Abstract
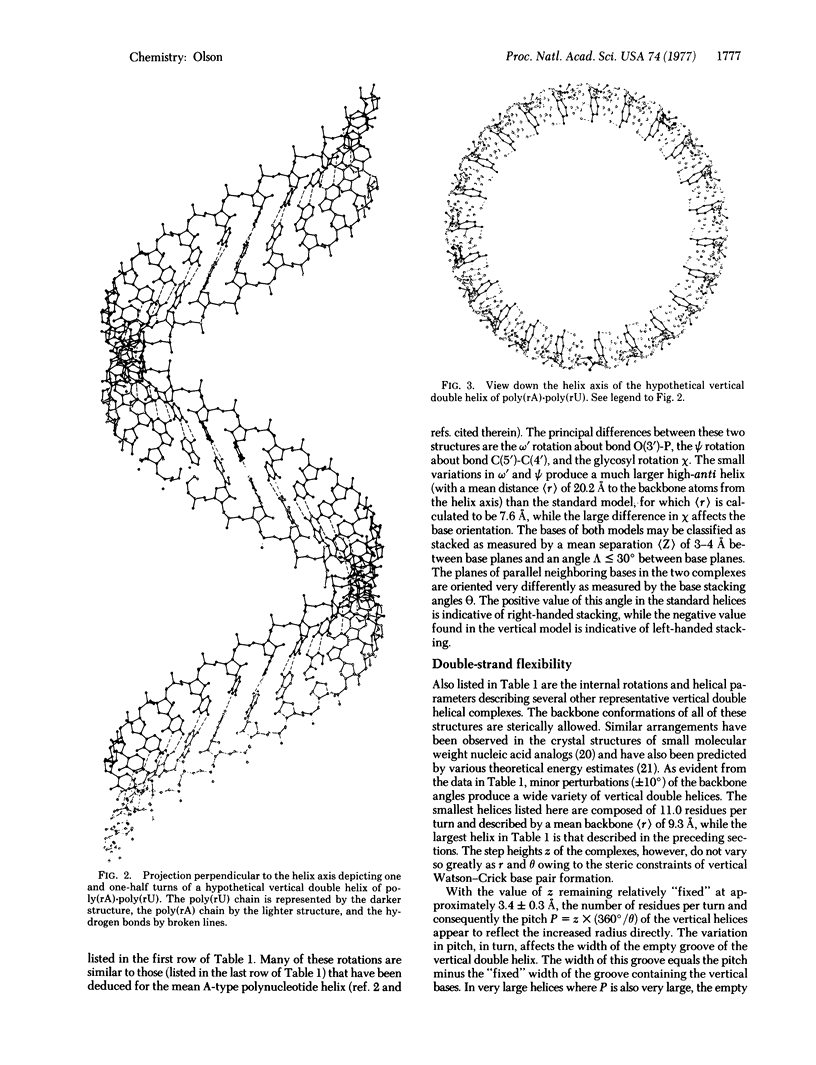
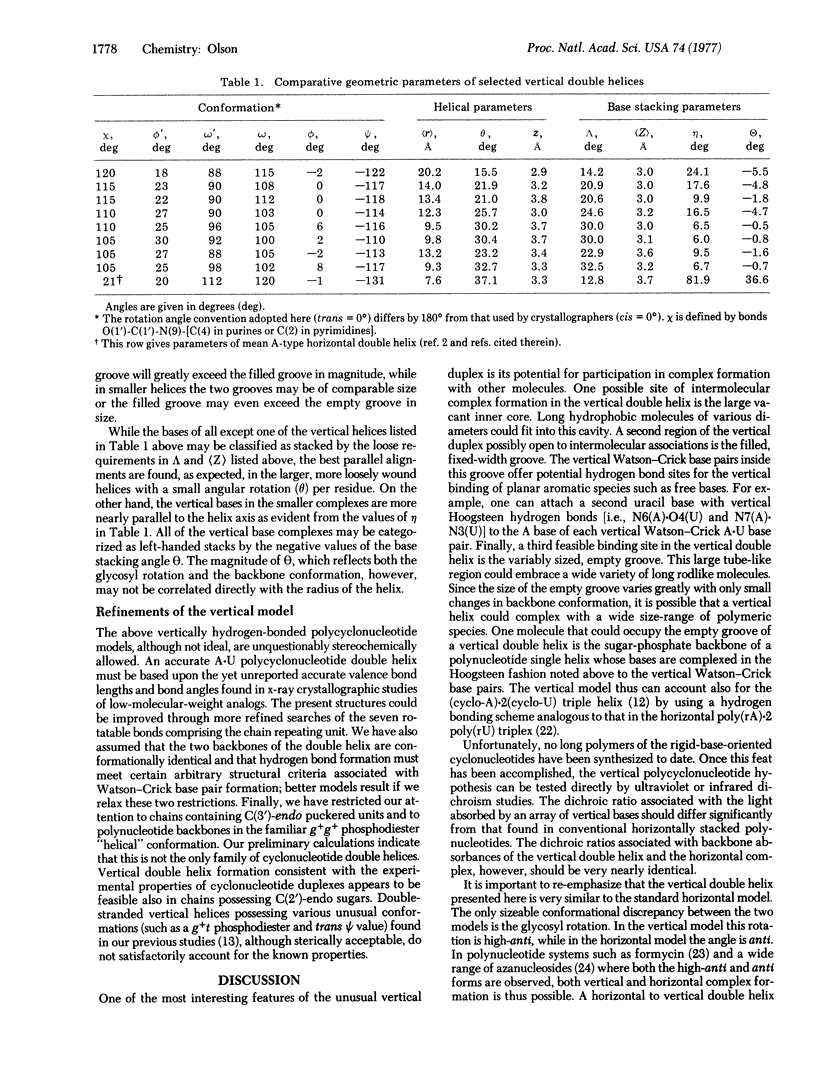
Structural details are reported for a novel right-handed polynucleotide double helix stabilized by vertical base stacking and hydrogen bonding. The primary difference between this duplex and the familiar Watson-Crick horizontally stabilized polynucleotide complex arises in the glycosyl rotation of the heterocyclic bases with respect to the sugar-phate backbone. In the vertical double helix the bases adopt a fairly unusual (although not sterically impossible) high-anti conformation, while in the horizontal models deduced from x-ray analyses the bases occur in the favored anti arrangement. The base pairing scheme in both duplexes is the standard Watson-Crick type. The vertical double helix is demonstrated to be a plausible model for the unusual complex formation observed between complementary synthetic polycyclonucleotides (the bases of which are fixed by a chemical linkage in the high-anti orientation) and also a potential alternative ordered structure available to naturally occurring nucleic acid systems that can adopt both anti and high-anti glycosyl arrangements.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Bond P. J. Structures for Poly(U)-poly(A)-poly(U)triple stranded polynucleotides. Nat New Biol. 1973 Jul 25;244(134):99–101. doi: 10.1038/newbio244099a0. [DOI] [PubMed] [Google Scholar]
- Fujii S., Tomita K. Conformational analysis of polynucleotides. I. The favorable left-handed helical model for the poly(8,2'-S-cycloadenylic acid) with high anti conformation. Nucleic Acids Res. 1976 Aug;3(8):1973–1984. doi: 10.1093/nar/3.8.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikchara M., Tezuka T. Synthesis of cyclouridine oligonucleotides forming a double stranded complex of left-handedness with cycloadenosine oligonucleotides. J Am Chem Soc. 1973 Jun 13;95(12):4054–4056. doi: 10.1021/ja00793a041. [DOI] [PubMed] [Google Scholar]
