Abstract
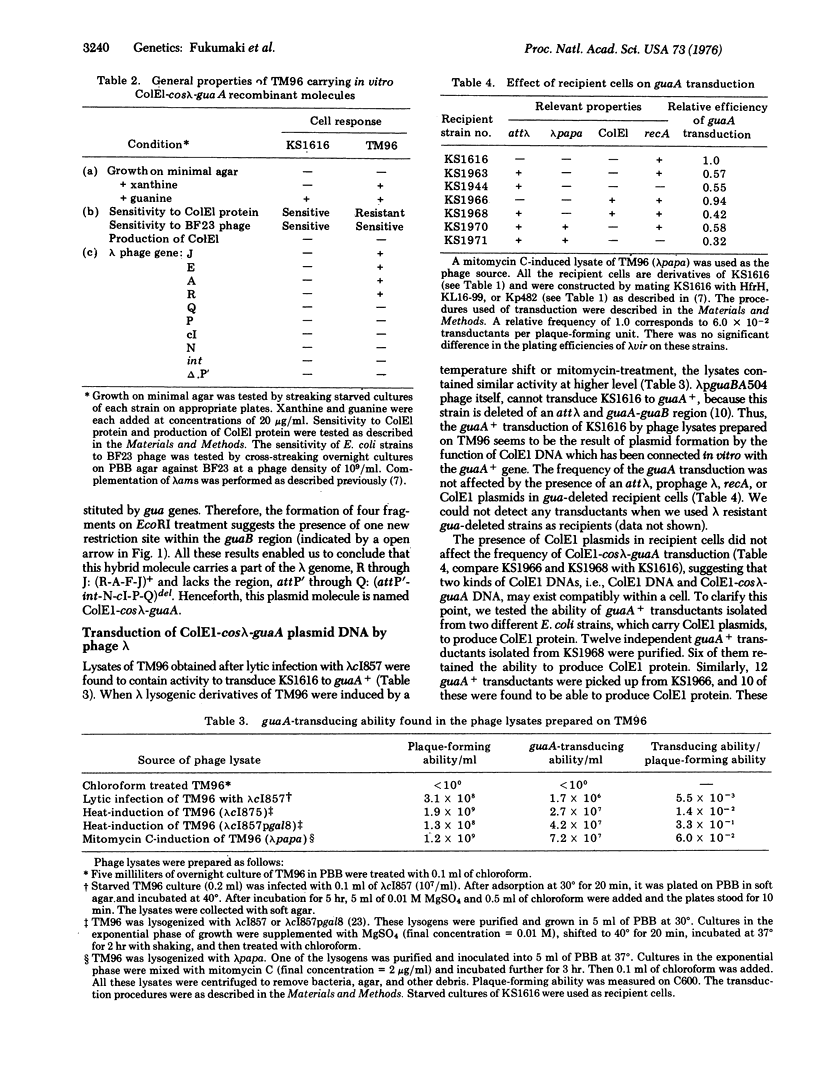
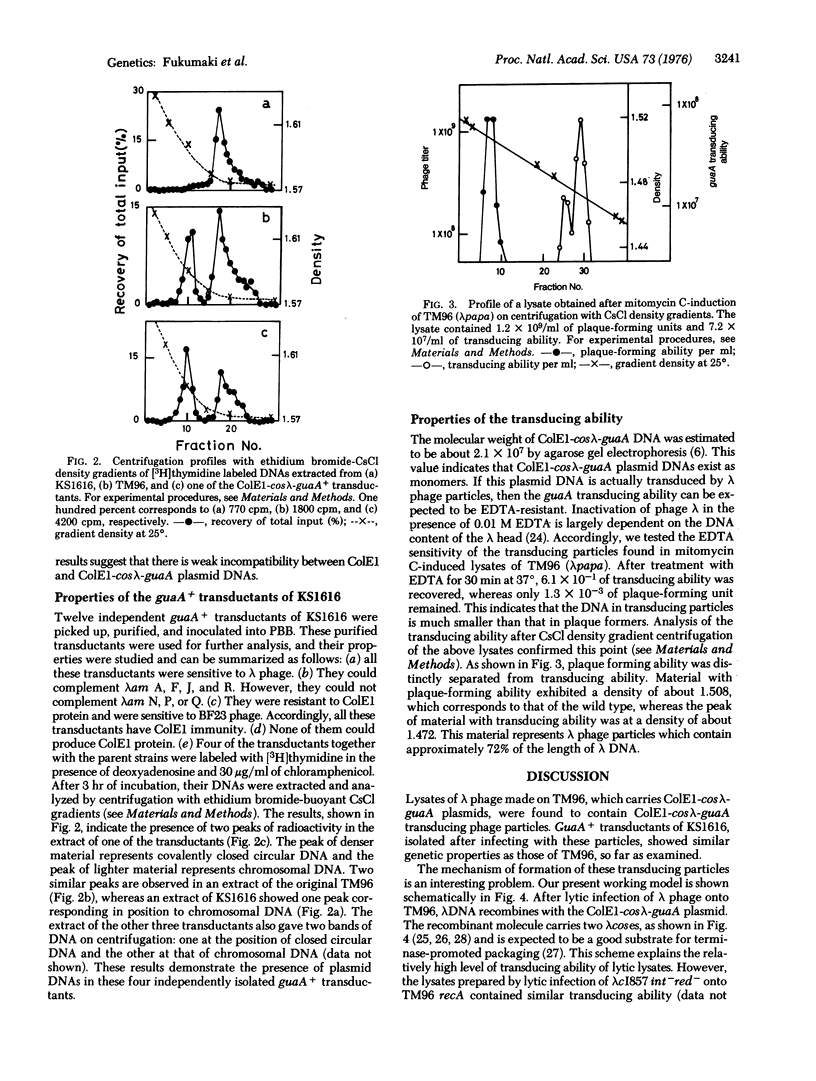
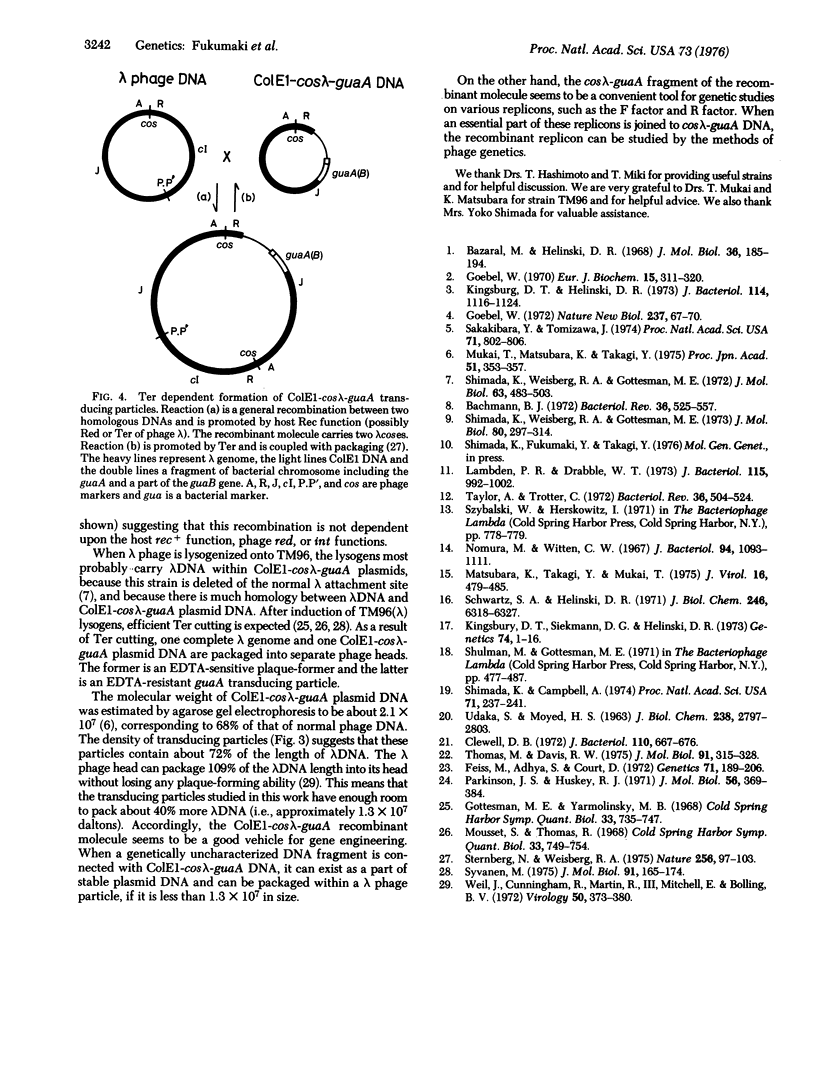
Genetic studies were made on E. coli K-12 TM96, which carries recombinant molecules constructed by in vitro combination of colicin E1 DNA and a DNA fragment of E. coli for guanine synthesis derived from transducing phage. The recombinant molecules existed as stable plasmids within the cell and contained genes for colicin E1 immunity and the guaA enzyme (xanthosine 5'-monophosphate aminase) together with a part of the lambda genome, R through J: (R-A-F-J)+. A block of the lambda genome, int through Q, was not detected in the recombinant molecule. Thus, this recombinant molecule was named ColEl-coslambda-guaA, and the specialized tranduction of the ColEl-coslambda-guA DNA into various E. coli K-12 cells by lambda phage was described. Lysates prepared by lytic infection of lambda phage onto TM96 or by induction of TM96(lambda) lysogens contained transducing particles which could transduce gua-deleted E. coli to stable guaA+ cells. These transductants were proved to have similar genetic properties as those of TM96. The frequency of transduction was not affected by the presence of an attachement site for lambda, prophage lambda, colicin E1 plasmids, or the recA property within gua-deleted recipient cells. Transducing particles were resistant to EDTA treatment and most of them had an average density of about 1.472. This value corresponds to that of lambda phage particles, which contain about 72% of the lenght of lambda DNA.