Abstract
Flavonoids, such as the tea catechin epigallocatechin-gallate (EGCG), can protect against atherosclerosis by decreasing vascular endothelial cell inflammation. Heme oxygenase (HO-1) is an enzyme that plays an important role in vascular physiology, and its induction may provide protection against atherosclerosis. HO-1 can be compartmentalized in caveolae in endothelial cells. Caveolae are plasma microdomains important in vesicular transport and the regulation of signaling pathways associated with the pathology of vascular diseases. We hypothesize that caveolae play a role in the uptake and transport of EGCG and mechanisms associated with the anti-inflammatory properties of this flavonoid. To test this hypothesis, we explored the effect of EGCG on the induction of Nrf2 and HO-1 in endothelial cells with or without functional caveolae. Treatment with EGCG activated Nrf2 and increased HO-1 expression and cellular production of bilirubin. In addition, EGCG rapidly accumulated in caveolae, which was associated with caveolin-1 displacement from the plasma membrane towards the cytosol. Similar to EGCG treatment, silencing of caveolin-1 by siRNA technique also resulted in upregulation of Nrf2, HO-1 and bilirubin production. These data suggest that EGCG-induced caveolin-1 displacement may reduce endothelial inflammation.
Keywords: EGCG, Nrf2, Caveolae, Endothelial Cells, Atherosclerosis
Introduction
Diets high in polyphenols (e.g., flavonoids) are associated with a reduced risk of chronic diseases, such as cardiovascular diseases, by affecting molecular mechanisms involved in the initiation and progression of these diseases [1]. Flavonoids constitute a subclass of bioactive compounds rich in fruits and vegetables, soy food, legumes, tea and cocoa [2]. Green tea consumption has been shown to be significantly greater in healthy subjects compared to those with coronary artery disease, suggesting that green tea might be protective against coronary atherosclerosis [3]. Catechins are the major constituents of the polyphenols in green tea, and the most abundant catechin in green tea is epigallocatechin-3-O-gallate (EGCG). Even though the consumption of flavonoids such as EGCG are known to improve endothelial cell function, and thus reduce cardiovascular risk [4], protective mechanisms are not clear but may be linked to caveolae signaling.
There is increasing evidence that caveolae play a critical role in the pathology of vascular diseases and that the lack of the caveolin-1 gene may provide protection against the development of atherosclerosis [5]. Caveolae are plasma-membrane domains that are highly enriched in cholesterol and sphingolipids. One of the functions attributed to endothelial caveolae is their ability to transfer molecules from the lumen of blood vessels to the sub-endothelial space [6]. Like clathrin-mediated endocytosis, internalization through caveolae involves complex signaling [7]. Caveolin-1 is a major scaffolding protein constituent of caveolae that participates in vesicular trafficking and signal transduction, and caveolin-1 can cycle between the plasma membrane and several intracellular compartments. Caveolae are particularly abundant in endothelial cells, where they are believed to play a major role in the regulation of endothelial vesicular trafficking as well as the uptake of lipids and related lipophilic compounds possibly including bioactive food components such as polyphenols by means of endocytosis [8, 9]. For example, it has been reported that caveolin-1 modulates cellular sensitivity to resveratrol through the enhancement of its internalization and trafficking [10].
Protective mechanisms of flavonoids may include upregulation of heme oxygenase-1 (HO-1) [11], an enzyme localized to plasma membrane caveolae [12]. HO-1 is an inducible enzyme which catalyzes the oxidative degradation of heme by degrading heme to iron, carbon monoxide and biliverdin, with the latter being quickly reduced to bilirubin. The heme oxygenase system is an important regulator of endothelial cell integrity and oxidative stress, and dysfunctional HO-1 signaling may be a pro-atherogenic event. In fact, the end products of HO-1 metabolism, such as bilirubin, can exert potent antioxidant and anti-inflammatory actions within the vascular system, including endothelial cells [13] HO-1 also can be upregulated by EGCG [11], and we have recently demonstrated that EGCG-mediated protection against TNF-α induced MCP-1 expression is HO-1 dependent [14].
Recent genome-wide analysis demonstrated that the transcription factor NF-E2-related factor (Nrf2) can regulate numerous genes that are involved in the cytoprotective response against oxidative stress [15]. For example, the induction of the antioxidant gene HO-1 in vascular cells is associated with nuclear translocation of Nrf2 and subsequent transactivation of an antioxidant response element in the promoter region of HO-1 [16]. There is evidence that flavonoids like EGCG can induce HO-1 via activation of Nrf2 [11, 17]. Since HO-1 is an enzyme localized to plasma membrane caveolae [12], we were interested in the possible involvement of functional caveolae in EGCG-mediated stimulation of Nrf2 and HO-1. We recently reported a role of caveolin-1 in EGCG-mediated protection against linoleic acid-induced endothelial cell activation [9], suggesting that caveolae may provide a regulatory platform of our observed effects of EGCG on stimulation of Nrf2 and HO-1. In the current study we provide evidence that EGCG stimulates Nrf2 and HO-1 via alteration in caveolae function associated with caveolin-1 displacement.
