Abstract
The mechanistic understanding of the dynamic processes linking nutrient acquisition and biomass production of competing individuals can be instructive in optimizing intercropping systems. Here, we examine the effect of inoculation with Funneliformis mosseae on competitive dynamics between wheat and faba bean. Wheat is less responsive to mycorrhizal inoculation. Both inoculated and uninoculated wheat attained the maximum instantaneous N and P capture approximately five days before it attained the maximum instantaneous biomass production, indicating that wheat detected the competitor and responded physiologically to resource limitation prior to the biomass response. By contrast, the instantaneous N and P capture by uninoculated faba bean remained low throughout the growth period, and plant growth was not significantly affected by competing wheat. However, inoculation substantially enhanced biomass production and N and P acquisition of faba bean. The exudation of citrate and malate acids and acid phosphatase activity were greater in mycorrhizal than in uninoculated faba bean, and rhizosphere pH tended to decrease. We conclude that under N and P limiting conditions, temporal separation of N and P acquisition by competing plant species and enhancement of complementary resource use in the presence of AMF might be attributable to the competitive co-existence of faba bean and wheat.
Intercropping is the simultaneous growing of two or more plant species in the same field for a significant period of their growth but without necessarily being sown and harvested together1. Intercropping is known to produce more biomass than the average of the monocultures (termed “overyielding”), particularly in low nitrogen (N) or phosphorus (P) input systems. Interspecific plant interactions, including above- and belowground competition, are an important factor determining facilitation and overyielding in intercropping systems in tropical and temperate habitats2,3,4. In the last decade mechanisms such as niche complementarity5 and positive interspecific interactions (facilitation) in resource use6,7 have been documented to contribute to overyielding. Interspecific plant interactions are influenced by abiotic factors such as nutrient availability, soil type and local climatic and growth conditions8 and by biotic factors such as pathogens and mutualists9,10. The effect of arbuscular mycorrhizal fungi on plant interspecific interactions is the focus of the present study.
Approximately 80% of terrestrial plant species establish mutualistic associations with arbuscular mycorrhizal fungi (AMF) which play a vital role in plant nutrition and ecosystem services11. AMF predominantly enhance plant uptake of P, but also N and other nutrients, and the plants in return provide carbon and energy to the fungi. AMF affect plant community productivity and plant-plant interactions12. Studies on the presence and/or identity of AMF in plant competition in grassland systems indicate that AMF change inter- and intra-specific plant interactions13. The outcome of the competition can be negative, neutral or positive14,15 depending on nutrient availability16, host identity17 and plant species interactions18. AMF taxa show host preferences and host species vary widely in AMF symbiosis in terms of changes in biomass and P acquisition19. Consequently, some plant species acquire more nutrients from AMF than do others20. Moreover, competing plant species can be interconnected by common mycorrhizal networks (CMNs) which may potentially influence the distribution of limiting mineral nutrients21 or carbon among interconnected individuals, potentially affecting competition22. Hence, AM fungi might change the competitive relationships between competing plants via the modification of plant nutritional levels23 although other mechanisms such as delivery of allelochemicals via CMNs have also been reported24. However, conclusions from previous studies have been based mainly on the end-point harvest measurement of biomass production. To fully understand plant competition as a process the dynamic patterns of both biomass production and resource capture by the competitors need to be elucidated25,26. A recent study clearly showed that the dynamic processes of competing individuals of Dactylis glomerata and Plantago lanceolata processed N and produced biomass differently, and a shift from competition to facilitation was observed during the experimental period25.
Cereal/legume combinations ususally represent very effective intercropping systems with the potential to provide overyielding due to the complementary N strategies of the partner species in the utilization of N resources27 or facilitation of P use7. The cereal partner is superior to the legume in the usage of N and the legumes are forced to rely on N2-fixation28. The different ecological niches of the two species lead to the domination of facilitation over competitive interactions with a consequent overall positive effect, especially on the part of the non-legume component of the mixture6. In addition, N transfer via CMNs between legume and non-legume has been shown in both pot29 and field30 experiments, although the amount of N transferred may have been minor compared to the N requirements of the plants. Moreover, interspecific facilitation of P acquisition by P-mobilizing species has been observed in several cereal-legume intercropping systems such as wheat/lupine and wheat/chickpea in pot experiments31 and in maize/faba bean under field conditions32. Hence, facilitation of both N and P usage is involved in overyielding in cereal-legume intercropping, and AMF may affect interspecific plant interactions by regulation of N and P uptake by the competing plants.
Recent studies show that sequential measurements of the processes linking biomass production and resource capture by the competitors are important in understanding the dynamics and mechanisms of competitive interactions26,33. The competitive outcomes depend crucially on when and how ‘competition’ is measured25. The dynamics of competition in cereal/legume mixtures is particularly interesting and will mirror the interactive processes of biomass production and nutrient acquisition in relation to N2-fixation by legumes and the P (N) contribution by AMF. In the present study the temporal patterns in the effects of inoculation with F. mosseae on competitive interactions between wheat and faba bean were tested. Wheat/faba bean is one of the main intercropping combinations adopted by farmers in northwest and southwest China. In these combinations wheat and faba bean respond differently to AMF, with faba bean typically benefiting from AMF and wheat being unresponsive. We hypothesized that (1) dynamic biomass production of competing wheat and faba bean is favorable for faba bean and the effect is significant at later growth stages, and (2) the influence of AMF on competitive interactions is correlated to N and/or P uptake dynamics. Rhizosphere carboxylates and pH were compared at early (after 30 days) and later (after 70 days) growth stages. Legumes have been shown to release large amounts of acid phosphatase to mobilize organic P31,34. In addition, AMF directly or indirectly affect acid phosphatase activity to mobilize soil organic P35. Acid phosphatase activity was therefore determined in the present study.
Results
Colonization by AMF
No colonization was observed in the uninoculated plants. Both wheat and faba bean inoculated with F. mosseae were well colonized (Fig. S1). The colonization percentages (A%, arbuscule abundance; M%, intensity of mycorrhizal colonization) of wheat roots were low at day 30, increased with time, and the highest values were observed at day 79. Similar results were found for A% in faba bean roots and the highest value of M% was observed at day 59. The presence of the competitor plant had no significant influence on colonization percentages of either plant species. Average percentage of root length colonized was significantly higher for faba bean than wheat at days 59 and 67. At day 59 the average M% values were 43.0 and 82.4% for wheat and faba bean and the corresponding A% values were 30.7 and 57.5%.
Cumulative biomass production and uptake of N and P
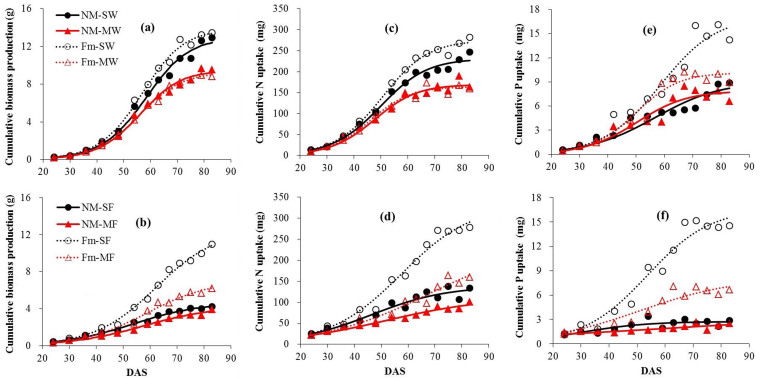
Cumulative biomass production and N and P uptake by plants were strongly affected by inoculation, growth in mixture, and their interactions (Table S1). The maximum biomass (Ymax) of both inoculated and uninoculated wheat and faba bean plants was influenced strongly by the presence of the competitor and it differed greatly between the species. Inoculation with F. mosseae did not have a significant influence on biomass accumulation in wheat either sole cropped or in mixture, but the presence of faba bean reduced Ymax of neighboring wheat by 30.0% on average. By contrast, inoculation greatly increased the growth of faba bean plants both separate and in mixture (Fig. 1a, b). The maximum biomass of inoculated sole and mixture faba bean plants were 169.4 and 66.0% higher than that those of uninoculated plants at harvest (Table 1). Divergences in the biomass trajectories between inoculated and uninoculated, separate and mixed treatments of faba bean plants were detectable at 54 and 42 d (P < 0.05) (Table S2). Competition reduced the maximum biomass of inoculated faba bean to 43.7% of that attained when faba bean was grown alone (Table 1).
Figure 1. Cumulative shoot biomass production (a, b), N (c, d) and P uptake (e, f) by wheat (a, c, e) and faba bean plants (b, d, f).
