Abstract
Background
Delirium can be hypothesized to be an extreme manifestation of sickness behavior in elderly persons with neurodegenerative disease. The purpose of this study was to investigate whether increased cerebral inflammation with microglial, astrocyte, and cytokine activation exists in patients with delirium compared to nondelirious patients.
Methods
Postmortem brain tissue from 9 cases with delirium was compared to 6 age-matched controls without delirium. Human leukocyte antigen-DR (HLA-DR) and CD68 cell count and glial fibrillary acidic protein (GFAP), interleukin-1β (IL-1β), IL-6,β-amyloid, and tau protein immunoreactivity were determined in hippocampus, frontal cortex, and white matter.
Results
There were no significant differences in the patients with delirium compared to the controls with respect to age 73 versus 70.5 years (p=0.72) or dementia (22% versus 0%, p=0.22). Both markers for microglial activity showed significantly higher scores in delirium brain specimens than controls in the total brain score (HLA-DR 129 vs. 20 and CD68 30 vs. 8.5) as well as in the various brain areas separately. The immunoreactivity of astrocyte activity (GFAP) was higher in the total brain score in patients with delirium (5.2 vs. 4.0, p=0.05), but in the various brain areas this was only significant in the dentate gyrus. IL-6 immunoreactivity was higher in patients with delirium in all brain areas and IL-1β was not detectable. Coexisting infectious disease or dementia did not influence the overall results.
Conclusions
These preliminary study results show an association between human brain activity of microglia, astrocytes, and IL-6 and delirium in elderly patients and add to the accumulating evidence that inflammatory mechanisms are involved in delirium.
Introduction
Delirium is the most frequent neuropsychiatric syndrome in the hospital, especially in older patients with pre-existent cognitive impairment.1 It is independently associated with impaired physical and cognitive recovery afterward, increased hospital costs, and a higher mortality. Despite these serious effects on outcome, the pathogenesis of delirium is still incompletely understood, but there is growing understanding of the role of inflammation in this condition.2–4 With aging and (concomitant) neurodegeneration, heightened inflammatory states are common in patients at risk for delirium, even under normal conditions.5,6 Additionally, an unlimited number of clinical conditions with systemic inflammatory reactions can induce delirium.
Rodent studies have shown that central nervous system (CNS) inflammatory responses to acute inflammatory insults are exaggerated in the aged or neurodegenerative brain.7,8 These inflammatory responses are accompanied by sickness behavioral responses. Apart from malaise, sleepiness, and apathy, this behavior is characterized by acute cognitive changes in these rodents,9,10 and recent studies have shown acute and transient working memory deficits, resembling the syndrome of delirium in humans.9 Microglial cells and astrocytes in the brain are suggested to play a role in the behavioral changes due to systemic inflammation.3 These cells can be changed from a resting state into an activated state with increasing age and in neurodegenerative disease, as evidenced by altered morphology and upregulated cell-surface antigens.6,10 Cytokines may be expressed as a consequence of this activation, or may be synthesized by ‘primed’ microglial cells upon further activation by systemic inflammatory signals arising from infections, injuries, or surgeries.11 Moreover, there is evidence that proinflammatory cytokines such as interleukin-1β (IL-1β) can inhibit acetylcholine release and cholinergic-dependent memory function, and thus these inflammatory mediators may be key determinants of cognitive dysfunction during episodes of delirium.12,13
Previously, in human brain tissue, an association between a robust systemic infection, as seen in sepsis, and activation of microglia in the brain was shown.14 It is well established that systemic cytokines can activate the CNS via: (1) Direct activation of the brain in areas where the blood–brain barrier (BBB) is altered or absent; (2) activation of the cerebral endothelium and/or transport systems for cytokines across the BBB; and (3) detection of peripheral immune activation by specialized sensory nerves, such as the vagus nerve, carrying information into the brain via autonomic nervous system.15,16 In patients with delirium, proinflammatory cytokines such as IL-1β and IL-6 were both found to be increased in blood and also in the cerebrospinal fluid of patients with systematic lupus erythematosus.17–21 However, to our knowledge, no human neuropathological studies have been published that directly examined whether patients in the acute phase of delirium showed evidence of increased microglial activity.22 The purpose of this exploratory study was to investigate whether delirium is associated with a glial response encompassing elevated glial expression of cytokines and enhanced astrocyte and microglial cell activation in postmortem human brain tissue.
Methods
Selection of cases and controls
Patients were selected by the following criteria: Permission for use of autopsy material for research purposes, autopsies performed within 24 hr after death, presence of frontal and hippocampal brain tissue, and absence of signs of CNS infection. From 2003 until 2008, all medical and surgical older patients that were known to the geriatric team to be delirious were followed during hospital admission. Delirium was assessed by a geriatrician using the Confusion Assessment Method and verified by the Diagnostic and Statistical Manual of Mental Disorders IV-Text Revision (DSM-IV-TR).23,24 The information that the scoring of the DSM-IV-TR criteria was based on was different for cases than for controls. We based our information for the patients with delirium on our psychiatric examination of the patient, medical and nursing records including the Delirium Observation Screening Scale, and information given by the patient's closest relative. In the control group, the information was based retrospectively on the medical and nursing records. Delirious patients that died during this episode were included as cases. Controls were obtained from all authorized brain autopsies that had been performed on patients in the Amsterdam Medical Center (AMC) during the period of 1992–2004. Next, controls were matched for age and presence of cognitive disorders or extracerebral infections. Controls were excluded if their medical records described neuropsychiatric symptoms that could have been related to the diagnosis of delirium. Informed consent was obtained from the next of kin for the use of brain tissue and for access to medical records for research purposes.