Materials and Methods
Cell culture and experimental media
Primary endothelial cells were isolated from porcine aortic arteries and cultured as previously described [18]. The basic culture medium consisted of medium 199 (M-199) (Cat. No. 31100-035; GIBCO Laboratories, NY) containing 10% (v/v) fetal bovine serum (FBS, HyClone Laboratories, UT). Experimental media contained 5% FBS and were supplemented with EGCG (Cayman Chemicals, Ann Arbor, MI; purity >98%). EGCG was dissolved in DMSO; at a final concentration less than 0.1%, DMSO did not affect cell viability.
Detergent-free purification of caveolae-rich membrane domains
Caveolae-rich membrane domains were isolated as previously reported [19], with minor modifications. Briefly, cells were plated in 150 mm plates. After treatment, cells were washed with PBS and lysed with 2 ml of ice-cold MES-buffered saline [MBS; 25mM MES (morpholineethanesulfonic acid, pH 6.5), 150 mM NaCl] containing 500 mM Na2CO3. Following homogenization and sonication, homogenates were adjusted to 45% sucrose by addition of 2 ml of 90% sucrose prepared in MBS and placed at the bottom of an ultracentrifuge tube, and overlaid with a discontinuous sucrose gradient. Subsequently, 5 ml of 35% sucrose and then 3 ml of 5% sucrose were added. The gradient samples were then centrifuged at 39,000 rpm (260,000g) for 16 h using a SW41 rotor (Beckman Instruments, Palo Alto, CA). Twelve 1 ml fractions were collected, and aliquots of each fraction were subjected to SDS-PAGE and immunoblotting to assess caveolin-1.
Immunoblot analysis of HO-1, caveolin-1 and Nrf2 protein expression
Cells were treated with either vehicle (0.1% DMSO) or EGCG (30 μM) followed by immunoblot analysis of HO-1 protein expression. To detect the effect of EGCG and caveolin-1 gene silencing on Nrf2 accumulation in nucleus, both nuclear and cytosol protein extracts were prepared from endothelial cells. Western blots were visualized using the appropriate horseradish peroxidase-conjugated secondary antibodies followed by ECL immunoblotting detection reagents (Amersham Biosciences, Buckinghamshire, England).
Caveolin-1 siRNA and transfection
The caveolin-1 gene silencer was designed by Dharmacon (Lafayette Colorado) according to previously described methods [20]. The sequence of the control gene silencer was 5′-AAAGAGCGACTTTACACACdTdT-3′; caveolin-1 gene silencers were 5′-CCAGAAGGGACACACAGUUdTdT-3′ and 5′-CAUCUACAAGCCCAACAACdT dT-3′. Cells were transfected with control siRNA or a mixture of two siRNAs targeted against caveolin-1 (40 nM each) using GeneSilencer (Genlantis, San Diego, CA) with Optimem I medium (Invitrogen, Carlsbad, CA). Cells were incubated with transfection mixtures for 4 h and then replaced with 10% serum medium. Cells were synchronized overnight after 48 h transfection, and then treated with EGCG.
Electrophoretic mobility shift assays of Nrf2-ARE-DNA binding
Nuclear extracts were prepared from endothelial cells. Synthetic 5′-biotinylated complementary oligonucleotides were purchased from Integrated DNA Technologies (Coralville, IA). Nuclear extracts were incubated at room temperature for 20 min with biotin-labeled oligonucleotide probes containing ARE, the enhancer DNA element for HO-1(5′ bio-AGATTTTGCTGAGTCACCAGTCCC-3′). Gel mobility shift assay was performed to demonstrate the shifted DNA-protein complexes Nrf2-ARE using a LightShiftTM chemiluminescent EMSA kit (Pierce, Rockford, IL). Reactions using 200-fold molar excess of unlabeled oligonucletide probes were performed to demonstrate the specificity of the shifted DNA-protein complexes for Nrf2. To further examine the presence of Nrf2 protein in the retarded bands, EMSA was performed and followed by Western blotting (shift-Western) using an antibody against Nrf2.
Quantification of EGCG by LC/MS/MS
After treatment with EGCG, cells were washed twice in cold PBS. Then cells were scraped with lysis buffer. Protein concentrations were measured by the Bradford method. Then, samples were diluted in 2 ml cold PBS containing 100 μl 20% ascorbic acid solution and 50 pmol internal standards (ethyl gallate). Cell suspensions or aqueous samples were diluted with 4 ml ethyl acetate, vortexed and then centrifuged for 10 min at 3,500 rpm. Organic layers were removed and evaporated to dryness under a gentle N2 stream in a water bath. Dry extracts were reconstituted with 50:50 water:acetonitrile and injected into LC/MS/MS. The mass spectrometer included an Applied Biosystems/MDS SCIEX 4000-Qtrap hybrid, linear ion trap, triple quadrupole MS (Applied Biosystems, Foster City, CA), and the liquid chromatograph was a Prominence UFLC from Shimadzu Corp., Columbia, MD. Polyphenols were separated using an Eclipse XDB C8, 5 μM, 4.6 X 150 mm (Agilent) column.