Each symbol represents a single harvest and is the mean of three replicates. Curves are derived from Eqn 1 using mean parameter estimates. NM-SW, uninoculated sole wheat; NM-MW, uninoculated wheat mixed with faba bean; Fm-SW, inoculated sole wheat; Fm-MW, inoculated wheat mixed with faba bean. Similar symbols are also used for faba bean plants.
Table 1. Mean values of the parameters in Eqn 1 fitted to temporal changes in cumulative shoot biomass production and N and P uptake of wheat and faba bean plants derived from Fig. 1.
| Wheat | Faba bean | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | NM-SW | NM-MW | Fm-SW | Fm-MW | NM-SF | NM-MF | Fm-SF | Fm-MF | |
| Biomass | r (d−1) | 0.122 | 0.128 | 0.126 | 0.124 | 0.081 | 0.077 | 0.089 | 0.085 |
| Ymax (g) | 12.94 | 9.49 | 13.84 | 9.24 | 4.61 | 4.21 | 12.42 | 6.99 | |
| R2 | 0.991 | 0.989 | 0.999 | 0.986 | 0.979 | 0.925 | 0.938 | 0.878 | |
| N | r (d−1) | 0.114 | 0.124 | 0.115 | 0.129 | 0.071 | 0.052 | 0.076 | 0.052 |
| Ymax (mg) | 231.1 | 169.3 | 275.1 | 164.3 | 138.3 | 111.7 | 331.3 | 236.0 | |
| R2 | 0.972 | 0.957 | 0.984 | 0.959 | 0.921 | 0.938 | 0.983 | 0.941 | |
| P | r (d−1) | 0.085 | 0.111 | 0.100 | 0.128 | 0.083 | 0.021 | 0.088 | 0.059 |
| Ymax (mg) | 9.03 | 7.87 | 17.04 | 10.14 | 2.74 | 3.92 | 16.62 | 8.12 | |
| R2 | 0.894 | 0.873 | 0.991 | 0.938 | 0.713 | 0.693 | 0.967 | 0.955 | |
NM-SW, uninoculated sole wheat; NM-MW, uninoculated wheat mixed with faba bean; Fm-SW, inoculated sole wheat; Fm-MW, inoculated wheat mixed with faba bean. Similar symbols are also used for faba bean plants. Logistic models accounted for 87.8–99.9% of the variation in biomass data, and for 92.1–98.4% and 69.3–99.1% of the variation in N and P uptake data. r, rate constant of per capita biomass production or net N and P uptake; Ymax, maximum attainable per capita biomass production or net N and P uptake; R2 indicates the goodness-of-fit determination coefficient accounted for by each linear model.
The maximum N uptake by both wheat and faba bean plants in response to inoculation and the competing plant species showed similar trends to the biomass production (Fig. 1c, d). One exception was that inoculation enhanced N uptake of sole wheat by 19.0% compared with uninoculated plants at harvest and the difference between sole and mixed treatments was detected at day 54. The maximum N uptake values by mycorrhizal sole and mixed faba bean plants were 139.6 and 111.3% higher than by uninoculated plants (Table 1). The difference in cumulative N uptake between sole and mixed treatments by inoculated plants was larger than that by uninoculated plants. The divergences of N uptake trajectories of faba bean plants between inoculated and uninoculated, sole and mixed treatments were detectable after 59 and 42 d respectively (Fig. 1c, d; Table S2).
Cumulative P uptake by wheat was promoted by mycorrhizal inoculation in contrast to biomass production and cumulative N uptake. The maximum P uptake of inoculated plants in sole and mixed treatments increased by 88.7 and 28.8% compared to uninoculated plants (Table 1). The corresponding values for faba bean were 506.6 and 107.1% at harvest. No difference in cumulative P uptake was observed between sole and mixed treatments for uninoculated wheat or faba bean (Fig. 1e, f). The divergences in P uptake trajectories between inoculated and uninoculated and sole and mixed treatments were detectable after 42 and 42 d for faba bean and 54 and 48 d for wheat (Table S2).
In general, N concentrations of shoots and seeds of wheat were unaffected by inoculation or by competing plants except that at some harvests shoot N concentrations decreased in the presence of the competing plant species (Table S1, S3). N concentrations of uninoculated sole faba bean were in general significantly higher than those of inoculated plants or uninoculated plants in mixture. In contrast, shoot P concentrations of inoculated wheat were significantly higher than those of uninoculated plants from 59 d on and this difference declined over the growing period (Table S4). P concentrations in seeds of inoculated wheat plants were significantly higher than those of uninoculated plants. Similarly, shoot P concentrations of inoculated faba bean were significantly higher than those of uninoculated plants from 42 d on and the difference was undetectable by the final harvest. Overall, inoculation increased both shoot and seed P concentrations. Competition decreased shoot and seed P concentrations of inoculated faba bean but had little or no effect on wheat.
Instantaneous biomass and N and P uptake
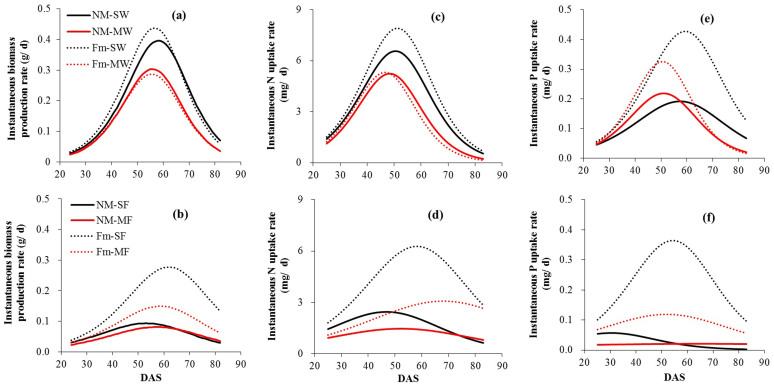
The time when wheat attained maximum instantaneous biomass production and N uptake was unaffected by competing faba bean and by inoculation (Fig. 2a, c; Table 2). Inoculation increased the maximum instantaneous biomass and N uptake by sole wheat but not by wheat plants in mixture. Competing wheat plants attained their maximum instantaneous P uptake rate 6 d (uninoculated) or 8 d (inoculated) prior to the corresponding sole plants. Inoculation had no significant effect on the date when wheat plants attained their maximum but increased the maximum instantaneous rates of P uptake by 122.4 and 48.4% for sole and mixed wheat, respectively (Fig. 2c; Table 2). Inoculation increased the maximum instantaneous biomass and N uptake by sole wheat but not by wheat in mixture. Competing wheat plants attained their maximum instantaneous P uptake rate 6 d (uninoculated) or 8 d (inoculated) prior to the corresponding sole plants. Inoculation had no significant effect on the date when they attained their maximum but did increase the maximum instantaneous rates of P uptake by 122.4 and 48.4% of sole and mixed wheat, respectively (Fig. 2c; Table 2).
Figure 2. Mean instantaneous per capita rates of shoot biomass production (a, b), N (c, d) and P production (e, f) by wheat (a, c, e) and faba bean plants (b, d, f) derived as the slopes of the logistic models fitted to cumulative data, derived using mean values of the rate constant, r (Table 1).
Statistical analysis of maximum instantaneous rates and their timings are presented in Table 2. NM-SW, uninoculated sole wheat; NM-MW, uninoculated wheat mixed with faba bean; Fm-SW, inoculated sole wheat; Fm-MW, inoculated wheat mixed with faba bean. Similar symbols are also used for faba bean plants.
Table 2. Estimated times (Tmax) and rates (Imax) of mean maximum instantaneous biomass production and uptake of N and P by wheat and faba bean plants. The values are derived from Eqn1.
| Wheat | Faba bean | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | NM-SW | NM-MW | Fm-SW | Fm-MW | NM-SF | NM-MF | Fm-SF | Fm-MF | |
| Biomass | Tmax (d) | 59 | 57 | 58 | 57 | 54 | 58 | 63 | 60 |
| Imax (g/d) | 0.396 | 0.303 | 0.437 | 0.286 | 0.093 | 0.081 | 0.277 | 0.149 | |
| N | Tmax (d) | 51 | 48 | 51 | 47 | 47 | 52 | 58 | 68 |
| Imax (mg/d) | 6.57 | 5.24 | 7.91 | 5.32 | 2.44 | 1.46 | 6.27 | 3.07 | |
| P | Tmax (d) | 57 | 51 | 59 | 51 | 30 | 46 | 54 | 52 |
| Imax (mg/d) | 0.192 | 0.219 | 0.427 | 0.325 | 0.057 | 0.020 | 0.364 | 0.119 | |
NM-SW, uninoculated sole wheat; NM-MW, uninoculated wheat mixed with faba bean; Fm-SW, inoculated sole wheat; Fm-MW, inoculated wheat mixed with faba bean. Similar symbols are also used for faba bean plants.