Medical records were searched for cause of death and signs of infection. Systemic infection was scored positive in cases of positive cultures of urine, sputum, or blood taken before death, use of antibiotics, or if the physician made notice of an infectious diagnosis. For controls, the information of pre-existent cognitive impairment was based upon medical history. For patients with delirium, additional information was also available from the Informant Questionnaire on Cognitive Decline short form taken at admission (IQCODE-sf).25 The caretaker was asked to recall the situation 2 weeks prior to the admission and compare it with the situation 10 years earlier. Patients with a mean score of 3.9 or higher were considered to have global cognitive impairment.26
Tissue preparation
Tissue was fixed in 10% buffered formalin and embedded in paraffin. Because key symptoms of delirium are emotional disinhibition and memory deficits, the five regions of interest that were selected were: frontal grey (CTX) and white matter (WM), hippocampus (cornu ammonis areas 1 [CA1] and 3 [CA3]), and dentate gyrus (DG). Two representative blocks per patient of each region were sectioned, stained, and assessed. The tissue was sectioned at 6μ m and mounted on precoated glass slides (Star Frost, Waldemar Knittel GmbH, Barunschweig, Germany). Sections of all specimens were processed for Hematoxylin & Eosin (H&E), Luxol Fast Blue (LFB), as well as for immunocytochemical stainings for a number of neuronal and glial markers described below.
Immunocytochemistry
For the detection of the inflammatory cells the following antibodies (Abs) were used: (glial fibrillary acidic protein [GFAP]; polyclonal rabbit, DAKO, Glostrup, Denmark; 1:4,000), anti-human leukocyte antigen (HLA)-DP, -DQ, -DR (mouse clone CR3/43; DAKO, Glostrup, Denmark; 1:400); anti-CD68 (mouse monoclonal, clone PG-M1; DAKO; Glostrup, Denmark 1:200); IL-1β (goat polyclonal, sc-1250, Santa Cruz Bio., CA; 1:70); and anti-human IL-6 (1:10, monoclonal mouse, clone 677B6A2, Biosource; Camarillo, CA).27,28
For single-label immunocytochemistry, paraffin-embedded sections were deparaffinized, rehydrated, and incubated for 20 min in 0.3% hydrogen peroxide (H2O2) diluted in methanol to quench the endogenous peroxidase activity. Antigen retrieval was performed by incubation for 10 min at 121°C in citrate buffer (0.01 M, pH 6.0). Sections were washed with phosphate-buffered saline (PBS), and incubated for 30 min in 10% normal goat serum (Harlan Sera-Lab, Loughborough, Leicestershire, UK). We incubated the sections with the primary antibodies overnight at 4°C. Thereafter, sections were washed in PBS; the ready-for-use Powervision peroxidase system (Immunologic, Duiven, The Netherlands) was used and 3,3′-diaminobenzidine (DAB; Sigma) was used as chromogen. Sections were counterstained with Hematoxylin, dehydrated, and coverslipped.
For double-labeling studies, IL-6 was combined with GFAP or Iba1 (ionized calcium binding adaptor molecule 1; polyclonal goat 1:100; Abcam, Cambridge, UK). After incubation with the primary antibodies overnight at 4°C, sections were, incubated for 2 hr at room temperature with Alexa Fluor® 568 conjugated and Alexa Fluor® 488 (anti-mouse immunoglobulin G [IgG] and anti-goat or anti-rabbit IgG; 1:100, Molecular Probes, The Netherlands). Sections were then analyzed by means of a laser scanning confocal microscope (Leica TCS Sp2, Wetzlar, Germany).
The degenerative changes of Alzheimer type were assessed using anti-tau (mouse monoclonal AT8 antibody, recognizing the phosphorylated Ser202 residue of the tau protein; 1:5,000 dilution; Innogenetics, Ghent, Belgium) and anti-β-amyloid (mouse monoclonal 6F/3D, raised against residues 8–17 of amyloid A4; 1:200; DAKO). Quantitative and semiquantitative immunoreactivity were evaluated by two independent observers blind to the clinical data. In case of disagreement, independent reevaluation was performed by both observers to define the final score.