- Ikehara M., Uesugi S. Polynucleotides. XVI. Oligomers of 8,2'-anhydro-8-mercapto-9-( -D-arabinofuranosyl)adennine 5'-monophosphate. J Am Chem Soc. 1972 Dec 27;94(26):9189–9193. doi: 10.1021/ja00781a033. [DOI] [PubMed] [Google Scholar]
- Ikehara M., Uesugi S., Yano J. Polynucleotides. XXIV. Synthesis and properties of a dinucleoside monophosphate derived from 8,2'-O-cycloadenosine. J Am Chem Soc. 1974 Jul 24;96(15):4966–4972. doi: 10.1021/ja00822a040. [DOI] [PubMed] [Google Scholar]
- Ladner J. E., Jack A., Robertus J. D., Brown R. S., Rhodes D., Clark B. F., Klug A. Atomic co-ordinates for yeast phenylalanine tRNA. Nucleic Acids Res. 1975 Sep;2(9):1629–1637. doi: 10.1093/nar/2.9.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson W. K., Dasika R. D. Spatial configuration of ordered polynucleotide chains. 3. Polycyclonucleotides. J Am Chem Soc. 1976 Aug 18;98(17):5371–5380. doi: 10.1021/ja00433a052. [DOI] [PubMed] [Google Scholar]
- Olson W. K. Syn-anti effects on the spatial configuration of polynucleotide chains. Biopolymers. 1973;12(8):1787–1814. doi: 10.1002/bip.1973.360120808. [DOI] [PubMed] [Google Scholar]
- Olson W. K. The spatial configuration of ordered polynucleotide chains. I. Helix formation and base stacking. Biopolymers. 1976 May;15(5):859–878. doi: 10.1002/bip.1976.360150505. [DOI] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Prusiner P., Brennan T., Sundaralingam M. Crystal structure and molecular conformation of formycin monohydrates. Possible origin of the anomalous circular dichroic spectra in formycin mono- and polynucleotides. Biochemistry. 1973 Mar 13;12(6):1196–1202. doi: 10.1021/bi00730a028. [DOI] [PubMed] [Google Scholar]
