Abstract
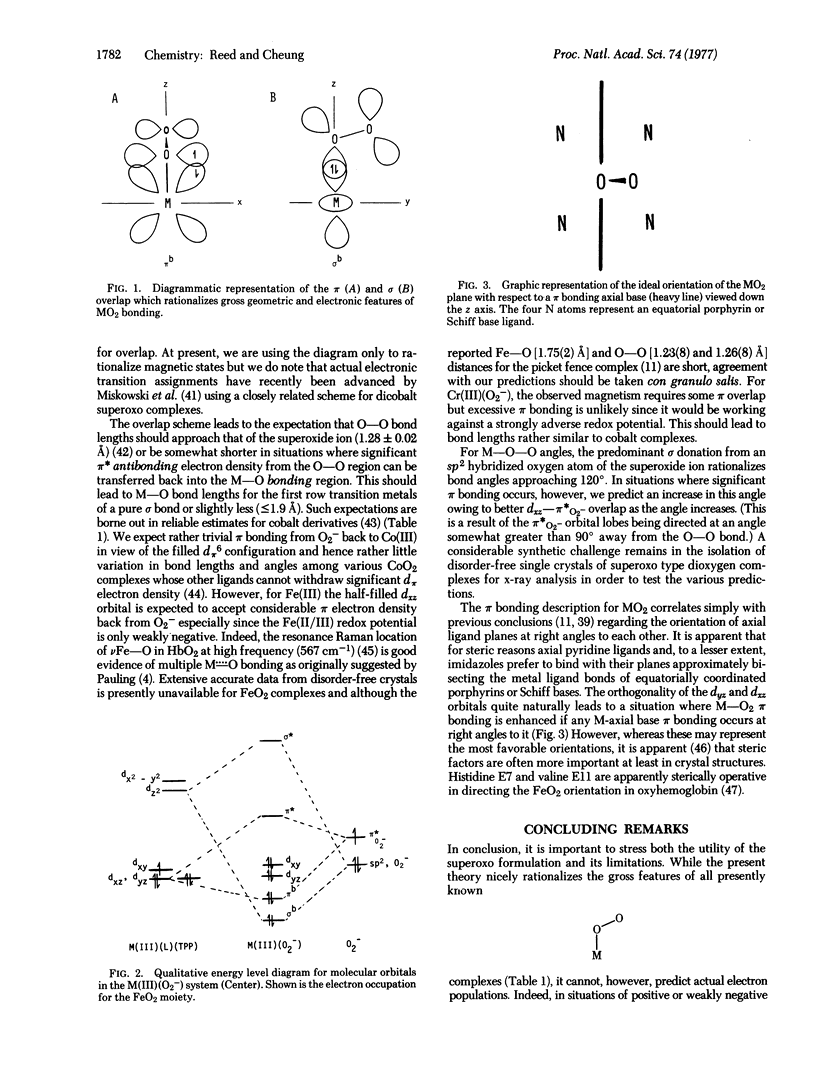
The celebrated Fe(II)0(2) versus Fe(III) (02-) debate over the formal representation of the Fe02 moiety in hemoglobin can be resolved by consideration of the utility of each formalism. In the context of rationalizing the gross structural and electronic features of end-bound dioxygen, particularly in light of a new closely related chromium complex, the M(III)-(02-) formulation is both chemically reasonable and most useful. In conjunction with a qualitative molecular orbital overlap picture, the differing magnetic states of enc-bound M02 complexes and other geometrical features can be rationalized or predicted.
