Abstract
The Drosophila antenna is a sophisticated structure that functions in both olfaction and audition. Previous studies have identified Homothorax, Extradenticle, and Distal-less, three homeodomain transcription factors, as required for specification of antennal identity. Antennal expression of cut is activated by Homothorax and Extradenticle, and repressed by Distal-less. cut encodes the Drosophila homolog of human CAAT-displacement protein, a cell cycle-regulated homeodomain transcription factor. Cut is required for normal development of external mechanosensory structures and Malphigian tubules (kidney analogs). The role of cut in the Drosophila auditory organ, Johnston's organ, has not been characterized. We have employed the FLP/FRT system to generate cut null clones in developing Johnston's organ. In cut mutants, the scolopidial subunits that constitute Johnston's organ differentiate abnormally and subsequently degenerate. Electrophysiological experiments confirm that adult Drosophila with cut null antennae are deaf. We find that cut acts in parallel to atonal, spalt-major, and spalt-related, which encode other transcription factors required for Johnston's organ differentiation. We speculate that Cut functions in conjunction with these factors to regulate transcription of as yet unidentified targets.
Keywords: ato, CDP, chordotonal organ, Cux1, ct, dll, hearing, exd, hth, Johnston's organ
INTRODUCTION
Chordotonal organs in Drosophila hearing
There are two classes of mechanoreceptive sense organs in Drosophila. Type 1 mechanosensors include chordotonal organs (CHOs) and external sensory (ES) organs. ES organs comprise bristles and campaniform sensillae. Type 2 sense organs are multiple dendritic (MD) neurons (reviewed in ref. 1). MD neurons derive from both ES and CHO lineages.2 Johnston's organ (JO) is the largest CHO in Drosophila, and is specialized for hearing. JO, which is housed in the second antennal segment (a2), is composed of approximately 200 multicellular subunits termed scolopidia (Fig. 1). Each scolopidium includes a scolopale cell, two or three sensory neurons,3 a cap cell and a ligament cell (reviewed in ref. 4). Cells of a scolopidium are clonally related and arise from a single sensory organ precursor (SOP).5 Each scolopidium is attached to cuticle both apically and basally. In JO, the basal attachment is made within a2 and the apical attachment is to the cuticle of the joint between a2 and the third antennal segment (a3). When a near-field sound wave strikes the distal arista, the force is conveyed via the fifth and fourth antennal segments (a5 and a4, respectively) to a3, which then rotates relative to a2. This leads to tension changes on JO scolopidia, the opening of mechanically-gated ion channels, and the firing of JO neurons (reviewed in refs. 4, 6 and 7).
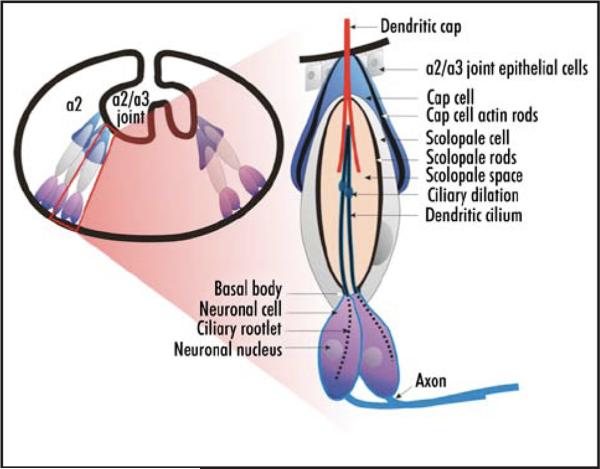
Figure 1.