- Quigley G. J., Seeman N. C., Wang A. H., Suddath F. L., Rich A. Yeast phenylalanine transfer RNA: atomic coordinates and torsion angles. Nucleic Acids Res. 1975 Dec;2(12):2329–2341. doi: 10.1093/nar/2.12.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg J. M., Seeman N. C., Day R. O., Rich A. RNA double helices generated from crystal structures of double helical dinucleoside phosphates. Biochem Biophys Res Commun. 1976 Apr 19;69(4):979–987. doi: 10.1016/0006-291x(76)90469-1. [DOI] [PubMed] [Google Scholar]
- Singh P., Hodgson D. J. 8-Azaadenosine. Crystallographic evidence for a "high-anti" conformation around a shortened glycosidic linkage. J Am Chem Soc. 1974 Aug 7;96(16):5276–5278. doi: 10.1021/ja00823a057. [DOI] [PubMed] [Google Scholar]
- Stout C. D., Mizuno H., Rubin J., Brennan T., Rao S. T., Sundaralingam M. Atomic coordinates and molecular conformation of yeast phenylalanyl tRNA. An independent investigation. Nucleic Acids Res. 1976 Apr;3(4):1111–1123. doi: 10.1093/nar/3.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman J. L., Kim S. H. Idealized atomic coordinates of yeast phenylalanine transfer RNA. Biochem Biophys Res Commun. 1976 Jan 12;68(1):89–96. doi: 10.1016/0006-291x(76)90014-0. [DOI] [PubMed] [Google Scholar]
- Uesugi S., Tezuka T., Ikehara M. Polynecleotides. XXX. Synthesis and properties of oligonucleotides of cyclouridine phosphate. Hybridization with the oligomer of S-cycloadenosine phosphate to form left-handed helical complexes. J Am Chem Soc. 1976 Feb 18;98(4):969–973. doi: 10.1021/ja00420a017. [DOI] [PubMed] [Google Scholar]
- Uesugi S., Yasumoto M., Ikehara M., Fang K. N., Ts'o P. O. Synthesis and properties of the dinucleoside monophosphate of adenine 8-thiocyclonucleoside. J Am Chem Soc. 1972 Jul 26;94(15):5480–5486. doi: 10.1021/ja00770a053. [DOI] [PubMed] [Google Scholar]
- Yathindra N., Sundaralingam M. Analysis of the possible helical structures of nucleic acids and polynucleotides. Application of (n-h) plots. Nucleic Acids Res. 1976 Mar;3(3):729–747. doi: 10.1093/nar/3.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]



