported as neutral polymorphisms with an allele frequency higher than 0.1% (Supplementary Table 4).
Among the 28 polymorphisms selected on the basis of their frequency, two were unambiguously reported to be pathological (T251L, A467T) (http://tools.niehs.nih.gov/Polg/) and for that reason they were excluded.
In order to analyze the level of conservation of the mutated residues, Pol γ protein sequences from different organisms were aligned in order to identify which of the polymorphisms involved residues conserved between humans and yeast. This analysis revealed that 18 mutations out of 26 involve amino acids which are not conserved or are located in a poorly conserved region. The remaining eight polymorphisms are variations of conserved (P241L, G268A, L392V, E1143G) or semi-conserved residues (R193Q, R964C, R1142W, R1146C), and are located in a conserved region (Supplementary Fig. 1).
P241L has been identified as a change present in 1 among 200 controls and as a polymorphism present in the Chinese population with an allelic frequency of 12–15% (Gui et al., 2012; Luoma et al., 2007). G268A has been considered associated with sporadic progressive external ophthalmoplegia (PEO) in several studies. On the basis of further analysis, and considering its high frequency in the population (0.5–3.5%), this change is unlikely to be a pathological SNP or a phenotypic modifier (Tang et al., 2011; http://jmg.bmj.com/content/48/10/669/reply#jmedgenet_el_989). R964C was considered a pathological mutation, causing severe ataxia in heterozygosity with a second detrimental mutation (Stricker et al., 2009; Tang et al., 2011; Wong et al., 2008). However, a patient homozygous for this mutation did not suffer from mitochondrial disorders, but developed lactic acidosis after a 1-year treatment with stavudine (Yamanaka et al., 2007). It was also demonstrated that the in vitro polymerase activity of Pol γ harboring R964C is reduced 5 to 9-fold (Bailey et al., 2009; Yamanaka et al., 2007), with a 3-fold decreased ability to discriminate between dTTP and stavudine triphosphate (d4TTP) compared to that of the wt, thus explaining the stavudine sensitivity of the subject. The involvement of the mutation E1143G with a pathological phenotype has been described with contradictory conclusions. In addition, in heterozygosity E1143G has been statistically associated with a higher probability of developing liver failure after treatment with valproic acid (Stewart et al., 2010) or stavudine-induced lipodystrophy (Chiappini et al., 2009). No information is available on the other polymorphisms taken into consideration.
At first, we performed an in silico prediction analysis of the phenotypic consequences of such polymorphisms, by using five different prediction tools. Although all the prediction algorithms are based principally on the conservation of the involved amino acid during the evolution and, for some programs, other parameters, the results obtained were not consistent (Supplementary Table 4). However, by performing a consensus of the results obtained via these five tools, two polymorphisms (R193Q and P241L) were predicted to be neutral, two polymorphisms (L392V and E1143G) possibly damaging and four polymorphisms (G268A, R964C, R1142W and R1146C) probably damaging (Supplementary Table 5).
3.2. Effect of the selected polymorphisms on mtDNA extended and point mutability
The effect of the selected POLG polymorphisms on the mtDNA stability was evaluated in vivo in yeast strains carrying MIP1 alleles containing mutations in the equivalent positions. In order to obtain these allelic variants, mutations which determine substitutions of conserved residues (P241L, G268A, L392V, E1143G) were inserted in wt MIP1 cloned in the centromeric vector pFL39, by site-directed mutagenesis. In the case of mutations in non-conserved positions (R193Q, R964C, R1142W, R1146C), we constructed two alleles for each polymorphism: in the former, the Mip1 amino acid was changed according to the wt Pol γ (humanized allele); in the latter, the Mip1 amino acid was changed according to the polymorphism (mutant allele). All mip1 alleles were introduced in the DWM-5A (mip1Δ) strain containing MIP1 wt cloned in the pFL38 vector (Baruffini et al., 2006), thus obtaining a first series of strains containing both the mutant and the wt alleles (heteroallelic strains), which were used in the case of dominance/recessive tests. By plasmid shuffling on 5-FOA, we then obtained a second series of strains containing only the mutant mip1 allelic variants (haploid strains).
We first evaluated the frequency of petite mutation in haploid strains, in order to determine whether polymorphisms behaved as neutral or if they altered the ability of DNA polymerase γ to fully replicate mtDNA. Analyses were performed at 28 °C, the optimal growth temperature for yeast, as well as at 37 °C, which is the temperature normally used to evaluate thermo-sensitivity of a given mutant in yeast.
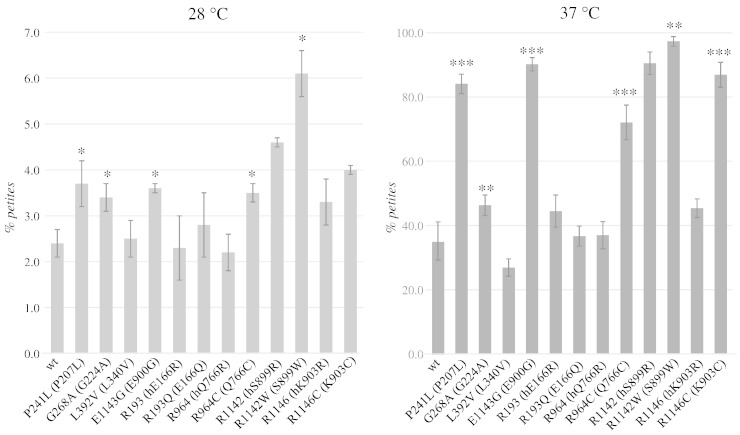
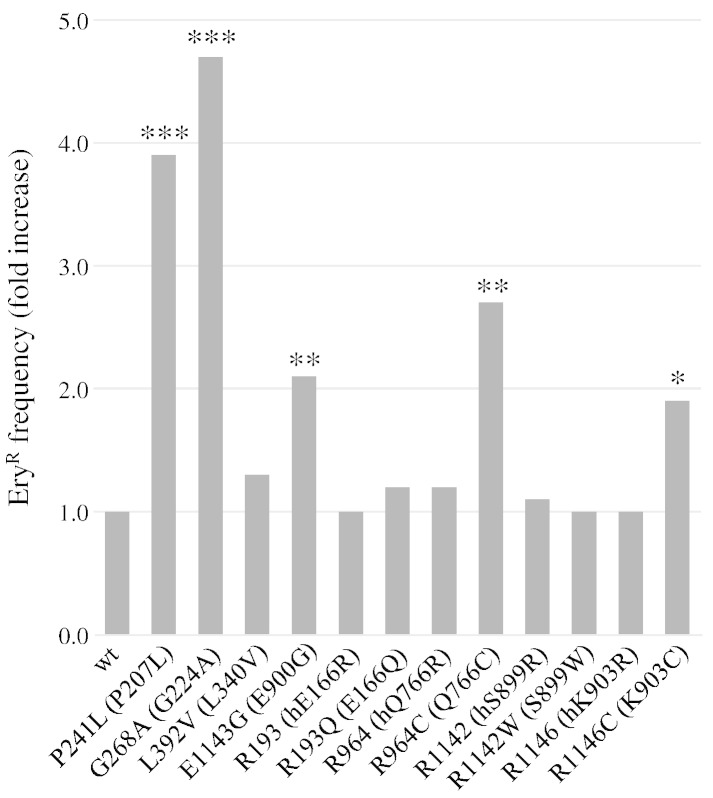
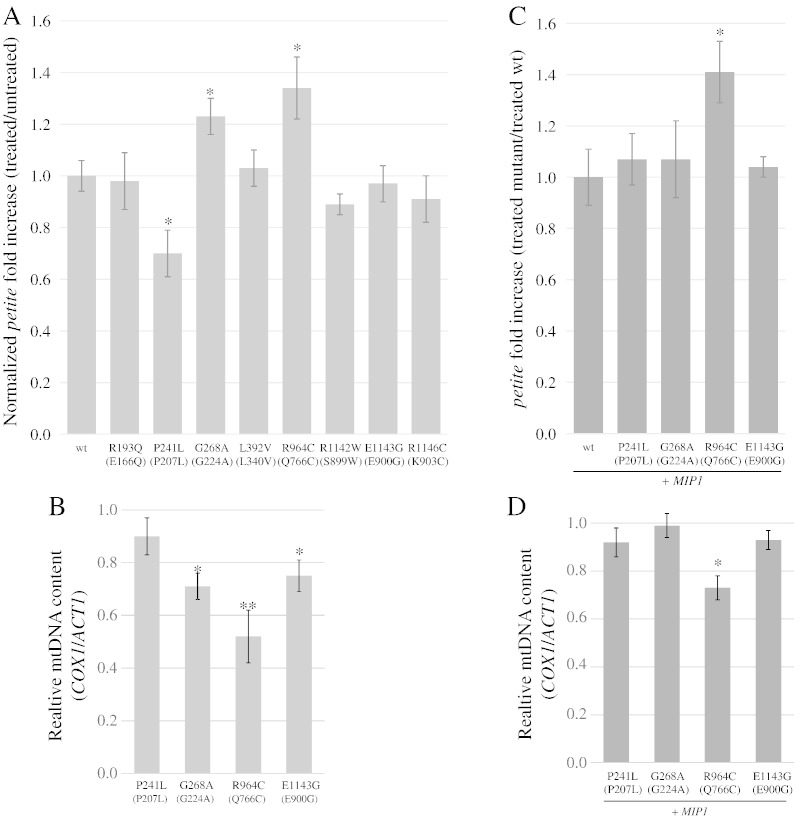
Five out of eight polymorphisms (P241L, G268A, R964C, R1142W and E1143G) showed increased petite frequency at 28 °C, suggesting that they affect mitochondrial stability and thus are not neutral substitutions (Fig. 1). As mentioned above, three of them (G268A, R964C and E1143G) were already known to alter the properties of Pol γ. In addition, the five polymorphisms, together with R1146C, showed a strong thermosensitivity, suggesting that the mutation altered the stability and/or the catalytic properties of Pol γ at higher temperatures. As regards point mutability, most polymorphisms increased the frequency of EryR point mutations, ranging from a ~ 2-fold increase for R1146C and E1143G mutations to ~ 4-fold increase for P241L and G268A mutations (Fig. 2).