Schematics of the second antennal segment and a Johnston's organ scolopidium. On the left is a schematic of the interior of a2 with a single scolopidium boxed in red. On the right is an enlarged view of that scolopidium. The neuronal cell bodies, axons, and ciliated dendrites are indicated, as is the dendritic cap, which is secreted by the scolopale cell. Together, the dendritic cap and the cap cell tether the scolopidium apically to the joint cuticle. The scolopale cell ensheathes the dendrites. It contains rigid actin rods that form the scolopales. The scolopale space is thought to contain a potassium rich endolymph.
cut is used throughout development in Drosophila
Cut (Ct) is a member of a large class of transcription factors characterized by the presence of both ct repeats and a homeodomain (reviewed in ref. 8). In Drosophila, overexpression of ct in larval pentascolopidial CHO (lch5) precursors results in their transformation towards an ES organ fate.9,10 Conversely, in ct mutants, ES organ precursors develop into CHOs.11-13 ct function therefore is necessary, and in some contexts sufficient, for the specification of an ES organ fate from a mechano-sensory precursor. An additional role for ct recently was described in a subclass of MD neurons, the dendritic arborization (da) neurons. The level of ct expression in da neurons is correlated positively with their degree of arborization.14 ct also is required for normal differentiation of the fly kidney analogs, the Malphigian tubules.15
Ct and its homologs are transcriptional regulators
The transcriptional activity of Drosophila Ct has not been tested. However, the human Ct homolog, CAAT-displacement protein (CDP; Cux1 in the mouse), is a potent repressor (reviewed in ref. 16). Vertebrate CDP/Cux1 represses gene transcription by two distinct mechanisms.17 Active repression by CDP/Cux1 occurs through DNA binding and the direct recruitment of histone deacetylases.17,18 Passive repression by CDP/Cux1 occurs via competition for binding sites with transcriptional activators.19-22 CDP/Cux1 is post-translationally regulated in a cell-cycle dependent fashion. Regulation includes both dephosphorylation by CDC25a23 and cleavage by cathepsin L at the G1/S transition.24,25 These modifications convert CDP/Cux1 from a transcriptional repressor to an activator.
Drosophila ct activates bereft (bft).26 Misexpression of bft suppresses an eye phenotype induced by the dominant negative mutations of kinase suppressor of ras (ksr).27,28 This indicates that bft functions either downstream of or in parallel to ksr in the Ras1/mitogen activated kinase (MAPK) signaling cascade. It is not known whether the regulation of bft by Ct is direct. Nor is it known whether Ct also functions within or in parallel to MAPK signaling.
Hth and n-Exd activate and Dll represses ct in the antennal imaginal disc
Gene products that have critical roles in specifying antenna fates include the transcription factors Distal-less (Dll), Homothorax (Hth), and Extradenticle (Exd).29-35 At late third instar, Dll expression is localized to the center of the disc that gives rise to distal structures (a2, a3, a4, a5, arista), and is absent from presumptive proximal (head capsule and a1) cells.36,37 Conversely, Hth expression at late third instar is more proximal (presumptive head cuticle, a1, a2 and a3) and is absent from presumptive a4-ar.32,33 Exd is constitutively expressed throughout the third larval instar antennal disc, though its nuclear localization requires Hth.33,38 dll, hth and exd mutants exhibit phenotypes that include antenna to leg transformation and distal truncations.29-35,39-41 In late third larval instar antennal discs, Hth and Dll expression overlap in a domain that corresponds to presumptive antennal segments a2 and a3.41 Within this domain, atonal (ato), dachshund (dac) and spalt-major/ spalt-related (salm/salr) are activated by dll, exd and hth.41,42 salm/salr and ato have described roles in JO development.43,44
In contrast, ct is activated by Hth42 and Exd (this work) and repressed by Dll.42 The repression by Dll is likely indirect and mediated by Spineless (Ss). Ss, in turn, may function via Distal antenna (Dan; also called Fernandez) and Distal antenna-related (Danr; also called Hernandez).45,46 The consequence of activation by Hth and Exd and repression by Dll is expression of Ct at late third instar that is confined to head capsule-a3. This expression is absent from the leg, where ct instead appears at third instar in isolated cellls of unknown fates and in tarsal claw precursors.47 The differences in ct expression between the two limb types indicate that ct is not generally required for ventral appendage development. Instead, ct is one of a growing suite of factors with distinct roles in different appendages. The expression pattern of ct in the developing antenna led us to hypothesize that ct might have a role in the development of antennal structures including JO.
