Abstract
The serotonin 5-hydroxytryptamine 2A (5-HT2A) receptor is a potential therapeutic target to a host of neuropsychiatric conditions, but agonist actions at this site are linked to abuse-related hallucinogenic effects that may limit therapeutic efficacy of chronic drug administration. Tolerance to some effects of hallucinogens has been observed in humans and laboratory animals, but the understanding of tolerance and cross-tolerance between distinct structural classes of hallucinogens is limited. Here, we used the drug-elicited head twitch response (HTR) in mice to assess the development of tolerance and cross-tolerance with two phenethylamine-derived [DOI (2,5-dimethoxy-4-iodoamphetamine) and 2C-T-7 (2,5-dimethoxy-4-propylthiophenethylamine)] and two tryptamine-derived [DPT (N,N-dipropyltryptamine) and DIPT (N,N-diisopropyltryptamine)] drugs with agonist affinity for 5-HT2A receptors. Tolerance developed to HTR elicited by daily DOI or 2C-T-7, but not to HTR elicited by DPT or DIPT. DOI-elicited tolerance was not surmountable with dose, and a similar insurmountable cross-tolerance was evident when DOI-tolerant mice were tested with various doses of 2C-T-7 or DPT. These studies suggest that the use of phenethylamine-derived hallucinogens as therapeutic agents may be limited not only by their abuse potential, but also by the rapid development of tolerance that would likely be maintained even if a patient were switched to a different 5-HT2A agonist medication from a distinct structural class. However, these experiments also imply that tryptamine-derived hallucinogens might have a reduced potential for tolerance development, compared with phenethylamine-derived 5-HT2A agonists, and might therefore be more suitable for chronic administration in a therapeutic context.
Introduction
Hallucinogenic effects exerted by classic ergoline-, phenethylamine-, or tryptamine-derived drugs are currently understood to be mediated by agonist actions at serotonin 5-hydroxytryptamine 2A (5-HT2A) receptors (Nichols, 2004; Halberstadt, 2014), a site also implicated in numerous neuropsychiatric conditions including migraine (Berger et al., 2009), eating disorders (Kaye et al., 2005), depression and bipolar disorder (Fountoulakis et al., 2012), anxiety and panic (Graeff and Zangrossi, 2010), psychosis and schizophrenia (Selvaraj et al., 2014), and substance use disorder (Bubar and Cunningham, 2008). As such, medications targeting 5-HT2A could potentially be useful in treating a wide range of conditions, although the hallucinogenic effects of agonist medications would likely limit their clinical utility if chronic administration were required. Nevertheless, interest in using hallucinogenic drugs as therapeutics (e.g., Griffths and Grob, 2010; Vollenweider and Kometer, 2010) has been growing in recent years; thus, a greater understanding of the pharmacologic mechanisms underlying hallucinogenic effects is warranted. In particular, a better understanding of hallucinogenic effects after chronic administration may be especially relevant to understanding the long-term efficacy of these drugs as therapeutic agents.
Tolerance to numerous effects of some hallucinogens develops rapidly, evidenced by a progressive decrease in effects over repeated administration of a constant dose. In humans, this phenomenon has mainly been studied using the ergoline hallucinogen lysergic acid diethylamide (e.g., Isbell et al., 1959, 1961), but tolerance to behavioral and physiologic effects of phenethylamine-derived hallucinogens can also be readily induced in humans (Wolbach et al., 1962; Hollister et al., 1969; Angrist et al., 1974), presumably mediated by downregulation and desensitization of serotonin 5-HT2A receptors (McKenna et al., 1989; Owens et al., 1991; Smith et al., 1999). Tolerance elicited by tryptamine-derived hallucinogens in humans is not as well characterized, with one early study reporting a lesser degree of tolerance to psilocybin-elicited effects than to lysergic acid diethylamide–elicited effects (Isbell et al., 1961), and more recent experiments failing to demonstrate the development of tolerance to effects of the structurally-related hallucinogen DMT (N,N-dimethyltryptamine; Gillin et al., 1976; Strassman et al., 1996), perhaps due to its apparent inability to desensitize 5-HT2A receptors (Smith et al., 1998).
Because the hallucinogen-induced head twitch response (HTR) in rodents is also mediated by agonist actions at 5-HT2A receptors (González-Maeso et al., 2007; Fantegrossi et al., 2008a), it is among the most useful and reliable rodent models for psychedelic effects. Nevertheless, the role of tolerance in HTR elicited by hallucinogens as a drug class also remains somewhat unclear because the vast majority of work in this area has used only a single compound, the phenethylamine-derived DOI (2,5-dimethoxy-4-iodoamphetamine; Fig. 1, top left; reviewed in Canal and Morgan, 2012). In this regard, Darmani et al. (1990, 1992) and Darmani and Gerdes (1995) repeatedly demonstrated both tolerance and supersensitivity to DOI-elicited HTR in male ICR mice, depending on the specific dose regimen employed. Similarly, male C57BL/6N mice administered DOI every day for 8 days exhibited a reduced HTR (compared with the initial effect) on each day of testing (Dougherty and Aloyo, 2011). Most recently, tolerance to HTR induced by DOI was observed in DBA/2J mice administered the drug every day, but not every other day or once per week (Rangel-Barajas et al., 2014). Finally, a remarkable stability in DOI-elicited HTR after weekly dosing has also been reported in male Sprague-Dawley rats (Gewirtz and Marek, 2000).
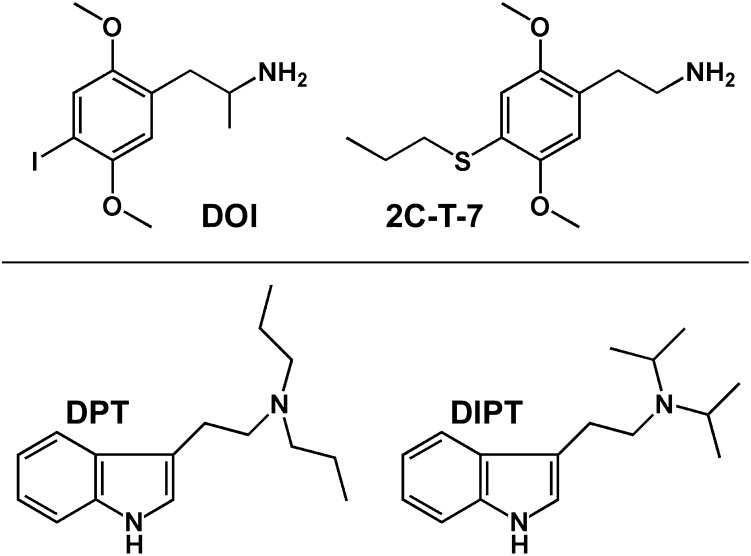
Fig. 1.