Fig. 1.
Petite mutant frequency in mip1 wt and mutant strains at 28 °C (left panel) and 37 °C (right panel). In brackets the corresponding substitution in yeast Mip1. The letter “h” before the substitution indicates the humanized wt allele of the corresponding human substitution. The values are mean of three independent experiments ± standard deviation. Statistical significance: *: p < 0.05; **: p < 0.01; ***: p < 0.001.
Fig. 2.
EryR mutant frequency in mip1 wt and mutant strains. The frequency of EryR mutants was calculated as the ratio of the number of EryR mutants to the number of rho+ cells plated on the Petri dish. The ‘fold increase’ was calculated by normalization of the wt MIP1 strain relative frequency, which was 2.3 × 10− 7, to 1. Legend and statistical significance are as in Fig. 1.
We then evaluated the dominance/recessivity of the phenotype. It has previously been demonstrated that a hemizygous diploid yeast strain MIP1/mip1Δ is haploinsufficient, i.e. it showed a ~ 2-fold increase in both extended and point mutability (Baruffini et al., 2006). Among the recessive mutants, it is thus possible to distinguish mutant alleles, which totally or partially complement the haploinsufficient phenotype of the MIP1/mip1Δ strain or which behave as a null allele. In the first case, the petite frequency and/or the EryR frequency are similar or slightly higher compared to those of the wt homozygous strain. In the second case, the petite frequency and/or the EryR frequency are similar to those of the hemizygous diploid strain. We found that all the polymorphisms are recessive concerning extended mutability and fully complement the MIP1/mip1Δ phenotype (data not shown). On the other hand, the two mutations in the exonuclease domains (P241L and G268A) are dominant in regard to the EryR mutant frequency, which was 2.3 to 3.2-fold higher, respectively, compared to that of the heterozygous diploid strain.
3.3. Effect of the selected polymorphisms in combination with a pathological mutation
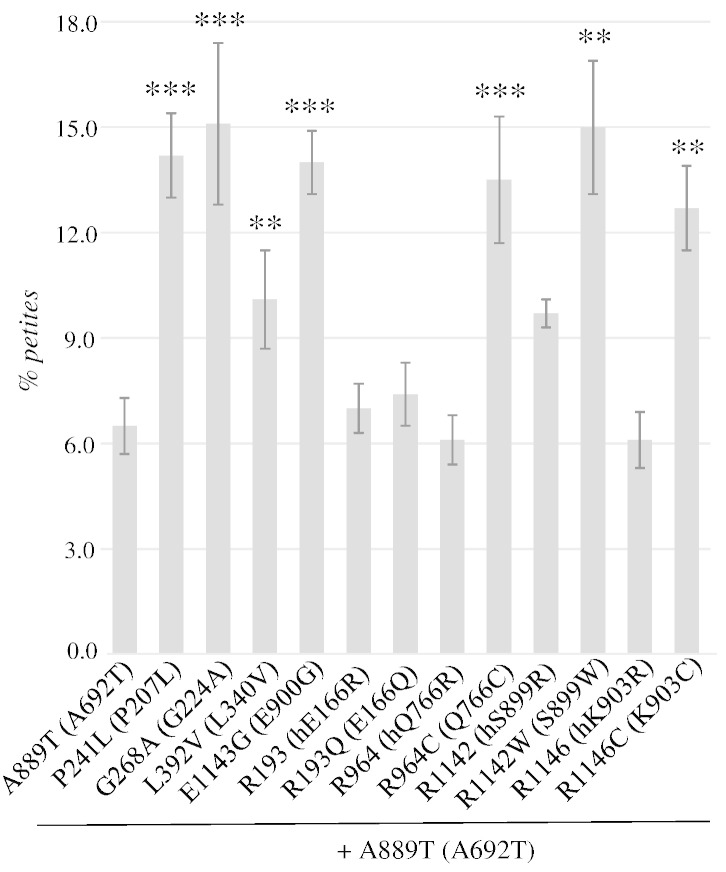
Several pathological mutations in POLG have been found in cis with one or more polymorphisms. However, in most cases, it is not clear whether the pathology and the severity of the disease are caused only by the pathological mutation(s), or if the polymorphism could behave as a phenotypic modifier which improves or worsens the pathological phenotype. We have previously demonstrated in yeast that mutation equivalent to E1143G polymorphism increased the petite frequency of the A889T-equivalent mutation in MIP1 when in cis, since the presence of the former mutation decreased soluble protein levels of Mip1 (Baruffini et al., 2007). In order to determine whether other polymorphisms have the capability to increase the mtDNA instability when in cis with a pathological mutation, we analyzed the effect of the selected polymorphisms in combination with the A889T-equivalent mutation A692T. We showed that all the polymorphisms except for R193Q behaved as phenotypic modifiers that increased the petite frequency of the mutant Mip1A692T (Fig. 3). For most polymorphisms, including E1143G, the effects were additive, i.e. the polymorphism increased the petite frequency by the same degree as in the presence of wt Mip1. Furthermore, two polymorphisms, L392V and R1146C, which were found to be neutral in the absence of other mutations at 28 °C, caused a 1.6 and a 2.1-fold increase in the petite frequency in the presence of the A889T mutation.
Fig. 3.
Petite frequency in mip1 mutants in combination with the human A889T (yeast A692T) mutation. Legend and statistical significance are as in Fig. 1.
3.4. Analysis of the toxicity induced by NRTI treatment in the presence of the selected polymorphisms: effect of stavudine
We then analyzed if the presence of mutations in DNA polymerase γ correlated with a variation of mitochondrial toxicity due to NRTI treatment, in particular stavudine or zalcitabine.