Full text
PDF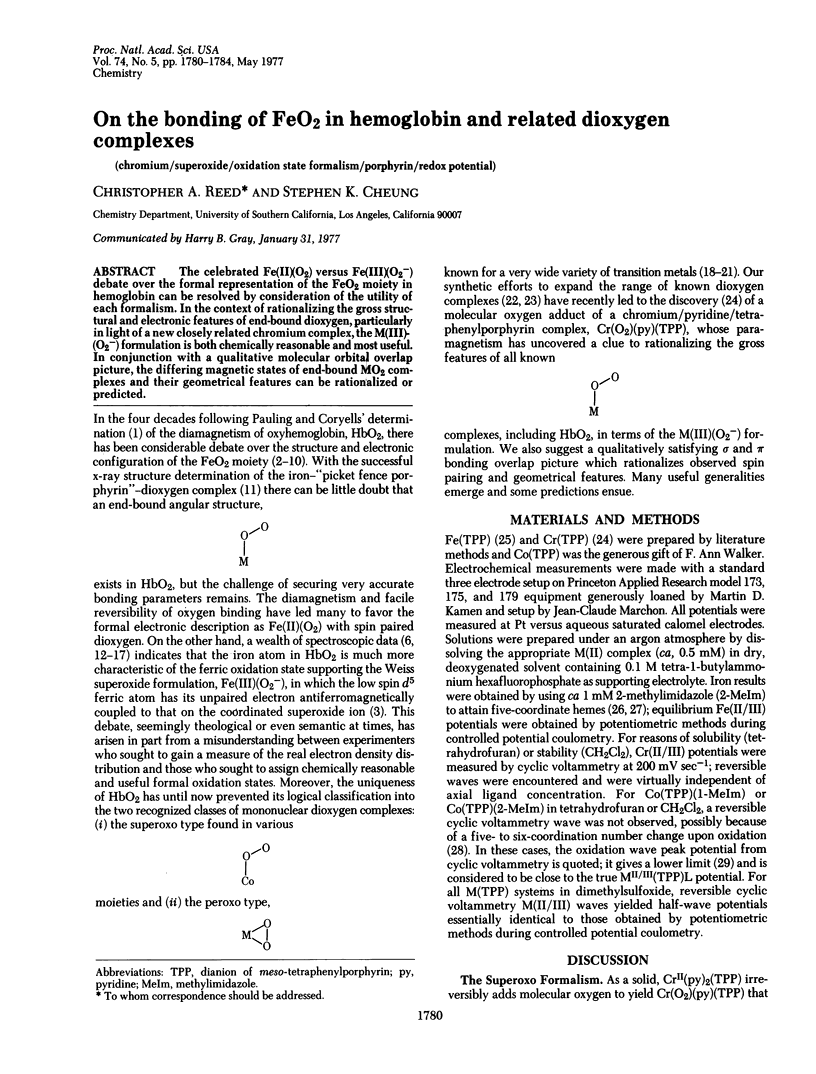



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow C. H., Maxwell J. C., Wallace W. J., Caughey W. S. Elucidation of the mode of binding of oxygen to iron in oxyhemoglobin by in frared spectroscopy. Biochem Biophys Res Commun. 1973 Nov 1;55(1):91–96. doi: 10.1016/s0006-291x(73)80063-4. [DOI] [PubMed] [Google Scholar]
- Brault D., Rougee M. Ferrous porphyrins in organic solvents. II. Optical spectra and paramagnetic susceptibilities. Biochemistry. 1974 Oct 22;13(22):4598–4602. doi: 10.1021/bi00719a020. [DOI] [PubMed] [Google Scholar]
- Collman J. P., Brauman J. I., Halbert T. R., Suslick K. S. Nature of O2 and CO binding to metalloporphyrins and heme proteins. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3333–3337. doi: 10.1073/pnas.73.10.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collman J. P., Brauman J. I., Suslick K. S. Letter: Oxygen binding to iron porphyrins. J Am Chem Soc. 1975 Nov 26;97(24):7185–7186. doi: 10.1021/ja00857a050. [DOI] [PubMed] [Google Scholar]
- Collman J. P., Gagne R. R., Reed C. A., Robinson W. T., Rodley G. A. Structure of an iron(II) dioxygen complex; a model for oxygen carrying hemeproteins. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1326–1329. doi: 10.1073/pnas.71.4.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collman J. P., Hoard J. L., Kim N., Lang G., Reed C. A. Synthesis, stereochemistry, and structure-related properties of alpha, beta, gamma, delta-tetraphenylporphinatoiron(II). J Am Chem Soc. 1975 May 14;97(10):2676–2681. doi: 10.1021/ja00843a015. [DOI] [PubMed] [Google Scholar]
- Collman J. P., Reed C. A. Syntheses of ferrous-porphyrin complexes. A hypothetical model for deoxymyoglobin. J Am Chem Soc. 1973 Mar 21;95(6):2048–2049. doi: 10.1021/ja00787a075. [DOI] [PubMed] [Google Scholar]
- Dedieu A., Rohmer M. M., Benard M., Veillard A. Letter: Oxygen binding to iron porphyrins. An ab initio calculation. J Am Chem Soc. 1976 Jun 9;98(12):3717–3718. doi: 10.1021/ja00428a060. [DOI] [PubMed] [Google Scholar]
- Fergusson J. E., Robinson W. T., Rodley G. A. Significance of the orientation of coordinated imidazole and benzimidazole groups in haemoprotein and vitamin B 12 structures. Aust J Biol Sci. 1972 Dec;25(6):1365–1371. doi: 10.1071/bi9721365. [DOI] [PubMed] [Google Scholar]
- Goddard W. A., 3rd, Olafson B. D. Ozone model for bonding of an O2 to heme in oxyhemoglobin. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2335–2339. doi: 10.1073/pnas.72.6.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidner E. J., Ladner R. C., Perutz M. F. Structure of horse carbonmonoxyhaemoglobin. J Mol Biol. 1976 Jul 5;104(3):707–722. doi: 10.1016/0022-2836(76)90130-3. [DOI] [PubMed] [Google Scholar]
