Abstract
Cell-based therapy has emerged as a promising approach to combat the myocyte loss and cardiac remodeling that characterize the progression of left ventricular dysfunction to heart failure. Several clinical trials conducted during the past decade have shown that a variety of autologous bone marrow- and peripheral blood-derived stem and progenitor cell populations can be safely administered to patients with ischemic heart disease and yield modest improvements in cardiac function. Concurrently, rapid progress has been made at the preclinical level to identify novel therapeutic cell populations, delineate the mechanisms underlying cell-mediated cardiac repair, and optimize cell-based approaches for clinical use.
The following review summarizes the progress that has been made in this rapidly evolving field over the past decade and examines how our current understanding of the mechanisms involved in successful cardiac regeneration should direct future investigation in this area. Particular emphasis is placed on discussion of the general hypothesis that the benefits of cell therapy primarily result from stimulation of endogenous cardiac repair processes that have only recently been identified in the adult mammalian heart, rather than direct differentiation of exogenous cells. Continued scientific investigation in this area will guide the optimization of cell-based approaches for myocardial regeneration, with the ultimate goal of clinical implementation and substantial improvement in our ability to restore cardiac function in ischemic heart disease patients.
Keywords: cardiac regeneration, stem cells, cell therapy
Introduction
Despite significant advances in the understanding and treatment of cardiovascular diseases, the development of heart failure secondary to coronary artery disease remains a significant cause of morbidity and mortality worldwide [1]. The evolution of left ventricular (LV) dysfunction to heart failure is characterized by chronic myocyte loss that is inadequately compensated for by cellular hypertrophy of remaining myocytes, ultimately leading to deterioration of cardiac contractile performance [2]. Unfortunately, the majority of available treatment options only delay the progression of disease without addressing the fundamental problem of cardiomyocyte loss, while the lone exception of organ transplantation is hampered by the limited availability of donor hearts. As a result, there is a critical need for the development of therapeutic strategies aimed at reversing myocyte loss, restoring cardiac function, and preventing the progression to clinical heart failure.
With this goal in mind, the administration of stem and progenitor cells has emerged as an innovative approach to replace lost myocytes and rejuvenate damaged and dysfunctional myocardium. Given the tremendous clinical potential of such efforts, it is not surprising that this field has evolved at a rapid pace since the publication of initial studies demonstrating that transplantation of skeletal myoblasts to infarcted myocardium could improve cardiac contractile performance [3, 4]. Indeed, the past decade has witnessed major advances in the development of cell-based approaches for cardiac repair, including the completion of several large clinical trials confirming the safety and efficacy of various autologous peripheral blood- and bone marrow-derived cell populations in patients with ischemic heart disease. For example, a recent meta-analysis of 50 studies including over 2,000 patients treated with autologous bone marrow-derived cells concluded that cell therapy leads to small but significant improvements in LV ejection fraction (~4%) and reduces infarct size (~4%), LV end-systolic volume (~9 mL), and LV end-diastolic volume (~5 mL) [5]. Moreover, minimal adverse events have been reported and several trials have shown beneficial effects of cell therapy on indices of patient performance such as exercise capacity and six-minute walk distance [6, 7]. These encouraging results have fueled interest in optimizing cell-based approaches to build upon the modest improvements observed in the first generation of clinical trials of cell therapy for cardiac repair.
Concurrent with the initial stages of clinical translation, rapid progress has been made at the pre-clinical level to improve our understanding of the mechanisms involved in successful cardiac regeneration after injury. Although the heart has traditionally been considered a terminally differentiated postmitotic organ incapable of cellular turnover, a growing body of evidence suggests that this is not the case[8]. Several recent studies utilizing various techniques of cellular dating have shown that considerable myocyte turnover occurs throughout life in the healthy and diseased heart [9–11]. Together with the seminal discovery of circulating[12] and resident[13, 14] endogenous cardiac stem cells, this finding has dramatically shifted our perspective regarding the potential for regeneration of the damaged human heart. While initial attempts at cell-mediated cardiac repair were performed with the goal of directly replacing lost myocytes via transdifferentiation of exogenous stem cells, the recognition of endogenous cardiac regenerative potential has led to new approaches involving the administration of cell populations derived from the heart itself and/or activation of the host’s own reparative responses to promote myocardial regeneration and improve cardiac function. In this review, we will summarize recent progress in our understanding of the heart’s capacity for repair, discuss how the recognition of endogenous regenerative potential has directed the development of novel cell-based therapeutic approaches, and examine the mechanisms by which these approaches appear to revitalize damaged and dysfunctional myocardium.
Endogenous Repair Capacity of the Mammalian Heart
For many decades, the mammalian heart has been considered a terminally differentiated organ without the intrinsic capacity for regeneration. Under this view, it was believed that the number of cardiomyocytes was established shortly after birth and remained largely unchanged throughout the adult lifespan, with changes in heart mass attributed exclusively to hypertrophy of existing myocytes [15]. However, accumulating evidence suggests that cellular turnover does in fact occur in the adult mammalian heart [8]. Using the innovative approach of radiocarbon DNA dating, Bergmann and colleagues[9] provided evidence of cardiomyocyte renewal in human adult hearts and estimated that ~50% of the cardiomyocyte compartment is replaced throughout a typical life span. This notion of cardiomyocyte proliferation was supported by estimates of cardiomyocyte DNA synthesis in post-mortem tissue from cancer patients who had been treated with the thymidine analog iododeoxyuridine, which is incorporated into the DNA of cycling cells and thus allows assessment of myocyte proliferation over time[16]. Mathematical modeling suggested that cardiomyocytes turn over at a much higher rate (~20% per year) than had been proposed by Bergmann et al., but nevertheless reinforced the emerging concept that myocyte turnover and tissue regeneration occur throughout adulthood. The discrepant findings regarding the magnitude of cardiomyocyte turnover may have been resolved by a recent collaborative effort involving investigators from each of the previous studies in which retrospective radiocarbon DNA dating was performed on fresh tissue samples from healthy and failing human hearts[11]. This method minimizes the requirement of mathematical modeling that was heavily relied upon in earlier reports and may have contributed to an underestimation of myocyte turnover [17]. Using this improved approach, the authors found that from 20 to 78 years of age, the entire cardiomyocyte compartment is replaced ~8 times in healthy hearts, with an even greater turnover rate apparent in patients with heart failure. This finding challenges the prevailing dogma of the heart as a postmitotic organ and indicates that the mammalian heart retains a significant degree of plasticity and cell turnover throughout adult life.
The recent discovery of cardiac stem cells (CSCs) has also played a pivotal role in promoting the modern view of the heart as an organ capable of regeneration. Although a specific hierarchy of cardiac stem and progenitor cells has yet to be defined, several groups of investigators have identified cell populations with characteristics of stem cells that reside in the postnatal heart [18]. The most extensively studied cell population is that characterized by expression of the tyrosine kinase receptor c-kit. Initially identified by Beltrami and colleagues[19], Lin−/c-kit+ cells are self-renewing, clonogenic, and multipotent, with the ability to differentiate into cardiomyocytes, smooth muscle cells, and endothelial cells[14]. These CSCs are thought to reside within specialized niches primarily located in the atrium and apex, where they maintain their “stemness” via connexin- and cadherin-mediated structural connections with mature cardiomyocytes, fibroblasts, and other stem cells [20]. Additional cell populations characterized by the expression of surface markers (stem cell antigen (SCA)-1 [21]), transcription factors (Islet-1 [22]), or the ability to efflux fluorescent dye (side population cells expressing ATP-binding cassette transporters [23]) have also been found in the heart and shown to possess characteristics of stem cells. More recently, the epicardium has been identified as a potential source of cardiac progenitor cells that are capable of regaining their embryonic potential to form new vascular cells and cardiomyocytes following stimulation by thymosin beta-4 [24]. Although initial reports suggested minimal overlap between these putative cardiac stem cell populations, subsequent studies have shown expression of some surface antigens in the same line of cells at different stages of maturation[18]. Regardless, it is clear that cells with the capacity to form cardiomyocytes exist in the postnatal mammalian heart, providing further evidence of endogenous cardiac regenerative potential.