Measurement of bilirubin production
Bilirubin was measured as previously described [14]. Briefly, after plating and gene silencing, cells were treated with EGCG or vehicle, and all further manipulations were carried out in a dark room. Absorbance of the organic layer containing bilirubin was measured at 450 nm with a reference wavelength at 600 nm using a SpectraMaxPro M2 spectrophotometer (Molecular Devices, Sunnyvale, CA). The quantity of bilirubin produced was calculated using a molar extinction coefficient of bilirubin dissolved in benzene, with the molar extinction coefficient being e450 =27.3 mmol−1·L. cm−1[21].
Statistical analysis
Values are reported as mean ± standard error of the mean (SEM) of at least three independent groups. Data were analyzed using Sigma Stat software (Jandel Corp., Wan Rafael, CA). One way ANOVA followed by post hoc least significant difference (LSD)’s pairwise multiple comparison procedure were used for statistical analysis of the original data. A statistical probability of P< 0.05 was considered significant.
Results
EGCG treatment stimulates caveolin-1 displacement
We tested the hypothesis that EGCG can co-localize with caveolae and displace caveolin-1. Caveolae enriched fractions were isolated using a detergent-free sucrose density gradient centrifugation method, followed by protein analysis using Western blot. In control cultures, and as expected, caveolin-1 protein was most enriched in fractions 4 and 5 (Figure 1). In contrast, treatment with EGCG induced displacement of caveolin-1 in endothelial cells. At 0.5 h and 1 h, caveolin-1 translocated from fractions 4 and 5 toward fractions 6 to 8, and after 2 h, caveolin-1 recycled back mainly in plasma membrane lipid rafts (fractions 4 and 5). These data suggest that exposure to EGCG leads to caveolin-1 displacement, and that caveolae may transport EGCG from the plasma membrane toward the cytosol or intracellular compartments.
Figure 1.
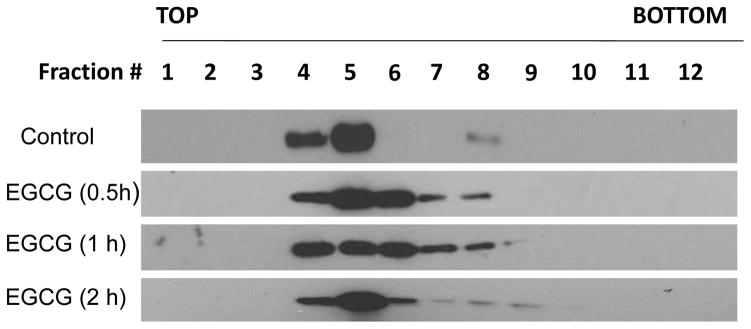
EGCG treatment stimulates caveolin-1 displacement. Endothelial cells were treated with vehicle (0.1% DMSO) or EGCG (30 μM) for 0.5–2 h. Caveolae enriched fractions were isolated by detergent-free sucrose gradient centrifugation method. Then the expression of caveolin-1 was measured by Western blot. Fractions 1 to 3 containing 5% sucrose, fractions 4 to 8 containing 35% sucrose, fractions 9 to 12 containing 45% sucrose. Result shown represents one of three independent experiments.
EGCG quantification in endothelial cells
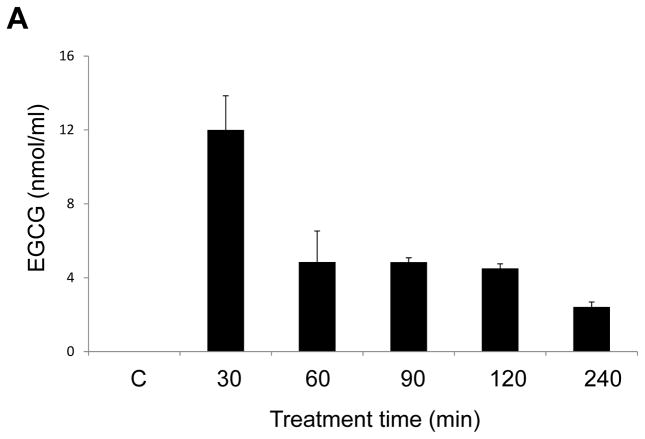
To further understand the stability and cell uptake of EGCG by endothelial cells, we quantified EGCG by LC/MS/MS analysis. Cells were exposed to EGCG for up to 4 h. After a peak at 30 min, EGCG levels in the media decreased, with only about 20% of the parent flavonoid remaining at 4 h (Figure 2A). Cell-associated levels were maximal at 30 min and then declined in parallel with observed media levels (Figure 2B). These results suggest that EGCG is easily auto-oxidized and quickly metabolized [22]. Results in Figure 2C demonstrate that endothelial cells can become enriched with EGCG in a dose-dependent manner.
Figure 2.