Quantitative analysis
Quantitative analysis was performed for HLA-DR and CD68, and the numbers of positive cells were quantified as previously described.27,29 Briefly, three representative digital photos were obtained from the white and grey matter (frontal lobe) and hippocampus of controls and cases using an Olympus microscope (BX41, Tokyo, Japan) equipped with a digital camera (DFC500, Leica Microsystems-Switzerland Ltd., Heerbrugg, Switzerland).30 The three images spanned 1-mm2 regions within the cortex and selected hippocampal fields (CA1, CA3, and DG) and included the areas of maximal cell infiltration. These images were collected using image acquisition and analysis software (Phase 3 Image System integrated with Image-Pro Plus, Media Cybernetics, Silver Spring, MD). Hippocampal sections were collected at the hippocampal midlevel of every subject. To normalize possible differences in anatomical level or hippocampal size, the surface area of the dentate region was determined in adjacent Nissl-stained sections. The operator outlined all individual cell types within the captured image, and an automated cell count was generated. The overall concordance between independent observers was >90% and the overall kappa value was >0.85. Data obtained in each of the three regions per section were averaged, thus providing a single value for each section, and this value was used for statistical analysis of data.
Semiquantitative evaluation of immunoreactivity
Labeled tissue sections were examined with respect to the presence or absence of specific immunoreactivity (IR) for GFAP, IL-1β, and IL-6. The analysis on specimens stained with DAB was performed using a light microscope as described before.31,32 The sections (two representative paraffin sections per case) were stained simultaneously with each antibody. Neuronal cell bodies were differentiated from glia on the basis of morphology. Two representative sections per patient were stained and assessed with each antibody. The intensity of immunoreactive staining was evaluated using a scale of 0–3 (0, no; 1, weak; 2, moderate; 3, strong staining). All areas of the specimen were examined, and the score represents the predominant cell staining intensity found in each section as averaged from the selected fields. The frequency of positive—(1) <5% positive cells; (2) 5–30% positive cells; (3) high, >30% positive cells—was evaluated to give information about the relative number of positive cells within the selected regions (cortex, gray and white matter or hippocampus, CA1, CA3, or dentate region). As proposed earlier, the product of these two values (intensity and frequency scores) was taken to give the overall score (immunoreactivity total score).28,29
Statistics
The Statistical Package for the Social Sciences (SPSS), v.16.0, was used for data analysis. Mean cell count or immunoreactivity score of the five brain regions was determined independently for each antigen. Mann–Whitney tests and chi-squared tests were used to assess differences between groups as a whole and stratified for infection. A two-tailed criterion of p<0.05 was considered statistically significant. Correlations between CD68, HLA-DR, GFAP, and IL-6 and S100B were determined using Spearman correlation tests.
Results
Table 1 gives an overview of the characteristics of the included 9 patients with delirium and 6 controls. There were no significant differences in the patients with delirium compared to the controls with respect to age 73 years (range 62–84 years) versus 70.5 years (68–82 years) (p=0.72) or male sex 44% versus 67% (p=0.40), or dementia 22% versus 0% (p=0.22). The frequency of patients dying with infection was not significantly higher in the delirious patients than in controls 78% versus 50% (p=0.26). Postmortem Interval was 12 hr (9–14 hr) for controls versus 11 hr (8–15) for delirium patients (p=0.77).
Table 1.
Patient Characteristics
| Age (years) | Sex | Cause of death | Infection | Cognitive disordera | Postmortem interval | Neuropathological findings |
|---|---|---|---|---|---|---|
| Cases | ||||||
| 84b | M | Leukoclastic vasculitis | Noc | No | 10 | Atherosclerosis; a small infarct in the thalamus |
| 81 | F | Intraventricular haemorrhage | Yes | No | 8 | Intraventricular haemorrhage |
| 73 | M | Respiratory insufficiency by pulmonic vasculitis | Yes | No | 14 | No disease-specific pathology |
| 62 | M | Respiratory insufficiency | Yes | No | 11 | Atherosclerosis and arteriolosclerosis |
| 62 | F | Hypovolemic shock, hemorrhagic gastritis, sepsis | Yes | No | 15 | No disease-specific pathology |
| 70 | F | Respiratory insufficiency | Yes | No | 11 | No disease-specific pathology |
| 81 | F | Respiratory insufficiency by pneumonia | Yes | Alzheimer disease | 9 | Alzheimer disease: Braak stadium I |
| 73 | F | Malignant neuropleptic syndrome | No | Frontotemporal dementia | 13 | Alzheimer disease: Braak stadium III |
| 70 | M | Respiratory insufficiency | Yes | No | 10 | Cerebellar hemorrhage |
| Controls | ||||||
| 73 | F | Cardiogenic shock | Yes | No | 11 | No disease-specific pathology |
| 68 | F | Acute myeloid leucemia | Yes | No | 10 | No disease-specific pathology |
| 82 | M | Myocardial infarction | No | No | 14 | No disease-specific pathology |
| 68b | M | Heart failure | No | No | 9 | No disease-specific pathology |
| 70 | M | Pseudomonas sepsis, myocardial infarction | Yes | No | 13 | No disease-specific pathology |
| 71 | M | Bowel ischaemia | No | No | 12 | No disease-specific pathology |
Clinical diagnosis.
Patients selected for Fig. 2
No evidence of vasculitis in the central nervous system.