The high rate of morbidity and mortality in patients with ischemic heart disease clearly demonstrates that the endogenous capacity for cardiac repair is insufficient to offset the loss of myocytes that typically occurs with acute or chronic myocardial ischemia. However, evidence of some degree of myocyte regeneration after injury has provided further support for the notion that the heart is capable of a limited amount of self-repair. For example, Richard Lee’s laboratory recently used genetic fate-mapping with stable isotope labeling and multi-isotope imaging mass spectrometry in mice to demonstrate that the formation of new cardiomyocytes occurs at a low rate during normal aging, but is increased in areas adjacent to infarcted myocardium[25]. Interestingly, the genesis of new myocytes primarily occurred via the division of pre-existing cardiomyocytes that had re-entered the cell cycle. While these results suggest that endogenous progenitors have a minimal role in repair after injury, other investigators have performed lineage-tracing experiments with transgenic mice to demonstrate that progenitor cells are mobilized and/or recruited to the heart and form cardiomyocytes after myocardial infarction or pressure overload[26]. To investigate the mechanisms involved in this process, specific growth factor signaling pathways have been examined based on immunohistochemical evidence that c-kit+ CSCs express the hepatocyte growth factor (HGF) receptor c-Met and the insulin-like growth factor (IGF)-1 receptor (IGF-R) [27]. In vitro experiments revealed that HGF promoted CSC migration and IGF-1 enhanced cell survival and proliferation, which lead the authors to administer these growth factors to the infarcted rat heart in an attempt to stimulate a regenerative response in vivo. Fluorescent labeling of CSCs in the atrio-ventricular groove enabled tracking of these cells, which migrated towards the infarcted area and appeared to begin differentiating into small myocytes. Moreover, the combined HGF/IGF-1 treatment resulted in a significant improvement in ventricular function and animal survival, supporting the idea that activation of endogenous CSCs may be a viable approach to promote cardiac recovery after injury.
Collectively, the documentation of cardiomyocyte turnover throughout adulthood, evidence that new cardiomyocytes can form from the division of pre-existing cardiomyocytes, and the identification of resident cardiac stem and progenitor cells that contribute to myocyte renewal have re-shaped our understanding of myocardial biology. It is now apparent that cardiac cellular homeostasis throughout life is a reflection of the balance between myocyte death and myocyte renewal, with profoundly negative implications for organ function when this balance is shifted in such a way that cell death exceeds cell regeneration. Although endogenous progenitor cells and division of pre-existing cardiomyocytes may contribute to cardiac regeneration after injury, the adult heart’s capacity for repair is clearly of insufficient magnitude to offset the dramatic loss of myocytes that characterizes acute or chronic ischemic heart disease. Nevertheless, the recognition of natural regenerative mechanisms in the mammalian heart has stimulated interest in the development of therapeutic approaches that exploit the endogenous potential for cardiac repair.
Experimental Cell Transplantation for Cardiac Repair: Emergence of Novel Cell Populations
Initial attempts at administering exogenous blood- and bone marrow-derived progenitor cells to repair damaged myocardium were motivated in large part by the findings of Orlic et al. [28], who reported that bone marrow hematopoietic stem cells could transdifferentiate into cardiomyocytes after transplantation to infarcted myocardium. However, subsequent studies [29–31] raised doubts regarding the transdifferentiation capacity of bone marrow-derived cells and it is now generally believed that these cells only rarely give rise to cardiomyocytes, if ever [18]. Nevertheless, clinical trials testing the safety and efficacy of these cell populations have moved forward, generally showing modest improvements in ventricular function despite the inability to directly generate a large number of new myocytes [5]. Driven by the discovery of endogenous cardiac progenitors, more recent studies have focused on novel cell populations that may possess superior cardiomyogenic potential given their apparent involvement in physiologic cardiomyocyte turnover.
Cardiac Stem Cells
Following the identification of stem cell populations resident in the adult heart, attempts at isolating these cells for the purpose of experimental cell therapy soon followed based on the notion that cardiac-derived cells may be particularly effective at regenerating myocardium. A series of preclinical studies involving the isolation of c-kit+ CSCs from fragments of cardiac tissue, ex vivo expansion in culture, and subsequent transplantation into damaged myocardium have provided encouraging results. For example, intramyocardial injection of human c-kit+ CSCs into the infarcted hearts of immunosuppressed rodents elicited significant improvements in cardiac function, with evidence that the exogenously delivered CSCs differentiated into cardiomyocytes, endothelial cells, and vascular smooth muscle[14]. These and other[32] positive results have facilitated the translation of this approach to human patients with the Stem Cell Infusion in Patients with Ischemic Cardiomyopathy (SCIPIO) trial, a phase I clinical trial of autologous c-kit+ CSCs. Although only a small number of patients have been studied, initial data indicate that CSC treatment improves regional and global LV function, reduces infarct size, and increases viable myocardium for up to 1 year after injection[33, 34].
Cardiosphere-Derived Cells
Soon after the discovery of resident CSCs, Messina and colleagues [35] described the isolation of undifferentiated cells from adult cardiac tissue specimens that would spontaneously form spherical clusters when placed in suspension culture. These clusters were termed ‘cardiospheres’ and were shown to consist of proliferating c-kit+ cells in their core, with differentiating cells expressing cardiac and endothelial cell markers in their periphery. Building on this finding, Marban’s laboratory modified the cardiosphere isolation procedure and used cardiospheres as the basis of cell expansion, ultimately yielding cardiosphere-derived cells (CDCs) [36]. It has been proposed that CDCs possess greater potential for repair because cardiospheres recapitulate the microenvironment of the cardiac stem cell niche, as evidenced by an elevated number of c-kit+ cells, upregulation of stem cell-related transcription factors such as Nanog and Sox2, and enhanced expression of extracellular matrix proteins and adhesion molecules [37]. In preclinical models of acute and chronic ischemic heart disease, administration of CDCs improves ventricular function, reduces infarct size, and increases viable myocardium [36, 38]. Interestingly, a direct comparison of CDCs with other stem and progenitor cell populations revealed that CDCs exhibit superior cardiomyogenic capacity, angiogenic potential, and release of paracrine factors in vitro [39]. Moreover, CDCs injected into infarcted mouse hearts yielded a greater improvement in cardiac function, higher cell engraftment, and superior attenuation of pathologic ventricular remodeling compared with other cell types. CDCs were even deemed superior to purified c-kit+ CSCs based on paracrine factor release and functional benefit after transplantation, suggesting that the therapeutic potential of CSCs may be enhanced by cardiosphere culture and/or administration in the context of a supportive mixed-cell milieu[39]. Preliminary results from the first clinical trial of CDCs have recently been published, demonstrating that intracoronary injection of autologous CDCs is safe and elicits significant improvements in regional contractility and viable heart mass, but not LV ejection fraction, 6-months after treatment [40].