- Hoffman B. M., Petering D. H. Coboglobins: oxygen-carrying cobalt-reconstituted hemoglobin and myoglobin. Proc Natl Acad Sci U S A. 1970 Oct;67(2):637–643. doi: 10.1073/pnas.67.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loew G. H., Kirchner R. F. Letter: Electronic structure and electric field gradients in oxyhemoglobin and -cytochrome P-450 model compounds. J Am Chem Soc. 1975 Dec 10;97(25):7388–7390. doi: 10.1021/ja00858a037. [DOI] [PubMed] [Google Scholar]
- Pauling L., Coryell C. D. The Magnetic Properties and Structure of Hemoglobin, Oxyhemoglobin and Carbonmonoxyhemoglobin. Proc Natl Acad Sci U S A. 1936 Apr;22(4):210–216. doi: 10.1073/pnas.22.4.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrock M., Münck E., Debrunner P. G., Marshall V., Lipscomb J. D., Gunsalus I. C. Mössbauer studies of cytochrome P-450 cam . Biochemistry. 1973 Jan 16;12(2):258–265. doi: 10.1021/bi00726a013. [DOI] [PubMed] [Google Scholar]
- Spiro T. G., Strekas T. C. Resonance Raman spectra of heme proteins. Effects of oxidation and spin state. J Am Chem Soc. 1974 Jan 23;96(2):338–345. doi: 10.1021/ja00809a004. [DOI] [PubMed] [Google Scholar]
- Truxillo L. A., Davis D. G. Electrochemistry of cobalt tetraphenylporphyrin in aprotic media. Anal Chem. 1975 Nov;47(13):2260–2267. doi: 10.1021/ac60363a052. [DOI] [PubMed] [Google Scholar]
- WEISS J. J. NATURE OF THE IRON-OXYGEN BOND IN OXYHAEMOGLOBIN. Nature. 1964 Apr 4;202:83–84. doi: 10.1038/202083b0. [DOI] [PubMed] [Google Scholar]
- WEISS J. J. NATURE OF THE IRON-OXYGEN BOND IN OXYHAEMOGLOBIN. Nature. 1964 Jul 11;203:182–183. [PubMed] [Google Scholar]
- WEISS J. J. NATURE OF THE IRON-OXYGEN BOND IN OXYHAEMOGLOBIN. Nature. 1964 Jul 11;203:182–183. [PubMed] [Google Scholar]
- Weschler C. J., Hoffman B. M., Basolo F. Letter: Synthetic oxygen carrier. A dioxygen adduct of a manganese porphyrin. J Am Chem Soc. 1975 Sep 3;97(18):5278–5280. doi: 10.1021/ja00851a043. [DOI] [PubMed] [Google Scholar]
- White S. J., Forward E. M. Serial changes in maximal isometric contraction of forearm flexor muscles. Phys Ther. 1966 Oct;46(10):1061–1067. doi: 10.1093/ptj/46.10.1061. [DOI] [PubMed] [Google Scholar]
- Wittenberg J. B., Wittenberg B. A., Peisach J., Blumberg W. E. On the state of the iron and the nature of the ligand in oxyhemoglobin. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1846–1853. doi: 10.1073/pnas.67.4.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yammoto T., Palmer G. The valence and spin state of iron in oxyhemoglobin as inferred from resonance Raman spectroscopy. J Biol Chem. 1973 Jul 25;248(14):5211–5213. [PubMed] [Google Scholar]