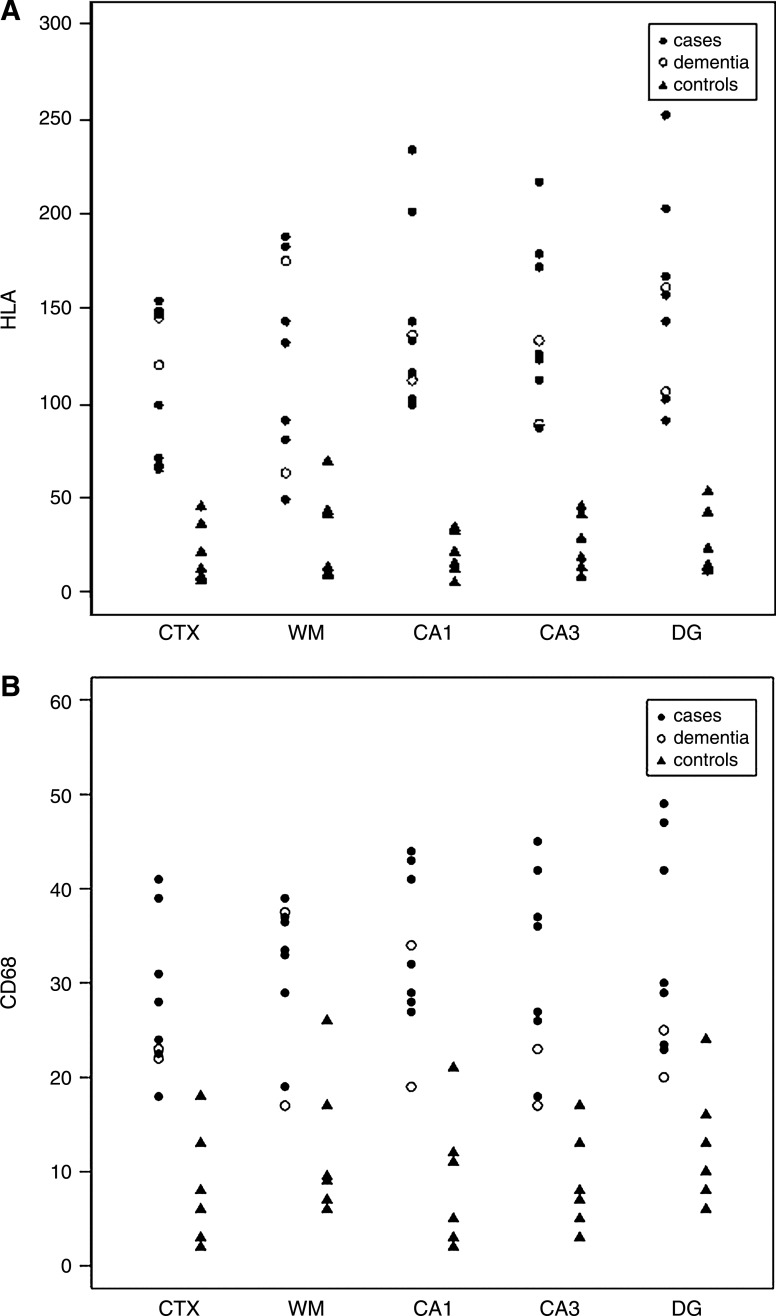
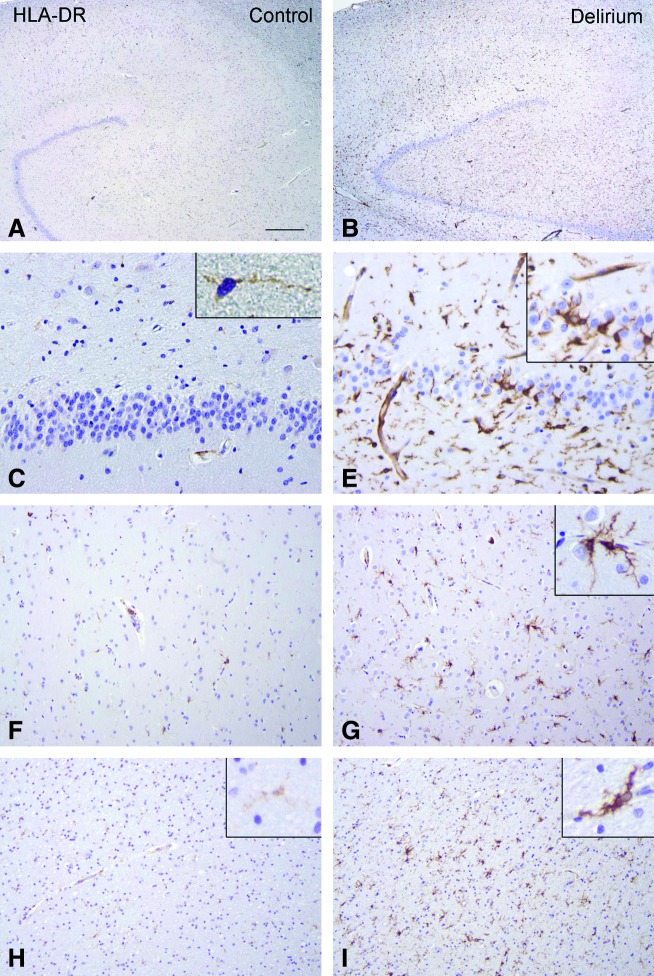
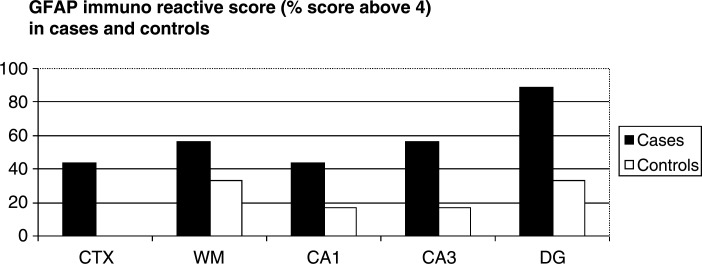
Figure 1 shows the microglial cell count (HLA-DR [Fig. 1A] and CD68 [Fig. 1B]) of the patients with delirium and the controls. Differences are significant between the two groups in all brain regions. A representative sample of a patient with delirium and a control without delirium are demonstrated in Fig. 2. The semiquantitative evaluation of immunoreactivity of the astrocytes (GFAP) is shown with the percentage of patients with delirium or controls above 4 in all brain areas separately (Fig. 3). The GFAP scores show only a significant difference for the DG (p=0.03). One hundred percent of the patients with delirium show IL-6 scores above 1 against 17% of controls for all different brain areas (results not shown) (p<0.001).