In contrast to wheat, the instantaneous biomass production and uptake of N and P by faba bean showed different patterns between inoculated and uninoculated plants. The mean instantaneous rates of biomass production of uninoculated plants remained relatively constant and low (~ 0.060 g d−1) and were not significantly influenced by the competing wheat. The dates when inoculated sole and mixed faba bean plants attained their maximum instantaneous biomass production were 63 and 60 d and the maximum biomass production in sole inoculated plants (0.277 g d−1) was about twice as high as in inoculated plants in mixture (0.149 g d−1) (Fig. 2b; Table 2). The instantaneous uptake rates of P showed similar patterns, e.g. the maximum P values in inoculated sole faba bean were three times as high as those in uninoculated sole plants, and maximum instantaneous P uptake rates occurred at 54 d (Fig. 2f; Table 2). The time taken for maximum N uptake by inoculated sole faba bean was 58 d while the corresponding value for inoculated plants in mixture was 68 d and the maximum N uptake by inoculated plants was about twice as high as in uninoculated faba bean (Fig. 2d; Table 2).
Plant development
Spike and pod weights were greatly affected by inoculation, growth in mixture and their interactions (Table 1). The cumulative spike and pod weights were influenced strongly by the presence of the competitor (except for uninoculated faba bean) and they differed between the two plant species (Fig. S2). Inoculation significantly enhanced the spike weight of sole wheat relative to uninoculated plants but there was no significant difference between inoculated and uninoculated treatments in wheat grown in mixture. Inoculation greatly enhanced pod weights of both sole and mix faba bean. The maximum pod weights of inoculated sole and mix faba bean were 407.9% and 111.0% higher than those of uninoculated plants at harvest.
The numbers of tillers and effective spikes in sole wheat were significantly higher than those in mixed plants. Inoculation did not significantly affect tillers or effective spikes (Table 3). By contrast, the flowers and pods of both sole and mix faba bean plants were significantly increased by inoculation. Inoculation increased the flowers by 104.4 and 105.0% in sole and mixed treatments, respectively, and the corresponding values for pods were 176.9 and 81.8%. By contrast, the flowers and pods of both sole and mix faba bean plants were significantly increased by inoculation. Inoculation increased the flowers by 104.4 and 105.0% in sole and mix treatments, respectively, and the corresponding values for pods were 176.9 and 81.8%.
Table 3. Growth and development of wheat and faba bean plants grown either separately or in mixture, inoculated with F. mosseae or uninoculated (mean ± SD, n = 18).
| Wheat | Faba bean | |||||||
|---|---|---|---|---|---|---|---|---|
| Index | NM-SW | NM-MW | Fm-SW | Fm-MW | NM-SF | NM-MF | Fm-SF | Fm-MF |
| Tillers (no. plant−1) | 5.9 ± 1.1a | 4.1 ± 0.5b | 5.6 ± 1.0a | 4.0 ± 0.7b | - | - | - | - |
| Effective spikes (no. plant−1) | 4.0 ± 0.6a | 3.2 ± 0.6b | 4.3 ± 0.6a | 3.1 ± 0.5b | - | - | - | - |
| Flowers (no. plant−1) | - | - | - | - | 8.9 ± 4.9c | 6.7 ± 3.2c | 18.2 ± 5.3a | 13.8 ± 4.0b |
| Pods (no. plant−1) | - | - | - | - | 1.3 ± 0.5c | 1.1 ± 0.3c | 3.6 ± 0.5a | 2.0 ± 0.5b |
| Stem diameter (mm) | 4.0 ± 0.2a | 3.9 ± 0.3a | 4.1 ± 0.3a | 3.9 ± 0.1a | 4.0 ± 0.2b | 3.7 ± 0.3b | 4.6 ± 0.4a | 3.9 ± 0.2b |
| Plant height (cm) | 86.4 ± 4.2a | 86.7 ± 2.7a | 82.2 ± 4.1a | 82.4 ± 5.8a | 57.2 ± 5.9c | 56.4 ± 4.3c | 73.9 ± 7.0a | 67.4 ± 7.7b |
NM-SW, uninoculated sole wheat; NM-MW, uninoculated wheat mixed with faba bean; Fm-SW, inoculated sole wheat;
Fm-MW, inoculated wheat mixed with faba bean. Similar symbols are also used for faba bean plants. Values with the same letter within each row are not significantly different for wheat and faba bean plants, respectively (one-way ANOVA, P< 0.05).
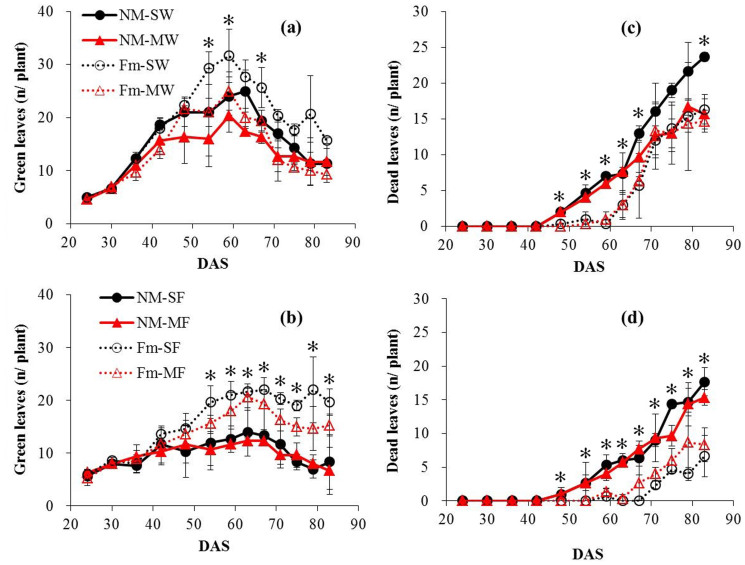
AMF inoculation and growth in mixture had a significant influence on stem diameter and plant height but no interaction was found (Table S1). Inoculation increased stem diameter and plant height of faba bean but the opposite trend in height of wheat occurred (Table 3). The green leaves of sole wheat plants were slightly longer than those of plants in mixture throughout the growing period and were not significantly affected by inoculation. Inoculation markedly decreased the occurrence of dead leaves of wheat compared to uninoculated plants, both sole and in mixture, and the divergence time was 48 d (Fig. 3a, c; Table S2). Inoculation significantly increased green leaves after 54 d and decreased dead leaves after 48 d in both sole and mixed faba bean and at the end of the experiment the number of total green leaves of sole and mixed faba bean plants were 2.36 and 2.50 times higher than those of the corresponding uninoculated plants (Fig. 3b, d; Table S2).
Figure 3. Green leaves and dead leaves per plant of wheat and faba bean plants (mean ± SD, n = 3) grown either separately or in mixture, inoculated with F. mosseae or uninoculated.
NM-SW, uninoculated sole wheat; NM-MW, uninoculated wheat mixed with faba bean; Fm-SW, inoculated sole wheat; Fm-MW, inoculated wheat mixed with faba bean. Similar symbols are also used for faba bean plants. * Indicates significant difference between inoculated and uninoculated treatments at P <0.05.
Soil Nmin and Olsen-P
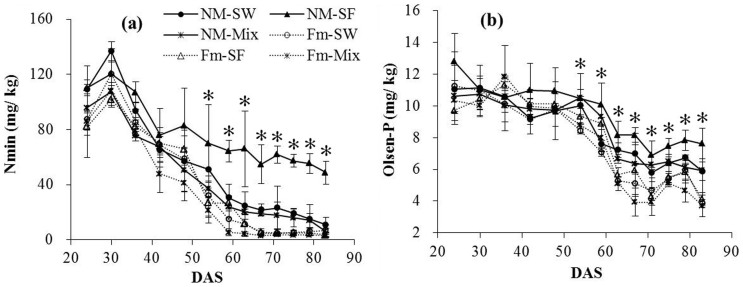
Soil Nmin concentrations decreased over the growing periods and the decline was rapid within 60 days. Soil Nmin in uninoculated sole faba bean showed the highest value over the whole growing period (Fig. 4a). Soil Nmin values of the inoculated treatments were lower than those of uninoculated treatments and the divergence was detectable after 54 d. We also calculated the N balance after harvest. Assuming that N losses via nitrate leaching and NOx emission were similar across all the treatments, N difference among treatments was mainly attributable to N2 fixation. Inoculation stimulated N2 fixation of faba bean and N in the mixture treatment increased by 81 mg pot−1 while the value in the uninoculated treatment was 24 mg pot−1 (Fig. S3).