Chordotonal development
The expression of ct highlights some differences between JO and canonical chordotonal models such as lch5. ato expression in lch5 precursors promotes CHO identity,48 while ct misexpression in lch5 precursors causes a transformation to ES fate.9,10 Conversely, either loss of ct in developing embryonic ES organs11-13 or misexpression of ato in developing notal or wing ES organs causes a transformation towards chordotonal identity.49 It was inferred from these experiments that the roles of ct and ato, if not directly antagonistic, were more or less mutually exclusive in the context of developing mechanosensors. Nonetheless, in the antenna, ct and ato expression overlap in the presumptive JO. This suggests that the relationship between these two genes in the antenna is distinct from that found either in ES organs or in other CHO's. ato is required for differentiation of JO scolopidia and for Drosophila hearing.43,50,51 Here we show that ct also is required for JO development, and that adult Drosophila lacking antennal ct function are deaf. The scolopidia of ct mutant JOs are disorganized and the cuticular structures associated with them are severely disrupted. The ct phenotype is distinct from that of ato mutants in which no scolopidia are formed and relatively mild cuticular defects are observed.52 The ct phenotype resembles that of salm/salr mutants44 at both pupal and adult stages, in that mutant scolopidia differentiate during pupation and degenerate by adulthood. We find that ct, ato, and salm/salr are independently regulated in the antenna, and likely to cooperate in the specification and maintenance JO fate.
MATERIALS AND METHODS
Generation of ct mutant antennae
To determine the role of ct(FBgn0004198) in the Drosophila antenna, we generated antennae that were partially or largely null for ct using the FRT/FLP method of Xu and Rubin (1993) and a FLP transgene regulated by the an eyeless enhancer (eyFLP;53 FBti0015983). The genotypes examined were: y w ctc145(FBal0001952) FRT19a(FBti0000870)/
UBI-GFP(FBti0015575) FRT19a; eyFLP and y w ctc145 FRT19a/M(1)15DEF (FBgn0015252) FRT19a; eyFLP
The P{GawB}elavC155 Gal4(FBti0002575) and the UAS-mCD8-GFP(FBti0012684) lines used in Figure 4 are available from the Bloomington Stock center.
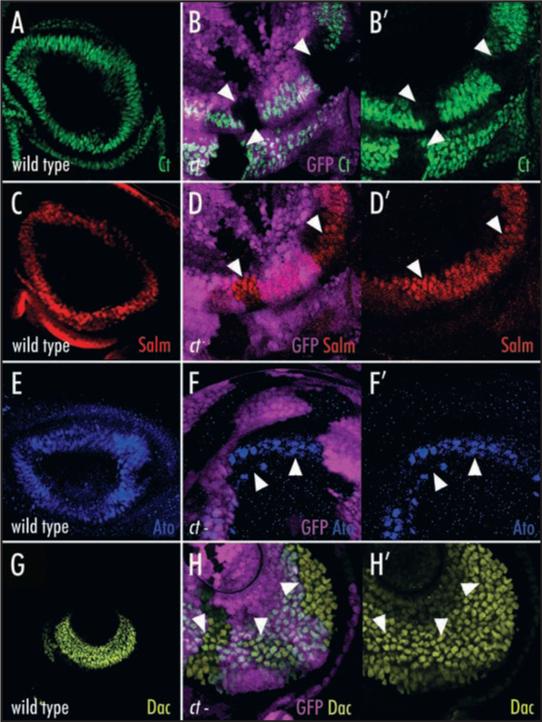
Figure 4.