FIG. 1.
Microglia activity (numbers of positive cells) for HLA-DR (A) and CD68 (B) in patients with (n=7) and without (n=6) delirium and patients with dementia and delirium (n=2) in frontal grey (CTX) and white matter (WM), hippocampus (cornu ammonis area 1 (CA1) and 3 (CA3), and dentate gyrus (DG).
FIG. 2.
Human leukocyte antigen-DR (HLA-DR) immunoreactivity (IR) in controls and patients with delirium. (A, C, F, H) HLA-DR IR in control hippocampus (A; C, dentate gyrus) and in control grey (F) and white matter (H, frontal lobe). Insert in C shows a resting microglial cell with light HLA-DR IR. (B, E, G, I) HLA-DR IR in the hippocampus (B; E, dentate gyrus) and in the grey (G) and white matter (I, frontal lobe) of patients with delirium, showing increased IR in activated migroglia. Inserts in E and G show higher magnifications of reactive microglial cells with strong HLA-DR IR. Scale bars: A and B, 500 μm; C–E, 60 μm; F–I, 120 μm. (Color images available online at www.liebertonline.com/rej).
FIG. 3.
Astrocyte immunoreactivity in patients with and without delirium. GFAP, Glial fibrillary acidic protein; CTX, frontal grey matter; WM, white matter; CA1 and CA3, cronu ammonis area 1 and 3; DG, dentate gyrus.
The mean score was significantly higher in patients with delirium for HLA-DR, CD68, GFAP, and IL-6 (Table 2). Only the 2 patients with known dementia showedβ-amyloid and tau positivity and also showed low IL-1β reactivity in the white matter. IL-1β immunoreactivity was absent in the other sections and patients. To exclude a technical problem with IL-1β staining, positive external controls were used; a case of encephalitis and 2 cases of focal cortical dysplasia showed adequate IL-1β expression.
Table 2.
Mean Activity Total Score (Range) in Patients with and without Deliriuma
| Variable | Delirium (n=10) | No delirium (n=6) | p value |
|---|---|---|---|
| HLA-DR | 129 (79–207) | 20.1 (10–49) | <0.001 |
| CD68 | 30 (20–41) | 8.5 (4–21) | 0.002 |
| GFAP | 5.2 (4–7) | 4.0 (4–6) | 0.05 |
| IL-6 | 1.6 (1–3) | 0.0 (0–1) | <0.001 |
| IL-1β | 0.0 (0–0) | 0.0 (0–0) | 0.86 |
Cell count for HLA-DR and CD68 and immunoreactivity score for GFAP, IL-6, and IL-1β.
HLA-DR, Human leukocyte antigen-DR; GFAP, glial fibrillary acidic protein; IL-6, interleukin-6.
Repeating the analyses in patients without known pre-existent cognitive impairment (7 cases and 6 controls) showed comparable results; higher scores were found in patients with delirium for HLA-DR (129 vs. 20.1, p=0.003), CD68 (30 vs. 8.5, p=0.003), GFAP (5.2 vs. 4.0, p=0.05), and IL-6 (1.6 vs. 0.0, p=0.002) but not for IL-1β (0.0 vs. 0.0, p=0.28). When patients without infection (3 controls and 2 cases) were excluded from the first analysis, the mean total score was still significantly higher in patients with delirium for HLA-DR (118 vs. 38, p=0.02), CD68 (30 vs. 14, p=0.03), and IL-6 (1.8 vs. 0, p=0.02) but not for GFAP (5 vs. 5, p=0.52), and IL-1β (0 vs. 0, p=0.67). Patients without infection showed no significant difference in all variables.