Figure 4. Soil Nmin and Olsen-P concentrations at each harvest of wheat and faba bean plants (mean ± SD, n = 3) grown in either separately or in mixture, inoculated with F. mosseae or uninoculated.
NM-SW, uninoculated sole wheat; NM-SF, uninoculated sole faba bean; NM-Mix, uninoculated mixture; Fm-SW, inoculated sole wheat; Fm-SF, inoculated sole faba bean; Fm-Mix, inoculated mixture. * Indicates significant difference between inoculated and uninoculated treatments at P< 0.05.
The temporal dynamics of soil available P showed a similar trend to Nmin concentration except that Olsen-P remained constant during the first 60 days (Fig. 4b). The influence of inoculation was detectable after 54 days and thereafter it remained relatively constant. The soil Olsen-P contents in uninoculated sole faba bean were higher than those in other treatments.
Rhizosphere pH, acid phosphatase activity and carboxylates
Rhizosphere soil was collected by the shaking method and determinations were made at 30 and 70 days. Rhizosphere pH and acid phosphatase activities were affected only by plant species (Table 1). The rhizosphere pH values of faba bean were significantly lower than those of wheat irrespective of sole and mixed treatments. Inoculation decreased soil pH in the rhizosphere of faba bean at 70 d while the competing plants did not have any significant effect on rhizosphere pH (Table 4). The acid phosphatase activities (APA) in the rhizosphere of wheat were much lower than those in the rhizosphere of faba bean. In general, inoculation did not have any significant influence on acid phosphatase activity in the rhizosphere. The APA activities of faba bean at 30 d were higher than those at 70 d (Table 4).
Table 4. Rhizosphere pH values, acid phosphatase activities and carboxylate concentrations of wheat and faba bean plants grown in either separately or in mixture, inoculated with F. mossese or uninoculated (mean ± SD, n = 6).
| Wheat | Faba bean | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Index | DAS | NM- SW | NM- MW | Fm- SW | Fm- MW | NM-SF | NM-MF | Fm- SF | Fm- MF |
| pH | 30d | 7.6 ± 0.1a | 7.6 ± 0.2a | 7.4 ± 0.1a | 7.8 ± 0.4a | 7.3 ± 0.1a | 7.1 ± 0.1a | 7.0 ± 0.3a | 7.3 ± 0.1a |
| 70d | 7.9 ± 0.0a | 8.1 ± 0.2a | 8.0 ± 0.1a | 8.1 ± 0.1a | 7.8 ± 0.0a | 7.8 ± 0.0a | 7.5 ± 0.2b | 7.4 ± 0.2b | |
| APA µmol PNP h−1(g−1 soil) | 30d | 1.1 ± 0.6a | 1.6 ± 0.7a | 3.2 ± 1.1a | 1.9 ± 1.6a | 11.9 ± 2.8b | 20.8 ± 2.5a | 28.5 ± 3.7a | 23.8 ± 6.3a |
| 70d | 1.8 ± 0.4a | 2.9 ± 2.6a | 3.7 ± 0.6a | 3.8 ± 2.2a | 10.6 ± 4.8a | 6.2 ± 2.1a | 10.4 ± 1.9a | 8.2 ± 4.6a | |
| Malate µmol (g−1 soil) | 30d | 0 | 0 | 0 | 0 | 2.7 ± 3.3a | 11.7 ± 10.6a | 6.8 ± 6.6a | 12.1 ± 7.2a |
| 70d | 1.8 ± 2.6b | 4.8 ± 4.5b | 10.3 ± 3.3ab | 37.8 ± 46.5a | 2.9 ± 2.8b | 40.6 ± 33.3b | 39.6 ± 21.6b | 103.5 ± 61.6a | |
| Citrate µmol (g−1 soil) | 30d | 2.0 ± 0.9a | 2.0 ± 0.6a | 1.0 ± 0.6b | 2.4 ± 1.0a | 7.1 ± 3.7b | 10.7 ± 5.0ab | 15.1 ± 4.3a | 13.6 ± 4.8a |
| 70d | 10.8 ± 2.8a | 9.2 ± 2.5a | 6.8 ± 1.8a | 19.2 ± 19.0a | 14.8 ± 9.2b | 55.9 ± 33.8a | 27.2 ± 11.3ab | 50.9 ± 33.6a | |
NM-SW, uninoculated sole wheat; NM-MW, uninoculated wheat mixed with faba bean; Fm-SW, inoculated sole wheat; Fm-MW, inoculated wheat mixed with faba bean. Similar symbols are also used for faba bean plants. APA, acid phosphatase activities. Values with the same letter within each row are not significantly different for wheat and faba bean plants, respectively (P<0.05).
Neither the competing plant nor inoculation had any significant effect on the exudation of carboxylates. Four different carboxylates (malate, citrate, fumarate and T-aconitate) were detected in the rhizosphere. As the concentrations of fumarate and T-aconitate were below the detection limits, only the malate and citrate results are presented. The exudation of carboxylates by faba bean was much higher than by wheat (~5–21 times at 30 d and ~2–7 times at 70 d) and the carboxylates at 70 days were much higher than at the earlier growth stage at 30 d (Table 4). At 30 days only citrate was detected in the rhizosphere of wheat while small amounts of malate were detected in that of faba bean. At 70 d the amount of malate and citrate were comparable, and both competing plants and inoculation tended to increase the exudation of the carboxylates.
Discussion
Using the approach of Trinder et al25 we were able to characterize how AMF regulated the competition process of wheat-faba bean in N and P limiting soils, based on the dynamic trajectories of biomass and N and P uptake by the plants. The main finding of the present study is that AMF mediated wheat-faba bean competition and acted asymmetrically in favor of faba bean. Wheat was a strong competitor and had the competitive advantage in both N and P acquisition whereas faba bean was a weak competitor which showed nutrient use complementarity to wheat. The experiment provides interesting results regarding the links between temporal cumulative and instantaneous biomass production and N and P capture to determine how these processes co-varied in competing individuals.
In the present study P was the major growth-limiting nutrient, followed by N. Adding 100 mg/kg N did not meet the requirement of the plants throughout the growth period (218.3 mg/kg soil available N vs about 250 mg/pot N taken up by the plants). In addition, the rapid decline in soil Nmin concentration during the 40–60 d period and values near zero from 60 d on, and the depletion of available P from 60 days on indicate that competition was initially due to N, followed by both N and P at later growth stages when the plants entered the reproductive phase (Fig. 4). Competition for N and P was further indicated by the early senescence of non-mycorrhizal faba bean and wheat plants, irrespective of sole growth or growth in mixture (Fig. 3). When in competition, wheat attained the maximum instantaneous N and P capture approximately five days before the point at which it attained maximum instantaneous biomass production. The results indicate that the plants might have detected the competitor and responded physiologically to resource limitation prior to the response of the biomass. Consequently, the numbers of tillers and effective spikes of wheat decreased significantly when competing with faba bean (Table 3). Our results highlight the notion proposed by Trinder et al26 and others33 that sequential measurements of the processes linking biomass production and resource capture by the competitors are important in understanding the dynamics and mechanisms of competitive interactions. Competitive capture of other resources (other nutrients and water) was partially excluded in the pot experiments as nutrients (other than N and P) and water were in sufficient supply. Under field conditions temporal competition and complementarity for light and N have been shown to affect the outcome of yield in a durum wheat-winter pea intercropping system36. However, the influence of shading cannot be evaluated using the available data although shading was carefully avoided by leaving a considerable space between adjacent experimental pots.
The present experiment indicates that wheat is a strong competitor for N and P (Fig. 1c, e). Cereals often have a stronger competitive ability to take up N than legumes37. When in competition, the maximum instantaneous N and P capture values of uninoculated wheat were 3.59 and 10.95 times higher than those of faba bean (Table 2). In addition, the maximum instantaneous N and P capture of wheat in mixture occurred 3–5 days earlier than those in isolation and the difference continued to the final harvest, particularly at later harvests (~ day 50 at booting stage), indicating a strong neighbor effect on N and P acquisition and the superior competition of wheat proceeding throughout the growth period when wheat shifted from vegetative to reproductive growth. Inoculation increased the magnitude of the difference in instantaneous P uptake by wheat between sole plants and plants in mixture, but did not change the time taken to attain the maximum instantaneous P capture (Table 2). When in competition the instantaneous N and P capture of both non-mycorrhizal and mycorrhizal faba bean remained relatively constant throughout the growth period (Fig. 2d, f).The productivity of the cereal relies heavily on N input38. The higher competitive ability might be due to the greater root length of wheat and faster root growth compared to faba bean. In addition, the inherited fast growth rate36 and consequently high demand for N and P by wheat might provide a strong sink to enforce nutrient acquisition.