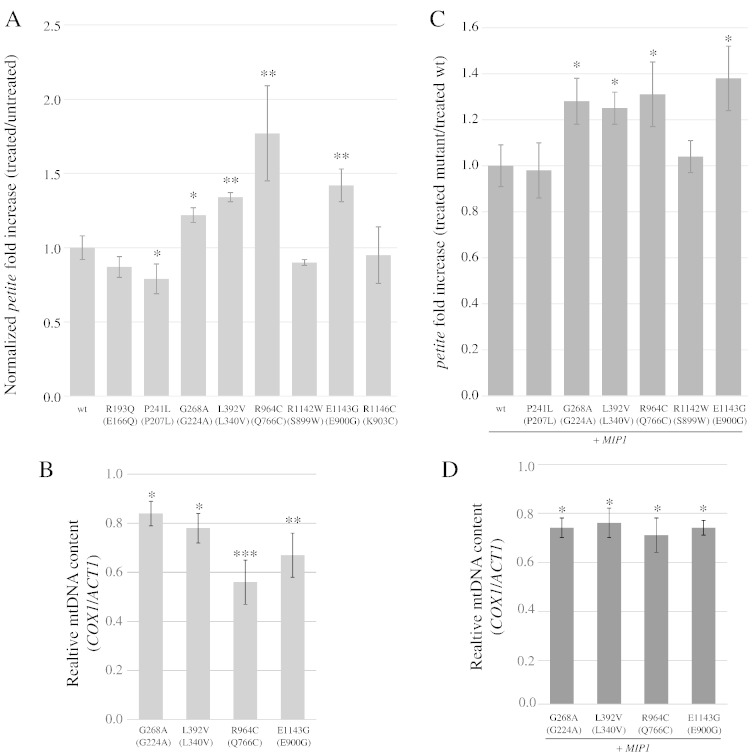
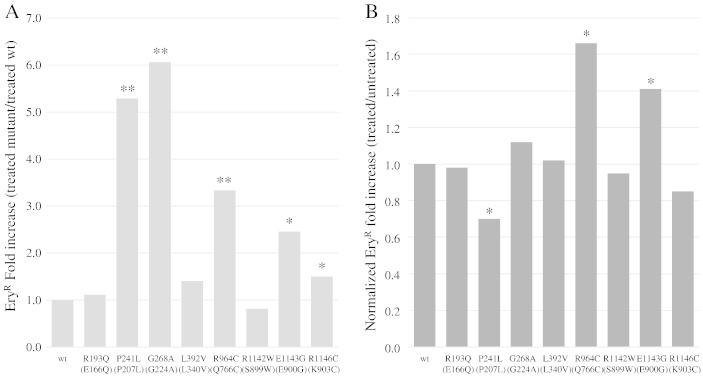
d4T is active as an inhibitor of Pol γ (and of HIV reverse transcriptase) after import into the cell and triphosphorylation by thymidine kinase and thymidylate kinase. S. cerevisiae is not able to mediate deoxynucleosides uptake and lacks the enzyme required to catalyze the first phosphorylation, and therefore it is unable to activate nucleosides from the environment, as well as to activate NRTI. For our experiments, we took advantage of a yeast strain expressing the human ENT1 (hENT1) gene, encoding the equilibrative nucleoside transporter, and the thymidine kinase gene from the herpes simplex virus (HSV-TK), which was integrated in the yeast genome under the control of GAL1-10 promoter (strain YLV3) (Vernis et al., 2003). The observation that the mtDNA mutability increased in the YLV3 strain after treatment with d4T in galactose, but not in glucose, demonstrated that the expression of hENT1 and HSV-TK mediated the uptake and the activation of d4T and the consequent mitochondrial toxicity due to incorporation of the NRTI in the S. cerevisiae mtDNA by Mip1. This model system has been validated by assessing the association between d4T toxicity and expression of mip1 alleles carrying mutations known to be sensitive to stavudine in human patients (Baruffini and Lodi, 2010). Moreover, by Northern analysis and RT-qPCR, we showed that the mRNA levels of hENT1, HSV-TK and MIP1 were influenced neither by the levels of d4T added to the medium nor by the concentration of galactose ranging from 0.3% to 2% (Supplementary Fig. 2). From YLV3 we obtained a series of strains deleted of the MIP1 gene (YLV3t3m3) and harboring mip1 alleles carrying the different selected polymorphisms, cloned in the centromeric vector pFL39. The mtDNA toxicity was studied in these strains after treatment with 1 mM d4T, by measuring the frequency of petite mutants. The frequency of petite mutants was increased by d4T treatment in YLV3t3m3 carrying both wt and mutant mip1 alleles (Supplementary Table 6). The Mip1 variant corresponding to human polymorphisms G268A, L392V, R964C and E1143G rendered the strain more susceptible to d4T than wt Mip1, since the ratio of petite frequency between treated and untreated mutant strain normalized to the wt strain was higher than 1 (Fig. 4A). In particular, data related to R964C and E1143G were in agreement with what has been observed in humans (Bailey et al., 2009; Yamanaka et al., 2007). On the contrary, variant P241L renders Mip1 less susceptible to the NRTI (ratio of petite frequency between treated and untreated mutant strains normalized to wt strain lower than 1). In the case of polymorphisms R193Q, R1142W and R1146C, the effect of stavudine and the effect of the polymorphism were additive (the ratio of petite frequency between treated and untreated mutant strains normalized to wt strain is not significantly different from 1).
Fig. 4.
(A) Normalized petite fold increase of strains treated with 1 mM d4T relative to untreated strains. For each mutant, the normalized petite fold increase is the mean of the ratios [(petite frequency of treated mutant strain)/(petite frequency of untreated mutant strain)]/[(petite frequency of treated wt strain)/(petite frequency of untreated wt strain)] (see also Supplementary Table 6). For mutant strains harboring human substitutions P193Q, R964C, R1142W and R1146C, the frequencies were compared to strains harboring the corresponding mip1 humanized allele. (B) mtDNA levels in strains with a higher normalized petite fold increase after treatment with 1 mM d4T. The ratio COX1/ACT1 was normalized to 1 for wild type strain. (C) Normalized petite fold increase of heteroallelic strains treated with 2 mM d4T compared to homoallelic strain treated with 2 mM d4T. For each mutant, the normalized petite fold increase is the ratio (petite frequency of treated mutant strain/petite frequency of treated wt strain) (See also Supplementary Table 7). For mutant MIP1/mip1 strains harboring human substitutions R964C, R1142W and R1146C, the frequencies were compared to strains harboring the corresponding MIP1/mip1 humanized allele. Statistical significance is as in Fig. 1. (D) mtDNA levels in heteroallelic strains with a higher petite fold increase after treatment with 2 mM d4T. The ratio COX1/ACT1 was normalized to 1 for homoallelic wild type strain.
We further measured through qPCR the mtDNA levels after treatment with d4T. We observed that in the YLV3t3m3 MIP1 strain the mtDNA copy number decreased approximately of 2.2-fold compared to the untreated condition. Since treatment with the same concentration of d4T resulted in a petite frequency, i.e. respiratory deficient cells without an intact mtDNA, of approximately 20% (Supplementary Table 6), the qPCR results clearly show that d4T treatment reduces the level of mtDNA to 50% in respiratory competent cells. Treatment with d4T in mip1 mutant strains with increased petite frequency results in decreased mtDNA levels compared to the treated wild type strain, indicating that, in presence of mip1 polymorphisms, d4T not only increased the number of respiratory deficient cells but also decreased the mtDNA levels in the whole cell population (Fig. 4B).
Due to the low frequency of Pol γ polymorphisms in the human population, the majority of subjects carrying these polymorphisms are heterozygous. To mimic this condition, we constructed YLV3t3m3 heteroallelic strains bearing a copy of both wt MIP1 and of one of each mutant allele, as well as a homoallelic strain bearing two copies of wt MIP1. Petite frequency was measured after treatment with 2 mM d4T, the optimal concentration to assess the dominant/recessive behavior of mutant alleles in the presence of stavudine (Baruffini and Lodi, 2010). Heteroallelic strains carrying Mip1 variants corresponding to G268A, L392V, R964C and E1143G Pol γ polymorphisms displayed a significant increase of mitochondrial mutability, compared to the homoallelic strain, indicating that these mutations behave as dominant concerning d4T toxicity (Fig. 4C and Supplementary Table 7). As for haploid strains, treatment with 2 mM d4T results in decreased mtDNA copy number for G268A, L392V, R964C and E1143G Pol γ polymorphisms in heterozygosis (Fig. 4D).
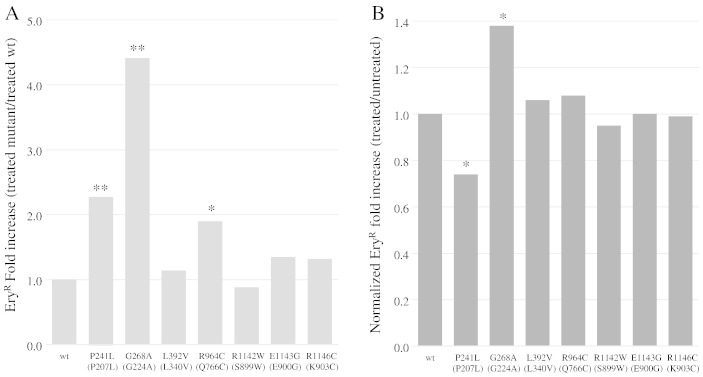
The effect of stavudine on point mutations was also analyzed in haploid strains carrying mutant alleles. After treatment with 1 mM d4T, the EryR mutant frequency increased in all strains, including those harboring wt MIP1 (Supplementary Table 8, columns 2 and 3). In addition, the fold increase, expressed as ratio between treated mutant strain and treated wt, is greater than 1 for most mutant strains, indicating that the simultaneous presence of a MIP1 mutator allele in and of d4T is detrimental for the fidelity of mtDNA replication (Fig. 5A). However, if the fold increase is further normalized with the untreated wt strain, the effect between the intrinsic mutability of mutant Mip1 and the susceptibility to d4T are synergistic only in the case of mutations R964C and E1143G (normalized fold increase higher than 1), whereas mutation P241L is less susceptible to the presence of d4T (normalized fold increase lower than 1) (Fig. 5B), as already observed in the case of extended mutability.