Mesenchymal Stem Cells
Friedenstein and colleagues[41] first identified mesenchymal stem cells (MSCs) as a rare population of plastic-adherent, bone marrow-derived cells capable of forming single-cell colonies. These cells have subsequently been shown to possess multi-lineage potential, with the ability to differentiate into chondrocytes, adipocytes, and osteoblasts[42]. In vitro experiments involving co-culture with mature ventricular myocytes have provided evidence that MSCs can transdifferentiate into cardiomyocytes in the appropriate microenvironment[43]. For example, mouse MSCs express alpha-actinin, form gap junctions, and synchronously contract when co-cultured with mature rat cardiomyocytes [44]. Interestingly, separation of MSCs and cardiomyocytes with a semi-permeable membrane prevented transdifferentiation, indicating that this process requires direct intercellular communication. The differentiation of MSCs is likely regulated by multiple signaling pathways, including the Wnt canonical pathway and the TGF-beta pathway, which each respond to a variety of growth factors to direct gene expression[45].
The in vitro cardiomyogenic potential of MSCs, as well as their accessibility from a number of tissues and capacity to undergo expansion in culture, facilitated the use of this cell population in attempts to promote cardiac repair in several pre-clinical models of ischemic heart disease. In a swine model of ischemic cardiomyopathy, transendocardially injected MSCs were shown to engraft in the heart, differentiate into cardiomyocytes, endothelial cells, and vascular smooth muscle cells, reduce infarct size, and improve ejection fraction for up to 12 weeks[46]. Although the trilineage differentiation potential of MSCs administered to the heart has been supported by other studies[47], the frequency of engraftment and differentiation is very low compared with the magnitude of functional recovery that is generally observed[48], suggesting that direct differentiation is not the predominant mechanism by which these cells promote cardiac repair (discussed below). Recent work by Chong and colleagues[49] describing a population of epicardium-derived cardiac MSCs raises the intriguing possibility that isolation, ex vivo expansion and therapeutic administration of MSCs native to the heart may offer a superior approach for cardiac regeneration, although this remains to be tested. Regardless, the clinical application of extra-cardiac-derived MSCs for myocardial repair has progressed, with initial evidence that MSCs derived from bone marrow[50] and adipose tissue[51] are safe and have favorable effects on cardiac structure and function in patients with ischemic heart disease.
Mechanisms Underlying Cell Therapy-Mediated Repair: Stimulation of Endogenous Cardiac Regeneration
In an effort to optimize the efficacy of cell-mediated approaches for cardiac repair, there has been significant interest in identifying the physiological, cellular, and molecular mechanisms underlying successful myocardial regeneration. Although initial investigation centered on the ability of injected stem cells to differentiate into cardiomyocytes, more recent studies have revealed that the functional benefits of cell therapy arise through several mechanisms of action that generally involve the potentiation of endogenous self-repair processes.
Paracrine Factor-Mediated Stimulation of Host Cardiac Repair Processes by Bone Marrow Precursors
Initial support for the notion that mechanisms other than cardiomyogenic differentiation of donor cells contributed to cell-mediated cardiac repair came from the finding that injection of bone marrow-derived MSCs overexpressing the survival gene Akt1 after myocardial infarction improved cardiac function in less than 72 hours[52]. Because this time-course of improvement was not consistent with direct differentiation of injected cells, the authors postulated that the beneficial effects were mediated by the release of paracrine factors (reviewed in [53, 54]. Subsequent studies showing a similar recovery of function following administration of concentrated conditioned medium from Akt1-modified MSCs supported this hypothesis[55]. Further insight into these processes was provided by Young-sup Yoon’s laboratory, who examined the expression patterns and sources of paracrine factors after transplantation of human bone marrow-derived endothelial progenitor cells (EPCs) into mice after myocardial infarction[56]. Cell injection elicited a significant elevation in circulating concentrations of various angiogenic and anti-apoptotic paracrine factors that persisted for at least 14 days, despite the disappearance of the injected EPCs after one week. Interestingly, the majority of these factors were of mouse origin, indicating that the sustained upregulation of paracrine factors could be attributed to host cells and/or tissues. Moreover, EPC transplantation enhanced mobilization of endogenous stem cells from the host’s bone marrow and recruitment of these cells to the ischemic myocardium, implicating the activation of endogenous repair processes in the functional improvements afforded by exogenous cell administration.
Peripheral Injection of Mesenchymal Stem Cells Improves Cardiac Function Via Paracrine-Mediated Myocardial Regeneration
To take advantage of the potent paracrine actions of bone marrow MSCs, colleagues from our department have completed a series of studies investigating the therapeutic benefits of extra-cardiac MSC injection in a hamster heart failure model. Bi-lateral hamstring muscle injection of MSCs significantly improved ventricular function 1-month after injection, despite the fact that the injected cells were trapped in the local skeletal musculature. Evidence of myocardial regeneration was provided by an ~80% increase in myocyte nuclear density, ~two-fold elevations in the expression of the cell-cycle markers Ki-67 and phospho-histone H3 (pHH3), and a reduction in mean myocyte diameter[57]. In addition, intramuscular MSC injection led to an increase in circulating growth factor concentrations, mobilization of c-kit+, CD31+, and CD133+ progenitor cells, and a subsequent increase in c-kit+ cells in the myocardium. Taken together, these results indicate that MSCs are capable of stimulating endogenous repair processes to promote cardiac regeneration, even when they are administered via extracardiac injection to remote skeletal muscle. To delineate the mechanisms involved in this therapeutic strategy, the activity of the skeletal muscle JAK-STAT signaling pathway was investigated[58]. Incubation of cultured skeletal myocytes with MSC-conditioned media induced phosphorylation of JAK1, JAK2, and STAT3, resulting in gp130 receptor-dependent production of HGF and vascular endothelial growth factor (VEGF). Similar responses were observed in MSC-injected hamstring muscle in vivo, including activation of JAK-STAT3 signaling and increased HGF and VEGF production. These host-derived factors promoted myocardial regeneration and improvements in cardiac function, a response that was abolished by the JAK-STAT3 inhibitor WP1066. Collectively, these results indicate that MSCs activate JAK-STAT3 signaling following skeletal muscle injection, causing the release of host tissue-derived growth factors and activation of endogenous self-repair mechanisms to promote cardiac regeneration.
Global Intracoronary Mesenchymal Stem Cell Infusion in Viable Dysfunctional Myocardium
Building on these findings, we investigated the effects of intracoronary autologous MSC injection in pigs with hibernating myocardium. In this model, regional ventricular dysfunction arises from repetitive ischemia in collateral-dependent myocardium resulting from a chronic stenosis of the left anterior descending (LAD) coronary artery. This condition is characterized by regional apoptosis-induced myocyte loss and compensatory hypertrophy that is stable between 3- and 5-months after instrumentation, allowing assessment of cell therapy-mediated cardiac regeneration without the confounding effects of ongoing cell death[59]. Four-weeks after MSC injection, regional LAD wall thickening was significantly improved despite a persistent impairment in resting and adenosine-dilated coronary blood flow [60]. Transient elevations in circulating c-kit+ and CD133+ cells were observed three-days after cell injection, with corresponding increases in c-kit+/CD45− and CD133+/CD45− cells in hibernating and remote areas of the heart. Approximately 60% of the c-kit+/CD45− cells found in the heart were CD133−, suggesting that a large proportion of c-kit+ cells were resident cardiac stem cells, with fewer than half of the c-kit+ cell population derived from extra-cardiac sources, such as the bone marrow. In addition, MSC-treated animals exhibited significantly greater expression of Ki-67 and pH-H3 in the hibernating region, indicating that cell therapy stimulated myocyte proliferation. These effects resulted in an increased myocyte nuclear density and a concomitant regression in average myocyte diameter in hibernating myocardium, supporting myocyte regeneration following cell injection. Importantly, ex vivo analysis of cell fate following injection of fluorescent-labeled MSCs demonstrated rare instances of engraftment, with fluorescent staining limited to endothelium and vascular smooth muscle but not cardiomyocytes. These data demonstrate that intracoronary injection of autologous bone marrow-derived MSCs improves regional contractile function in swine with hibernating myocardium via myocardial regeneration that appears to result from stimulation of myocyte proliferation and mobilization of endogenous progenitor cells (Figure 1).