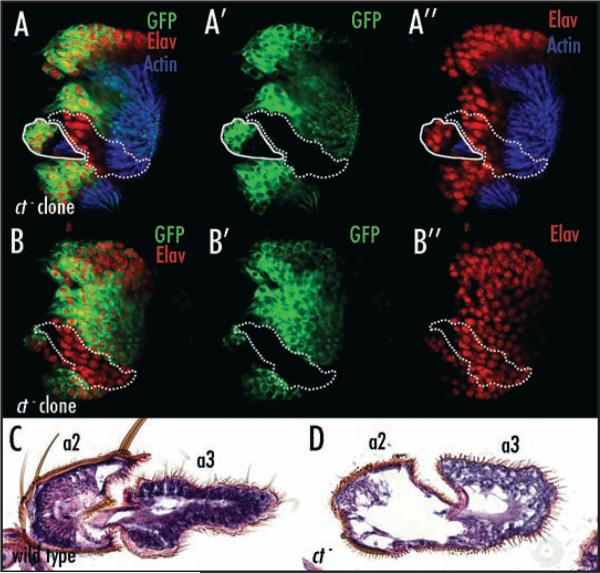
cut is not required for the activation or repression of known antennal patterning genes. (A) Ct (green) is expressed in presumptive head capsule, a1 and a2 in late third instar antennal imaginal discs. (B and B′) Ct (green) expression is lost cell autonomously from ct null clones (arrowheads). The clones are marked by the absence of GFP (purple) expressed ubiquitously from a construct on the ct+ chromosome (genotype is y, w, ctc145, FRT19a/ Ubi-GFP FRT19a; eyFLP). (C) Salm (red) is expressed in presumptive a2. (D and D’) Salm (red) expression persists in ct null clones (arrowheads) marked by the absence of GFP (purple). (E) Ato (blue) is expressed in presumptive a2. (F and F′) Ato (blue) expression persists in ct null clones (arrowheads) marked by the absence of GFP (purple). (G) Dac (yellow) is expressed in presumptive a3. (H and H′). The Dac (yellow) expression domain does not expand in ct null clones (arrowheads) marked by the absence of GFP (purple).
Immunohistochemistry, cuticle preparations and histology
Antibody staining of third instar antennal discs was carried out as previously described.41 A detailed pupal antibody staining protocol will be provided elsewhere (Ebacher and Boekhoff-Falk, in preparation). Secondary antibodies and stains were all Alexa-conjugated (488, 568, 633) from Invitrogen with the exception of goat anti-HRP and rhodamine-conjugated phalloidin that were obtained from Jackson ImmunoResearch Laboratories. Paraffin sections and hematoxylin and eosin staining of adult Oregon R and y w ctc145 FRT19a/M(1), FRT19a; eyFLP were carried out as described by Downs et al. Imaging was carried out on BioRad MRC1024 and Zeiss confocal microscopes, and a Zeiss Axioplan microscope equipped with an Axiocam.
All of the monoclonal antibodies used in this paper were from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA 52242. These antibodies include murine anti-Cut (Ct), murine anti-Dachshund (Dac; FBgn0005677) and rat anti-Embryonic lethal, abnormal vision (Elav; FBgn0000570). The rabbit anti-Spalt (FBgn0004579) antibody was a generous gift from Dr. Bertrand Mollereau. The guinea pig anti-Atonal (FBgn0010433) antibody was a generous gift from Dr. Hugo Bellen.
Electrophysiology
Electrophysiology was performed as described.44 Briefly, auditory stimuli consisted of computer-generated pulse song as well as 160- and 250-Hz sine songs. The acoustic signals from a loudspeaker were delivered frontally to the fly's head through Tygon tubing. An electrolytically sharpened tungsten electrode was inserted between the first and second antennal segments to record extracellularly from the antennal nerve. A similar, reference electrode was inserted into the dorsal side of the head. The differential signal (DAM 50 differential amplifier; World Precision Instruments, Sarasota, FL) was amplified 1,000-fold and routed to an InstruNet 100B (GW Instruments) AD/DA converter connected to a Mac PowerBook 1400. We used SUPERSCOPE II software (GW Instruments) to generate the stimuli and to record the signals. Each trial consisted of the auditory stimulus presented 10 consecutive times. Amplitudes are an average of the 10 recordings.