Structures of the phenethylamine-derived (top) and tryptamine-derived (bottom) hallucinogens used in these studies.
To better understand the ubiquity of tolerance to HTR elicited by distinct structural classes of hallucinogens, as well as the potential for cross-tolerance among these chemically diverse 5-HT2A agonists, we studied the effects of acute and chronic administration of DOI, the structurally similar phenethylamine 2C-T-7 (2,5-dimethoxy-4-propylthiophenethylamine; Fig. 1, top right), and two tryptamines [DPT (N,N-dipropyltryptamine; Fig. 1, bottom left) and DIPT (N,N-diisopropyltryptamine; Fig. 1, bottom right)] on HTR in mice. Acute, single-exposure dose-effect curves were determined for all compounds, and maximally effective doses were then chronically administered to study the capacity of each compound to induce tolerance to HTR. Mice made tolerant to HTR elicited by DOI were subsequently tested with various doses of DOI, 2C-T-7, or DPT to examine the surmountability of tolerance or cross-tolerance within and across structural classes. The specific hypotheses tested were that tolerance would develop to chronic administration of all compounds, and that reciprocal cross-tolerance would be observed among the phenethylamines and tryptamines. The major findings of these studies are as follows: phenethylamine-derived 5-HT2A agonists induce tolerance to HTR, whereas tryptamine-based compounds do not; and tolerance to behavioral effects induced by DOI is maintained when DPT is substituted, indicating cross-tolerance between these distinct structural classes.
Materials and Methods
Animals.
Male NIH Swiss mice (Harlan Laboratories Inc., Indianapolis, IN) weighing 20–25 g on delivery were housed three animals per cage in temperature-controlled rooms maintained at an ambient temperature of 22 ± 2°C at 45–50% humidity. Lights were set to a 12-hour light/dark cycle (experiments were conducted 3–5 hours into the light phase), and animals were fed ad libitum with standard rodent chow (Laboratory Rodent Diet #5001; LabDiet, St. Louis, MO) and water for at least 3 days after arrival. Mice were randomly assigned to experimental groups (n = 5 or 6 per group), and were free fed for the duration of all experimental manipulations. For all dose-effect curve determinations, each animal was used in only one experimental observation; however, for tolerance studies, the same animals were repeatedly injected, as described below.
Procedure.
On experimental days, mice were weighed, marked, and returned to the home cage. Doses were then calculated and prepared for intraperitoneal injection. For initial dose-effect determinations, individual animals were removed from the home cage, injected with saline (0.01 ml/g), a dose of DOI, 2C-T-7, DPT, or DIPT, and then placed into an observation cage containing fresh bedding. Ten minutes after this injection, an overhead camera was activated and behavior was recorded for 10 minutes. For tolerance studies, maximally effective doses of each compound were selected for chronic administration based on the initial dose-effect determinations presented in Fig. 2. Each animal was removed from the home cage and injected with DOI (1.0 mg/kg), 2C-T-7 (1.0 mg/kg), DPT (3.0 mg/kg), or DIPT (10.0 mg/kg), and then treated as described above. Injections were administered every 24 hours for 5 days (all compounds), every other day for five total injections (DOI only), or every 7 days for four total injections (DOI only). To determine whether tolerance to DOI-elicited HTR was surmountable with dose, mice were treated every 24 hours with either saline or 1.0 mg/kg DOI for 3 days, and then tested on day 4 with various doses of DOI. For cross-tolerance experiments, mice were treated every 24 hours with either saline or 1.0 mg/kg DOI for 3 days, and then tested on day 4 with various doses of 2C-T-7 or DPT. No food or water was available during experimental sessions. In all cases, videotapes were scored for drug-elicited HTR (defined as a rapid rotational jerk of the head that can be distinguished from species-appropriate grooming or scratching behaviors) by at least one observer blind to drug treatment.
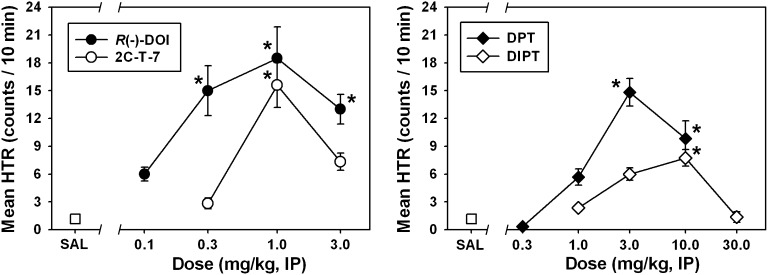
Fig. 2.
Biphasic dose-dependent effects of R(−)-DOI (●), 2C-T-7 (○), DPT (♦), and DIPT (⋄) on head twitch behavior in NIH Swiss mice. Asterisks indicate significant differences from saline (P < 0.05). Abscissae show the dose of drug, expressed as mg/kg on a log scale. Ordinates show the mean head twitches recorded over a 10-minute observation period. IP, intraperitoneal; SAL, saline.
Data Analysis.
Data are presented as the mean ± S.E.M. In all figures, points without error bars indicate instances in which the variance is contained within the data point. For drug-elicited HTR dose-effect determinations (Fig. 2), data were not normally distributed and were therefore analyzed by a Kruskal–Wallis one-way analysis of variance (ANOVA) on ranks, followed by Dunn’s post hoc test to compare all drug doses to saline. Tolerance development data were statistically analyzed using a repeated-measures one-way ANOVA followed by Tukey’s Honestly Significant Difference test for all pairwise comparisons (Figs. 3 and 4). For tolerance surmountability and cross-tolerance studies, data were not normally distributed, and were thus analyzed by a Kruskal–Wallis one-way ANOVA on ranked data, followed by Tukey’s Honestly Significant Difference test for all pairwise comparisons (Figs. 5 and 6). In all cases, significance was judged at the level of P < 0.05.