Figure 1. Intracoronary mesenchymal stem cells elicit myocardial regeneration that arises from mobilization of endogenous progenitor cells and stimulation of myocyte proliferation.
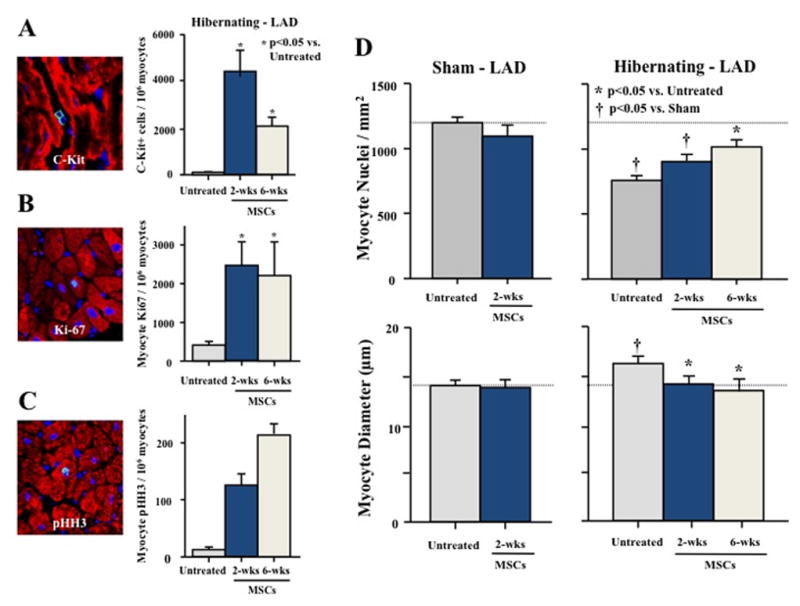
Autologous bone marrow-derived MSCs (44×106) were administered via intracoronary injection to swine with chronic hibernating myocardium (n=10). Animals were studied either 2-weeks (n=6) or 6-weeks later (n=4) and compared with untreated animals with hibernating myocardium (n=7) or sham-normal animals receiving MSCs (n=6). (A) Myocardial c-kit+ cells were increased two-weeks after MSC treatment and remained elevated six-weeks after injection. In addition, intracoronary MSC administration resulted in persistent elevations in (B) Ki-67+ and (C) phospho-histone H3 (pHH3)+ myocytes, indicating that cell therapy stimulated myocyte proliferation. (D) Untreated hibernating myocardium was characterized by a reduction in myocyte nuclear density and compensatory myocyte hypertrophy. MSC-treated animals exhibited a progressive increase in myocyte nuclear density and a concomitant reduction in myocyte diameter, consistent with significant myocardial regeneration. (Modified from [60]).
Studies Supporting Mesenchymal Stem Cell-Mediated Stimulation of Endogenous Repair
The notion that the benefits of cell therapy are mediated by mobilization of endogenous progenitor cells is further supported by studies using genetic lineage tracing approaches to demonstrate that administration of bone marrow-derived c-kit+ cells promotes cardiac regeneration by augmenting the formation of new cardiomyocytes from endogenous progenitors after myocardial infarction[61]. These results are concordant with those of Hatzistergos and colleagues[62], who injected bone marrow-derived MSCs into the infarct area and border zone of pigs three days after myocardial infarction. MSC-treated animals exhibited reduced infarct size and an improvement in ejection fraction compared with vehicle-treated controls. Although evidence of MSC engraftment and differentiation to cardiomyocytes and blood vessels was reported, activation of endogenous c-kit+ CSCs and stimulation of cardiomyocyte cell-cycling were implicated as the primary mechanisms responsible for the beneficial effects of cell therapy. Interestingly, the same degree of tissue recovery was not observed following injection of MSC-conditioned media, suggesting that cell-to-cell interactions are necessary to permit activation of endogenous repair mechanisms. Follow-up in vitro experiments showed that MSCs stimulated c-kit+ CSC proliferation and increased CSC expression of Nkx2.5 and troponin I, suggesting that MSCs may promote CSC differentiation to cardiomyocytes. Taken together, these observations reinforce the importance of host-derived regenerative responses in mediating the reparative effects of MSC therapy.
Cardiac Stem Cells and Cardiosphere-Derived Cells as Platforms for Cardiac Repair
Activation of endogenous regeneration as a mechanism underlying cell therapy-mediated repair is not limited to cell populations derived from the bone marrow. Although stem cells derived from the heart itself possess superior cardiomyogenic potential in vitro, recent studies suggest that direct differentiation following transplantation is not the primary mechanism by which these cell populations promote cardiac recovery. For example, significant improvements in cardiac structure (increased viable heart mass) and function (increased ejection fraction and fractional shortening) have been observed following intracoronary injection of c-kit+ CSCs to rats thirty-days after myocardial infarction[32]. Interestingly, injected cells were detected in the hearts of only 7 of 17 CSC-treated rats one-month after cell transplantation, although all treated animals exhibited similar increases in viable myocardium and ventricular function. Immunohistochemical staining against c-kit revealed an ~10-fold increase in the number of c-kit+ cells in both the risk area and non-infarcted regions of CSC-treated rat hearts. To determine the source of these cells, the authors administered the thymidine analogue 5-bromo-2′deoxyuridine (BrdU) for 2 weeks prior to euthanasia and co-stained myocardial tissue sections for c-kit and BrdU to identify newly-formed (endogenous) c-kit+ CSCs. While ~40% of the total c-kit+ population was also BrdU+ in vehicle-treated animals, the percentage of double-positive cells was significantly higher in the cell-treated animals, indicating activation of endogenous CSCs following injection of exogenous c-kit+ cells[32]. Similarly, Chimenti et al. [63] injected human adult-derived CDCs into the infarct border zone of immunodeficient mice and quantified the percentage of human nuclear antigen (HNA)+ (i.e., exogenous cell-derived) myocytes and capillaries one-week later. Although cell treatment doubled the number of myocytes and capillaries, only ~12% of cardiomyocytes and ~10% of capillaries were also HNA+, indicating that indirect mechanisms contribute to a significant portion of cell therapy-mediated repair. Further analyses revealed that CDC treatment increased tissue levels of exogenous cell-derived growth factors, enhanced expression of the pro-survival signaling kinase Akt, and reduced apoptosis, suggesting that paracrine-mediated effects on cell survival may have contributed to functional recovery as well.
In summary, data from our laboratory and others indicate that direct differentiation of exogenous cells into cardiomyocytes following transplantation may not be the primary mechanism underlying the regenerative benefits of many cell-based therapeutic approaches. Instead, it appears that direct and paracrine factor-mediated activation of recently recognized endogenous mechanisms of repair, such as mobilization of bone marrow- and cardiac-resident progenitor cells and stimulation of cardiomyocyte cell cycle re-entry, are likely responsible for myocardial regeneration and functional improvements derived from cell therapy (Figure 2).
Figure 2. Proposed paradigm of cell therapy-mediated cardiac repair via stimulation of endogenous repair processes.