Fig. 5.
(A) EryR fold increase of mutant strains treated with 1 mM d4T relative to treated wt strain. For each mutant, the fold increase (treated mutant/treated wt) is the mean of the ratios (EryR frequency of treated mutant strain/EryR frequency of treated wt strain). (B) EryR normalized fold increase of mutant strains treated with 1 mM d4T compared to untreated strains. For each mutant, the normalized fold increase (treated/untreated) is the mean of the ratios [(EryR frequency of treated mutant strain)/(EryR frequency of untreated mutant strain)]/[(EryR frequency of treated wt strain)/(EryR frequency of untreated wt strain)] (See also Supplementary Table 8). For mutant strains harboring human substitutions P193Q, R964C and R1142W, the frequencies were compared to strains harboring the corresponding mip1 humanized allele. Statistical significance is as in Fig. 1.
3.5. Analysis of the toxicity induced by NRTI treatment in the presence of the selected polymorphisms: effect of zalcitabine
Zalcitabine (ddC), similarly to stavudine, is active after phosphorylation in the cell. Strains derived from YLV3t3m3 were found insensitive to the toxicity induced by this NRTI (data not shown), probably either because hENT1 is unable to transport zalcitabine, or because HSV-TK, which is reported to phosphorylate other deoxypyrimidines (Chen et al., 1979), is unable to phosphorylate it, or both. In order to improve the import of ddC, hENT1 was deleted in the YLV3t3m3 strain and substituted with human CNT3 cDNA. hCNT3, together with hCNT1 and hCNT2, belongs to the SCL28 family of Na-coupled concentrative nucleoside transporters, responsible for the high affinity transport of both nucleosides and synthetic nucleoside analogs in mammals (Gray et al., 2004). hCNT3 was chosen since it was characterized as broadly selective for both purine and pyrimidine nucleosides. hCNT3 is also able to transport a number of anticancer and antiviral nucleoside analogs, including ddC (Gray et al., 2004). hCNT3 cDNA was cloned under the control of the GAL1-10 promoter both in the centromeric pUSG-E12 and in the multicopy pYES2 vectors. These recombinant plasmids were then introduced into YLV3t3m3 devoid of hENT1, and the correct expression and galactose induction of hCNT3 were verified by Northern blot (data not shown). Strains expressing hCNT3, both in monocopy and in multicopy, treated with ddC, did not exhibit mitochondrial toxicity (data not shown), suggesting that, in this case also, the nucleoside analog was not correctly transported and/or phosphorylated to the metabolically active form.
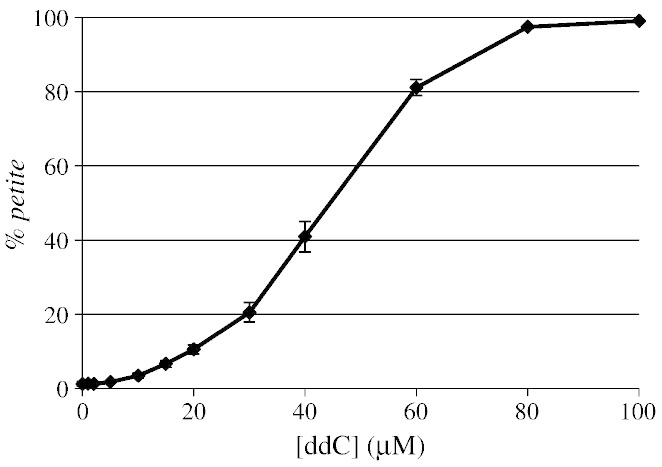
A new strain was then constructed by genomic integration of the human deoxycytidine kinase cDNA (DCK1), under the control of a constitutive PGK promoter, thus obtaining the W1BCK1 strain. To test the ddC sensitivity of this recombinant strain, we treated it with increasing concentrations of ddC. Mitochondrial toxicity was then analyzed by measuring the frequency of petite mutants. As reported in Fig. 6, the frequency of petite mutants increased in a dose dependent manner, indicating that: i) ddC was transported into the yeast cell, probably by aspecific transporters; ii) ddC was phosphorylated and activated to the metabolic active form by hDCK1 in yeast. Moreover, we demonstrated by RT-qPCR that the MIP1 and hDCK1 mRNA levels were not influenced by the presence and the concentration of ddC (Supplementary Fig. 3).
Fig. 6.
Susceptibility to ddC of W1BCK1 strain treated with ddC from 0 (petite frequency equal to 1.2%) to 100 μM (petite frequency equal to 99.8%).
From W1BCK1 we obtained a series of strains deleted of the MIP1 gene (W1BCK1-10) and harboring mip1 alleles carrying the different selected polymorphisms, cloned in the centromeric vector pFL39.
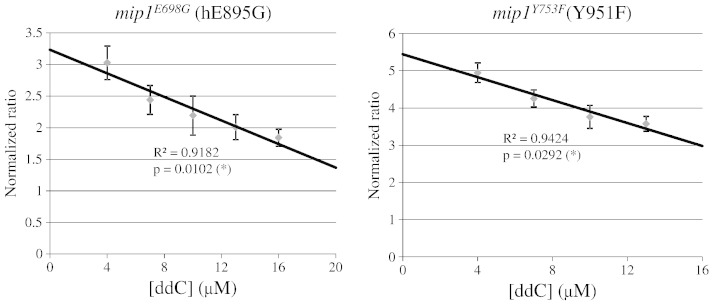
In order to validate the system, we took advantage of biochemical data previously obtained on human Pol γ mutants. It has been reported that human Pol γ harboring E895A or Y951F mutations showed an ~ 18-fold and 2400-fold decrease respectively, in incorporation of ddCTP relative to dCTP compared to wt Pol γ, indicating a lower susceptibility to ddC toxicity (Lim et al., 2003). Thus, we tested whether we could observe a similar effect in the W1BCK1-10 yeast strain carrying mutations E698G and Y753F in the equivalent positions. In the haploid strain these mutations induce 100% petite; in the heteroallelic strain, both mutant mip1 alleles behaved as negative dominant, increasing the petite frequency compared to a strain harboring a copy of wt MIP1, i.e. the hemiallelic strain (11.6% and 18.7% respectively, vs 2.0%). This effect is coherent with the dominant pathological phenotype observed in patients heterozygous for mutations in the same position (Spinazzola et al., 2009; Tang et al., 2011). We found that, following treatment with ddC, the petite frequency of both heteroallelic and hemiallelic strains increased (data not shown) in the case of both mutations E895G and Y951F, but the ratios of petite frequency of the heteroallelic strain to petite frequency of the hemialleic strain decreased significantly by increasing ddC concentration (Fig. 7). This result indicated that, when E895G and Y951F mutant isoforms are present, Mip1 is less susceptible to ddC toxicity, thus parallelizing with the biochemical results previously obtained in Pol γ.
Fig. 7.
Susceptibility to ddC of W1BCK1-10 mip1 heteroallelic mutant strains compared to hemiallelic strain. In order to normalize the data to the zero value, at each concentration tested, the ratio of petite frequency of the heteroallelic strain to petite frequency of hemiallelic strain was calculated as [(petite frequency of treated heteroallelic strain) – (petite frequency of untreated heteroallelic strain)]/[(petite frequency of treated hemiallelic strain) – (petite frequency of untreated hemiallelic strain)]. Cells were treated with ddC ranging from 4 to 20 μM, and results were included if petite frequency of heteroallelic strain was lower than the threshold value of 50%. A linear regression curve was plotted by using the Excel linear regression function. Statistical analysis was performed using the ANOVA test of linearity. Significance was calculated by using the F distribution with one degree of freedom at the numerator and three or two degrees of freedom at the denominator.
Once we had validated the model, we tested extended and point mutability in W1BCK1-10 harboring the mip1 alleles that carry the different selected polymorphisms. Regarding petite frequency in haploid strains (Fig. 8A and Supplementary Table 9), only the Mip1 variants corresponding to human polymorphisms G268A and R964C were more susceptible to ddC than wt Mip1. Mip1 mutants harboring substitutions L392V and E1143G, which were sensitive to d4T, were not sensitive to ddC compared to the wt, as well as R193Q, R1142W and R1146C variants. As observed for d4T, treatment with ddC results in a 3.2-fold decrease of mtDNA levels in MIP1 wild type strain, indicating that respiratory competent cells retain approximately 30% of the mtDNA levels. Again, strains with increased petite frequency (Supplementary Table 9) display also a reduced mtDNA copy number after treatment with ddC (Fig. 8B). Among the heteroallelic strains, only the strain harboring R964C showed a dominant effect in the presence of ddC (Fig. 8C and D and Supplementary Table 10).