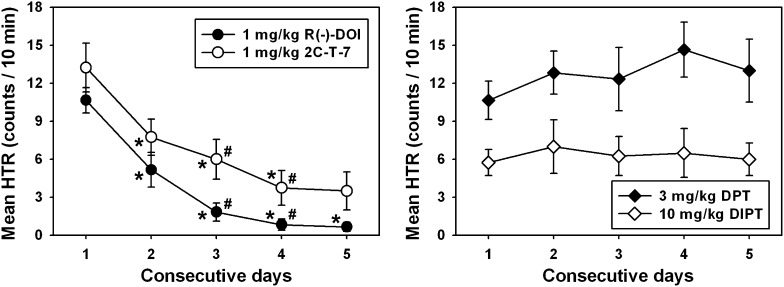
Fig. 3.
Tolerance development to HTR elicited by daily administration of the most effective doses of DOI (●) and 2C-T-7 (○), but not to HTR induced by the most effective doses of DPT (♦) or DIPT (⋄). Asterisks indicate significant differences (P < 0.05) from HTR observed on day 1, whereas hash marks indicate significant differences (P < 0.05) from HTR quantified the previous day. Abscissae show the consecutive day of drug administration. Ordinates are as described in Fig. 2.
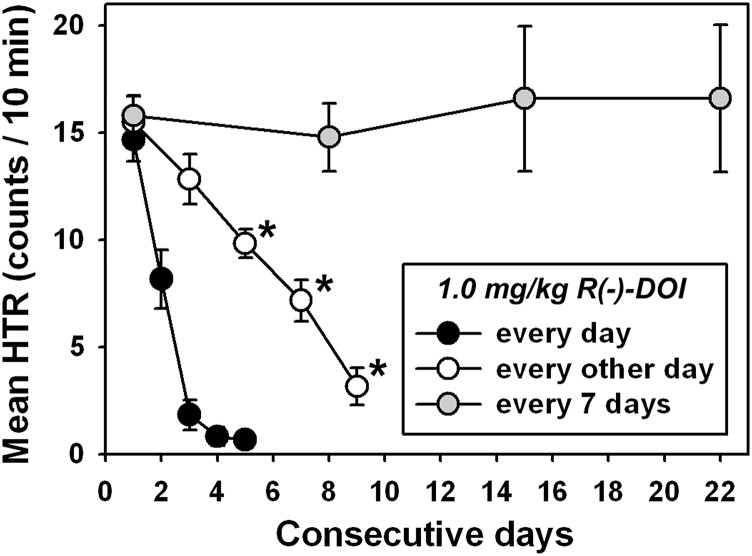
Fig. 4.
Tolerance development to HTR elicited by different frequencies of 1.0 mg/kg DOI administration. For ease of comparison, data from daily administration of 1.0 mg/kg DOI have been replotted from Fig. 3 (●) without indications of statistical significance, whereas open circles represent administration of 1.0 mg/kg DOI every other day, and gray circles represent administration of 1.0 mg/kg DOI every 7 days. All other graph properties are as described in Fig. 3. *P < 0.05.
Fig. 5.
Tolerance to DOI-elicited head twitch behavior is not surmounted by a higher DOI dose. Filled circles represent mice treated with saline on days 1–3 and tested with various DOI doses on day 4, whereas open circles represent mice treated with 1.0 mg/kg DOI on days 1–3, and then tested with various DOI doses on day 4. Asterisks indicate significant differences (P < 0.05) from HTR elicited by saline administration, whereas hash marks indicate significant differences (P < 0.05) from saline-treated for each DOI test dose. The abscissa is as described in Fig. 2. The ordinate is as described in Fig. 2. IP, intraperitoneal; SAL, saline.
Fig. 6.
Cross-tolerance to head twitch behavior is observed in mice treated with 1.0 mg/kg DOI on days 1–3, and then tested with various doses of 2C-T-7 (left) or DPT (right) on day 4. As with DOI itself (Fig. 5), cross-tolerance is insurmountable with increasing 2C-T-7 or DPT dose. All other graph properties are as described in Fig. 5. *P < 0.05; #P < 0.05. IP, intraperitoneal; SAL, saline.
Drugs.
R(−)-DOI was purchased from Sigma Aldrich (St. Louis, MO). 2C-T-7 was obtained from the National Institute on Drug Abuse drug supply program (Research Technology Branch, Research Triangle Park, NC). DPT was synthesized in the Laboratory of Medicinal Chemistry at the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (Bethesda, MD) and was supplied as a generous gift from Dr. Kenner C. Rice. DIPT was synthesized at the Research Technology Branch of the Research Triangle Institute and was supplied as a generous gift by Dr. Bruce Blough. All drugs were dissolved in physiologic saline, which, along with all other experimental supplies, was obtained from standard commercial sources. Sonication in warm water was required to dissolve some of the drug concentrations used in these studies, and all injections were administered at a constant volume of 0.01 ml/g.
Results
Dose-Effect Determinations.