Although the initial application of cell-based therapies for myocardial repair was motivated by the idea that the delivery of exogenous stem and/or progenitor cells could promote myocyte regeneration via direct differentiation to cardiac cells, studies to date suggest that this rarely occurs, if at all. Instead, it appears that cell administration stimulates endogenous cardiac repair processes, possibly via paracrine signaling, direct cell-to-cell interactions, and/or transfer of micro-RNAs that influence the transcriptional activity of host cells. Data from experimental studies in our laboratory indicate that the repair process involves mobilization of c-kit+ resident cardiac stem cells in both healthy (sham control) and diseased hearts. However, only animals with viable, dysfunctional (hibernating) myocardium exhibit increased markers of cell cycle activation (such as Ki-67 and phospho-histone H3), indicative of myocyte proliferation. Collectively, the mobilization of endogenous progenitors and stimulation of myocyte cell cycle re-entry result in myocardial regeneration, characterized by an increased density of myocyte nuclei and a reduction in mean myocyte diameter.
Implications for Future Research
Our evolving understanding of the processes involved in successful cardiomyocyte regeneration after injury has important implications for the development of novel strategies to optimize cell-mediated repair of the heart. Identification of specific molecular and cellular mechanisms promoting the formation of new myocytes from endogenous progenitor cells and/or the proliferation of pre-existing myocytes has directed experimental approaches aiming to target these pathways to enhance regeneration. In addition, recognition of the important role played by the host’s own self-repair processes in the success of cell-based therapies has encouraged researchers and clinicians to consider avenues of myocardial repair beyond direct differentiation of exogenous cells delivered to the damaged heart.
One active area of investigation involves the development of techniques to modify stem and progenitor cell populations ex vivo prior to administration in an attempt to improve cell survival, proliferation, engraftment, and/or production of paracrine factors after delivery. Several approaches have shown promising results, including cell preconditioning with hypoxic culture conditions, genetic modifications, and pharmacologic augmentation of reparative capacity[64]. For example, incubation of cardiosphere-derived c-kit+ progenitor cells in a hypoxic environment for six hours prior to delivery enhanced their therapeutic efficacy, as these cells elicited a larger reduction in infarct size and greater improvement in ventricular function than cells cultured under normoxic conditions[65]. This effect was abrogated by the addition of a CXC chemokine receptor (CXCR)-4 inhibitor, indicating that the benefits of hypoxic preconditioning occur via upregulated activity of CXCR4 and/or its ligand stromal cell-derived factor (SDF)-1. The SDF-1/CXCR4 axis plays an important role in progenitor cell homing to damaged tissue and has also been shown to be involved in the increased mobilization of bone marrow cells to the damaged heart after injection of MSCs genetically modified to overexpress IGF-1[66]. As an alternative method to target the IGF system without ex vivo genomic manipulation of cells prior to injection, combination therapy involving administration of c-kit+ CSCs and biotinylated IGF-1 nanofibers has also been tested as a way to enhance cell-mediated cardiac regeneration. Compared with either CSCs or IGF-1 nanofibers alone, combination therapy elicited a greater recovery of cardiac function and superior myocardial regeneration in large part via enhanced activation of endogenous resident cardiac progenitor cells[67]. Augmentation of endogenous repair was also implicated as a mechanism underlying the superior reparative efficacy of c-kit+ CSCs genetically engineered to express Pim-1 kinase, a downstream target of Akt that promotes cell survival and proliferation[68]. Thus, several cell modification techniques have been proven to successfully enhance the regenerative capacity of cell-based therapies in preclinical experiments. While many of these approaches aim to enhance the survival and/or retention of cells after injection, the vast majority of exogenously delivered cells still do not persist several weeks after delivery yet elicit persistent functional benefit, further reinforcing the importance of the endogenous repair system in this process.
As an alternative to modifying cells ex vivo prior to autologous administration, there is interest in allogeneic transplantation of cell populations that may offer superior myocardial regenerative potential. The finding that stimulation of endogenous repair processes is a primary mechanism underlying cell therapy-mediated cardiac regeneration facilitates this approach, since long-term engraftment and immune tolerance to administered cells is not mandatory to obtain structural and functional benefits. This idea is particularly attractive given that the majority of patients that would benefit from cell therapy may possess a stem cell population that is compromised by age and/or disease, thereby limiting the quantity and quality of cells available for isolation and ultimately hampering the effectiveness of autologous cell transplantation[69]. In this context, evidence that allogeneic transplantation of several prospective cell populations, including MSCs[50] and CDCs[70], is safe and effective at improving ventricular function in preclinical models of ischemic heart disease is particularly exciting. Further validation of allogeneic cell transfer approaches in large animals and humans will motivate future investigation aimed at identifying sources of functionally superior stem and progenitor cells. Along these lines, accumulating evidence supports the notion that cardiac stem cells isolated at an early stage of postnatal development may be particularly well-suited for therapeutic use. Multiple studies demonstrate that cardiac c-kit+ cells derived during the first week of postnatal life exhibit superior cardiomyogenic capacity in vitro compared with cells isolated from adult hearts[71, 72], which may contribute to the transient regenerative potential of mammalian hearts at this stage of life[73]. The recent finding that human neonatal-derived CDCs elicit greater myocardial repair than adult-derived CDCs when administered after infarction provides early support for the potential translation of a new therapeutic paradigm in which cardiac stem cells derived from young donors are utilized to promote myocardial regeneration in older patients with ischemic heart disease[74]. Alternatively, non-viable (i.e., lethally irradiated) stem cells could be used as in vivo “feeder layers” to promote endogenous myocardial repair since long-term cell survival is not necessary, an approach supported by recent data demonstrating activation of endogenous regeneration and improvements in myocardial function following injection of mitotically inactivated embryonic stem cells[75]. However, it must be kept in mind that potential age- or disease-related deficits in recipient repair processes may ultimately hinder the effectiveness of cell transplantation, regardless of the source of administered cells, given the important role of endogenous regenerative responses in mediating repair after exogenous cell injection.
In addition to strategies focusing on amplifying the reparative capacity of cells prior to delivery, efforts have also been made to identify the optimal myocardial substrate and patient population to maximize the efficacy of cell-based therapy. This may be particularly important given the prominent role that the host cell and tissue repair mechanisms play in mediating cell therapy-stimulated regeneration. From this perspective, it is tempting to speculate that targeting dysfunctional but viable myocardium, rather than infarct and border zone areas, may facilitate greater regeneration after cell administration. This notion is supported by data from our laboratory documenting the ability of MSCs[60], CDCs [76], and other interventions [77] to promote myocyte regeneration in swine with viable dysfunctional (“hibernating”) myocardium. Although there are many causes of hibernating myocardium, they typically reflect the consequences of cardiomyocyte loss and compensatory cellular hypertrophy arising from overload [78]. This hypertrophied cellular phenotype arises from many of the pathophysiological stimuli encountered in heart failure, including ischemia, chronically elevated preload, and neurohormonal activation, all of which increase over time during disease progression. Importantly, a large amount of chronically dysfunctional myocardium in patients with ischemic cardiomyopathy is generally viable [79, 80] and is thought to be a major contributor to the progression of global ventricular dysfunction. Therefore, focusing cell-based therapies on regenerating myocytes in viable dysfunctional tissue may be a more effective strategy than targeting fibrotic tissue or the relatively small border zone adjacent to the infarct, which has been the aim of many studies. Mechanistically, chronic hibernating myocardium may be amenable to therapeutic regeneration as a result of chronic cellular remodeling that may facilitate myocyte proliferation. Originally described by Borgers and colleagues [81], hibernating myocytes are characterized by a loss of myofibrils, mini-mitochondria, and a reversion to a fetal phenotype, indicative of cellular dedifferentiation. We have previously demonstrated that this dedifferentiated cellular phenotype is present in remote, normally perfused regions of the heart as well as in dysfunctional, chronically ischemic hibernating myocardium in swine with a chronic LAD stenosis [82], a pattern that has also been observed in patients with ischemic cardiomyopathy undergoing coronary revascularization [83]. Recently, Zhang et al. [84] have shown that dedifferentiation of mature mammalian cardiomyocytes in vitro results in downregulation of cell-cycle inhibitors and re-expression of cardiac progenitor cell markers including c-kit, ultimately promoting cell proliferation and the formation of new myocytes. These results raise the intriguing possibility that myocyte dedifferentiation may be involved in facilitating cell proliferation in hearts with hibernating myocardium. Future studies will be necessary to directly test this hypothesis, with the results likely to provide insight regarding how different pathophysiological substrates respond to therapies aimed at stimulating myocyte regeneration. Ultimately, this information will have significant implications for the clinical translation of cell therapy and the identification of patient populations that may receive the largest benefit from these therapeutic approaches.