Fig. 8.
Legends and statistical analysis are as in Fig. 4 except that 30 μM ddC and 60 μM ddC were used in (A) and (B), and in (C) and (D) respectively (see also Supplementary Tables 9 and 10).
All strains showed an increase of EryR mutant frequency after treatment with ddC (Supplementary Table 11, columns 2 and 3). The fold increase was significantly greater than 1 only for three mutations (Fig. 9A). The fold increase normalized with the untreated wt strain showed a synergistic effect of the intrinsic mutability of mutant Mip1 and of the susceptibility to ddC only for the G268A mutation (Fig. 9B). On the contrary, the P241L mutation was less susceptible to the presence of ddC, as already observed in the case of d4T.
Fig. 9.
Legends and statistical analysis are as in Fig. 5 except that 20 μM ddC was used (see also Supplementary Table 11).
4. Discussion
Mutations in amino acid residues which have a relatively high frequency in the population are generally considered neutral. However, several amino acid substitutions, despite not being the cause of pathology, may modulate the effect of pathological mutations or alter the effect of a drug. In recent years, the use of next generation sequencing has strongly increased the number of sequenced genomes or exomes, revealing the existence of a high number of mutations in the population, or in specific ethnic groups, most of which have a low frequency and are not characterized.
Pol γ, the mitochondrial replicase, is among the most studied mitochondrial proteins and its activity is fundamental for the maintenance of adequate levels of mtDNA. Physiological, biochemical and phenotypic consequences of pathological mutations have been described, whereas little is reported concerning amino acid substitutions which have low frequency and are considered neutral. Firstly, if the biochemical effect of a mutation is small, kinetic studies may not point out the defects caused by this mutation, such as changes in the Km, in the kcat or in the processivity of Pol γ. Secondly, if the mutation frequency is low, it could be difficult to find a statistical correlation between the presence of the mutation and a pathological phenotype or an altered response to a drug in the population. Thus, the use of an in vivo system with high sensitivity, such as the model organism S. cerevisiae which we are proposing, can sharpen the putative defects caused by mutations/polymorphisms, in particular on extended mitochondrial mutability or on point mutability. We introduced eight polymorphisms in the yeast MIP1 gene, which was chosen on the basis of the frequency in the population and of the conservation between yeast and human Pol γ. Quite surprisingly, six among them (75%) increased the petite frequency or the EryR frequency. The observed variations were modest, indicating that the polymorphisms should not be pathological alone, but suggesting that their presence can contribute to increasing the levels of mutant mtDNA in the cell. The two mutations in the exonuclease (exo) domain, P241L and G268A, increased the point mutability at higher levels, suggesting that mutant Pol γ harboring these mutations, as well as other exo domain mutations lying in the protein surface (Euro et al., 2011), have a reduced ability to remove mismatched nucleotides. In addition, G268A is predicted to lie in a cluster, which comprises residues 268–277, for which a decrease in exonuclease activity is predicted if mutated (Farnum et al., 2014). Six mutations also determine a strong thermosensitivity. Among them is the E1143G mutation, which has been reported to have a decreased in vitro activity at high temperatures (Chan et al., 2006). Interestingly, R1142, E1143 and R1146 are located in a β-sheet that surrounds the catalytic site in the palm subdomain and can therefore maintain the architecture of the active site (Euro et al., 2011), suggesting that mutations in these amino acids can alter the tertiary structure, especially at high temperatures.
In many patients, several pathological mutations have been identified together with one or more polymorphisms considered as neutral. This suggests that, at least in some cases, a neutral polymorphism can modify the phenotype associated with a pathological mutation. Little information is known in this regard, with the exception of the E1143G mutation. Biochemical studies on human Pol γ harboring this mutation have been contradictory. Chan et al. (2006) found that mutant E1143G Pol γ has a 1.4-fold higher catalytic activity than wt Pol γ, and that this mutation can partially rescue the strong biochemical defects of the W748S mutation in cis. On the contrary, Palin et al., 2010, showed that human Pol γ harboring the W748S mutation does not show any biochemical defects and behaves like wt Pol γ in vivo, and that the presence in cis of the E1143G mutation does not alter the in vivo behavior of the mutant protein. We previously showed that the presence of the E1143G-equivalent mutation in yeast decreases the mtDNA stability by 2-fold because of the A889T mutation, due to the reduced stability of the protein harboring both mutations compared to a protein harboring only the latter mutation (Baruffini et al., 2007). Therefore, this mutation, identified at the beginning as a neutral polymorphism, is now considered a phenotypic modulator of pathological mutations in cis.
In order to evaluate the possible role of the selected polymorphisms as phenotypic modifiers, we measured the petite frequency in strains harboring the A889T-equivalent mutation in cis with the polymorphism under analysis. This mutation was chosen as a reference since, to our knowledge, this is the only pathological mutation, besides the non-conserved substitution W748S, that has been shown in vivo to have a worse phenotype when in cis with a polymorphism (Baruffini et al., 2007). We showed that all the polymorphisms, except for E193Q, had negative effect, indicating that they could potentially modulate the pathological phenotype. For two polymorphisms, L392V and R1146C, the effect in combination with the A889T mutation was synergistic.
A limitation to the use of yeast MIP1 to study the effects of mutations is that only conserved or semi-conserved residues can be studied. During the preparation of this manuscript, Qian and co-authors created a yeast model system in which both human Pol γ subunit genes, cloned under the yeast MIP1 promoter and in frame with the MIP1 fragment encoding the mitochondrial targeting signal, complement the absence of MIP1, indicating that human Pol γ can replicate yeast mtDNA (Qian et al., 2014). Interestingly, a comparison between the effects of four human mutations which have been studied both in the human POLG and in the MIP1 gene showed very similar results concerning mtDNA stability, mtDNA point mutability and dominance/recessivity in the two systems, indicating that the use of yeast MIP1 has a good predictive ability for conserved and semi-conserved residues. However, the creation of a yeast strain expressing human POLG will be an unequaled model for the in vivo studies of non-conserved mutations.
An additional point addressed in this work concerns the role of the yeast model in predicting the possible correlation between specific mutations in Mip1, corresponding to human mutations, and mtDNA mutability induced by treatment with nucleoside reverse transcriptase inhibitors (NRTI), used in the highly active antiretroviral therapy (HAART), i.e. d4T and ddC. These molecules are inhibitors of Pol γ, at least in their triphosphorylated forms, as observed in different studies (Johnson et al., 2001; Lim and Copeland, 2001; Martin et al., 1994). We showed that, as for HIV reverse transcriptase and human Pol γ, yeast Mip1 is inhibited more by ddC than d4T, because 30 μM of ddC are enough to increase the petite frequency to 20% compared to 1 mM of d4T. Based on the “Pol γ hypothesis” of NRTI toxicity, each mutation/SNP which changes the Pol γ affinity for the incoming NRTI-TP, the discrimination between the NRTI-TP and the corresponding dNTP, or the NRTI excision efficiency in the mtDNA could alter the NRTI induced toxicity. To date, an association between NRTI-induced mitochondrial toxicity and SNPs/mutations in Pol γ has been reported for two mutations, R964C and E1143G (Chiappini et al., 2009; Yamanaka et al., 2007).
Our previous and present results showed that mutant versions of Mip1 harboring four polymorphisms (G268A, L392V, R964C and E1143G) are more sensitive to d4T-induced mitochondrial toxicity, resulting in higher petite frequency and EryR mutant frequency, and lower mtDNA levels, than those observed in Mip1 wt strain treated with d4T. In addition, for all these polymorphisms, the effects of stavudine toxicity on mtDNA stability are dominant, i.e. a heteroallelic strain harboring a wt copy of Mip1 and a mutant copy of Mip1 showed higher petite frequency in the presence of d4T as well as a decrease in total mtDNA levels compared to a strain harboring two copies of wt Mip1. This result indicates that also heterozygous subjects, who are more frequent than homozygous ones due to the relative low frequency of these polymorphisms, are susceptible to d4T toxicity, as already observed in patients heterozygous for E1143G or R964C.
Interestingly, Mip1 harboring a P241L mutation is less susceptible to d4T-induced extended and point mutability, suggesting that mutant polymerase either binds with a lower affinity d4T-TP or has an increased ability to remove incorporated d4T. Additionally, P241L is part of a cluster which also includes residues 224–244 and which is predicted to decrease polymerase activity and to increase exonuclease activity if mutated (Farnum et al., 2014).