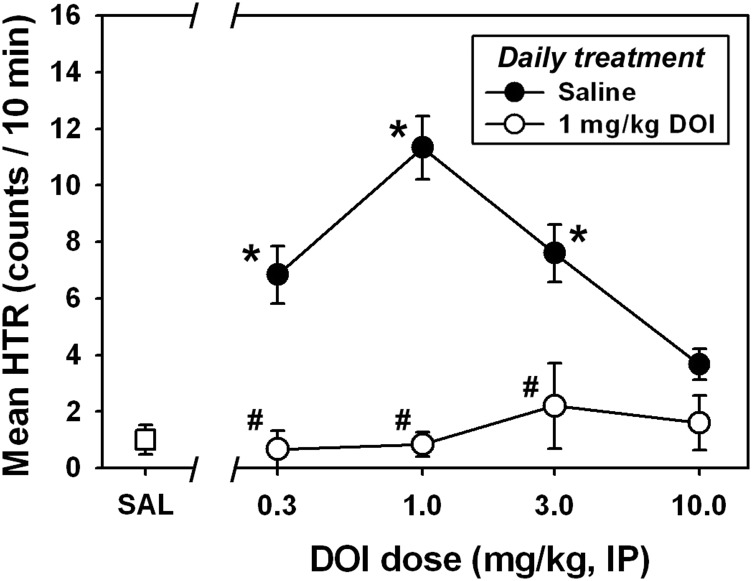
Similar to what we previously observed using this assay, administration of saline elicited very few head twitches (Fig. 2, □), whereas DOI elicited a characteristic biphasic dose-effect function (Fig. 2, ●). DOI-elicited HTR was significantly different from that observed after saline administration at doses of 0.3 mg/kg (Q = 4.033), 1.0 mg/kg (Q = 4.427), and 3.0 mg/kg (Q = 3.864; P < 0.05 in all cases). A maximum of approximately 18 head twitches was quantified in the 10-minute observation period at a dose of 1.0 mg/kg DOI, which is typically the most effective dose using our procedure (Fantegrossi et al., 2010). 2C-T-7 also elicited a dose-dependent and biphasic HTR (Fig. 2, ○), and the 1.0 mg/kg dose was significantly different (Q = 4.230; P < 0.05) from saline. A maximum of approximately 16 head twitches was quantified in the 10-minute observation period at a dose of 1.0 mg/kg 2C-T-7, which was also the most effective dose in our previous HTR studies with this drug (Fantegrossi et al., 2005). Similar to the phenethylamine-derived hallucinogens, DPT induced a dose-dependent and biphasic HTR (Fig. 2, ♦) with effects significantly different from those elicited by saline at doses of 3.0 (Q = 4.244) and 10.0 mg/kg (Q = 3.108; P < 0.05 for both doses). A maximum of approximately 15 head twitches was quantified in the 10-minute observation period at a dose of 3.0 mg/kg DPT, which was also the most effective dose in our previous HTR studies with this drug (Fantegrossi et al., 2008b). Finally, DIPT administration also produced a biphasic dose-effect curve for HTR, but this drug was less effective than all other compounds studied, eliciting significant effects only at the 10.0 mg/kg dose (Q = 3.545; P < 0.05). A maximum of less than eight head twitches was quantified in the 10-minute observation period at a dose of 10.0 mg/kg DIPT.
Tolerance Development.
Progressive tolerance to DOI-elicited HTR developed with daily administration of 1.0 mg/kg (Fig. 3, ●), with HTR quantified on days 2–5 significantly different from that observed on day 1 (Q = 6.786, 11.875, 16.116, and 21.205, respectively; P < 0.05 for all comparisons), day 3 significantly different from day 2 (Q = 5.089; P < 0.05), and day 4 significantly different from day 3 (Q = 4.241; P < 0.05). A similar result was obtained with daily administration of 1.0 mg/kg 2C-T-7 (Fig. 3, ○), with HTR quantified on days 2–5 significantly different from that observed on day 1 (Q = 12.623, 16.831, 21.974, and 22.909, respectively; P < 0.05 for all comparisons), day 3 significantly different from day 2 (Q = 4.308; P < 0.05), and day 4 significantly different from day 3 (Q = 5.143; P < 0.05). In contrast with the phenethylamine-derived hallucinogens, HTR elicited by daily administration of 3.0 mg/kg DPT or 10.0 mg/kg DIPT did not change over the treatment interval (P < 0.05). No significant differences from HTR induced on day 1 were achieved on any other day of testing with either tryptamine-derived compound. Importantly, DPT induced an HTR that was approximately equal in magnitude of that of DOI and 2C-T-7 on day 1.
Because different regimens of DOI administration have previously been shown to modulate tolerance development (see Introduction), the most effective DOI dose was also administered every other day, or every 7 days, to further characterize the effects of injection frequency on tolerance to DOI-induced HTR. Interestingly, progressive tolerance to DOI-elicited HTR also developed when 1.0 mg/kg was administered every other day (Fig. 3, ○), with HTR quantified after the third, fourth, and fifth injections (i.e., days 5, 7, and 9) significantly different from that observed after the first injection (Q = 5.787, 9.042, and 13.201, respectively; P < 0.05 for all comparisons), but no two consecutive observations were significantly different from one another (P > 0.05 for all comparisons). By contrast, no tolerance to DOI-elicited HTR developed when 1.0 mg/kg was administered every 7 days (Fig. 3, gray circles) (P > 0.05 for all comparisons).
Tolerance to DOI-Elicited HTR Is Insurmountable with DOI Test Dose.
As demonstrated in Fig. 3, daily administration of 1.0 mg/kg DOI for 3 consecutive days produced almost complete tolerance to DOI-elicited HTR on day 4, and this tolerance was maintained on day 5. Therefore, daily administration of 1.0 mg/kg DOI (or saline) for 3 consecutive days was used to induce tolerance to DOI-elicited HTR (or to control for repeated handling and injection), and then 0.3, 1.0, 3.0, or 10.0 mg/kg DOI was administered on day 4. A typical biphasic dose-effect function was generated in mice receiving daily saline injections for 3 days, and then tested with various doses of DOI on day 4 (Fig. 5, ●). In these animals, DOI-elicited HTR was significantly different from that observed after saline administration at doses of 0.3 mg/kg (Q = 3.996), 1.0 mg/kg (Q = 5.307), and 3.0 mg/kg (Q = 4.101; P < 0.05 in all cases), and the 1.0 mg/kg dose was the most effective dose for eliciting HTR, as usual. By contrast, no HTR significantly different from that observed after saline administration was observed in mice receiving daily 1.0 mg/kg DOI injections for 3 days (Fig. 5, ○) at any tested dose of DOI on day 4 (P > 0.05 for all comparisons). The tolerance to HTR induced by daily 1.0 mg/kg DOI injection for 3 consecutive days was apparent at all active DOI test doses, because HTR observed after administration of 0.3 mg/kg (Q = 4.437), 1.0 mg/kg (Q = 5.424), and 3.0 mg/kg DOI (Q = 4.411) was significantly different between daily saline-treated and daily DOI-treated animals (P < 0.05 for all comparisons).
Cross-Tolerance to DOI-Elicited HTR Is Insurmountable with Dose.