Summary
Tremendous progress has been made in the field of cell-based therapeutics for cardiac repair and regeneration in the past fifteen years, with encouraging results from initial clinical trials supporting the safety of treatment approaches involving the administration of various stem and progenitor cell populations. Looking forward, it is imperative to expand our current knowledge of the mechanisms involved in successful myocardial regeneration to guide future basic and translational investigation aimed at optimizing cell therapy for clinical use. A growing body of evidence clearly demonstrates that a primary mechanism underlying the beneficial effects of cell transplantation is activation of endogenous cardiac repair processes, the details of which remain poorly understood. It is anticipated that future studies will continue to advance our understanding of these self-repair mechanisms and lead to the discovery of new and effective methods of cell-based myocardial repair, ultimately resulting in an improved ability to restore cardiac function in heart disease patients and reduce the substantial rate of morbidity and mortality associated with this condition throughout the world.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diwan A, Dorn GW., 2nd Decompensation of cardiac hypertrophy: cellular mechanisms and novel therapeutic targets. Physiology (Bethesda) 2007;22:56–64. doi: 10.1152/physiol.00033.2006. [DOI] [PubMed] [Google Scholar]
- 3.Marelli D, Desrosiers C, el-Alfy M, Kao RL, Chiu RC. Cell transplantation for myocardial repair: an experimental approach. Cell Transplant. 1992;1:383–390. doi: 10.1177/096368979200100602. [DOI] [PubMed] [Google Scholar]
- 4.Taylor DA, Atkins BZ, Hungspreugs P, Jones TR, Reedy MC, Hutcheson KA, Glower DD, Kraus WE. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat Med. 1998;4:929–933. doi: 10.1038/nm0898-929. [DOI] [PubMed] [Google Scholar]
- 5.Jeevanantham V, Butler M, Saad A, Abdel-Latif A, Zuba-Surma EK, Dawn B. Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: a systematic review and meta-analysis. Circulation. 2012;126:551–568. doi: 10.1161/CIRCULATIONAHA.111.086074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strauer BE, Steinhoff G. 10 years of intracoronary and intramyocardial bone marrow stem cell therapy of the heart: from the methodological origin to clinical practice. J Am Coll Cardiol. 2011;58:1095–1104. doi: 10.1016/j.jacc.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 7.Vrtovec B, Poglajen G, Lezaic L, Sever M, Domanovic D, Cernelc P, Socan A, Schrepfer S, Torre-Amione G, Haddad F, Wu JC. Effects of Intracoronary CD34+ Stem Cell Transplantation in Nonischemic Dilated Cardiomyopathy Patients: 5-Year Follow-Up. Circ Res. 2013;112:165–173. doi: 10.1161/CIRCRESAHA.112.276519. [DOI] [PubMed] [Google Scholar]
- 8.Leri A, Kajstura J, Anversa P. Role of cardiac stem cells in cardiac pathophysiology: a paradigm shift in human myocardial biology. Circ Res. 2011;109:941–961. doi: 10.1161/CIRCRESAHA.111.243154. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kajstura J, Gurusamy N, Ogorek B, Goichberg P, Clavo-Rondon C, Hosoda T, D’Amario D, Bardelli S, Beltrami AP, Cesselli D, Bussani R, del Monte F, Quaini F, Rota M, Beltrami CA, Buchholz BA, Leri A, Anversa P. Myocyte turnover in the aging human heart. Circ Res. 2010;107:1374–1386. doi: 10.1161/CIRCRESAHA.110.231498. [DOI] [PubMed] [Google Scholar]
- 11.Kajstura J, Rota M, Cappetta D, Ogorek B, Arranto C, Bai Y, Ferreira-Martins J, Signore S, Sanada F, Matsuda A, Kostyla J, Caballero MV, Fiorini C, D’Alessandro DA, Michler RE, del Monte F, Hosoda T, Perrella MA, Leri A, Buchholz BA, Loscalzo J, Anversa P. Cardiomyogenesis in the aging and failing human heart. Circulation. 2012;126:1869–1881. doi: 10.1161/CIRCULATIONAHA.112.118380. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Cesselli D, Beltrami AP, Rigo S, Bergamin N, D’Aurizio F, Verardo R, Piazza S, Klaric E, Fanin R, Toffoletto B, Marzinotto S, Mariuzzi L, Finato N, Pandolfi M, Leri A, Schneider C, Beltrami CA, Anversa P. Multipotent progenitor cells are present in human peripheral blood. Circ Res. 2009;104:1225–1234. doi: 10.1161/CIRCRESAHA.109.195859. [DOI] [PubMed] [Google Scholar]
- 13.Beltrami AP, Urbanek K, Kajstura J, Yan S-M, Finato N, Bussani R, Nadal-Ginard B, Silvestri F, Leri A, Beltrami CA, Anversa P. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 14.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D’Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci USA. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anversa P, Kajstura J, Leri A, Bolli R. Life and death of cardiac stem cells: a paradigm shift in cardiac biology. Circulation. 2006;113:1451–1463. doi: 10.1161/CIRCULATIONAHA.105.595181. [DOI] [PubMed] [Google Scholar]
- 16.Kajstura J, Urbanek K, Perl S, Hosoda T, Zheng H, Ogorek B, Ferreira-Martins J, Goichberg P, Rondon-Clavo C, Sanada F, D’Amario D, Rota M, Del Monte F, Orlic D, Tisdale J, Leri A, Anversa P. Cardiomyogenesis in the adult human heart. Circ Res. 2010;107:305–315. doi: 10.1161/CIRCRESAHA.110.223024. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Raval Z, Losordo DW. On the Fabric of the Human Body. Circulation. 2012 doi: 10.1161/CIRCULATIONAHA.112.136127. [DOI] [PubMed] [Google Scholar]
- 18.Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473:326–335. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 20.Urbanek K, Cesselli D, Rota M, Nascimbene A, De Angelis A, Hosoda T, Bearzi C, Boni A, Bolli R, Kajstura J, Anversa P, Leri A. Stem cell niches in the adult mouse heart. Proc Natl Acad Sci USA. 2006;103:9226–9231. doi: 10.1073/pnas.0600635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, Entman ML, Schneider MD. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A. 2003;100:12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, Platoshyn O, Yuan JX, Evans S, Chien KR. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin CM, Meeson AP, Robertson SM, Hawke TJ, Richardson JA, Bates S, Goetsch SC, Gallardo TD, Garry DJ. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev Biol. 2004;265:262–275. doi: 10.1016/j.ydbio.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 24.Smart N, Bollini S, Dube KN, Vieira JM, Zhou B, Davidson S, Yellon D, Riegler J, Price AN, Lythgoe MF, Pu WT, Riley PR. De novo cardiomyocytes from within the activated adult heart after injury. Nature. 2011;474:640–644. doi: 10.1038/nature10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP, Lee RT. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2012 doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, Robbins J, Lee RT. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970–974. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Urbanek K, Rota M, Cascapera S, Bearzi C, Nascimbene A, De Angelis A, Hosoda T, Chimenti S, Baker M, Limana F, Nurzynska D, Torella D, Rotatori F, Rastaldo R, Musso E, Quaini F, Leri A, Kajstura J, Anversa P. Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circ Res. 2005;97:663–673. doi: 10.1161/01.RES.0000183733.53101.11. [DOI] [PubMed] [Google Scholar]