Regarding ddC, we observed that only two polymorphisms, G268A and R964C, determined an increased sensitivity to the NRTI, and only for the latter the effects are dominant. This suggests that this NRTI could be better tolerated compared to d4T in HIV patients harboring polymorphisms. Again, P241L is less sensitive to ddC toxicity.
We have previously demonstrated that extended mtDNA mutability (petite frequency) and EryR point mutability due to mutations in Mip1 are derived from different mechanisms, since for most mutations there is no correlation between increase in the former and increase in the latter. It is worth noticing that, for both the NRTIs analyzed, we showed in this study that there was a significant correlation between induced extended mutability (petite frequency) and induced point mutability (EryR mutant frequency), suggesting, but not demonstrating, a cause/effect relationship. Although this observation cannot exclude that the NRTI caused, as a direct effect, both an increase in petite frequency and in point mutability, the results are coherent with those of Payne et al. (2011), who demonstrated that mtDNA mutations accumulated in cells from patients treated with NRTIs are not due to de novo point mutations induced by the NRTI, but might instead be caused by the clonal expansion of preexisting mutant mtDNA particles, needed to restore a proper mtDNA level in case of mtDNA depletion consequent to the NRTI treatment.
5. Conclusions
In this work we studied the effects of eight polymorphisms in the DNA polymerase γ gene, which are one third of the known human polymorphisms with a frequency higher than 0.1%, on the mtDNA stability and point mutability in an in vivo system. This study suggests that many human SNPs/mutations in POLG (i) are not neutral, (ii) could potentially behave as phenotypic modulators of pathological mutations and (iii) can increase the mitochondrial dysfunction induced by treatment with NRTIs. The analysis is based on the general assumption that if an amino acid is conserved between two homologous proteins, such as Mip1 and human Pol γ, the substitution of that amino acid in one protein can predict the effect of the mutation in the second protein. However, it must be underlined that this assumption is not applicable for any proteins/amino acids, especially if the amino acid lies far from the active site. In the case of Pol γ, the sequence identity between human and yeast enzymes is approximately 45%, with peaks of 65–70% in the exonuclease and polymerase domains, and lows in the linker domain, which in yeast is shorter, lacking the POLG2 subunit binding subdomain. For these reasons, our results should be confirmed by studies of the polymorphisms in mammalian Pol γ, either in the yeast model system expressing human Pol γ or in mammalian cells or in a mammalian model system, especially in the case of substitutions of amino acids which contribute to the binding of the POLG2 subunit, absent in yeast.
From a pharmacogenetic point of view, we found that, in the presence of polymorphisms in POLG, zalcitabine should be better tolerated than stavudine since a lower number of polymorphisms determined ddC-induced toxicity and the detrimental effects are less significant in the case of ddC. These results agree, in general, with clinical observations, which showed that d4T is commonly less tolerated than ddC, and in particular with the observation that there is a correlation between the presence of E1143G polymorphism and induced lipodystrophy in the case of d4T treatment, but not of ddC treatment (Chiappini et al., 2009). Hence, our analysis supports the need of developing novel NRTIs which inhibit HIV reverse transcriptase but not Pol γ, and stresses the importance of monitoring the effects of such NRTIs by biochemical and/or in vivo analysis on wt and mutant DNA polymerase γ. Such an approach has been recently applied for the novel NRTIs 3′-fluoro-3′-deoxythymidine (FLT) and 2′,3′-didehydro-3′-deoxy-4′-ethynylthymidine (Ed4T) (Sohl et al., 2012), which showed that the former, once triphosphorylated, showed only a 35-fold discrimination compared to dTTP, whereas Ed4T-tryphospate showed a 6200-fold discrimination. However, DNA polymerase γ harboring R964C may lead to a higher degree of mitochondrial toxicity in the presence of Ed4T-tryphospate, as observed previously in vivo (Yamanaka et al., 2007), in vitro (Bailey et al., 2009) and in this study for d4T. Therefore, it is necessary to consider the presence of SNPs that can lead to unexpected side effects when developing new NRTIs.
Acknowledgments
We wish to thank Beatrice Martinelli (Kelima Translations, Sheffield, UK) for proofreading the manuscript; Alberto Inga (University of Trento, Italy) and Roberta Ruotolo (University of Parma) for the gift of pUSG-E12 and pYES2, respectively; Claudio Casoli (GEMIB S.R.L., Parma, Italy) for the gift of zalcitabine and stavudine; and Antonietta Cirasolo (University of Parma) for the skillful technical assistance. The project was funded by Telethon (GGP11011).
Appendix A. Supplementary data
Supplementary Fig. 1. Alignment of DNA polymerase γ protein sequences from different organisms. Hs: Homo sapiens, Pt: Pan troglodytes, Mm: Mus musculus, Xl: Xenopus laevis, Dr: Danio rerio, Sc: Saccharomyces cerevisiae.
Supplementary Fig. 2. (A) Northern blot on hENT1 and HSV-TK mRNA. For each gene, strain and condition, the signal was normalized for the signal of ACT1 mRNA. An arbitrary value of 1 has been assigned to the YLV3 strain grown on 2% galactose. The values are mean of two independent experiments. (B) RT-qPCR on MIP1 mRNA. An arbitrary value of 1 has been assigned to the YLV3 strain grown on 2% galactose. The values are mean of three independent experiments ± standard deviation.
Supplementary Fig. 3. RT-qPCR on MIP1 and hENT1 mRNAs. An arbitrary value of 1 has been assigned to the W1BCK1 strain in the absence of ddC. The values are mean of three independent experiments ± standard deviation.
Supplementary tables.
References
- Ausubel F.M., Brent R., Kingston R.E., Moore D.D., Seidman J.G., Smith J.A., Struhl K. vol. 2. Wiley; NY: 1994. Saccharomyces cerevisiae. (Current protocols in molecular biology). [Google Scholar]
- Azrak S., Ayyasamy V., Zirpoli G., Ambrosone C., Bandera E.V., Bovbjerg D.H., Jandorf L., Ciupak G., Davis W., Pawlish K.S., Liang P., Singh K. CAG repeat variants in the POLG1 gene encoding mtDNA polymerase-gamma and risk of breast cancer in African–American women. PLoS One. 2012;7:e29548. doi: 10.1371/journal.pone.0029548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baile M.G., Claypool S.M. The power of yeast to model diseases of the powerhouse of the cell. Front. Biosci. (Landmark Ed.) 2013;18:241–278. doi: 10.2741/4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey C.M., Kasiviswanathan R., Copeland W.C., Anderson K.S. R964C mutation of DNA polymerase gamma imparts increased stavudine toxicity by decreasing nucleoside analog discrimination and impairing polymerase activity. Antimicrob. Agents Chemother. 2009;53:2610–2612. doi: 10.1128/AAC.01659-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruffini E., Lodi T. Construction and validation of a yeast model system for studying in vivo the susceptibility to nucleoside analogues of DNA polymerase gamma allelic variants. Mitochondrion. 2010;10:183–187. doi: 10.1016/j.mito.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Baruffini E., Lodi T., Dallabona C., Puglisi A., Zeviani M., Ferrero I. Genetic and chemical rescue of the Saccharomyces cerevisiae phenotype induced by mitochondrial DNA polymerase mutations associated with progressive external ophthalmoplegia in humans. Hum. Mol. Genet. 2006;15:2846–2855. doi: 10.1093/hmg/ddl219. [DOI] [PubMed] [Google Scholar]