EGCG quantification in endothelial cells was performed by LC/MS/MS analysis. Endothelial cells were treated with EGCG (30 μM) for up to 240 min, and EGCG levels were measure by LC/MS/MS in the media (Figure 2A) and cells (Figure 2B). In separate experiments, endothelial cells were treated with increasing concentrations of EGCG (0–180 μM) for 1h, before measuring cellular levels of EGCG (Figure 2C). Results shown represent the mean ± SEM of three independent experiments.
EGCG accumulates in caveolae-enriched fractions
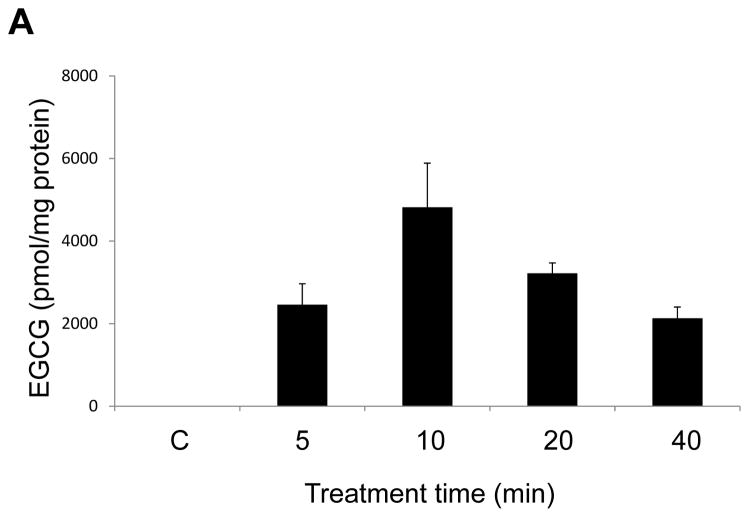
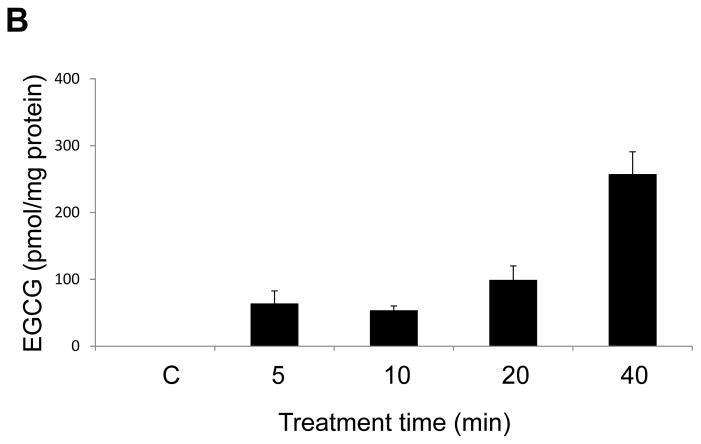
To further investigate the role of caveolae in the uptake and transport of EGCG, we measured EGCG levels in both caveolae-rich membrane fractions and non-caveolae fractions after treatment with EGCG for 5, 10, 20 or 40 min. EGCG markedly accumulated in caveolae-rich membrane domains at 10 min (Figure 3A), with a subsequent relocation into the non-caveolae cell fractions. At 40 min, the uptake of EGCG into the non-caveolae fractions was increased 1.6 fold compared to the 20 min time point (Figure 3B).
Figure 3.
EGCG accumulates in caveolae-enriched fractions. Endothelial cells were treated with EGCG (30 μM) for 5 – 40 min. Caveolae were isolated by detergent-free sucrose density gradient centrifugation method. EGCG levels in caveolae-rich domains (Figure 3A) and non-caveolae fractions (Figure 3B) were measured by LC/MS/MS. EGCG levels shown in the figure were normalized against protein levels in caveolae or non-caveolae fractions, respectively. Results shown represent the mean ± SEM of three independent experiments.
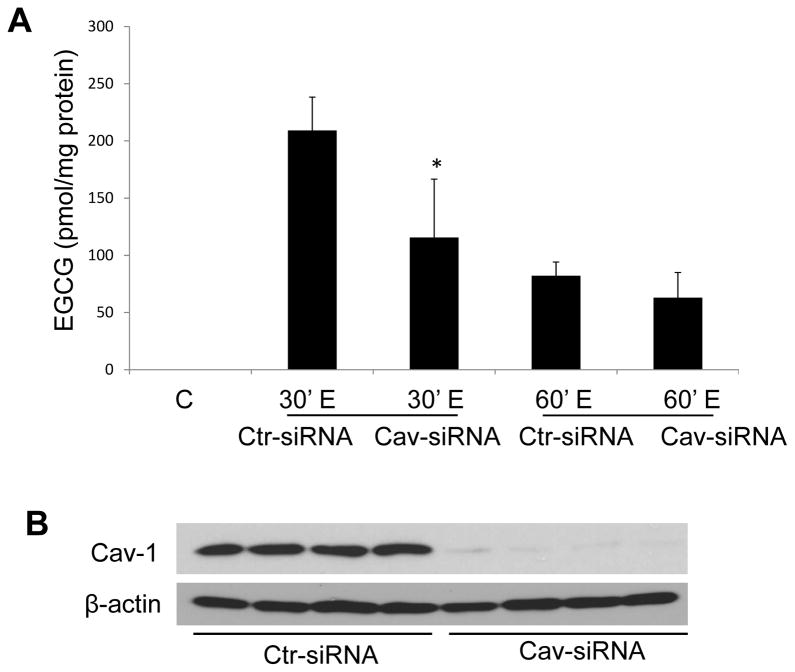
Caveolae silencing reduces cellular EGCG uptake
To explore a potential role of caveolae in EGCG uptake, cells were transfected with caveolin-1 specific siRNAs. Compared to cells with functional caveolae, cellular levels of EGCG at 30 min were significantly decreased after silencing caveolin-1 (Figure 4A). This trend continued but was not significant at 1 h after EGCG exposure. As illustrated in Figure 4B, caveolin-1 was successfully silenced.