AM fungi are known to affect plant growth and nutrition and thus alter competitive relationships13,39,40. In the presence of AMF the biomass of faba bean increased markedly from day 54 until the final harvest, especially when grown separately. The biomass of wheat remained constant regardless of whether it was grown separately or in mixture (Fig. 1a). This study confirms previous studies indicating that wheat is less AMF-dependent41, although AMF mediated P uptake might be still active with no increase in P content and biomass42,43. By contrast, a strong positive response by faba bean to AMF inoculation was observed (Fig. S1), as indicated by the substantial promotion of plant growth (Fig. 1b) and increase in flowers and pods (Fig. S2) of faba bean in the presence of F. mosseae when grown separately. Our results indicate that when in competition AMF asymmetrically favored faba bean (Fig. 1b). These results are partly in agreement with previous studies showing that AMF favored legumes when competing with grass species15,40. However, these studies and other AMF-related plant competition experiments44,45 suggest that species that perform better in association with AMF are able to increase their own nutrient uptake and lead to a growth depression in the neighboring competing plant species. In the present study, in the presence of AMF the increased growth of faba bean did not inhibit wheat growth, and the competitive advantages of wheat persisted throughout the growing period. It is unlikely that inoculation with F. mosseae asymmetrically supplied soil nutrients to faba bean, as both instantaneous P and cumulative P capture by wheat were also greatly enhanced (Fig. 2c, e). Thus, root competition alone cannot explain the pattern of plant growth, and mycorrhiza-mediated resource competition is important. The most likely explanation for competitive enhancement of mycorrhizal faba bean is that complementarity resource use between faba bean and wheat is enforced by AMF. This is reflected in the dynamic patterns of both N and P acquisition. In the absence of AMF, Nmin and available P were mostly taken up by wheat while soil N and P were less available for faba bean (Fig. 4). In the presence of AMF, although wheat was still able to take up Nmin and P, AMF might have increased N2 fixation and mobilized P sources otherwise not available to wheat, and thus increased N and P uptake by faba bean. AMF are known to affect N2 fixation in legumes by increasing the numbers of nodules, nitrogenase activity, the leghaemoglobin content of nodules, and shoot biomass46. The increase in N-content may be a consequence of a P-mediated stimulation of N2-fixation by mycorrhiza47. In the present study the calculated N derived from N2 fixation at harvest was greatly increased in the mycorrhizal faba bean (Fig. S3). However there was no significant difference in nodule numbers between inoculated and uninoculated faba bean (Fig. S4). With respect to P, accumulating evidence shows that faba bean is effective in P acquisition and thus is less dependent on P fertilization or P sources7,48. Compared to wheat, the large seeds of faba bean contain high P concentrations (Tab. S4) and thus can provide sufficient P for faba bean growth at early growth stages. Faba bean shows strong rhizosphere activities including the exudation of carboxylates48 and acid phosphatase activity, and soil acidification7 which help the plants to mobilize P under P limiting conditions. In the present study, as summarized in Fig. 5, AMF might increase P acquisition of faba bean by enhanced exploration of soil P not available to plant roots by the external mycelium, or increase the exudation of citrate and malate and decrease the rhizosphere pH (Table 4). Faba bean has been shown to take up P from organic-P49 and AMF might increase P uptake from organic P via enhanced ALP activity of the host plants50. It is also likely that mycorrhiza-mediated rhizosphere changes might facilitate P uptake by competing wheat and this needs further investigation. Interspecific P facilitation is highlighted to contribute to overyielding in intercropped maize and faba bean in both pot48,49 and field32 experiments. In addition, mycorrhizal faba bean requires extra P for nitrogen fixation, and N2 fixation might further affect P mobilization in the rhizosphere of faba bean.
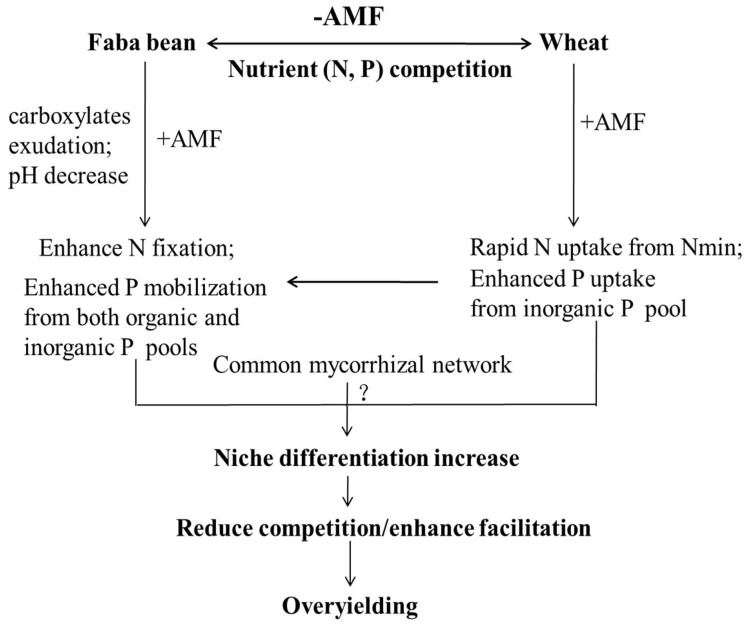
Figure 5. Belowground interspecific competitive/facilitative processes in mixed crop production systems.
The diagram shows how AMF alter interspecific plant interactions directly or indirectly via their effects on nutrient acquisition to attain mutually beneficial strategies: (1) Depletion of Nmin by wheat plants facilitating N2 fixation of mycorrhizal faba bean plants; Promotion of N2 fixation as a result of improved P nutrition by inoculation; Enhanced P uptake of wheat plants by inoculation; (2) Uptake of inorganic P by wheat plants enhancing the exploration of inorganic and possibly also inorganic P by mycorrhizal faba bean plants; (3) Enhancement of rhizosphere activity (pH, carboxylates and acid phosphatase activities) of faba bean by inoculation; (4) possible N (P?) transfer by common mycorrhizal networks (CMNs) between faba bean and wheat plants.
Faba bean and wheat are interconnected by mycorrhizal networks. Previous studies have shown that N fixed by legumes can be transferred to neighboring cereal crops51. Trivial N transfer has also been reported52. In addition, interspecific P facilitation from larger plants can relieve P deficiency in smaller plants12,23. However, recent studies show that competition for P favors the larger and older plants (Cucumis sativus) over smaller and younger seedlings (Solanum lycopersicon)19, and P limitation between interspecific (Linumusitatis simum vs Sorghum bicolor)53 and intraspecific plants (Andropogon gerardii) led to size inequality54. We did not directly demonstrate N and P transfer between faba bean and wheat. However, on the basis of the results of the present experiments, direct transfer of N and P via CMNs may be minor as wheat and faba bean are in strong competition for limiting soil N and P throughout the growth period. Mechanisms underlying CMNs in plant competitive outcomes are highly complex19. Facilitation and/or competition may depend on the identity and diversity of AM fungal species53, the age and size of the plants12, and the supply levels of important nutrients.
A number of studies on legume-cereal interactions have demonstrated that temporal niche separation may be attributable to competition dynamics55. In the present study the temporal dynamics of growth and nutrient uptake by wheat and faba bean showed some complementarity. In the absence of AMF, pre-emption of soil nutrients by wheat led to relatively low and constant instantaneous N and P acquisition by faba bean. Consequently, low P and high cost for N2 fixation inhibited the growth of faba bean throughout the growth period. Our results support the notion that a small difference in early competition exerts a large effect on overall competition during the whole crop development phase56. In the presence of AMF the significant correlations between biomass production of faba bean and N and P uptake throughout the growth period indicate that N and P are synergistically enhanced by AMF and consequently lead to increased competitive ability of faba bean (Fig. 1b, d, f). The time taken to attain maximum instantaneous P uptake by mycorrhizal faba bean was very similar to that taken by competing wheat and 16 days prior to maximum N uptake by faba bean. Maximum N uptake by faba bean occurred six days later than by competing wheat (Table 2). These results indicate that faba bean depends on AMF to supply the extra P required for nitrogen fixation, and temporal separation of N and P acquisition by wheat and faba bean is important for the competitive co-existence of faba bean and wheat (Fig. 5). Future experiments should incorporate sequential measurement of N2 fixation activity and P requirement of the root nodules on the legume.