- Baruffini E., Ferrero I., Foury F. Mitochondrial DNA defects in Saccharomyces cerevisiae caused by functional interactions between DNA polymerase gamma mutations associated with disease in human. Biochim. Biophys. Acta. 2007;1772:1225–1235. doi: 10.1016/j.bbadis.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Baruffini E., Serafini F., Lodi T. Construction and characterization of centromeric, episomal and GFP-containing vectors for Saccharomyces cerevisiae prototrophic strains. J. Biotechnol. 2009;143:247–254. doi: 10.1016/j.jbiotec.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Baruffini E., Ferrero I., Foury F. In vivo analysis of mtDNA replication defects in yeast. Methods. 2010;51:426–436. doi: 10.1016/j.ymeth.2010.02.023. [DOI] [PubMed] [Google Scholar]
- Baruffini E., Horvath R., Dallabona C., Czermin B., Lamantea E., Bindoff L., Invernizzi F., Ferrero I., Zeviani M., Lodi T. Predicting the contribution of novel POLG mutations to human disease through analysis in yeast model. Mitochondrion. 2011;11:182–190. doi: 10.1016/j.mito.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Blomberg Jensen M., Leffers H., Petersen J.H., Daugaard G., Skakkebaek N.E., Rajpert-De Meyts E. Association of the polymorphism of the CAG repeat in the mitochondrial DNA polymerase gamma gene (POLG) with testicular germ-cell cancer. Ann. Oncol. 2008;19:1910–1914. doi: 10.1093/annonc/mdn407. [DOI] [PubMed] [Google Scholar]
- Bonneaud N., Ozier-Kalogeropoulos O., Li G.Y., Labouesse M., Minvielle-Sebastia L., Lacroute F. A family of low and high copy replicative, integrative and single-stranded S. cerevisiae/E. coli shuttle vectors. Yeast. 1991;7:609–615. doi: 10.1002/yea.320070609. [DOI] [PubMed] [Google Scholar]
- Chan S.S., Longley M.J., Copeland W.C. Modulation of the W748S mutation in DNA polymerase gamma by the E1143G polymorphism in mitochondrial disorders. Hum. Mol. Genet. 2006;15:3473–3483. doi: 10.1093/hmg/ddl424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.S., Walker J., Prusoff W.H. Kinetic studies of herpes simplex virus type 1-encoded thymidine and thymidylate kinase, a multifunctional enzyme. J. Biol. Chem. 1979;254:10747–10753. [PubMed] [Google Scholar]
- Chiappini F., Teicher E., Saffroy R., Debuire B., Vittecoq D., Lemoine A. Relationship between polymerase gamma (POLG) polymorphisms and antiretroviral therapy-induced lipodystrophy in HIV-1 infected patients: a case–control study. Curr. HIV Res. 2009;7:244–253. doi: 10.2174/157016209787581409. [DOI] [PubMed] [Google Scholar]
- Cui Z., Mason T.L. A single nucleotide substitution at the rib2 locus of the yeast mitochondrial gene for 21 S rRNA confers resistance to erythromycin and cold-sensitive ribosome assembly. Curr. Genet. 1989;16:273–279. doi: 10.1007/BF00422114. [DOI] [PubMed] [Google Scholar]
- Dujon B. Molecular Biology of the Yeast Saccharomyces: Life Cycle and Inheritance. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1981. Mitochondrial genetics and functions. [Google Scholar]
- Euro L., Farnum G.A., Palin E., Suomalainen A., Kaguni L.S. Clustering of Alpers disease mutations and catalytic defects in biochemical variants reveal new features of molecular mechanism of the human mitochondrial replicase. Pol γ Nucleic Acids Res. 2011;39:9072–9084. doi: 10.1093/nar/gkr618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnum G.A., Nurminen A., Kaguni L.S. Mapping 136 pathogenic mutations into functional modules in human DNA polymerase γ establishes predictive genotype–phenotype correlations for the complete spectrum of POLG syndromes. Biochim. Biophys. Acta. 2014;1837:1113–1821. doi: 10.1016/j.bbabio.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz R.D., Schiestl R.H. High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat. Protoc. 2007;2:31–34. doi: 10.1038/nprot.2007.13. [DOI] [PubMed] [Google Scholar]
- Gietz R.D., Schiestl R.H. Quick and easy yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat. Protoc. 2007;2:35–37. doi: 10.1038/nprot.2007.14. [DOI] [PubMed] [Google Scholar]
- Gray J.H., Owen R.P., Giacomini K.M. The concentrative nucleoside transporter family, SLC28. Pflugers Arch. 2004;447:728–734. doi: 10.1007/s00424-003-1107-y. [DOI] [PubMed] [Google Scholar]
- Gui Y.X., Xu Z.P., Lv W., Liu H.M., Zhao J.J., Hu X.Y. Association of mitochondrial DNA polymerase γ gene POLG1 polymorphisms with parkinsonism in Chinese populations. PLoS One. 2012;7:e50086. doi: 10.1371/journal.pone.0050086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güldener U., Heinisch J., Köhler G.J., Voss D., Hegemann J.H. A second set of loxP marker cassettes for Cre-mediated multiple gene knockouts in budding yeast. Nucleic Acids Res. 2002;30:e23. doi: 10.1093/nar/30.6.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S.N., Hunt H.D., Horton R.M., Pullen J.K., Pease L.R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Hoffman C.S., Winston F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene. 1987;57:267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- Johnson A.A., Ray A.S., Hanes J., Suo Z., Colacino J.M., Anderson K.S., Johnson K.A. Toxicity of antiviral nucleoside analogs and the human mitochondrial DNA polymerase. J. Biol. Chem. 2001;276:40847–40857. doi: 10.1074/jbc.M106743200. [DOI] [PubMed] [Google Scholar]
- Kaiser C., Michaelis S., Mitchell A. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1994. Methods in Yeast Genetics: A Laboratory Course Manual. [Google Scholar]
- Koczor C.A., Lewis W. Nucleoside reverse transcriptase inhibitor toxicity and mitochondrial DNA. Expert Opin. Drug Metabol. Toxicol. 2010;6:1493–1504. doi: 10.1517/17425255.2010.526602. [DOI] [PubMed] [Google Scholar]
- Kohler J.J., Lewis W. A brief overview of mechanisms of mitochondrial toxicity from NRTIs. Environ. Mol. Mutagen. 2007;48:166–172. doi: 10.1002/em.20223. [DOI] [PubMed] [Google Scholar]
- Lea D.E., Coulson C.A. The distribution of the numbers of mutants in bacterial populations. J. Genet. 1949;49:264–285. doi: 10.1007/BF02986080. [DOI] [PubMed] [Google Scholar]
- Lee H., Hanes J., Johnson K.A. Toxicity of nucleoside analogues used to treat AIDS and the selectivity of the mitochondrial DNA polymerase. Biochemistry. 2003;42:14711–14719. doi: 10.1021/bi035596s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.S., Kennedy W.D., Yin Y.W. Structural insight into processive human mitochondrial DNA synthesis and disease-related polymerase mutations. Cell. 2009;16:312–324. doi: 10.1016/j.cell.2009.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis W. Pharmacogenomics, toxicogenomics, and DNA polymerase gamma. J. Infect. Dis. 2007;195:1399–1401. doi: 10.1086/513879. [DOI] [PubMed] [Google Scholar]
- Lewis W., Kohler J.J., Hosseini S.H., Haase C.P., Copeland W.C., Bienstock R.J., Ludaway T., McNaught J., Russ R., Stuart T., Santoianni R. Antiretroviral nucleosides, deoxynucleotide carrier and mitochondrial DNA: evidence supporting the DNA pol gamma hypothesis. AIDS. 2006;20:675–684. doi: 10.1097/01.aids.0000216367.23325.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S.E., Copeland W.C. Differential incorporation and removal of antiviral deoxynucleotides by human DNA polymerase gamma. J. Biol. Chem. 2001;276:23616–23623. doi: 10.1074/jbc.M101114200. [DOI] [PubMed] [Google Scholar]
- Lim S.E., Ponamarev M.V., Longley M.J., Copeland W.C. Structural determinants in human DNA polymerase gamma account for mitochondrial toxicity from nucleoside analogs. J. Mol. Biol. 2003;329:45–57. doi: 10.1016/s0022-2836(03)00405-4. [DOI] [PubMed] [Google Scholar]
- Luoma P.T., Eerola J., Ahola S., Hakonen A.H., Hellström O., Kivistö K.T., Tienari P.J., Suomalainen A. Mitochondrial DNA polymerase gamma variants in idiopathic sporadic Parkinson disease. Neurology. 2007;69:1152–1159. doi: 10.1212/01.wnl.0000276955.23735.eb. [DOI] [PubMed] [Google Scholar]
- Martin J.L., Brown C.E., Matthews-Davis N., Reardon J.E. Effects of antiviral nucleoside analogs on human DNA polymerases and mitochondrial DNA synthesis. Antimicrob. Agents Chemother. 1994;38:2743–2749. doi: 10.1128/aac.38.12.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minet M., Dufour M.E., Lacroute F. Complementation of Saccharomyces cerevisiae auxotrophic mutants by Arabidopsis thaliana cDNAs. Plant J. 1992;2:417–422. doi: 10.1111/j.1365-313x.1992.00417.x. [DOI] [PubMed] [Google Scholar]