Figure 4.
Caveolae silencing reduces cellular EGCG uptake. Endothelial cells were transfected with siRNA for caveolin-1 (Cav-1 siRNA) or with control siRNA (Ctr- siRNA) and treated with EGCG (30 μM) for 30 min and 1 h. EGCG levels in whole cells were measured by LC/MS/MS (Figure 4A). Results shown represent the mean ± SEM of three independent experiments. Figure 4B shows successful silencing of caveolin-1.
Cavelin-1 silencing induces upregulation of HO-1 and bilirubin production
HO-1 has potent anti-inflammatory effects, which may be exerted through the generation of bilirubin. Therefore, we tested the effects of caveolin-1 gene silencing on HO-1 expression and bilirubin production in endothelial cells. The results showed that both EGCG treatment and caveolin-1 gene silencing significantly induced HO-1 levels (Figure 5A). Similar to the HO-1 data, both EGCG and caveolin-1 gene silencing significantly induced the cellular secretion of bilirubin (Figures 5B).
Figure 5.
Cavelin-1 silencing induces upregulation of HO-1 and bilirubin production. Endothelial cells were transfected with siRNA for caveolin-1 (Cav-1 siRNA) or with control siRNA (Ctr- siRNA) and then treated with vehicle (0.1% DMSO) or EGCG (30 μM) for 6 h, before determining HO-1 protein expression (Figure 5A) and bilirubin production (Figure 5B). Results shown represent the mean ± SEM of three independent experiments. *Significantly different compared to control cultures.
Caveolin-1 silencing increases both nuclear accumulation of Nrf2 and Nrf2-ARE binding
It has been suggested that the regulation of Nrf2 transcriptional activation of phase II antioxidant enzymes (e.g., HO-1) relies on subcellular distribution rather than induction of this transcription factor through de novo synthesis [23]. Activation of Nrf2 results in increased accumulation of Nrf2 in the nucleus. Thus, we determined nuclear levels of Nrf2 in both control and EGCG-treated cells with or without functional caveolae to investigate whether induction of HO-1 is associated with nuclear translocation of Nrf2. As shown by immunoblot analysis in Figure 6A, Nrf2 protein levels in the nucleus significantly increased in cells treated with EGCG. Caveolin-1 silencing independently increased Nrf2, with no additive effects due to cellular exposure to EGCG. Nrf2 protein in the cytosol was unaffected by EGCG treatment or caveolin-1 silencing (Figure 6B). Since Nrf2 activates transcription activities of its genes through binding specifically to the ARE found in the promoters of target genes, we also studied the effects of EGCG on Nrf2-ARE binding. In agreement with the observed nuclear accumulation of Nrf2, Nrf2-ARE specific HO-1 promoter binding was also significantly enhanced both in EGCG treated or caveolin-1 silenced cells (Figure 6 C).
Figure 6.
Caveolin-1 silencing increases both nuclear accumulation of Nrf2 and Nrf2-ARE binding. Endothelial cells were transfected with siRNA for caveolin-1 (Cav-1 siRNA) or with control siRNA (Ctr- siRNA) and then treated with vehicle (0.1% DMSO) or EGCG (30 μM) for 3 h, before determining Nrf2 protein expression both in nucleus (Figure 6A) and cytosol (Figure 6B). Electrophoretic mobility shift assay for Nrf2-ARE binding was performed with nuclear proteins extracted from these endothelial cells (Figure 6C). Following the EMSA assay, Western blotting with the Nrf2 antibody demonstrated the upper band to be the Nrf2-ARE complex. Results shown represent the mean ± SEM of three independent experiments. *Significantly different compared to control cultures.
Discussion
There is evidence that catechins derived from green tea, such as EGCG, have antioxidant, anti-inflammatory and anti-angiogenesis properties and thus can provide protection against inflammatory diseases such as atherosclerosis [24]. In the current study we provide evidence that the vascular antioxidant and anti-inflammatory properties of EGCG may be regulated through the interaction of this flavonoid with caveolae. Lipid rafts, and especially caveolae, have been recently recognized as signal transduction hubs, and may be involved in the selective cellular uptake of plasma-derived material [25] and possibly resveratrol [10] and other polyphenols such as EGCG.