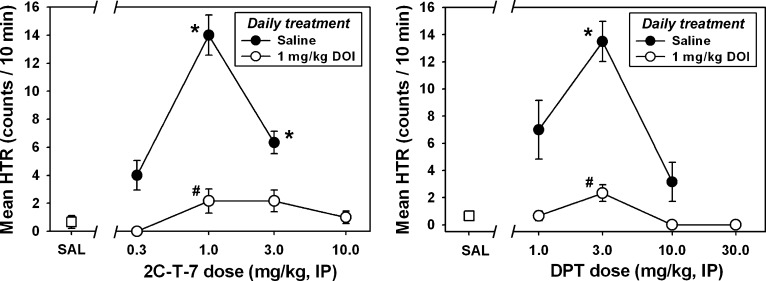
Daily administration of 1.0 mg/kg DOI (or saline) for 3 consecutive days was used to induce tolerance to DOI-elicited HTR (or to control for repeated handling and injection), and then 0.3, 1.0, 3.0, or 10.0 mg/kg 2C-T-7 was administered on day 4 to assess cross-tolerance within the phenethylamine-derived structural class of hallucinogens. A typical biphasic dose-effect function was generated in mice receiving daily saline injections for 3 days, and then tested with various doses of 2C-T-7 on day 4 (Fig. 6, left, ●). In these animals, 2C-T-7–elicited HTR was statistically different from that observed after saline administration at doses of 1.0 (Q = 6.561) and 3.0 mg/kg (Q = 5.059; P < 0.05 in both cases), and the 1.0 mg/kg 2C-T-7 dose was again the most effective dose for eliciting HTR. By contrast, no HTR significantly different from that observed after saline administration was observed in mice receiving daily 1.0 mg/kg DOI injections for 3 days (Fig. 6, left, ○) at any tested dose of 2C-T-7 on day 4 (P > 0.05 for all comparisons). HTR observed after administration of 1.0 mg/kg 2C-T-7 was significantly different between daily saline-treated and daily DOI-treated animals (Q = 5.628; P < 0.05), but no other between-group comparisons reached statistical significance.
To assess cross-tolerance across the phenethylamine-derived and the tryptamine-derived structural classes of hallucinogens, 1.0 mg/kg DOI (or saline) was administered every day for 3 consecutive days to induce tolerance to DOI-elicited HTR (or to control for repeated handling and injection), and then 1.0, 3.0, 10.0, or 30.0 mg/kg DPT was administered on day 4. In mice receiving daily saline injections for 3 days, and then tested with various doses of DPT on day 4 (Fig. 6, right, ●), a characteristic biphasic dose-effect function was produced, with DPT-elicited HTR significantly different from that observed after saline administration at the 3.0 mg/kg dose (Q = 5.584; P < 0.05). By contrast, no HTR significantly different from that observed after saline administration was observed in mice receiving daily 1.0 mg/kg DOI injections for 3 days (Fig. 6, right, ○) at any tested dose of DPT on day 4 (P > 0.05 for all comparisons). HTR observed after administration of 3.0 mg/kg DPT was significantly different between daily saline-treated and daily DOI-treated animals (Q = 4.491; P < 0.05), but no other between-group comparisons reached statistical significance.
Discussion
We initially hypothesized that all four compounds selected for study would produce tolerance to HTR over repeated administrations, and that reciprocal cross-tolerance would be observed between the phenethylamine- and tryptamine-derived drugs. Neither of these hypotheses was confirmed by the present studies. We observed dramatic tolerance to HTR elicited by daily administration of DOI or 2C-T-7, but no evidence of tolerance when either DPT or DIPT was administered according to the same schedule. Only a single dose of each compound was studied under conditions of chronic administration, so it is possible that other doses might induce greater or lesser tolerance to HTR than observed here. However, each dose studied under the present conditions was the maximally effective dose for each compound; in the case of DOI, 2C-T-7, and DPT, these doses induced roughly equivalent HTR. Thus, during chronic administration of these three compounds, HTR among all groups was essentially equal on the first day, making it very unlikely that a nonspecific “floor effect” limited tolerance development (although this possibility probably cannot be ruled out for DIPT).
Dose is always an important variable to manipulate in pharmacology, but another important contributor to the different profiles exhibited by the four compounds presented in these studies may be duration of action. Unfortunately, no systematic observations of time course of HTR induced by these substances are available, but effects of phenethylamine-derived hallucinogens are anecdotally longer lasting than those of tryptamine-derived hallucinogens. For example, Shulgin and Shulgin (1991) estimated the duration of action of DOI to be between 16 and 30 hours, and 2C-T-7 effects to last between 8 and 15 hours in humans. By contrast, using these same methods, the duration of DPT effects was estimated to be between 2 and 4 hours, and the effects of DIPT were estimated to last between 6 and 8 hours in humans (Shulgin and Shulgin, 1997). It may therefore be the case that the shorter-acting tryptamines could induce tolerance to HTR if administered more frequently than once per day, because drugs with shorter durations of action may require more frequent administration than drugs with longer durations of action to elicit tolerance to their effects. However, it should be noted that Strassman et al. (1996) did not observe tolerance to the subjective effects of short-acting DMT in humans even when administered intravenously every 30 minutes for four total injections.
In addition to differences in duration of action, the four drugs used in these studies may also differ in terms of their relative efficacies to activate 5-HT2A receptors. Among other drug classes (i.e., opioids), repeated treatment with equally effective doses of drugs that differ in intrinsic efficacy typically produce different degrees of tolerance (Duttaroy and Yoburn, 1995; Allen and Dykstra, 2000), with low-efficacy opioids eliciting greater tolerance than high-efficacy opioids (Paronis and Holtzman, 1992, 1994). Unfortunately, efficacy estimates for 5-HT2A agonists are rare, and the techniques used to determine relative efficacy at this receptor vary widely. To our knowledge, none of the four drugs used in the present studies have ever been directly compared in the same assay. However, a study using a fluorometric imaging plate reader technique in Chinese hamster ovary-K1 cells reported that racemic DOI had 61% the efficacy of 5-HT in this assay (Porter et al., 1999). A more recent study using an assay of nonspecific G protein activation in rat brain membranes reported that DPT was 46% as efficacious as 5-HT using these methods, which was identical to the relative efficacy obtained with the DOI analog 2,5-dimethoxy-4-iodophenethylamine (Nonaka et al., 2007). Thus, based on the available evidence, it is difficult to interpret the present results through the lens of intrinsic efficacy. However, it would appear that DPT is unlikely to exhibit a substantially greater 5-HT2A efficacy than DOI, which would suggest (based on what has been learned through the study of opioid tolerance) that its capacity to elicit tolerance to 5-HT2A–mediated effects, such as HTR, should be greater than or equal to that of DOI, which is the opposite of what we observed in the present studies.