RESULTS
ct is required for the development of the Drosophila auditory organ
To determine the role of ct in the Drosophila antenna, we generated antennae that were partially or largely null for ct using the FRT/FLP method of Xu and Rubin.54 When the aristae of these mutants are stimulated with a paintbrush or forceps, a3 does not rotate within a2 (not shown). Since rotation is required for hearing,6,50 this indicates that these animals are likely to be deaf. In antennae harboring large ct null clones, the internal cuticular structures associated with the a2/a3 joint are completely absent (Fig. 2A, B, E and F), while in antennae with smaller clones some joint structures remain (Fig. 2C and D). This implicates Ct in the development of a2/a3 joint cuticle. Another defect associated with ct mutant antennae is an a2 cuticular clefting phenotype (not shown). Both the loss of a2/a3 joint tissue and the clefting are similar to that observed in salm/salr mutants.44
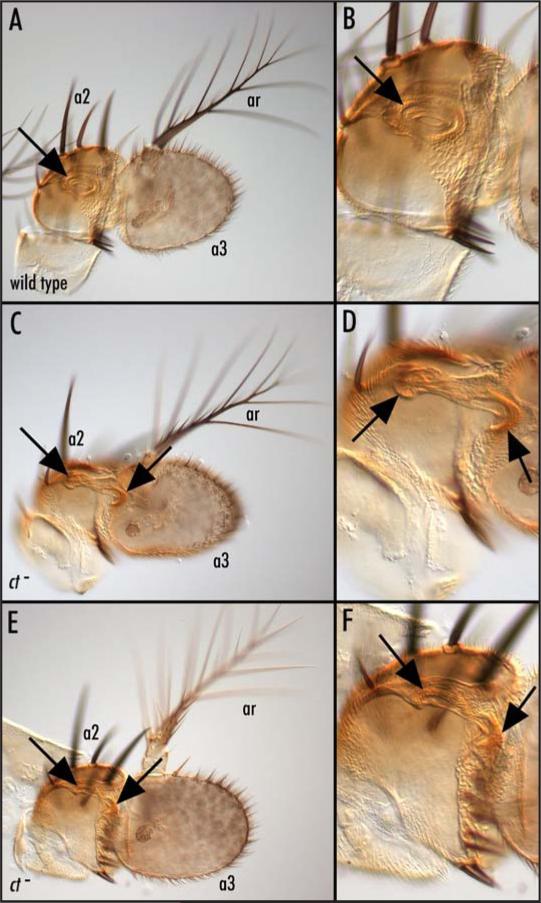
Figure 2.
cut mutants exhibit cuticular defects in the a2/a3 joint required for hearing. Low (A) and high (B) magnification internal optical sections of a wild type adult antenna; the arrow marks the a2/a3 joint cuticle where JO scolopidia attach. Low (C) and high (D) magnification internal optical sections of an antenna carrying a ct- clone in which there is moderate disruption of the joint cuticle (arrows). It is not possible to see the individual scolopidia in these images. The circular profile indicated by the arrows in (A) and (B) is that of the joint. Low (E) and high (F) magnification internal optical sections of joint cuticle from an antenna carrying an even larger ct− clone (genotype of C-F is: y, w, ctc145, FRT19a/M(1), FRT19a; eyFLP) in which there are more severe defects in the joint cuticle (arrows). Although the ct null clones in these images are marked with y, it is not possible to distinguish the y tissue in these images. a2 = second antennal segment; a3 = third antennal segment; ar = arista.
Drosophila with ct null antennae are deaf
ct mutant antennae show a2/a3 joint defects that include an inability for a2 to rotate relative to a3. We therefore anticipated that these animals would have impaired or diminished hearing and performed electrophysiological analyses to test this. This analysis reveals whether or not an antenna exhibits a sound-evoked potential when exposed to a recorded pulse song (the vibrations emitted by a male fly when attracting and courting a female fly).55 ct antennal mosaics (genotype is y w ctc145FRT19a/M(1) FRT19a; eyFLP) showed an average amplitude of 60 μV (similar to background noise) while wild type animals assayed at the same time showed an average amplitude of 1206 μV when presented with a recorded pulse song (Fig. 3). This indicates that ct mutants are deaf.
