Abstract
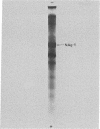
Antibodies to chromatin proteins of Novikoff hepatoma cells formed precipitin bands in the double-diffusion immunoprecipitation assay with chromatin proteins of Novikoff hepatoma, Walker 256 carcinosarcoma, and 18-day fetal rat liver. The antigen used for preparation of antiserum was the chromatin proteins initially extracted with 3 M NaCl-7 M urea and soluble after dialysis to 0.14 M NaCl-0.35 M urea. The chromatin proteins used for analytical studies were extracted with 0.6 M NaCl containing 0.01 M Tris-HCl (pH 8) and 100 muM phenylmethylsulfonyl fluoride. Corresponding chromatin proteins of normal and 18-hr regenerating rat liver, heart, and kidney did not form precipitin bands. The antigen was purified from the chrmatin of Novikoff hepatoma cells by exclusion chromatography on Sephadex G-150 and preparative nondenaturing polyacrylamide gel electrophoresis. Its migration on denaturing sodium dodecyl sulfate-polyacrylamide gels corresponded to a molecular weight of 26,000. Amino acid analysis showed that the ratio of acidic to basic amino acids was 1.4 to 1.0. Evidence for its homogeneity included its migration as a single protein spot on two-dimensional polyacrylamide gel electrophoresis and its single lysine amino-terminal amino acid. This protein is a glycoprotein, as shown by the presence of 15 moles of galactosamine per mole of antigen. These studies demonstrate the presence of a fetal glycoprotein in the chromatin of two tumors that may have an important role in determining their gene products.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin R. W., Barker C. R. Antigenic composition of transplanted rat hepatomas originally induced by 4-dimethylaminoazobenzene. Br J Cancer. 1967 Jun;21(2):338–345. doi: 10.1038/bjc.1967.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch G. I., Yeoman L. C., Taylor C. W., Busch H. Modified two-dimensional polyacrylamide gel electrophoresis systems for higher molecular weight nonhistone chromatin proteins from normal rat liver and Novikoff hepatoma ascites cells. Physiol Chem Phys. 1974;6(1):1–10. [PubMed] [Google Scholar]
- Chong M. T., Garrard W. T., Bonner J. Purification and properties of a neutral protease from rat liver chromatin. Biochemistry. 1974 Dec 3;13(25):5128–5134. doi: 10.1021/bi00722a012. [DOI] [PubMed] [Google Scholar]
- Chytil F., Spelsberg T. C. Tissue differences in antigenic properties of non-histone protein-DNA complexes. Nature. 1971 Oct 13;233(5320):215–218. [PubMed] [Google Scholar]
- Furlan M., Jericijo M., Suhar A. Purification and properties of a neutral protease from alf thymus nuclei. Biochim Biophys Acta. 1968 Aug 27;167(1):154–160. doi: 10.1016/0005-2744(68)90285-4. [DOI] [PubMed] [Google Scholar]
- Gold P., Freedman S. O. Specific carcinoembryonic antigens of the human digestive system. J Exp Med. 1965 Sep 1;122(3):467–481. doi: 10.1084/jem.122.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht M. E., Busch H. Isolation of an electrophoretically homogeneous nonhistone nucleolar protein. Life Sci II. 1971 Nov 22;10(22):1297–1309. doi: 10.1016/0024-3205(71)90237-2. [DOI] [PubMed] [Google Scholar]
- Kostraba N. C., Montagna R. A., Wang T. Y. Study of the loosely bound non-histone chromatin proteins. Stimulation of deoxyribonucleic acid-templated ribonucleic acid synthesis by a specific deoxyribonucleic acid-binding phosphoprotein fraction. J Biol Chem. 1975 Feb 25;250(4):1548–1555. [PubMed] [Google Scholar]
- Kostraba N. C., Wang T. Y. Non-histone proteins and gene activation in regenerating rat liver. Exp Cell Res. 1973 Aug;80(2):291–296. doi: 10.1016/0014-4827(73)90299-1. [DOI] [PubMed] [Google Scholar]
- Liu T. Y., Chang Y. H. Hydrolysis of proteins with p-toluenesulfonic acid. Determination of tryptophan. J Biol Chem. 1971 May 10;246(9):2842–2848. [PubMed] [Google Scholar]
- MANSI W. Slide gel diffusion precipitin test. Nature. 1958 May 3;181(4618):1289–1290. doi: 10.1038/1811289b0. [DOI] [PubMed] [Google Scholar]
- Margolis J., Kenrick K. G. Polyacrylamide gel electrophoresis in a continuous molecular sieve gradient. Anal Biochem. 1968 Oct 24;25(1):347–362. doi: 10.1016/0003-2697(68)90109-7. [DOI] [PubMed] [Google Scholar]
- Marushige K., Bonner J. Template properties of liver chromatin. J Mol Biol. 1966 Jan;15(1):160–174. doi: 10.1016/s0022-2836(66)80218-8. [DOI] [PubMed] [Google Scholar]