Regarding cross-tolerance, intrinsic efficacy is also a critical determinant among the opioids (Paronis and Holtzman, 1992), but has not been adequately explored with serotonergics. Nevertheless, the finding that a drug that produces no apparent tolerance to a behavioral effect (such as DPT in the present studies) still exhibits a reduced effectiveness in animals made tolerant to DOI-elicited HTR seems to be consistent with mechanistic explanations for tolerance to hallucinogen-induced effects involving downregulation and desensitization of 5-HT2A receptors. A previous study reported that even a single injection of DOI is sufficient to significantly reduce Bmax for receptors labeled by 1-(2,5-dimethoxy-4-bromophenyl)-2-aminopropane or ketanserin in rat cortex (Pranzatelli, 1991), and chronic DOI administration has repeatedly been demonstrated to downregulate 5-HT2A receptor expression in vivo (McKenna et al., 1989; Owens et al., 1991; Smith et al., 1999). However, in vitro, DOI may produce 5-HT2A upregulation (Akiyoshi et al., 1993), and it appears that the regulatory effects of chronic agonist exposure on 5-HT2A receptors may critically depend on the specific cell line studied (Grotewiel and Sanders-Bush, 1994). Indeed, Roth et al. (1995) reported that receptor downregulation and desensitization are dissociable phenomena in vitro. Despite the obvious complexity of 5-HT2A receptor regulation, it seems likely that the tolerance to HTR induced by DOI (and perhaps by 2C-T-7 as well) is due to progressively reduced receptor expression over the treatment period. In this dampened neuropharmacologic context, administration of any 5-HT2A agonist would be expected to produce a reduced behavioral effect; thus, the presently reported cross-tolerance experiments with DOI and DPT would not be inconsistent with what is known about 5-HT2A receptor regulation. Interestingly, the capacity of DPT and DIPT to downregulate or desensitize 5-HT2A receptors has not previously been determined, but would provide a mechanistic explanation for the lack of tolerance observed in the present studies if, like the structurally related DMT (Smith et al., 1998), these tryptamines also prove incapable of inducing 5-HT2A downregulation.
These studies also provide a systematic characterization of tolerance to DOI-elicited head twitch in another mouse strain, the NIH Swiss mouse. It seems apparent that large strain differences are likely to modulate tolerance development to DOI-elicited HTR, and this may be a fruitful area for further research. As noted in Introduction, ICR mice exhibit tolerance to DOI-elicited HTR when the drug is administered daily, but supersensitivity when the drug is administered every 48 hours (Darmani et al., 1990, 1992; Darmani and Gerdes, 1995). In the present studies, we used both of these dose regimens, and saw no signs of supersensitivity in the NIH Swiss mice, similarly to what has been reported in C57BL/6N mice receiving daily DOI (Dougherty and Aloyo, 2011). In DBA/2J mice, tolerance to HTR induced by DOI was observed when the drug was administered every day, but not every other day or once per week (Rangel-Barajas et al., 2014). This too contrasts with the present profile in NIH Swiss mice, in which tolerance was observed with DOI administration occurring every 24 or 48 hours, although the lack of tolerance development with weekly DOI administration is consistent across strains. To our knowledge, there have been no systematic studies of tolerance to hallucinogen-induced HTR across multiple mouse strains, although we previously reported similar dose-effect curves for DOI-elicited HTR between NIH Swiss mice (from two different vendors) and Swiss Webster mice (Fantegrossi et al., 2010). Indeed, the role of strain differences in serotonin pharmacology has not been adequately characterized. In two early studies, BALB/c mice were found to have more 5-HT per gram of dissected brain than C57bl/10 mice (Maas, 1962), whereas no differences in brain 5-HT were found when C57B1/6J and DBA/2J mice were compared (Ho et al., 1975). Of particular relevance to the present studies, ddY, ICR, DBA, C3H, and C57BL/6 mice were directly compared for HTR induced by tryptamine, and large strain differences were found such that all DBA mice exhibited marked tryptamine-elicited HTR, whereas only 1 of 20 ddY mice exhibited HTR, and there was no correlation between HTR and tryptamine levels in the brain (Yamada et al., 1987). Most recently, C57BL/6 and DBA/2 mice were both successfully trained to discriminate clozapine from saline, but the nonselective 5-HT2 receptor antagonist ritanserin elicited clozapine-like stimulus effects only in C57BL/6 mice (Porter et al., 2008), perhaps implying differences in 5-HT2 receptor expression and function in these two strains. Further research in this area is clearly warranted to help synthesize disparate results across laboratories utilizing distinct mouse strains.
In summary, these data suggest that the use of phenethylamine-derived hallucinogens as potential therapeutics may be limited not only by their abuse potential, but also by the rapid development of tolerance that would likely be maintained even if a patient were switched to a different 5-HT2A agonist medication from a distinct structural class. On the other hand, these experiments imply that tryptamine-derived hallucinogens might have a reduced potential for tolerance development, compared with phenethylamine-derived 5-HT2A agonists. As research into the therapeutic utility of hallucinogenic drugs proceeds (Nichols, 2014), further characterization of the acute and chronic pharmacologic effects of these substances from a basic sciences perspective will likely be required to guide drug development and chronic treatment protocols.
Acknowledgments
The authors thank the University of Arkansas for Medical Sciences Division of Laboratory Animal Medicine for expert husbandry services. The authors also thank the late Dr. Alexander Shulgin for a lifetime of inspirational research with the compounds used in this report, as well as his synthesis and characterization of hundreds of other equally fascinating molecules. He was a true pioneer, lending his unique voice to science and society, and his thoughts on chemistry, psychopharmacology, and neurotheology will be missed. The views expressed herein are those of the authors and do not necessarily represent the views of the University of Arkansas for Medical Sciences.