It has been reported that EGCG is taken up by some lipid raft proteins in the membrane, such as laminin receptor (LamR), and that it further alters the composition of membrane domains as well as changes in some signaling pathways [26]. Besides specific interactions with some genes and proteins, another important pathway that may function in EGCG action is direct targeting on lipid rafts. Structure and composition alterations of the lipid raft by polyphenols can dramatically affect signaling pathways, such as MAP kinase pathways [27]. Induction of MAP kinases is critical in the regulation of inflammatory pathways, and inhibition of MAP kinase signaling may in part explain the anti-inflammatory effects of polyphenols [28]. Our LC/MS/MS results suggest that EGCG can concentrate in the caveolae fractions before further cellular redistribution. In fact, following cellular exposure, EGCG quickly enriched in caveolae membrane domains, with a subsequent relocation to non-caveolae fractions. This suggests that caveolae may play a role in the uptake and transport of EGCG from plasma membrane lipid rafts towards the cytosol in endothelial cells. To confirm the role of caveolae in the uptake of EGCG, endothelial cells were silenced with caveolin-1-siRNA. Results showed that caveolin-1 gene silencing significantly decreased cellular uptake of EGCG at 30 min, i.e., at a time point when EGCG was markedly enriched in the caveolae fractions of normal endothelial cells. Thus, these data suggest that EGCG uptake into endothelial cells may be in part dependent on functional caveolae.
Caveolae usually have a specific lipid composition, which appears to be required for the specific functional relevance of caveolae-associated proteins [29]. In line with these functional characteristics, caveolae can provide a regulatory platform for proinflammatory signaling associated with vascular diseases such as atherosclerosis [30]. For example, enriching endothelial cells with docosahexaenoic acid can affect caveolae-associated nitric oxide synthase activity [31], a process which may be linked to caveolae-mediated endocytosis [32]. We also found previously that pretreatment with EGCG can block fatty acid-induced caveolin-1 expression in a time and concentration dependent manner [9]. In the current study, we provide evidence that EGCG can stimulate caveolin-1 displacement from the plasma membrane towards the cytosol. Most importantly, the ability of EGCG to displace caveolin-1 was associated with its ability to activate Nrf2 and to increase HO-1 expression and cellular production of bilirubin.
We have reported recently that EGCG-mediated protection against TNF-α-induced monocyte chemoattractant protein-1 expression is HO-1 dependent [14], and we now provide data which demonstrate that caveolae play an important role in the uptake and transport of EGCG, as well as protection against endothelial inflammation through the induction of Nrf2-dependent HO-1. Our results clearly show that in addition to EGCG, caveolin-1 gene silencing can induce HO-1 protein expression and thus up-regulate the production of bilirubin. Both HO-1 and bilirubin have been reported to play critical roles in cellular and tissue defenses against oxidative stress and inflammation [33]. HO-1 over-expression can inhibit pathological activities, including inflammation, vascular proliferation, and chronic transplant rejection. It has been reported that over-expression of the HO-1 protein inhibits lipopolysaccharide-induced iNOS expression [34]. Furthermore, the HO-1 protein is essential for the anti-inflammatory effects of IL-10 and 15-deoxy-delta 12, 14-prostaglandin J2 [35].
Since Nrf2 is the major transcription factor of HO-1, we investigated the role of Nrf2 in EGCG or caveolin-1 gene silencing mediated activation of HO-1. Nrf2 is a leucine zipper transcription factor which plays an essential role in the up-regulation of phase II anti-oxidant genes, including HO-1. Once migrated to the nucleus, Nrf2 forms heterodimers with small Maf proteins and subsequently binds to the cis-acting antioxidant response element (ARE). This leads to the transcriptional activation of a number of genes that encode the phase II detoxifying or antioxidant enzymes, such as NQO1, GST, GCL, and HO-1 [36]. In the current study, EGCG treatment significantly induced nuclear accumulation of Nrf2 as well as enhancement of Nrf2-ARE binding at the HO-1 promoter site. In contrast, in caveolin-1-silenced cells, both accumulation of Nrf2 as well as enhancement of Nrf2-ARE binding at the HO-1 promoter site were already elevated in untreated cells, with EGCG having no additional effect on stimulation of Nrf2. This suggests that caveolin-1 displacement within functional caveolae may be associated with EGCG-mediated induction of Nrf2 in endothelial cells. To support this hypothesis, it was shown that other nutrients can contribute to caveolin-1 displacement. For example, Li et al [37] found that displacement of caveolin-1 from the plasma membrane by treatment with EPA or DHA [31] is an important mechanism in the prevention against atherosclerosis.