- Morris A. D., Littleton C., Corman L. C., Esterly J., Sharp G. C. Extractable nuclear antigen effect on the DNA anti-DNA reaction and NZB/NZW mouse nephritis. J Clin Invest. 1975 May;55(5):903–907. doi: 10.1172/JCI108018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooden L. D., Van Den Broek H. W.J., Sevall J. S. Stabilization of histones from rat liver. FEBS Lett. 1973 Feb 1;29(3):326–328. doi: 10.1016/0014-5793(73)80050-x. [DOI] [PubMed] [Google Scholar]
- Okita K., Kligman L. H., Farber E. A new common marker for premalignant and malignant hepatocytes induced in the rat by chemical carcinogens. J Natl Cancer Inst. 1975 Jan;54(1):199–202. doi: 10.1093/jnci/54.1.199. [DOI] [PubMed] [Google Scholar]
- Panem S., Schauf V. Cell-cycle dependent appearance of murine leukemia-sarcoma virus antigens. J Virol. 1974 Jun;13(6):1169–1175. doi: 10.1128/jvi.13.6.1169-1175.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penke B., Ferenczi R., Kovács K. A new acid hydrolysis method for determining tryptophan in peptides and proteins. Anal Biochem. 1974 Jul;60(1):45–50. doi: 10.1016/0003-2697(74)90129-8. [DOI] [PubMed] [Google Scholar]
- Rule A. H., Goleski-Reilly C. Proceedings: Phase-specific oncocolon antigens: a theoretical framework for "carcinoembryonic antigen" specificities. Cancer Res. 1974 Aug;34(8):2083–2087. [PubMed] [Google Scholar]
- Schechter I. Biologically and chemically pure mRNA coding for a mouse immunoglobulin L-chain prepared with the aid of antibodies and immobilized oligothymidine. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2256–2260. doi: 10.1073/pnas.70.8.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligy V. L., Miyagi M. Comparison of template-property changes after salt extraction of avian erythrocyte and liver chromatin. Eur J Biochem. 1974 Jul 15;46(2):259–269. doi: 10.1111/j.1432-1033.1974.tb03618.x. [DOI] [PubMed] [Google Scholar]
- Shapiro D. J., Taylor J. M., McKnight G. S., Palacios R., Gonzalez C., Kiely M. L., Schimke R. T. Isolation of hen oviduct ovalbumin and rat live albumin polysomes by indirect immunoprecipitation. J Biol Chem. 1974 Jun 25;249(12):3665–3671. [PubMed] [Google Scholar]
- Suzuki M., Hinuma Y. Evaluation of Epstein-Barr virus-associated nuclear antigen with various human cell lines. Int J Cancer. 1974 Dec 15;14(6):753–761. doi: 10.1002/ijc.2910140609. [DOI] [PubMed] [Google Scholar]
- Taylor C. W., Yeoman L. C., Daskal I., Busch H. Two-dimensional electrophoresis of proteins of citric acid nuclei prepared with aid of a Tissumizer. Exp Cell Res. 1973 Nov;82(1):215–226. doi: 10.1016/0014-4827(73)90264-4. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Habel K., Green H. Antigenic and cultural properties of cells doubly transformed by polyoma virus and SV40. Virology. 1965 Oct;27(2):179–185. doi: 10.1016/0042-6822(65)90157-1. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Platt T., Weber K. Amino-terminal sequence analysis of proteins purified on a nanomole scale by gel electrophoresis. J Biol Chem. 1972 May 25;247(10):3242–3251. [PubMed] [Google Scholar]
- Weisenthal L. M., Ruddon R. W. Catabolism of nuclear proteins in control and phytohemagglutinin-stimulated human lymphocytes, leukemic leukocytes, and Burkitt lymphoma cells. Cancer Res. 1973 Nov;33(11):2923–2935. [PubMed] [Google Scholar]
- Yeoman L. C., Taylor C. W., Busch H. Two-dimensional gel electrophoresis of acid-extractable nuclear proteins of regenerating and thioacetamide-treated rat liver, Morris 9618a hepatoma, and Walker 256 carcinosarcoma. Cancer Res. 1974 Feb;34(2):424–428. [PubMed] [Google Scholar]
- Yeoman L. C., Taylor C. W., Jordan J. J., Busch H. Differences in chromatin proteins of growing and non-growing tissues. Exp Cell Res. 1975 Mar 1;91(1):207–215. doi: 10.1016/0014-4827(75)90159-7. [DOI] [PubMed] [Google Scholar]
- Yeoman L. C., Taylor C. W., Jordan J. J., Busch H. Early and late changes in nonhistone chromatin proteins accompanying rat liver regeneration. Cancer Res. 1975 May;35(5):1249–1255. [PubMed] [Google Scholar]
- Zardi L., Lin J. C., Baserga R. Immunospecificity to non-histone chromosomal proteins of anti-chromatin antibodies. Nat New Biol. 1973 Oct 17;245(146):211–213. doi: 10.1038/newbio245211a0. [DOI] [PubMed] [Google Scholar]