Conclusions
This study demonstrates that temporal cumulative and instantaneous biomass production of wheat and faba bean were closely correlated with dynamic N and P capture by the competing individuals. Wheat is a strong competitor and showed competitive advantages in both N and P acquisition. Faba bean was inferior to wheat in this respect throughout the growth period, possibly due to the early competitive disadvantages of N and P acquisition. Inoculation with AMF mediated wheat-faba bean competition and acted asymmetrically in favor of faba bean. The greater N and P acquisition by mycorrhizal faba bean indicates that AMF enforce complementary resource use between faba bean and wheat, possibly by increasing nitrogen fixation and enhancing P mobilization processes in the rhizosphere. The temporal separation of N and P uptake is also attributable to competitive co-existence of wheat and faba bean. Our results highlight the importance of studying competition processes rather than the single outcome of the final harvest. The mechanistic understanding of the dynamic patterns linking the nutrient acquisition and physiological processes of competing plants can be instructive for optimizing intercropping systems in terms of maximizing both nutrient capture and biomass yields. Recent accumulating evidence indicates that the diversity and richness of AMF are increased in intercropping vs monocropping systems, thus a favorable intercropping system should take account of the indigenous communities of AMF to boost productivity in a sustainable way.
Methods
Experimental design
The soil used was collected from the top 10 cm of a soil profile at the Shangzhuang Experimental Station (39°59′N, 116°17′E) of China Agricultural University, Beijing, China. The soil type was silt loam with a bulk density of 1.44 g cm−3 and a pH of 7.86 (1: 5, soil: water suspension). The soil had an organic carbon content of 10.1 g kg−1, total N of 0.63 g kg−1, mineral N content (Nmin) of 18.3 mg kg−1, NH4Ac-exchangeable K of 74.1 mg kg−1 and low available P (Olsen-P) of 6.72 mg·kg−1. The soil was air-dried, sieved (2 mm), and sterilized by γ-radiation (>25 k Gray, Beijing Radiation Application Research Center). Mineral nutrients other than P were mixed thoroughly into the soil at the following concentrations (mg kg−1dry soil): 100 N as Ca(NO3)2 and 200 K as K2SO4.
The inoculum of F. mosseae BGC YN05 consisted of spores, mycelium and fine root segments, and was propagated in a 1:1 (v/v) mixture of zeolite and river sand with Zea mays L. for four months in a growth chamber. Each mycorrhizal pot received 60 g of inoculum (approximately 2000 spores per pot) and the non-mycorrhizal pots were supplied with an equal amount of sterilized (105°C for 3 hours) inoculum. To minimize differences in microbial communities in mycorrhizal and non-mycorrhizal soils, 5 mL of AMF-free soil filtrate was added to each non-mycorrhizal pot. The filtrate was prepared from 100 g of a mixture containing equal parts of each inoculum (non-sterile) in 500 mL of reverse osmosis (RO) water, filtered through filter paper (No. 4) and diluted to 800 mL. Each pot contained 2 kg soil. Plastic pots (260 mm diameter and 175 mm high) were firstly filled with 1.4 kg soil at the bottom and then 60 g of AM fungal inoculum and 400 g soil were added. The seeds were then sown and covered with 140 g of sterilized soil. Seeds of wheat (Triticum aestivum L. cv. Yunmai-42) and faba bean (Vicia faba L. cv. 89-147) were surface-sterilized with 10% H2O2 for 30 min and rinsed thoroughly in de-ionized water. Seeds were germinated on moist filter paper in the dark at 25/20°C (day/night) for 60 h (faba bean) or 36 h (wheat) before sowing. Germinated seeds were grown solely (one individual per pot, either wheat or faba bean) or mixed (one wheat and one faba bean) in the pot. In the mixture treatment wheat and faba bean seeds were sown in the center of the pots and 2.5 cm apart. Faba bean plants were inoculated with R. leguminosarum bv. NM353 (provided by the Culture Collection of Beijing Agricultural University, CCBAU) by seed inoculation. The germinated seeds of faba bean were soaked with NM353 inoculation liquid for 30 min and then sown in each pot and 5 mL liquid inoculum was also added to each pot. Thus, the two plant species were grown separately or together in mixture, with or without inoculation with F. mosseae. There were 39 replicates of each treatment and 13 destructive harvests were taken every 4–6 days from days 24 to 83. Each plant combination was set up in triplicate.
Under field conditions the sowing density ratio of faba bean to wheat is 1: 3. Our preliminary experiments indicated that in pot experiments the total dry weights of three wheat seedlings were equivalent to that of one individual seedling (Li et al., unpublished). We therefore decided to keep one individual of each species in order to simulate the effect of the presence of the competing plant and avoid complex intra- and inter-specific interactions between the two plant species. Window screens were used to prevent shading from faba bean at later growth stages. The experiment was carried out in a glasshouse at China Agricultural University, Beijing, China. The light intensity was approximately 800–1200 µmol·m−2·s−1 with a night-day temperature range of 18–28°C. All pots were irrigated with deionized water daily and the pots were weighed every three days to maintain the soil moisture content at 20% (w/w).
Plant harvest, sampling and analysis
At each harvest shoots and roots were separated and care was taken to separate the roots of the two plants in each mixture pot. Subsamples of approximately 10 g or 30 g fresh roots of wheat and faba bean were used for determination of AM colonization. The remaining roots and the shoot samples were heated at 105°C for 30 min and then oven-dried (72 h, 75°C). Ground dry samples were digested in a mixture of concentrated H2SO4 and H2O2. Plant N concentrations were determined using the micro-Kjeldahl method and P concentrations colorimetrically by the molybdo-vanado-phosphate method57.
AM colonization
The percentage root length colonized by AMF was estimated visually after clearing washed roots in 10% KOH and staining with 0.05% (v/v) trypan blue in lactic acid according to Phillips and Hayman58. The root segments were checked under a compound microscope at 200 × magnification. Thirty pieces of root from each sample were randomly selected and mounted on microscope slides to assess mycorrhizal root length (M%) and arbuscule abundance (A%). The M% was an estimation of the amount of root cortex that was colonized by AM fungi relative to the whole root system, and A% was the arbuscule frequency across the entire root system.
Soil Nmin and Olsen-P analysis
The concentration of soil mineral N (Nmin, NH4+ + NO3−) was measured by a continuous-flow technique (Bran and Luebbe, Norderstedt, Germany) after extracting 12 g fresh soil with 100 mL 0.01 M CaCl2 on a shaker (180 rpm) for 1 h at 25°C. Soil samples were air-dried, ground, sieved (2.0 mm) and stored for further analysis. Soil was analyzed for 0.5 mol L−1 NaHCO3 (pH 8.5)-extractable P (Olsen-P)57.
Rhizosphere sampling and analysis
Rhizosphere soil was collected by the shaking method. Rhizosphere carboxylates, pH and acid phosphatase activity were determined at 30 days (when faba bean was at the seedling stage and wheat was at the tillering stage) and at 70 days when faba bean was at pod-filling stage and wheat was at filling stage. Roots from intact plants were rinsed for 1 min with 200 μmol L−1 CaCl2 to collect exudates. Three subsamples (10 mL) were used for the measurement of rhizosphere carboxylates, pH and acid phosphatase activity. For the measurement of carboxylates a microbial inhibitor (Micropur, Sicheres Trinkwasser, Germany, at 0.01 g L−1) was transferred into the collection solution to prevent microbial degradation before measurement. Carboxylates in the root exudates were analyzed by high-performance liquid chromatography (HPLC) in ion suppression mode. Separation was conducted on a 250 mm × 4.6 mm reversed-phase column (Alltima C18, Alltech Associates, Deerfield, MA). The mobile phase was 25 mmol L−1 KH2PO4 (pH 2.25) with a flow rate of 1 mL min−1 at 31°C and UV detection at 214 nm. The sample injection volume was 20 μL. Identification of carboxylates was carried out by comparing retention time and absorption spectra with those of known standards59.
Acid phosphatase activity of rhizosphere soil was determined according to Alvey et al60. The analysis involved colorimetric estimation of the p-nitrophenol released by phosphatase activity after incubation of some soil with 4 mL of 0.04 M sodium malate buffer (pH 5.3) at 28°C for 30 minutes. The reaction was stopped with 0.5 M NaOH and absorbance was measured spectrophotometrically at 405 nm. One unit of acid phosphatase activity was defined as the activity per gram soil which produced 1 μmol p-nitrophenol per hour.