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazaral M., Helinski D. R. Circular DNA forms of colicinogenic factors E1, E2 and E3 from Escherichia coli. J Mol Biol. 1968 Sep 14;36(2):185–194. doi: 10.1016/0022-2836(68)90374-4. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feiss M., Adyha S., Court D. L. Isolation of plaque-forming, galactose-transducing strains of phage lambda. Genetics. 1972 Jun;71(2):189–206. doi: 10.1093/genetics/71.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel W. Replication of the DNA of the colicinogenic factor E 1 (Col E 1 ) at the restrictive temperature in a DNA replication mutant thermosensitive for DNA polymerase. 3. Nat New Biol. 1972 May 17;237(72):67–70. doi: 10.1038/newbio237067a0. [DOI] [PubMed] [Google Scholar]
- Goebel W. Studies on extrachromosomal DNA elements. Replication of the colicinogenic factor Col E1 in two temperature sensitive mutants of Escherichia coli defective in DNA replication. Eur J Biochem. 1970 Aug;15(2):311–320. doi: 10.1111/j.1432-1033.1970.tb01009.x. [DOI] [PubMed] [Google Scholar]
- Gottesman M. E., Yarmolinsky M. B. The integration and excision of the bacteriophage lambda genome. Cold Spring Harb Symp Quant Biol. 1968;33:735–747. doi: 10.1101/sqb.1968.033.01.084. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. T., Helinski D. R. Temperature-sensitive mutants for the replication of plasmids in Escherichia coli: requirement for deoxyribonucleic acid polymerase I in the replication of the plasmid ColE 1 . J Bacteriol. 1973 Jun;114(3):1116–1124. doi: 10.1128/jb.114.3.1116-1124.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. T., Sieckmann D. G., Helinski D. R. Temperature-sensitive mutants for the replication of plasmids in Escherichia coli. II. Properties of host and plasmid mutations. Genetics. 