Abbreviations
- 2C-T-7
2,5-dimethoxy-4-propylthiophenethylamine
- 5-HT2A
5-hydroxytryptamine 2A
- ANOVA
analysis of variance
- DIPT
N,N-diisopropyltryptamine
- DMT
N,N-dimethyltryptamine
- DOI
2,5-dimethoxy-4-iodoamphetamine
- DPT
N,N-dipropyltryptamine
- HTR
head twitch response
Authorship Contributions
Participated in research design: Smith, Fantegrossi.
Conducted experiments: Smith, Bailey, Williams.
Performed data analysis: Smith, Bailey, Williams, Fantegrossi.
Wrote or contributed to the writing of the manuscript: Smith, Bailey, Williams, Fantegrossi.
Footnotes
This research was supported in part by the National Institutes of Health National Institute of General Medical Sciences [Institutional Development Award GM110702]; the University of Arkansas for Medical Sciences Translational Research Institute [Grant UL1-TR000039]; and the American Society for Pharmacology and Experimental Therapeutics [Summer Undergraduate Research Fellowship].
References
- Akiyoshi J, Hough C, Chuang DM. (1993) Paradoxical increase of 5-hydroxytryptamine2 receptors and 5-hydroxytryptamine2 receptor mRNA in cerebellar granule cells after persistent 5-hydroxytryptamine2 receptor stimulation. Mol Pharmacol 43:349–355. [PubMed] [Google Scholar]
- Allen RM, Dykstra LA. (2000) Attenuation of mu-opioid tolerance and cross-tolerance by the competitive N-methyl-D-aspartate receptor antagonist LY235959 is related to tolerance and cross-tolerance magnitude. J Pharmacol Exp Ther 295:1012–1021. [PubMed] [Google Scholar]
- Angrist B, Rotrosen J, Gershon S. (1974) Assessment of tolerance to the hallucinogenic effects of DOM. Psychopharmacology (Berl) 36:203–207. [DOI] [PubMed] [Google Scholar]
- Berger M, Gray JA, Roth BL. (2009) The expanded biology of serotonin. Annu Rev Med 60:355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. (2008) Prospects for serotonin 5-HT2R pharmacotherapy in psychostimulant abuse. Prog Brain Res 172:319–346. [DOI] [PubMed] [Google Scholar]
- Canal CE, Morgan D. (2012) Head-twitch response in rodents induced by the hallucinogen 2,5-dimethoxy-4-iodoamphetamine: a comprehensive history, a re-evaluation of mechanisms, and its utility as a model. Drug Test Anal 4:556–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmani NA, Gerdes CF. (1995) Temporal differential adaptation of head-twitch and ear-scratch responses following administration of challenge doses of DOI. Pharmacol Biochem Behav 50:545–550. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Martin BR, Glennon RA. (1990) Withdrawal from chronic treatment with (+/−)-DOI causes super-sensitivity to 5-HT2 receptor-induced head-twitch behaviour in mice. Eur J Pharmacol 186:115–118. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Martin BR, Glennon RA. (1992) Behavioral evidence for differential adaptation of the serotonergic system after acute and chronic treatment with (+/−)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) or ketanserin. J Pharmacol Exp Ther 262:692–698. [PubMed] [Google Scholar]
- Dougherty JP, Aloyo VJ. (2011) Pharmacological and behavioral characterization of the 5-HT2A receptor in C57BL/6N mice. Psychopharmacology (Berl) 215:581–593. [DOI] [PubMed] [Google Scholar]
- Duttaroy A, Yoburn BC. (1995) The effect of intrinsic efficacy on opioid tolerance. Anesthesiology 82:1226–1236. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Harrington AW, Eckler JR, Arshad S, Rabin RA, Winter JC, Coop A, Rice KC, Woods JH. (2005) Hallucinogen-like actions of 2,5-dimethoxy-4-(n)-propylthiophenethylamine (2C-T-7) in mice and rats. Psychopharmacology (Berl) 181:496–503. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Murnane KS, Reissig CJ. (2008a) The behavioral pharmacology of hallucinogens. Biochem Pharmacol 75:17–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Reissig CJ, Katz EB, Yarosh HL, Rice KC, Winter JC. (2008b) Hallucinogen-like effects of N,N-dipropyltryptamine (DPT): possible mediation by serotonin 5-HT1A and 5-HT2A receptors in rodents. Pharmacol Biochem Behav 88:358–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Simoneau J, Cohen MS, Zimmerman SM, Henson CM, Rice KC, Woods JH. (2010) Interaction of 5-HT2A and 5-HT2C receptors in R(-)-2,5-dimethoxy-4-iodoamphetamine-elicited head twitch behavior in mice. J Pharmacol Exp Ther 335:728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountoulakis KN, Kelsoe JR, Akiskal H. (2012) Receptor targets for antidepressant therapy in bipolar disorder: an overview. J Affect Disord 138:222–238. [DOI] [PubMed] [Google Scholar]
- Gewirtz JC, Marek GJ. (2000) Behavioral evidence for interactions between a hallucinogenic drug and group II metabotropic glutamate receptors. Neuropsychopharmacology 23:569–576. [DOI] [PubMed] [Google Scholar]
- Gillin JC, Kaplan J, Stillman R, Wyatt RJ. (1976) The psychedelic model of schizophrenia: the case of N,N-dimethyltryptamine. Am J Psychiatry 133:203–208. [DOI] [PubMed] [Google Scholar]
- González-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, et al. (2007) Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron 53:439–452. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Zangrossi H., Jr (2010) The dual role of serotonin in defense and the mode of action of antidepressants on generalized anxiety and panic disorders. Cent Nerv Syst Agents Med Chem 10:207–217. [DOI] [PubMed] [Google Scholar]
- Griffths RR, Grob CS. (2010) Hallucinogens as medicine. Sci Am 303:76–79. [PubMed] [Google Scholar]