Rhizosphere pH was determined with a PB-20 pH meter (Sartorius AG, Goettingen, Germany). Because the amount of rhizosphere soil collected differed greatly among treatments, the following equation was derived from a preliminary experiment to calculate the modified pH (soil: solution = 1: 2.5) from the measured pH: y = 0.886 w0.037, R2 = 0.85, where y is the ratio of modified pH to the original pH and w is the dry weight of the rhizosphere soil. The modified pH was regarded as the soil pH in the rhizosphere48.
Statistical analysis
The biomass (Y; g) of crop plants grown from seed to maturity is typically a sigmoid function of time, t. We employed the calculation of Trinder et al25 to compute the shoot biomass, N and P uptake, and weight of spikes and pods during plant growth. Root biomass and N and P uptake were not included due to the difficulty of isolating the intermingled roots of the two plant species. The logistic equation is:
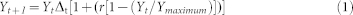 |
(r is the rate constant (d−1) for per capita biomass production; Ymaximum is a maximum to which Y tends asymptotically) was used to separately fit the data of shoot biomass and N and P uptake by simultaneously adjusting r and Ymaximum to maximize R2 with the SOLVER in Microsoft Excel 2010. The instantaneous rates were derived as the slopes of the logistic models fitted to the cumulative biomass and N and P content data.
N fixation was calculated after harvest according to the equation 2:
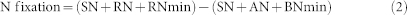 |
(SN, shoot N capture; RN, root N capture; RNmin, residual soil Nmin after harvest; SN, seed N; AN, added fertilizer N; BNmin, basal soil Nmin).
Two-way analysis of variance was performed on all datasets and treatments (except Nmin and Olsen-P) to evaluate the effects of inoculation and competition and their interactions for each plant species (wheat and faba bean) using SPSS 16.0 for Windows (SPSS Inc., Chicago, IL). Significant differences between pairs of mean values were determined with Duncan's multiple range test at the 5% level. All data were checked for normality and homogeneity of variances prior to analysis of variance. One way analysis of variance was performed when significant P values for factors or their interaction were obtained from two-way analysis of variance to analyze inoculation and competition effects on growth parameters (tillers, effective spikes, flowers, pods, stem diameter, and plant height), soil pH and APA, colonization indexes (M%, A%), and shoot N and P concentrations, within the same plant species. One-way analysis of variance was also performed to compare the divergence time of biomass, N and P uptake, number of green and dead leaves, and N and P concentration within the same plant species as affected by AMF and competition treatments.
Author Contributions
X.Q., H.G.L., F.S.Z. and J.L.Z. designed the research; X.Q., S.K.B., C.J.L. and Y.D. performed the research; X.Q., H.G.L. and J.L.Z. analyzed the data; and X.Q., H.G.L., P.C. and J.L.Z. wrote the paper.
Supplementary Material
supplementary tables/figures
Acknowledgments
This work was funded by the State Key Basic Research and Development Plan of China (2013CB127402), the Projects of International Cooperation and Exchanges NSFC (31210103906), and the National Natural Science Foundation of China (Grant Nos. 31272251, 31421092). The inoculum was kindly provided by Professor Youshan Wang at the Institute of Plant Nutrition and Resources, Beijing Academy of Agriculture and Forestry Research, China.
References
- Willey R. Intercropping-its importance and research needs. 1. Competition and yield advantages. Field Crop Abs. 32, 1–10 (1979). [Google Scholar]
- Vandermeer J. The Ecology of Intercropping. [Vandermeer, J. (ed.) (Cambridge, UK: Cambridge University Press, 1989). [Google Scholar]
- Zhang F. & Li L. Using competitive and facilitative interactions in intercropping systems enhances crop productivity and nutrient-use efficiency. Plant Soil 248, 305–312 (2003). [Google Scholar]
- Chen Y. et al. Wheat powdery mildew and foliar N concentrations as influenced by N fertilization and belowground interactions with intercropped faba bean. Plant Soil 291, 1–13 (2007). [Google Scholar]
- Hector A. et al. Plant diversity and productivity experiments in European grasslands. Science 286, 1123–1127 (1999). [DOI] [PubMed] [Google Scholar]
- Hauggaard-Nielsen H. & Jensen E. S. Facilitative root interactions in intercrops. Plant Soil 274, 237–250 (2005). [Google Scholar]
- Li L. et al. Diversity enhances agricultural productivity via rhizosphere phosphorus facilitation on phosphorus-deficient soils. Proc. Natl. Acad. Sci. USA 104, 11192–11196 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Marschner P., Solaiman Z. & Rengel Z. Belowground interactions between intercropped wheat and Brassicas in acidic and alkaline soils. Soil Biol. Biochem. 39, 961–971 (2007). [Google Scholar]
- Li L. et al. Root distribution and interactions between intercropped species. Oecologia 147, 280–290 (2006b). [DOI] [PubMed] [Google Scholar]
- Fernández-Aparicio M., Amri M., Kharrat M. & Rubiales D. Intercropping reduces Mycosphaerella pinodes severity and delays upward progress on the pea plant. Crop Prot. 29, 744–750 (2010). [Google Scholar]
- Smith S. E. & Read D. J. [The symbionts forming arbuscular mycorrhizas]. Mycorrhizal Symbiosis, 3rd edn [Smith, S.E. & Read, D.J. (ed.)] [13–41] (Academic Press, Cambridge, UK, 2008). [Google Scholar]
- van der Heijden M. G. A. & Horton T. R. Socialism in soil? The importance of mycorrhizal fungal networks for facilitation in natural ecosystems. J. Ecol. 97, 1139–1150 (2009). [Google Scholar]
- Mariotte P. et al. Arbuscular mycorrhizal fungi reduce the differences in competitiveness between dominant and subordinate plant species. Mycorrhiza 23, 267–277 (2013). [DOI] [PubMed] [Google Scholar]
- Veiga R. S., Jansa J., Frossard E. & van der Heijden M. G. A. Can arbuscular mycorrhizal fungi reduce the growth of agricultural weeds? PloS one 6, e27825. 10.1371/journal.pone.0027825 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagg C., Jansa J., Schmid B. & van der Heijden M. G. A. Belowground biodiversity effects of plant symbionts support aboveground productivity. Ecol. Lett. 14, 1001–1009 (2011). [DOI] [PubMed] [Google Scholar]
- Nazeri N. K., Lambers H., Tibbett M. & Ryan M. H. Moderating mycorrhizas: arbuscular mycorrhizas modify rhizosphere chemistry and maintain plant phosphorus status within narrow boundaries. Plant, Cell Environ. 37, 911–921 (2014). [DOI] [PubMed] [Google Scholar]
- Gosling P., Mead A., Proctor M., Hammond J. P. & Bending G. D. Contrasting arbuscular mycorrhizal communities colonizing different host plants show a similar response to a soil phosphorus concentration gradient. New Phytol. 198, 546–556 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teste F. P., Veneklaas E. J., Dixon K. W. & Lambers H. Complementary plant nutrient-acquisition strategies promote growth of neighbour species. Funct. Ecol. 10.1111/1365-2435.12270 (2014). [DOI] [Google Scholar]
- Merrild M. P., Ambus P., Rosendahl S. & Jakobsen I. Common arbuscular mycorrhizal networks amplify competition for phosphorus between seedlings and established plants. New Phytol. 200, 229–240 (2013). [DOI] [PubMed] [Google Scholar]
- Montesinos-Navarro A., Segarra-Moragues J. G., Valiente-Banuet A. & Verdú M. Plant facilitation occurs between species differing in their associated arbuscular mycorrhizal fungi. New Phytol. 196, 835–844 (2012). [DOI] [PubMed] [Google Scholar]
- Hodge A., Campbell C. D. & Fitter A. H. An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Letters to nature 413, 297–299 (2001). [DOI] [PubMed] [Google Scholar]
- Teste F. P., Simard S. W., Durall D. M., Guy R. D. & Berch S. M. Net carbon transfer between Pseudotsuga menziesii var. glauca seedlings in the field is influenced by soil disturbance. J. Ecol. 98, 429–439 (2010). [Google Scholar]
- Deslippe J. R. & Simard S. W. Below-ground carbon transfer among Betula nana may increase with warming in Arctic tundra. New Phytol. 192, 689–698 (2011). [DOI] [PubMed] [Google Scholar]