1973 May;74(1):1–16. doi: 10.1093/genetics/74.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambden P. R., Drabble W. T. The gua operon of Escherichia coli K-12: evidence for polarity from guaB to guaA. J Bacteriol. 1973 Sep;115(3):992–1002. doi: 10.1128/jb.115.3.992-1002.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara K., Takagi Y., Mukai T. In vitro construction of different oligomeric forms of lambdadv DNA and studies of their transforming activities. J Virol. 1975 Sep;16(3):479–485. doi: 10.1128/jvi.16.3.479-485.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousset S., Thomas R. Dilysogenic excision: an accessory expression of the termination function? Cold Spring Harb Symp Quant Biol. 1968;33:749–754. doi: 10.1101/sqb.1968.033.01.085. [DOI] [PubMed] [Google Scholar]
- Nomura M., Witten C. Interaction of colicins with bacterial cells. 3. Colicin-tolerant mutations in Escherichia coli. J Bacteriol. 1967 Oct;94(4):1093–1111. doi: 10.1128/jb.94.4.1093-1111.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J. S., Huskey R. J. Deletion mutants of bacteriophage lambda. I. Isolation and initial characterization. J Mol Biol. 1971 Mar 14;56(2):369–384. doi: 10.1016/0022-2836(71)90471-2. [DOI] [PubMed] [Google Scholar]
- Sakakibara Y., Tomizawa J. I. Replication of colicin E1 plasmid DNA in cell extracts. Proc Natl Acad Sci U S A. 1974 Mar;71(3):802–806. doi: 10.1073/pnas.71.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S. A., Helinski D. R. Purification and characterization of colicin E1. J Biol Chem. 1971 Oct 25;246(20):6318–6327. [PubMed] [Google Scholar]