Correlation between the mean immunoreactivity score for the different antigens was present between CD68 and HLA-DR (r=0.92, p<0.001), CD68 and GFAP (r=0.65, p=0.02), CD68 and IL-6 (r=0.85, p<0.001), HLA-DR and IL-6 (r=0.85, p<0.001), GFAP and IL-6 (r=0.76, p=0.003), and HLA-DR and GFAP (r=0.61, p<0.03).
Discussion
Recent observations indicated that microglial and astrocyte cell activation in the brain play a role in sickness behavior in the rodent. In the present study, the results that both markers for microglial activity, HLA-DR and CD68, showed higher scores in frontal cortex, white matter, and hippocampus of patients who died with delirium are consistent with the hypothesis that neuroinflammation in delirium is increased. The immunoreactivity of astrocyte activity (GFAP) was slightly higher in patients with delirium, especially in the DG. Cytokine immunoreactivity was higher in patients with delirium in all brain areas for IL-6, but not for IL-1β. A coexisting infection or pre-existent cognitive disorders did not influence the overall results. The immunoreactivity score showed a satisfactory level of reproducibility in intraobserver analyses with overall concordance of over 90% and the overall kappa ranged from 0.89 to 0.96.30
A limitation of our study is the sample size and the retrospective data collection. Presence of delirium in controls and presence of infection in patients with delirium and controls were all collected from the available data in the patient charts. Specifically, the hypoactive subtype of delirium could have been missed. However, it is to be expected that if we had accidently included a patient with delirium as a control, this would probably have reduced the differences we found. Including patients prospectively to obtain the diagnosis during lifetime would be preferable. However, in the light of the low frequency of permission for brain autopsies in elderly patients without cerebral infection, a large prospective study is impossible at present. Moreover, many patients do not give permission for sample withdrawal for cultures or questionnaires in this last phase of their life. Although patients were matched in terms of age, cognitive impairment, and infection, delirium cases show a trend toward higher age, more dementia, and more infection. It was difficult to find elderly controls without any premortem confusion according to the medical charts.
We realize that the risk of a Type I error is increased by comparing five markers measured in five areas between the two groups, but the application of the common multiple test correction methods (for instance, Bonferroni) is invalid because those methods assume independence between the markers and the areas, which is clearly not the case here. Use of permutations to obtain a valid estimate of the family-wise error rate or a false discovery rate is not possible because of the small numbers of patients. Making an adjustment for multiple testing also leads to underestimation of the true effect.
Finally, the determination of IL-1β was too low for quantification, consistent with previous studies of serum IL-1β and its association with delirium.18 Thus, despite the suspected important role of IL-1β as a key proinflammatory cytokine in acute cognitive dysfunction,33 the data here indicate that either IL-1β is too low to measure or has no essential role in delirium. In our patients with Alzheimer disease, there was some IL-1β activity shown in the white matter in concordance with our expectations.
The syndrome of delirium is the result of a wide variety of combinations of predisposing and precipitating factors.34 Previous observations suggest that inflammatory markers, especially the microglial markers CD68 and HLA-DR, show higher activity in two of the most important predisposing factors for delirium, i.e., higher age and cognitive impairment.14,35 The question emerges whether this relationship between delirium and a higher level of inflammation in the brain can be attributed solely to the prior activation of microglia in the brain associated with these predisposing factors for delirium. In opposition to this hypothesis is the fact that excluding patients with dementia in the analysis did not change our results.
With these caveats on the contribution of the predisposing factors for delirium to increased inflammation in mind, experimental studies showing that the acute inflammatory responses lead to the rapid start of sickness behavior support a role for the acute precipitating factor for delirium.22 The question of how the precipitating factor for delirium may contribute to the higher level of cerebral inflammation remains to be answered. Importantly, our delirium cases showed a trend toward more frequent coexisting infection than controls, so the association between microglia activation and symptoms of delirium may be related to the presence of systemic infection. In patients with a severe systemic infection, activation of microglia in the brain was shown,14 but also in most other important causes for death, such as cardiovascular events, systemic inflammation plays a role.36 It is known that peripheral inflammation can activate the CNS by a number of routes, including the circumventricular organs, vagal afferents, and the brain endothelium.16 Currently, it is unknown whether altered cytokine levels in blood in delirium are actively produced in the periphery, derived from the CNS, or both.35 Uchikado et al. have suggested that the vulnerability of neurological patients to delirium is explained by the fact that cerebral vascular endothelial cells respond to systemic inflammation and upregulate the expression of molecules involved in inflammatory processes.37 In support of a peripheral origin of cerebral inflammation, systemically administered lipopolysaccharide (LPS) induces CNS cytokine expression specifically by microglial cells proximal to hippocampal blood vessels in mice.38
Conclusions
This study suggests for the first time an association between delirium in elderly patients and activity of astrocytes, microglia, and IL-6 in the human brain and adds to the accumulating evidence that inflammatory mechanisms seem to be involved in the pathogenesis of delirium. In conclusion, this upregulation of CNS inflammation seems related to both the predisposing and precipitating factors for delirium. A better understanding of the underlying pathophysiological mechanism is crucial for the development of new prophylactic and therapeutic interventions of delirium and improving the prognosis of acutely ill elderly patients.