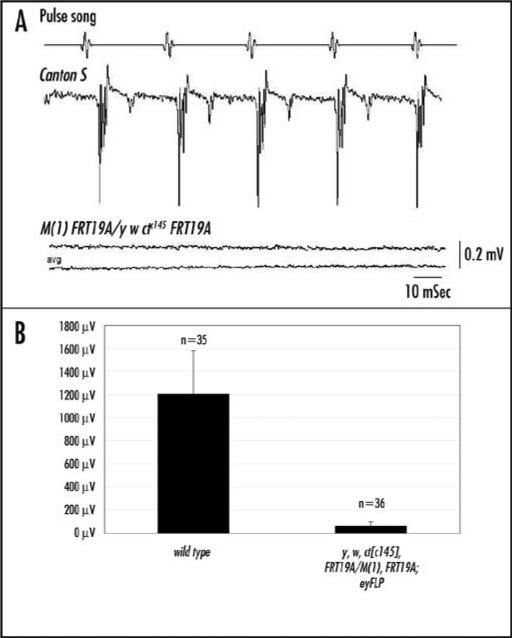
Figure 3.
Drosophila with cut null antennae are deaf. (A) In response to the pulse-song, strong sound-evoked potentials are generated within the antennal nerve between the antenna and the brain. ct mutants (genotype is: y, w, ctc145, FRT19a/M(1), FRT19a; eyFLP) exhibit a lower amplitude response than wild type animals in response to a pulse-song. Each trace is an average of 10. (B) A histogram showing the response amplitudes of wild type and mutant genotypes. The average control amplitude was 1206 μV, whereas the average amplitude of the ct mutants was 60 μV. p < 0.01, using Students t test.
ct does not regulate known antennal patterning genes
Because other genes expressed in the antenna have mutant phenotypes that overlap those of ct mutants, we investigated the relationships betweenct and other genes required for JO development. We first determined that ct expression is autonomously lost from ct-null clones in third larval instar antennal discs (Fig. 4A, B and B’). This loss of ct does not affect the expression of other antennal genes such as salm/salr, ato, dac (Fig. 4C–H’), bric-a-brac (bab), dll, exd, hth, pox-neuro (poxn) or senseless (sens) (not shown). We conclude that ct does not regulate any known antennal transcription factors at third instar and that ct therefore is regulating unidentified antennal genes or known genes at later stages.
The specification of JO neurons and scolopale cells does not require ct
Having established that ct is required for formation of a2/ a3 joint cuticle and for Drosophila hearing we investigated whether ct is required for JO specification. Immunohistochemical stainings of ct mutant pupal antennae reveal that scolopidia form, but are abnormal in their organization (Fig. 5A–B”) and diminished in number (data not shown). In pupal antennae harboring large ct null clones, one sees scolopidia with dysfunctional or missing attachments at either their basal or apical ends) (Fig. 5A, A’ and A”). Nonetheless, markers for neuronal (Elav and anti-HRP) and scolopale (NompA-GFP) cell types are expressed and scolopales are made and can be visualized with phalloidin staining (Fig. 5 and not shown). We conclude that although ct is required for the development of a functional hearing organ, it is not required for the specification of either JO neurons or scolopale cells. This contrasts with the situation in ES organs where ct is required for differentiation of all cells derived from the SOP.11 Instead, in the JO, ct is required for the differentiation of the cuticular structures to which the scolopidia attach. Consistent with this, ct is strongly expressed in all of the epidermal cells of a2. In addition, as described below, ct is involved either in the differentiation or maintenance of a subset of scolopidial cells.
Figure 5.