- Palin E.J., Lesonen A., Farr C.L., Euro L., Suomalainen A., Kaguni L.S. Functional analysis of H. sapiens DNA polymerase gamma spacer mutation W748S with and without common variant E1143G. Biochim. Biophys. Acta. 2010;1802:545–551. doi: 10.1016/j.bbadis.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne B.A., Wilson I.J., Hateley C.A., Horvath R., Santibanez-Koref M., Samuels D.C., Price D.A., Chinnery P.F. Mitochondrial aging is accelerated by anti-retroviral therapy through the clonal expansion of mtDNA mutations. Nat. Genet. 2011;43:806–810. doi: 10.1038/ng.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popanda O., Seibold P., Nikolov I., Oakes C.C., Burwinkel B., Hausmann S., Flesch-Janys D., Plass C., Chang-Claude J., Schmezer P. Germline variants of base excision repair genes and breast cancer: a polymorphism in DNA polymerase gamma modifies gene expression and breast cancer risk. Int. J. Cancer. 2013;132:55–62. doi: 10.1002/ijc.27665. [DOI] [PubMed] [Google Scholar]
- Qian Y., Kachroo A.H., Yellman C.M., Marcotte E.M., Johnson K.A. Yeast cells expressing the human mitochondrial DNA polymerase reveal correlations between polymerase fidelity and human disease progression. J. Biol. Chem. 2014;289:5970–5985. doi: 10.1074/jbc.M113.526418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saneto R.P., Lee I.C., Koenig M.K., Bao X., Weng S.W., Naviaux R.K., Wong L.J. POLG DNA testing as an emerging standard of care before instituting valproic acid therapy for pediatric seizure disorders. Seizure. 2010;19:140–146. doi: 10.1016/j.seizure.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F., Fink G.R., Hicks J.B. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1986. Laboratory Course Manual For Methods in Yeast Genetics. [Google Scholar]
- Singh K.K., Ayyasamy V., Owens K.M., Koul M.S., Vujcic M. Mutations in mitochondrial DNA polymerase-gamma promote breast tumorigenesis. J. Hum. Genet. 2009;54:516–524. doi: 10.1038/jhg.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohl C.D., Kasiviswanathan R., Kim J., Pradere U., Schinazi R.F., Copeland W.C., Mitsuya H., Baba M., Anderson K.S. Balancing antiviral potency and host toxicity: identifying a nucleotide inhibitor with an optimal kinetic phenotype for HIV-1 reverse transcriptase. Mol. Pharmacol. 2012;82:125–133. doi: 10.1124/mol.112.078758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sor F., Fukuhara H. Identification of two erythromycin resistance mutations in the mitochondrial gene coding for the large ribosomal RNA in yeast. Nucleic Acids Res. 1982;10:6571–6577. doi: 10.1093/nar/10.21.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sor F., Fukuhara H. Erythromycin and spiramycin resistance mutations of yeast mitochondria: nature of the rib2 locus in the large ribosomal RNA gene. Nucleic Acids Res. 1984;12:8313–8318. doi: 10.1093/nar/12.22.8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinazzola A., Invernizzi F., Carrara F., Lamantea E., Donati A., Dirocco M., Giordano I., Meznaric-Petrusa M., Baruffini E., Ferrero I., Zeviani M. Clinical and molecular features of mitochondrial DNA depletion syndromes. J. Inherit. Metab. Dis. 2009;32:143–158. doi: 10.1007/s10545-008-1038-z. [DOI] [PubMed] [Google Scholar]
- Stewart J.D., Horvath R., Baruffini E., Ferrero I., Bulst S., Watkins P.B., Fontana R.J., Day C.P., Chinnery P.F. Polymerase γ gene POLG determines the risk of sodium valproate-induced liver toxicity. Hepatology. 2010;52:1791–1796. doi: 10.1002/hep.23891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker S., Prüss H., Horvath R., Baruffini E., Lodi T., Siebert E., Endres M., Zschenderlein R., Meisel A. A variable neurodegenerative phenotype with polymerase gamma mutation. J. Neurol. Neurosurg. Psychiatry. 2009;80:1181–1182. doi: 10.1136/jnnp.2008.166066. [DOI] [PubMed] [Google Scholar]
- Stuart G.R., Santos J.H., Strand M.K., Van Houten B., Copeland W.C. Mitochondrial and nuclear DNA defects in Saccharomyces cerevisiae with mutations in DNA polymerase gamma associated with progressive external ophthalmoplegia. Hum. Mol. Genet. 2006;15:363–374. doi: 10.1093/hmg/ddi454. [DOI] [PubMed] [Google Scholar]
- Stumpf J.D., Copeland W.C. The exonuclease activity of the yeast mitochondrial DNA polymerase γ suppresses mitochondrial DNA deletions between short direct repeats in Saccharomyces cerevisiae. Genetics. 2013;194:519–522. doi: 10.1534/genetics.113.150920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf J.D., Bailey C.M., Spell D., Stillwagon M., Anderson K.S., Copeland W.C. mip1 containing mutations associated with mitochondrial disease causes mutagenesis and depletion of mtDNA in Saccharomyces cerevisiae. Hum. Mol. Genet. 2010;19:2123–2133. doi: 10.1093/hmg/ddq089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepanowska K., Foury F. A cluster of pathogenic mutations in the 3′–5′ exonuclease domain of DNA polymerase gamma defines a novel module coupling DNA synthesis and degradation. Hum. Mol. Genet. 2010;19:3516–3529. doi: 10.1093/hmg/ddq267. [DOI] [PubMed] [Google Scholar]
- Tang S., Wang J., Lee N.C., Milone M., Halberg M.C., Schmitt E.S., Craigen W.J., Zhang W., Wong L.J. Mitochondrial DNA polymerase gamma mutations: an ever expanding molecular and clinical spectrum. J. Med. Genet. 2011;48:669–681. doi: 10.1136/jmedgenet-2011-100222. [DOI] [PubMed] [Google Scholar]
- Taylor S.D., Zhang H., Eaton J.S., Rodeheffer M.S., Lebedeva M.A., O'rourke T.W., Siede W., Shadel G.S. The conserved Mec1/Rad53 nuclear checkpoint pathway regulates mitochondrial DNA copy number in Saccharomyces cerevisiae. Mol. Biol. Cell. 2005;16:3010–3018. doi: 10.1091/mbc.E05-01-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas B.J., Rothstein R. Elevated recombination rates in transcriptionally active DNA. Cell. 1989;56:619–630. doi: 10.1016/0092-8674(89)90584-9. [DOI] [PubMed] [Google Scholar]
- Vanderstraeten S., Van den Brule S., Hu J., Foury F. The role of 39–59 exonucleolytic proofreading and mismatch repair in yeast mitochondrial DNA error avoidance. J. Biol. Chem. 1998;273:23690–23697. doi: 10.1074/jbc.273.37.23690. [DOI] [PubMed] [Google Scholar]
- Vernis L., Piskur J., Diffley J.F. Reconstitution of an efficient thymidine salvage pathway in Saccharomyces cerevisiae. Nucleic Acids Res. 2003;31:e120. doi: 10.1093/nar/gng121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong L., Naviaux R., Brunetti-Pierri N., Zhang Q., Schmitt E., Truong C., Milone M., Cohen B., Wical B., Ganesh J., Basinger A., Burton B., Swoboda K., Gilbert D., Vanderver A., Saneto R., Maranda B., Arnold G., Abdenur J., Waters P., Copeland W. Molecular and clinical genetics of mitochondrial diseases due to POLG mutations. Hum. Mutat. 2008;29:E150–E172. doi: 10.1002/humu.20824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakubovskaya E., Chen Z., Carrodeguas J.A., Kisker C., Bogenhagen D.F. Functional human mitochondrial DNA polymerase gamma forms a heterotrimer. J. Biol. Chem. 2006;6:374–382. doi: 10.1074/jbc.M509730200. [DOI] [PubMed] [Google Scholar]
- Yamanaka H., Gatanaga H., Kosalaraksa P., Matsuoka-Aizawa S., Takahashi T., Kimura S., Oka S. Novel mutation of human DNA polymerase gamma associated with mitochondrial toxicity induced by anti-HIV treatment. J. Infect. Dis. 2007;15:1419–1425. doi: 10.1086/513872. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Alignment of DNA polymerase γ protein sequences from different organisms. Hs: Homo sapiens, Pt: Pan troglodytes, Mm: Mus musculus, Xl: Xenopus laevis, Dr: Danio rerio, Sc: Saccharomyces cerevisiae.
Supplementary Fig. 2. (A) Northern blot on hENT1 and HSV-TK mRNA. For each gene, strain and condition, the signal was normalized for the signal of ACT1 mRNA. An arbitrary value of 1 has been assigned to the YLV3 strain grown on 2% galactose. The values are mean of two independent experiments. (B) RT-qPCR on MIP1 mRNA. An arbitrary value of 1 has been assigned to the YLV3 strain grown on 2% galactose. The values are mean of three independent experiments ± standard deviation.
Supplementary Fig. 3. RT-qPCR on MIP1 and hENT1 mRNAs. An arbitrary value of 1 has been assigned to the W1BCK1 strain in the absence of ddC. The values are mean of three independent experiments ± standard deviation.
Supplementary tables.