Acknowledgments
The authors wish to thank Miranda Mandjes for her contribution in the drafting of the figures. We are grateful to J.T. van Heteren and J.J. Anink for their technical help.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Young J. Inouye SK. Delirium in older people. BMJ. 2007;334:842–846. doi: 10.1136/bmj.39169.706574.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maldonado JR. Pathoetiological model of delirium: A comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24:789–856. doi: 10.1016/j.ccc.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Eikelenboom P. Hoogendijk WJ. Jonker C. van Tilburg W. Immunological mechanisms and the spectrum of psychiatric syndromes in Alzheimer's disease. J Psychiatr Res. 2002;36:269–280. doi: 10.1016/s0022-3956(02)00006-7. [DOI] [PubMed] [Google Scholar]
- 4.Cerejeira J. Firmino H. Vaz-Serra A. Mukaetova-Ladinska EB. The neuroinflammatory hypothesis of delirium. Acta Neuropathol. 2010;119:737–754. doi: 10.1007/s00401-010-0674-1. [DOI] [PubMed] [Google Scholar]
- 5.Luz C. Dornelles F. Preissler T. Collaziol D. da Cruz IM. Bauer ME. Impact of psychological and endocrine factors on cytokine production of healthy elderly people. Mech Ageing Dev. 2003;124:887–895. doi: 10.1016/s0047-6374(03)00148-9. [DOI] [PubMed] [Google Scholar]
- 6.Eikelenboom P. Veerhuis R. Scheper W. Rozemuller AJM. van Gool WA. Hoozemans JJM. The significance of neuroinflammation in understanding Alzheimer's disease. J Neural Transm. 2006;113:1685–1695. doi: 10.1007/s00702-006-0575-6. [DOI] [PubMed] [Google Scholar]
- 7.Godbout JP. Chen J. Abraham J. Richwine AF. Berg BM. Kelley KW. Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- 8.Combrinck MI. Perry VH. Cunningham C. Peripheral infection evokes exaggerated sickness behaviour in pre-clinical murine prion disease. Neuroscience. 2002;112:7–11. doi: 10.1016/s0306-4522(02)00030-1. [DOI] [PubMed] [Google Scholar]
- 9.Murray C. Sanderson DJ. Barkus Cm. Deacon RM. Rawlins JN. Bannerman DM. Cunningham C. Systemic inflammation induces acute working memory deficits in the primed brain: Relevance for delirium. Neurobiol Aging. 2010 May 12; doi: 10.1016/j.neurobiolaging.2010.04.002. [e-pub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godbout JP. Johnson RW. Age and neuroinflammation: A lifetime of psychoneuroimmune consequences. Immunol Allergy Clin N Am. 2009;29:321–337. doi: 10.1016/j.iac.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Perry VH. Cunningham C. Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7:161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- 12.Taepavarapruk P. Song C. Reductions of acetylcholine release and nerve growth factor expression are correlated with memory impairment induced by interleukin-1beta administrations: effects of omega-3 fatty acid EPA treatment. J Neurochem. 2010;112:1054–1064. doi: 10.1111/j.1471-4159.2009.06524.x. [DOI] [PubMed] [Google Scholar]
- 13.Rada P. Mark GP. Vitek MP. Mangano RM. Blume AJ. Beer B. Hoebel BG. Interleukin-1β decreases acetylcholine measured by microdialysis in the hippocampus of freely moving rats. Brain Res. 1991;550:287–290. doi: 10.1016/0006-8993(91)91330-4. [DOI] [PubMed] [Google Scholar]
- 14.Lemstra AW. Groen in't Woud JC. Hoozemans JJM. van Haastert ES. Rozemuller EJM. Eikelenboom P. van Gool WA. Microglia activation in sepsis: A case-control study. J Neuroinflammation. 2007;4:4. doi: 10.1186/1742-2094-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hopkins SJ. Central nervous system recognition of peripheral inflammation: A neural, hormonal collaboration. Acta Biomed. 2007;78(Suppl 1):231–247. [PubMed] [Google Scholar]
- 16.Dantzer R. O'Connor JC. Freund G. Johnson RW. Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kudoh A. Takase H. Katagai H. Takazawa T. Postoperative interleukin-6 and cortisol concentrations in elderly patients with postoperative confusion. Neuroimmunomodulation. 2005;12:60–66. doi: 10.1159/000082365. [DOI] [PubMed] [Google Scholar]
- 18.van Munster BC. Korevaar JC. Zwinderman AH, et al. Time-course of cytokines during delirium in elderly patients with hip fractures. J Am Geriatr Soc. 2008;56:1704–1709. doi: 10.1111/j.1532-5415.2008.01851.x. [DOI] [PubMed] [Google Scholar]
- 19.de Rooij SE. van Munster BC. Korevaar JC. Levi M. Cytokines and acute phase response in delirium. J Psychosom Res. 2007;62:521–525. doi: 10.1016/j.jpsychores.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Marcantonio ER. Rudolph JL. Culley D. Corsby G. Alsop D. Inouye SK. Serum biomarkers for delirium. J Gerontol A Biol Sci Med Sci. 2006;61:1281–1286. doi: 10.1093/gerona/61.12.1281. [DOI] [PubMed] [Google Scholar]