- Shimada K., Campbell A. Int-constitutive mutants of bacteriophage lambda. Proc Natl Acad Sci U S A. 1974 Jan;71(1):237–241. doi: 10.1073/pnas.71.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Weisberg R. A., Gottesman M. E. Prophage lambda at unusual chromosomal locations. I. Location of the secondary attachment sites and the properties of the lysogens. J Mol Biol. 1972 Feb 14;63(3):483–503. doi: 10.1016/0022-2836(72)90443-3. [DOI] [PubMed] [Google Scholar]
- Shimada K., Weisberg R. A., Gottesman M. E. Prophage lambda at unusual chromosomal locations. II. Mutations induced by bacteriophage lambda in Escherichia coli K12. J Mol Biol. 1973 Oct 25;80(2):297–314. doi: 10.1016/0022-2836(73)90174-5. [DOI] [PubMed] [Google Scholar]
- Sternberg N., Weisberg R. Packaging of prophage and host DNA by coliphage lambda. Nature. 1975 Jul 10;256(5513):97–103. doi: 10.1038/256097a0. [DOI] [PubMed] [Google Scholar]
- Syvanen M. Processing of bacteriophage lambda DNA during its assembly into heads. J Mol Biol. 1975 Jan 15;91(2):165–174. doi: 10.1016/0022-2836(75)90157-6. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]
- UDAKA S., MOYED H. S. INHIBITION OF PARENTAL AND MUTANT XANTHOSINE 5'-PHOSPHATE AMINASES BY PSICOFURANINE. J Biol Chem. 1963 Aug;238:2797–2803. [PubMed] [Google Scholar]
- Weil J., Cunningham R., Martin R., 3rd, Mitchell E., Bolling B. Characteristics of lambda p4, a lambda derivative containing 9 per cent excess DNA. Virology. 1972 Nov;50(2):373–380. doi: 10.1016/0042-6822(72)90388-1. [DOI] [PubMed] [Google Scholar]