In summary, we provide evidence that caveolae play a role in the uptake and transport of EGCG and mechanisms, which may be associated with the anti-inflammatory properties of this flavonoid. Displacement of caveolin-1 may be necessary for EGCG to to initiate the protective signaling cascade. Similar to EGCG treatment, silencing of caveolin-1 by siRNA also resulted in upregulation of Nrf2, HO-1 and bilirubin production. These data suggest that EGCG-induced caveolin-1 displacement, and subsequent alteration in caveolae function, may be necessary for the activation of Nrf2/HO-1 cellular protection system.
References
- 1.Munoz-Espada AC, Watkins BA. Cyanidin attenuates PGE2 production and cyclooxygenase-2 expression in LNCaP human prostate cancer cells. J Nutr Biochem. 2006;17(9):589–96. doi: 10.1016/j.jnutbio.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Kris-Etherton PM, et al. Bioactive compounds in nutrition and health-research methodologies for establishing biological function: the antioxidant and anti-inflammatory effects of flavonoids on atherosclerosis. Annu Rev Nutr. 2004;24:511–38. doi: 10.1146/annurev.nutr.23.011702.073237. [DOI] [PubMed] [Google Scholar]
- 3.Sasazuki S, et al. Relation between green tea consumption and the severity of coronary atherosclerosis among Japanese men and women. Ann Epidemiol. 2000;10(6):401–8. doi: 10.1016/s1047-2797(00)00066-1. [DOI] [PubMed] [Google Scholar]
- 4.Shenouda SM, Vita JA. Effects of flavonoid-containing beverages and EGCG on endothelial function. J Am Coll Nutr. 2007;26(4):366S–372S. doi: 10.1080/07315724.2007.10719625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frank PG, et al. Genetic ablation of caveolin-1 confers protection against atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24(1):98–105. doi: 10.1161/01.ATV.0000101182.89118.E5. [DOI] [PubMed] [Google Scholar]
- 6.Frank PG, Pavlides S, Lisanti MP. Caveolae and transcytosis in endothelial cells: role in atherosclerosis. Cell Tissue Res. 2009;335(1):41–7. doi: 10.1007/s00441-008-0659-8. [DOI] [PubMed] [Google Scholar]
- 7.Pelkmans L, Helenius A. Endocytosis via caveolae. Traffic. 2002;3(5):311–20. doi: 10.1034/j.1600-0854.2002.30501.x. [DOI] [PubMed] [Google Scholar]
- 8.Matveev S, et al. The role of caveolae and caveolin in vesicle-dependent and vesicle-independent trafficking. Adv Drug Deliv Rev. 2001;49(3):237–50. doi: 10.1016/s0169-409x(01)00138-7. [DOI] [PubMed] [Google Scholar]
- 9.Zheng Y, et al. Role of caveolin-1 in EGCG-mediated protection against linoleic-acid-induced endothelial cell activation. J Nutr Biochem. 2009;20(3):202–9. doi: 10.1016/j.jnutbio.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang HL, et al. Caveolin-1 enhances resveratrol-mediated cytotoxicity and transport in a hepatocellular carcinoma model. J Transl Med. 2009;7:22. doi: 10.1186/1479-5876-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu CC, et al. Upregulation of heme oxygenase-1 by Epigallocatechin-3-gallate via the phosphatidylinositol 3-kinase/Akt and ERK pathways. Life Sci. 2006;78(25):2889–97. doi: 10.1016/j.lfs.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Kim HP, et al. Caveolae compartmentalization of heme oxygenase-1 in endothelial cells. FASEB J. 2004;18(10):1080–9. doi: 10.1096/fj.03-1391com. [DOI] [PubMed] [Google Scholar]
- 13.Durante W. Targeting heme oxygenase-1 in vascular disease. Curr Drug Targets. 2010;11(12):1504–16. doi: 10.2174/1389450111009011504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng Y, Toborek M, Hennig B. Epigallocatechin gallate-mediated protection against tumor necrosis factor-alpha-induced monocyte chemoattractant protein-1 expression is heme oxygenase-1 dependent. Metabolism. 2010;59(10):1528–35. doi: 10.1016/j.metabol.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 15.Itoh K, Mimura J, Yamamoto M. Discovery of the negative regulator of Nrf2, Keap1: a historical overview. Antioxid Redox Signal. 2010;13(11):1665–78. doi: 10.1089/ars.2010.3222. [DOI] [PubMed] [Google Scholar]
- 16.Siow RC, Ishii T, Mann GE. Modulation of antioxidant gene expression by 4-hydroxynonenal: atheroprotective role of the Nrf2/ARE transcription pathway. Redox Rep. 2007;12(1):11–5. doi: 10.1179/135100007X162167. [DOI] [PubMed] [Google Scholar]
- 17.Romeo L, et al. The major green tea polyphenol, (−)-epigallocatechin-3-gallate, induces heme oxygenase in rat neurons and acts as an effective neuroprotective agent against oxidative stress. J Am Coll Nutr. 2009;28(Suppl):492S–499S. doi: 10.1080/07315724.2009.10718116. [DOI] [PubMed] [Google Scholar]