- 28.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 29.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 30.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 31.Nygren JM, Jovinge S, Breitbach M, Sawen P, Roll W, Hescheler J, Taneera J, Fleischmann BK, Jacobsen SE. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004;10:494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- 32.Tang XL, Rokosh G, Sanganalmath SK, Yuan F, Sato H, Mu J, Dai S, Li C, Chen N, Peng Y, Dawn B, Hunt G, Leri A, Kajstura J, Tiwari S, Shirk G, Anversa P, Bolli R. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation. 2010;121:293–305. doi: 10.1161/CIRCULATIONAHA.109.871905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bolli R, Chugh AR, D’Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Chugh AR, Beache GM, Loughran JH, Mewton N, Elmore JB, Kajstura J, Pappas P, Tatooles A, Stoddard MF, Lima JA, Slaughter MS, Anversa P, Bolli R. Administration of Cardiac Stem Cells in Patients With Ischemic Cardiomyopathy: The SCIPIO Trial: Surgical Aspects and Interim Analysis of Myocardial Function and Viability by Magnetic Resonance. Circulation. 2012;126:S54–64. doi: 10.1161/CIRCULATIONAHA.112.092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronico MV, Coletta M, Vivarelli E, Frati L, Cossu G, Giacomello A. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 36.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marban E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 37.Li TS, Cheng K, Lee ST, Matsushita S, Davis D, Malliaras K, Zhang Y, Matsushita N, Smith RR, Marban E. Cardiospheres recapitulate a niche-like microenvironment rich in stemness and cell-matrix interactions, rationalizing their enhanced functional potency for myocardial repair. Stem Cells. 2010;28:2088–2098. doi: 10.1002/stem.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnston PV, Sasano T, Mills K, Evers R, Lee ST, Smith RR, Lardo AC, Lai S, Steenbergen C, Gerstenblith G, Lange R, Marban E. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation. 2009;120:1075–1083. doi: 10.1161/CIRCULATIONAHA.108.816058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li TS, Cheng K, Malliaras K, Smith RR, Zhang Y, Sun B, Matsushita N, Blusztajn A, Terrovitis J, Kusuoka H, Marban L, Marban E. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J Am Coll Cardiol. 2012;59:942–953. doi: 10.1016/j.jacc.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marban L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marban E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 42.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 43.Xu W, Zhang X, Qian H, Zhu W, Sun X, Hu J, Zhou H, Chen Y. Mesenchymal stem cells from adult human bone marrow differentiate into a cardiomyocyte phenotype in vitro. Exp Biol Med. 2004;229:623–631. doi: 10.1177/153537020422900706. [DOI] [PubMed] [Google Scholar]
- 44.Xu M, Wani M, Dai YS, Wang J, Yan M, Ayub A, Ashraf M. Differentiation of bone marrow stromal cells into the cardiac phenotype requires intercellular communication with myocytes. Circulation. 2004;110:2658–2665. doi: 10.1161/01.CIR.0000145609.20435.36. [DOI] [PubMed] [Google Scholar]
- 45.Augello A, De Bari C. The regulation of differentiation in mesenchymal stem cells. Hum Gene Ther. 2010;21:1226–1238. doi: 10.1089/hum.2010.173. [DOI] [PubMed] [Google Scholar]
- 46.Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, Pattany PM, Zambrano JP, Hu Q, McNiece I, Heldman AW, Hare JM. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci U S A. 2009;106:14022–14027. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Makkar RR, Price MJ, Lill M, Frantzen M, Takizawa K, Kleisli T, Zheng J, Kar S, McClelan R, Miyamota T, Bick-Forrester J, Fishbein MC, Shah PK, Forrester JS, Sharifi B, Chen PS, Qayyum M. Intramyocardial injection of allogenic bone marrow-derived mesenchymal stem cells without immunosuppression preserves cardiac function in a porcine model of myocardial infarction. J Cardiovasc Pharmacol Ther. 2005;10:225–233. doi: 10.1177/107424840501000403. [DOI] [PubMed] [Google Scholar]
- 48.Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923–940. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chong JJ, Chandrakanthan V, Xaymardan M, Asli NS, Li J, Ahmed I, Heffernan C, Menon MK, Scarlett CJ, Rashidianfar A, Biben C, Zoellner H, Colvin EK, Pimanda JE, Biankin AV, Zhou B, Pu WT, Prall OW, Harvey RP. Adult cardiac-resident MSC-like stem cells with a proepicardial origin. Cell Stem Cell. 2011;9:527–540. doi: 10.1016/j.stem.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB, Jr, Reisman MA, Schaer GL, Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Houtgraaf JH, den Dekker WK, van Dalen BM, Springeling T, de Jong R, van Geuns RJ, Geleijnse ML, Fernandez-Aviles F, Zijlsta F, Serruys PW, Duckers HJ. First experience in humans using adipose tissue-derived regenerative cells in the treatment of patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2012;59:539–540. doi: 10.1016/j.jacc.2011.09.065. [DOI] [PubMed] [Google Scholar]
- 52.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 53.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mirotsou M, Jayawardena TM, Schmeckpeper J, Gnecchi M, Dzau VJ. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J Mol Cell Cardiol. 2011;50:280–289. doi: 10.1016/j.yjmcc.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 56.Cho HJ, Lee N, Lee JY, Choi YJ, Ii M, Wecker A, Jeong JO, Curry C, Qin G, Yoon YS. Role of host tissues for sustained humoral effects after endothelial progenitor cell transplantation into the ischemic heart. J Exp Med. 2007;204:3257–3269. doi: 10.1084/jem.20070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shabbir A, Ziza D, Suzuki G, Lee T. Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: a noninvasive therapeutic regimen. Am J Physiol Heart Circ Physiol. 2009;296:H1888–H1897. doi: 10.1152/ajpheart.00186.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shabbir A, Zisa D, Lin H, Mastri M, Roloff G, Suzuki G, Lee T. Activation of host tissue trophic factors through JAK-STAT3 signaling: a mechanism of mesenchymal stem cell-mediated cardiac repair. Am J Physiol Heart Circ Physiol. 2010;299:H1428–1438. doi: 10.1152/ajpheart.00488.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lim H, Fallavollita JA, Hard R, Kerr CW, Canty JM., Jr Profound apoptosis-mediated regional myocyte loss and compensatory hypertrophy in pigs with hibernating myocardium. Circulation. 1999;100:2380–2386. doi: 10.1161/01.cir.100.23.2380. [DOI] [PubMed] [Google Scholar]