- Babikova Z. et al. Underground signals carried through common mycelial networks warn neighbouring plants of aphid attack. Ecol. Lett. 16, 835–843 (2013). [DOI] [PubMed] [Google Scholar]
- Trinder C. J., Brooker R. W., Davidson H. & Robinson D. Dynamic trajectories of growth and nitrogen capture by competing plants. New Phytol. 193, 948–958 (2012). [DOI] [PubMed] [Google Scholar]
- Trinder C. J., Brooker R. W. & Robinson D. Plant ecology's guilty little secret: understanding the dynamics of plant competition. Funct. Ecol. 27, 918–929 (2013). [Google Scholar]
- Inal A., Gunes A., Zhang F. & Cakmak I. Peanut/maize intercropping induced changes in rhizosphere and nutrient concentrations in shoots. Plant Physiol. Bioch. 45, 350–356 (2007). [DOI] [PubMed] [Google Scholar]
- Temperton V. M., Mwangi P. N., Scherer-Lorenzen M., Schmid B. & Buchmann N. Positive interactions between nitrogen-fixing legumes and four different neighbouring species in a biodiversity experiment. Oecologia 151, 190–205 (2007). [DOI] [PubMed] [Google Scholar]
- Li Y., Ran W., Zhang R., Sun S. & Xu G. Facilitated legume nodulation, phosphate uptake and nitrogen transfer by arbuscular inoculation in an upland rice and mung bean intercropping system. Plant Soil 315, 285–296 (2009). [Google Scholar]
- Moyer-Henry K. A., Burton J. W., Israel D. W. & Rufty T. W. Nitrogen transfer between plants: A 15N natural abundance study with crop and weed species. Plant Soil 282, 7–20 (2006). [Google Scholar]
- Li L., Tang C. X., Rengel Z. & Zhang F. S. Chickpea facilitates phosphorus uptake by intercropped wheat from an organic phosphorus source. Plant Soil 248, 297–303 (2003a). [Google Scholar]
- Li L. et al. Interspecific facilitation of nutrient uptake by intercropped maize and faba bean. Nutr. Cyc. Agroecosys. 65, 61–71 (2003b). [Google Scholar]
- Andersen M. K., Hauggaard-Nielsen H., Ambus P. & Jensen E. S. Biomass production, symbiotic nitrogen fixation and inorganic N use in dual and tri-component annual intercrops. Plant Soil 266, 273–287 (2004). [Google Scholar]
- Tang H. L., Shen J. B., Zhang F. S. & Rengel Z. Interactive effects of phosphorus deficiency and exogenous auxin on root morphological and physiological traits in white lupin (Lupinus albus L.) Sci China Life Sci. 56, 313–323 (2013). [DOI] [PubMed]
- Zhang L. et al. Hyphosphere interactions between an arbuscular mycorrhizal fungus and a phosphate solubilizing bacterium promote phytate mineralization in soil. Soil Biol. Biochem. 74, 177–183 (2014). [Google Scholar]
- Bedoussac L. & Justes E. Dynamic analysis of competition and complementarity for light and N use to understand the yield and the protein content of a durum wheat-winter pea intercrop. Plant Soil 330, 37–54 (2010). [Google Scholar]
- Jensen E. Grain yield, symbiotic N2 fixation and interspecific competition for inorganic N in pea-barley intercrops. Plant Soil 182, 25–38 (1996). [Google Scholar]
- Ofori F. & Stern W. R. Maize cowpea intercrop system-Effect of nitrogen-fertilizer on productivity and efficiency. Field Crops Res. 14, 247–261 (1986). [Google Scholar]
- Hart M. M., Reader R. J. & Klironomos J. N. Plant coexistence mediated by arbuscular mycorrhizal fungi. Trends Ecol. Evol. 18, 418–423 (2003). [Google Scholar]
- Scheublin T. R., van Logtestijn R. S. P. & van der Heijden M. G. A. Presence and identity of arbuscular mycorrhizal fungi influence competitive interactions between plant species. J. Ecol. 95, 631–638 (2007). [Google Scholar]
- Li H., Smith S. E., Holloway R. E., Zhu Y. & Smith F. A. Arbuscular mycorrhizal fungi contribute to phosphorus uptake by wheat grown in a phosphorus-fixing soil even in the absence of positive growth responses. New Phytol. 172, 536–543 (2006a). [DOI] [PubMed] [Google Scholar]
- Li H., Smith F. A., Dickson S., Holloway R. E. & Smith S. E. Plant growth depressions in arbuscular mycorrhizal symbiosis: not just caused by carbon drain? New Phytol. 178, 852–862 (2008). [DOI] [PubMed] [Google Scholar]
- Grace E. J., Cotsaftis O., Tester M., Smith F. A. & Smith S. E. Arbuscular mycorrhizal inhibition of growth in barley cannot be attributed to extent of colonization, fungal phosphorus uptake or effects on expression of plant phosphate transporter genes. New Phytol. 181, 938–949 (2009). [DOI] [PubMed] [Google Scholar]
- Moora M. & Zobel M. Effect of arbuscular mycorrhiza on inter- and intraspecific competition of two grassland species. Oecologia 108, 79–84 (1996). [DOI] [PubMed] [Google Scholar]
- Zabinski C. A., Quinn L. & Callaway R. M. Phosphorus uptake, not carbon transfer, explains arbuscular mycorrhizal enhancement of Centaurea maculosa in the presence of native grassland species. Funct. Ecol. 16, 758–765 (2002). [Google Scholar]
- Abd-Alla M. H., El-Enany A.-W. E., Nafady N. A., Khalaf D. M. & Morsy F. M. Synergistic interaction of Rhizobium leguminosarum bv. viciae andarbuscular mycorrhizal fungi as a plant growth promoting biofertilizers for faba bean (Vicia faba L.) in alkaline soil. Microbiol. Res. 169, 49–58 (2014). [DOI] [PubMed] [Google Scholar]
- Azcón B. R., Rubio R. & Barea J. M. Selective interactions between different species of mycorrhizal fungi and Rhizobium meliloti strains, and their effects on growth, N2-fixation (15N) and nutrition of Medicago sativa L. New Phytol. 117, 399–404 (1991). [DOI] [PubMed] [Google Scholar]
- Li H. et al. Phosphorus uptake and rhizosphere properties of intercropped and monocropped maize, faba bean, and white lupin in acidic soil. Biol. Fert. Soils 46, 79–91 (2010). [Google Scholar]
- Li S. & Li L. Effect of inoculating rhizobium and arbuscular mycorrhiza on organic P uptake in faba bean/maize intercropping system. Res. Agri. Modern. 32, 243–247 (2011). [Google Scholar]
- Feng G. et al. Histochemical visualization of phosphatase released by arbuscular mycorrhizal fungi in soil. J. Plant Nutr. 25, 969–980 (2002). [Google Scholar]
- He X., Xu M., Qiu G. Y. & Zhou J. Use of 15N stable isotope to quantify nitrogen transfer between mycorrhizal plants. J. Plant Ecol. 2, 107–118 (2009). [Google Scholar]
- Jalonen R., Nygren P. & Sierra J. Transfer of nitrogen from a tropical legume tree to an associated fodder grass via root exudation and common mycelial networks. Plant, Cell and Environ. 32, 1366–1376 (2009). [DOI] [PubMed] [Google Scholar]
- Walder F. et al. Mycorrhizal networks: common goods of plants shared under unequal terms of trade. Plant Physiol. 159, 789–797 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weremijewicz J. & Janos D. P. Common mycorrhizal networks amplify size inequality in Andropogon gerardii monocultures. New Phytol. 198, 203–213 (2013). [DOI] [PubMed] [Google Scholar]
- Postma J. A. & Lynch J. P. Complementarity in root architecture for nutrient uptake in ancient maize/bean and maize/bean/squash polycultures. Ann. Bot. 110, 521–534 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen S. N., Dresbøll D. B. & Thorup-Kristensen K. Root interactions between intercropped legumes and non-legumes — a competition study of red clover and red beet at different nitrogen levels. Plant Soil 378, 59–72 (2014). [Google Scholar]
- Fixen P. E. & Grove J. H. [Testing soils for phosphorus] Soil Testing and Plant Analysis. 3rd edn [Westerman, R.L. (ed.)] [141–180] (American Society of Agronomy and Soil Science Society of America: Madison, Wisconsin., 1990).
- Phillips J. & Hayman D. Improved procedures for cleaning and staining parasitic and vesicular arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 55, 158–160 (1970). [Google Scholar]
- Wang B. L. et al. Nitric oxide is involved in phosphorus deficiency-induced cluster-root development and citrate exudation in white lupin. New Phytol. 187, 1112–1123 (2010). [DOI] [PubMed] [Google Scholar]
- Alvey S., Bagayoko M., Neumann G. & Buerkert A. Cereal/legume rotations affect chemical properties and biological activities in two West African soils. Plant Soil 231, 45–54 (2001). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary tables/figures