- 18.Hennig B, et al. Exposure to free fatty acid increases the transfer of albumin across cultured endothelial monolayers. Arteriosclerosis. 1984;4(5):489–97. doi: 10.1161/01.atv.4.5.489. [DOI] [PubMed] [Google Scholar]
- 19.Lim EJ, et al. Coplanar polychlorinated biphenyl-induced CYP1A1 is regulated through caveolae signaling in vascular endothelial cells. Chem Biol Interact. 2008;176(2–3):71–8. doi: 10.1016/j.cbi.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Repetto S, et al. Insulin and IGF-I phosphorylate eNOS in HUVECs by a caveolin-1 dependent mechanism. Biochem Biophys Res Commun. 2005;337(3):849–52. doi: 10.1016/j.bbrc.2005.09.125. [DOI] [PubMed] [Google Scholar]
- 21.Clark JE, et al. Dynamics of haem oxygenase-1 expression and bilirubin production in cellular protection against oxidative stress. Biochem J. 2000;348(Pt 3):615–9. [PMC free article] [PubMed] [Google Scholar]
- 22.Sang S, et al. Stability of tea polyphenol (−)-epigallocatechin-3-gallate and formation of dimers and epimers under common experimental conditions. J Agric Food Chem. 2005;53(24):9478–84. doi: 10.1021/jf0519055. [DOI] [PubMed] [Google Scholar]
- 23.Dinkova-Kostova AT, et al. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A. 2002;99(18):11908–13. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramesh E, Geraldine P, Thomas PA. Regulatory effect of epigallocatechin gallate on the expression of C-reactive protein and other inflammatory markers in an experimental model of atherosclerosis. Chem Biol Interact. 2010;183(1):125–32. doi: 10.1016/j.cbi.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 25.Meijering BD, et al. Ultrasound and microbubble-targeted delivery of macromolecules is regulated by induction of endocytosis and pore formation. Circ Res. 2009;104(5):679–87. doi: 10.1161/CIRCRESAHA.108.183806. [DOI] [PubMed] [Google Scholar]
- 26.Patra SK, et al. Molecular targets of (−)-epigallocatechin-3-gallate (EGCG): specificity and interaction with membrane lipid rafts. J Physiol Pharmacol. 2008;59(Suppl 9):217–35. [PubMed] [Google Scholar]
- 27.Khan N, et al. Targeting multiple signaling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate. Cancer Res. 2006;66(5):2500–5. doi: 10.1158/0008-5472.CAN-05-3636. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez-Gallego J, et al. Fruit polyphenols, immunity and inflammation. Br J Nutr. 2010;104(Suppl 3):S15–27. doi: 10.1017/S0007114510003910. [DOI] [PubMed] [Google Scholar]
- 29.Li XA, Everson WV, Smart EJ. Caveolae, lipid rafts, and vascular disease. Trends Cardiovasc Med. 2005;15(3):92–6. doi: 10.1016/j.tcm.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Majkova Z, Toborek M, Hennig B. The role of caveolae in endothelial cell dysfunction with a focus on nutrition and environmental toxicants. J Cell Mol Med. doi: 10.1111/j.1582-4934.2010.01064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Q, et al. Docosahexaenoic acid affects endothelial nitric oxide synthase in caveolae. Arch Biochem Biophys. 2007;466(2):250–9. doi: 10.1016/j.abb.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 32.Maniatis NA, et al. Novel mechanism of endothelial nitric oxide synthase activation mediated by caveolae internalization in endothelial cells. Circ Res. 2006;99(8):870–7. doi: 10.1161/01.RES.0000245187.08026.47. [DOI] [PubMed] [Google Scholar]
- 33.Chung HT, Pae HO, Cha YN. Role of heme oxygenase-1 in vascular disease. Curr Pharm Des. 2008;14(5):422–8. doi: 10.2174/138161208783597335. [DOI] [PubMed] [Google Scholar]
- 34.Lin HY, Shen SC, Chen YC. Anti-inflammatory effect of heme oxygenase 1: glycosylation and nitric oxide inhibition in macrophages. J Cell Physiol. 2005;202(2):579–90. doi: 10.1002/jcp.20160. [DOI] [PubMed] [Google Scholar]
- 35.Lee TS, Tsai HL, Chau LY. Induction of heme oxygenase-1 expression in murine macrophages is essential for the anti-inflammatory effect of low dose 15-deoxy-Delta 12,14-prostaglandin J2. J Biol Chem. 2003;278(21):19325–30. doi: 10.1074/jbc.M300498200. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen T, et al. Increased protein stability as a mechanism that enhances Nrf2-mediated transcriptional activation of the antioxidant response element. Degradation of Nrf2 by the 26 S proteasome. J Biol Chem. 2003;278(7):4536–41. doi: 10.1074/jbc.M207293200. [DOI] [PubMed] [Google Scholar]
- 37.Li Q, et al. Eicosapentaenoic acid modifies lipid composition in caveolae and induces translocation of endothelial nitric oxide synthase. Biochimie. 2007;89(1):169–77. doi: 10.1016/j.biochi.2006.10.009. [DOI] [PubMed] [Google Scholar]