- 60.Suzuki G, Iyer V, Lee TC, Canty JM., Jr Autologous Mesenchymal Stem Cells Mobilize cKit+ and CD133+ Bone Marrow Progenitor Cells and Improve Regional Function in Hibernating Myocardium. Circ Res. 2011;109:1044–1054. doi: 10.1161/CIRCRESAHA.111.245969. [DOI] [PubMed] [Google Scholar]
- 61.Loffredo FS, Steinhauser ML, Gannon J, Lee RT. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell. 2011;8:389–398. doi: 10.1016/j.stem.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP, Rodriguez JE, Dulce R, Pattany PM, Valdes D, Revilla C, Heldman AW, McNiece I, Hare JM. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chimenti I, Smith RR, Li TS, Gerstenblith G, Messina E, Giacomello A, Marban E. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106:971–980. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mohsin S, Siddiqi S, Collins B, Sussman MA. Empowering adult stem cells for myocardial regeneration. Circ Res. 2011;109:1415–1428. doi: 10.1161/CIRCRESAHA.111.243071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tang YL, Zhu W, Cheng M, Chen L, Zhang J, Sun T, Kishore R, Phillips MI, Losordo DW, Qin G. Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ Res. 2009;104:1209–1216. doi: 10.1161/CIRCRESAHA.109.197723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haider H, Jiang S, Idris NM, Ashraf M. IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1alpha/CXCR4 signaling to promote myocardial repair. Circ Res. 2008;103:1300–1308. doi: 10.1161/CIRCRESAHA.108.186742. [DOI] [PubMed] [Google Scholar]
- 67.Padin-Iruegas ME, Misao Y, Davis ME, Segers VF, Esposito G, Tokunou T, Urbanek K, Hosoda T, Rota M, Anversa P, Leri A, Lee RT, Kajstura J. Cardiac progenitor cells and biotinylated insulin-like growth factor-1 nanofibers improve endogenous and exogenous myocardial regeneration after infarction. Circulation. 2009;120:876–887. doi: 10.1161/CIRCULATIONAHA.109.852285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mohsin S, Khan M, Toko H, Bailey B, Cottage CT, Wallach K, Nag D, Lee A, Siddiqi S, Lan F, Fischer KM, Gude N, Quijada P, Avitabile D, Truffa S, Collins B, Dembitsky W, Wu JC, Sussman MA. Human Cardiac Progenitor Cells Engineered With Pim-I Kinase Enhance Myocardial Repair. J Am Coll Cardiol. 2012 doi: 10.1016/j.jacc.2012.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dimmeler S, Leri A. Aging and disease as modifiers of efficacy of cell therapy. Circ Res. 2008;102:1319–1330. doi: 10.1161/CIRCRESAHA.108.175943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Malliaras K, Li TS, Luthringer D, Terrovitis J, Cheng K, Chakravarty T, Galang G, Zhang Y, Schoenhoff F, Van Eyk J, Marban L, Marban E. Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere-derived cells. Circulation. 2012;125:100–112. doi: 10.1161/CIRCULATIONAHA.111.042598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zaruba MM, Soonpaa M, Reuter S, Field LJ. Cardiomyogenic potential of C-kit(+)-expressing cells derived from neonatal and adult mouse hearts. Circulation. 2010;121:1992–2000. doi: 10.1161/CIRCULATIONAHA.109.909093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jesty SA, Steffey MA, Lee FK, Breitbach M, Hesse M, Reining S, Lee JC, Doran RM, Nikitin AY, Fleischmann BK, Kotlikoff MI. c-kit+ precursors support postinfarction myogenesis in the neonatal, but not adult, heart. Proc Natl Acad Sci USA. 2012;109:13380–13385. doi: 10.1073/pnas.1208114109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Simpson DL, Mishra R, Sharma S, Goh SK, Deshmukh S, Kaushal S. A strong regenerative ability of cardiac stem cells derived from neonatal hearts. Circulation. 2012;126:S46–53. doi: 10.1161/CIRCULATIONAHA.111.084699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Burt RK, Chen YH, Verda L, Lucena C, Navale S, Johnson J, Han X, Lomasney J, Baker JM, Ngai KL, Kino A, Carr J, Kajstura J, Anversa P. Mitotically Inactivated Embryonic Stem Cells can be Utilized as an In Vivo Feeder Layer to Nurse Damaged Myocardium Following Acute Myocardial Infarction: A Pre-Clinical Study. Circ Res. 2012 doi: 10.1161/CIRCRESAHA.111.262584. [DOI] [PubMed] [Google Scholar]
- 76.Suzuki G, Leiker M, Cimato T, Canty JM., Jr Intracoronary Infusion of Cardiosphere-Derived Cells (icCDCs) Improves Cardiac Function by Stimulating Myocyte Proliferation in Non-Infarcted Hibernating Myocardium with No Effect in Normal Myocardium. Circulation. 2011;124:A8851. [Google Scholar]
- 77.Suzuki G, Iyer V, Cimato T, Canty JM., Jr Pravastatin improves function in hibernating myocardium by mobilizing CD133+ and cKit+ hematopoietic progenitor cells and promoting myocytes to reenter the growth phase of the cardiac cell cycle. Circ Res. 2009;104:255–264. doi: 10.1161/CIRCRESAHA.108.188730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Canty JM., Jr . Coronary blood flow and myocardial ischemia. In: Bonow RO, Mann DL, Zipes DP, Libby P, editors. Braunwald’s Heart Disease. Elsevier; Philadelphia: 2012. pp. 1049–1075. [Google Scholar]
- 79.Sawada S, Elsner G, Segar DS, O’Shaughnessy M, Khouri S, Foltz J, Bourdillon PD, Bates JR, Fineberg N, Ryan T, Hutchins GD, Feigenbaum H. Evaluation of patterns of perfusion and metabolism in dobutamine-responsive myocardium. J Am Coll Cardiol. 1997;29:55–61. doi: 10.1016/s0735-1097(96)00430-5. [DOI] [PubMed] [Google Scholar]
- 80.Melon PG, de Landsheere CM, Degueldre C, Peters JL, Kulbertus HE, Pierard LA. Relation between contractile reserve and positron emission tomographic patterns of perfusion and glucose utilization in chronic ischemic left ventricular dysfunction: implications for identification of myocardial viability. J Am Coll Cardiol. 1997;30:1651–1659. doi: 10.1016/s0735-1097(97)00373-2. [DOI] [PubMed] [Google Scholar]
- 81.Dispersyn GD, Ramaekers FCS, Borgers M. Clinical pathophysiology of hibernating myocardium. Coron Artery Dis. 2001;12:381–385. doi: 10.1097/00019501-200108000-00007. [DOI] [PubMed] [Google Scholar]
- 82.Thomas SA, Fallavollita JA, Borgers M, Canty JM., Jr Dissociation of regional adaptations to ischemia and global myolysis in an accelerated swine model of chronic hibernating myocardium. Circ Res. 2002;91:970–977. doi: 10.1161/01.res.0000040396.79379.77. [DOI] [PubMed] [Google Scholar]
- 83.Gunning MG, Kaprielian RR, Pepper J, Pennell DJ, Sheppard MN, Severs NJ, Fox KM, Underwood SR. The histology of viable and hibernating myocardium in relation to imaging characteristics. J Am Coll Cardiol. 2002;39:428–435. doi: 10.1016/s0735-1097(01)01766-1. [DOI] [PubMed] [Google Scholar]
- 84.Zhang Y, Li TS, Lee ST, Wawrowsky KA, Cheng K, Galang G, Malliaras K, Abraham MR, Wang C, Marban E. Dedifferentiation and proliferation of mammalian cardiomyocytes. PLoS ONE. 2010;5:e12559. doi: 10.1371/journal.pone.0012559. [DOI] [PMC free article] [PubMed] [Google Scholar]