- 21.Katsumata Y. Harigai M. Kawaguchi Y. Fukasawa C. Soejima M. Takagi K. Tanaka M. Ichida H. Tochimoto A. Kanno T. Nishmura K. Kamatani N. Hara M. Diagnostic reliability of cerebral spinal fluid tests for acute confusional state (delirium) in patients with systemic lupus erythematosus: interleukin 6 (IL-6), IL-8, interferon-alpha, IgG index, and Q-albumin. J Rheumatol. 2007;34:2010–2017. [PubMed] [Google Scholar]
- 22.Dantzer R. Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inouye SK. van Dyck CH. Alessi CA. Balkin S. Siegal AP. Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 24.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders IV-Text Revision (DSM IV-TR) American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 25.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): Development and cross-validation. Psychol Med. 1994;24:145–153. doi: 10.1017/s003329170002691x. [DOI] [PubMed] [Google Scholar]
- 26.de Jonghe JF. Differentiating between demented and psychiatric patients with the Dutch version of the IQCODE. Int J Geriatr Psychiatry. 1997;12:462–465. doi: 10.1002/(sici)1099-1166(199704)12:4<462::aid-gps510>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 27.Ravizza T. Boer K. Redeker S. Spliet WGM. van Rijen PC. Troost D. Vezzani A. Aronica E. The IL-1beta system in epilepsy-associated malformations of cortical development. Neurobiol Dis. 2006;24:128–143. doi: 10.1016/j.nbd.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 28.van Heteren JT. Rozenberg F. Aronica E. Troost D. Lebon P. Kuijpers TW. Astrocytes produce interferon-alpha and CXCL10, but not IL-6 or CXCL8, in Aicardi-Goutieres syndrome. Glia. 2008;56:568–578. doi: 10.1002/glia.20639. [DOI] [PubMed] [Google Scholar]
- 29.Maldonado M. Baybis M. Newman D. Kolson DL. Chen W. McKhann G. Gutmann DH. Crino PB. Expression of ICAM-1, TNF-alpha, NF kappa B, and MAP kinase in tubers of the tuberous sclerosis complex. Neurobiol Dis. 2003;14:279–290. doi: 10.1016/s0969-9961(03)00127-x. [DOI] [PubMed] [Google Scholar]
- 30.Aronica E. Gorter JA. Redeker S. Ramkema M. Spliet WG. van Rijen PC. Leenstra S. Troost D. Distribution, characterization and clinical significance of microglia in glioneuronal tumours from patients with chronic intractable epilepsy. Neuropathol Appl Neurobiol. 2005;31:280–291. doi: 10.1111/j.1365-2990.2004.00636.x. [DOI] [PubMed] [Google Scholar]
- 31.Kwan P. Li HM. Al-Jufairi E. Abdulla R. Gonzales M. Kaye AH. Szoeke C. Ng HK. Wong KS. O'Brien TJ. Association between temporal lobe P-glycoprotein expression and seizure recurrence after surgery for pharmacoresistant temporal lobe epilepsy. Neurobiol Dis. 2010;39:192–197. doi: 10.1016/j.nbd.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 32.Boer K. Troost D. Spliet WG. van Rijen PC. Gorter JA. Aronica E. Cellular distribution of vascular endothelial growth factor A (VEGFA) and B (VEGFB) and VEGF receptors 1 and 2 in focal cortical dysplasia type IIB. Acta Neuropathol. 2008;115:683–696. doi: 10.1007/s00401-008-0354-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cunningham C. Sanderson DJ. Malaise in the water maze: untangling the effects of LPS and IL-1beta on learning and memory. Brain Behav Immun. 2008;22:1117–1127. doi: 10.1016/j.bbi.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Munster BC. de Rooij SE. Korevaar JC. The role of genetics in delirium in the elderly patient. Dement Geriatr Cogn Disord. 2009;28:187–195. doi: 10.1159/000235796. [DOI] [PubMed] [Google Scholar]
- 35.Lucin KM. Wyss-Coray T. Immune activation in brain aging and neurodegeneration: Too much or too little? Neuron. 2009;64:110–122. doi: 10.1016/j.neuron.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaysen GA. Biochemistry biomarkers of inflamed patients: Why look, what to assess. Clin J Am Soc Nephrol. 2009;4(Suppl 1):S56–S63. doi: 10.2215/CJN.03090509. [DOI] [PubMed] [Google Scholar]
- 37.Uchikado H. Akiyama H. Kondo H. Ikeda K. Tsuchiya K. Kato M. Oda T. Togo T. Iseki E. Kosaka K. Activation of vascular endothelial cells and perivascular cells by systemic inflammation-an immunohistochemical study of postmortem human brain tissues. Acta Neuropathol (Berl) 2004;107:341–351. doi: 10.1007/s00401-003-0815-x. [DOI] [PubMed] [Google Scholar]
- 38.Cunningham C. Wilcockson DC. Campion S. Lunnon K. Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25:9275–9284. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]