- Grotewiel MS, Sanders-Bush E. (1994) Regulation of serotonin2A receptors in heterologous expression systems. J Neurochem 63:1255–1260. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL. (2014) Recent advances in the neuropsychopharmacology of serotonergic hallucinogens. Behav Brain Res DOI: [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho AK, Tsai CS, Kissin B. (1975) Neurochemical correlates of alcohol preference in inbred strains of mice. Pharmacol Biochem Behav 3:1073–1076. [DOI] [PubMed] [Google Scholar]
- Hollister LE, Macnicol MF, Gillespie HK. (1969) An hallucinogenic amphetamine analog (DOM) in man. Psychopharmacology (Berl) 14:62–73. [DOI] [PubMed] [Google Scholar]
- Isbell H, Miner EJ, Logan CR. (1959) Cross tolerance between D-2-brom-lysergic acid diethylamide (BOL-148) and the D-diethylamide of lysergic acid (LSD-25). Psychopharmacology (Berl) 1:109–116. [DOI] [PubMed] [Google Scholar]
- Isbell H, Wolbach AB, Wikler A, Miner EJ. (1961) Cross tolerance between LSD and psilocybin. Psychopharmacology (Berl) 2:147–159. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Frank GK, Bailer UF, Henry SE, Meltzer CC, Price JC, Mathis CA, Wagner A. (2005) Serotonin alterations in anorexia and bulimia nervosa: new insights from imaging studies. Physiol Behav 85:73–81. [DOI] [PubMed] [Google Scholar]
- Maas JW. (1962) Neurochemical differences between two strains of mice. Science 137:621–622. [DOI] [PubMed] [Google Scholar]
- McKenna DJ, Nazarali AJ, Himeno A, Saavedra JM. (1989) Chronic treatment with (+/-)DOI, a psychotomimetic 5-HT2 agonist, downregulates 5-HT2 receptors in rat brain. Neuropsychopharmacology 2:81–87. [DOI] [PubMed] [Google Scholar]
- Nichols DE. (2004) Hallucinogens. Pharmacol Ther 101:131–181. [DOI] [PubMed] [Google Scholar]
- Nichols DE. (2014) The Heffter Research Institute: past and hopeful future. J Psychoactive Drugs 46:20–26. [DOI] [PubMed] [Google Scholar]
- Nonaka R, Nagai F, Ogata A, Satoh K. (2007) In vitro screening of psychoactive drugs by [(35)S]GTPgammaS binding in rat brain membranes. Biol Pharm Bull 30:2328–2333. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Knight DL, Ritchie JC, Nemeroff CB. (1991) The 5-hydroxytryptamine2 agonist, (+-)-1-(2,5-dimethoxy-4-bromophenyl)-2-aminopropane stimulates the hypothalamic-pituitary-adrenal (HPA) axis. II. Biochemical and physiological evidence for the development of tolerance after chronic administration. J Pharmacol Exp Ther 256:795–800. [PubMed] [Google Scholar]
- Paronis CA, Holtzman SG. (1992) Development of tolerance to the analgesic activity of mu agonists after continuous infusion of morphine, meperidine or fentanyl in rats. J Pharmacol Exp Ther 262:1–9. [PubMed] [Google Scholar]
- Paronis CA, Holtzman SG. (1994) Sensitization and tolerance to the discriminative stimulus effects of mu-opioid agonists. Psychopharmacology (Berl) 114:601–610. [DOI] [PubMed] [Google Scholar]
- Porter JH, Walentiny DM, Philibin SD, Vunck SA, Crabbe JC. (2008) A comparison of the discriminative stimulus properties of the atypical antipsychotic drug clozapine in DBA/2 and C57BL/6 inbred mice. Behav Pharmacol 19:530–542. [DOI] [PubMed] [Google Scholar]
- Porter RH, Benwell KR, Lamb H, Malcolm CS, Allen NH, Revell DF, Adams DR, Sheardown MJ. (1999) Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells. Br J Pharmacol 128:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranzatelli MR. (1991) Regulation of 5-HT2 receptors in rat cortex. Studies with a putative selective agonist and an antagonist. Biochem Pharmacol 42:1099–1105. [DOI] [PubMed] [Google Scholar]
- Rangel-Barajas C, Malik M, Vangveravong S, Mach RH, Luedtke RR. (2014) Pharmacological modulation of abnormal involuntary DOI-induced head twitch response in male DBA/2J mice: I. Effects of D2/D3 and D2 dopamine receptor selective compounds. Neuropharmacology 83:18–27. [DOI] [PubMed] [Google Scholar]
- Roth BL, Palvimaki EP, Berry S, Khan N, Sachs N, Uluer A, Choudhary MS. (1995) 5-Hydroxytryptamine2A (5-HT2A) receptor desensitization can occur without down-regulation. J Pharmacol Exp Ther 275:1638–1646. [PubMed] [Google Scholar]
- Selvaraj S, Arnone D, Cappai A, Howes O. (2014) Alterations in the serotonin system in schizophrenia: a systematic review and meta-analysis of postmortem and molecular imaging studies. Neurosci Biobehav Rev 45:233–245. [DOI] [PubMed] [Google Scholar]
- Shulgin A, Shulgin A. (1991) PiHKAL–A Chemical Love Story, Transform Press, Berkeley, CA. [Google Scholar]
- Shulgin A, Shulgin A. (1997) TiHKAL–The Continuation, Transform Press, Berkeley, CA. [Google Scholar]
- Smith RL, Barrett RJ, Sanders-Bush E. (1999) Mechanism of tolerance development to 2,5-dimethoxy-4-iodoamphetamine in rats: down-regulation of the 5-HT2A, but not 5-HT2C, receptor. Psychopharmacology (Berl) 144:248–254. [DOI] [PubMed] [Google Scholar]
- Smith RL, Canton H, Barrett RJ, Sanders-Bush E. (1998) Agonist properties of N,N-dimethyltryptamine at serotonin 5-HT2A and 5-HT2C receptors. Pharmacol Biochem Behav 61:323–330. [DOI] [PubMed] [Google Scholar]
- Strassman RJ, Qualls CR, Berg LM. (1996) Differential tolerance to biological and subjective effects of four closely spaced doses of N,N-dimethyltryptamine in humans. Biol Psychiatry 39:784–795. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Kometer M. (2010) The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat Rev Neurosci 11:642–651. [DOI] [PubMed] [Google Scholar]
- Wolbach AB, Jr, Isbell H, Miner EJ. (1962) Cross tolerance between mescaline and LSD-25, with a comparison of the mescaline and LSD reactions. Psychopharmacology (Berl) 3:1–14. [DOI] [PubMed] [Google Scholar]
- Yamada J, Sugimoto Y, Horisaka K. (1987) Pharmacological analysis of the variation in behavioural responses to tryptamine in five strains of mice. Eur J Pharmacol 140:323–330. [DOI] [PubMed] [Google Scholar]