cut mutants differentiate scolopidia that degenerate. High magnification views of a ct mutant pupal antenna (genotype is: y, w, ctc145, FRT19a/elav- Gal4, UAS-mCD8-GFP, FRT19a; eyFLP) ~40 hours post-pupariation. (A, A′ and A″) is a more apical section of the same antenna shown in (B, B′ B″). This antenna was stained with anti-Elav (red, A, A″, B and B″) to mark neuronal nuclei and phalloidin (blue, A and A″) to visualize the actin rods (scolopales) within the scolopale cells. In addition elav-Gal4 was used to drive expression of a membrane-bound GFP in wild type neurons (green, A, A′, B and B′). ct null clones lack GFP expression (tissue outlined by dotted lines in A–B″), but express Elav (red) and produce scolopales (blue). Thus both neurons and scolopale cells differentiate in ct null clones. Also shown in (A, A′ and A″) is a wild type cluster of cells (marked by GFP-expression and outlined with a solid line) that are detached from the cuticle. This indicates that ct function is required in cells other than the neurons and scolopale cells for normal JO morphology. (C) Histological section of part of a wild type adult antenna showing the scolopidia arrayed within a2. (D) Adult antenna carrying a large ct null clone in which the scolopidia degenerated during late pupal or early adult stages (genotype is: y, w, ctc145, FRT19a/M(1), FRT19a; eyFLP). Vacuolated, apparently degenerating cells also are present in a3. It is not possible for us to simultaneously mark both internal and external portions of these clones. However, based on parallel experiments where either the cuticle or the interior of the antenna was marked, the antenna shown here probably contains few, if any, ct+ cells.
ct mutant scolopidia degenerate by adulthood
To examine the fate of the abnormal scolopidia that differentiate in ct mutant JOs, we carried out a histological analysis of adult antennae. In adult animals carrying large ct null clones in the antennae, neurons and scolopale cells were rarely observed within a2 (Fig. 5C and D). This indicates that sometime between their differentiation in the pupae and eclosion of the adult flies, the mutant scolopidia degenerate.
ct and other antennal targets of Hth activation are also activated by Exd
To test whether ct and other genes regulated by Hth also are regulated by the Hth putative obligate binding partner, Exd, we examined the expression of Hth target genes in antennae harboring exd null clones (genotype is: y w exdxp11 FRT19a/UBI-GFP FRT19a; eyFLP). We find that ct, salm, and ato (Fig. 6), all of which are regulated by Hth,41,42 also require Exd for their antennal expression. This supports the hypothesis that hth and exd are binding partners during antennal development.
Figure 6.
Extradenticle is required for antennal cut, spalt-major and atonal expression. (A, A′ and A″) Ct (red) and Salm (blue) are lost cell-autonomously from exd null clones (arrowheads) marked by the lack of GFP (green) (genotype is: y, w, exdxp11, FRT19a/UBI-GFP, FRT19a; eyFLP). (B, B′ and B″) Ct (red) and Ato (blue) are lost cell-autonomously from exd null clones (arrowheads) marked by the lack of GFP (green). This indicates a requirement for Exd in the activation of two antennal genes previously shown to be downstream of hth. The arrow in (A) and (A′) indicates Ct expression within an exd null clone. exd null clones are transformed toward leg, thus it is possible that this cluster of cells represents ‘leg’ bristle precursors.
ct is required for arista specification
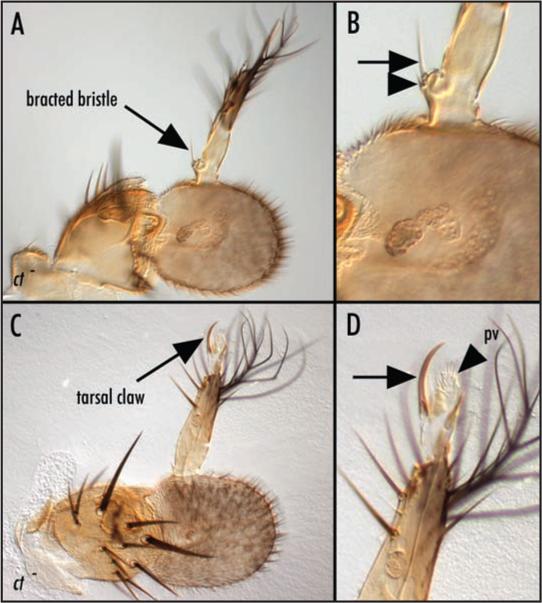
In animals carrying large ct null clones in the antennae, we observed an incompletely penetrant phenotype involving transformation of arista to tarsal (leg) identity (Fig. 7A–D). In some cases (8%, Fig. 8), the arista was partially transformed toward distal leg. In other cases (11%, Fig. 8), the transformation was more complete and included tarsal claws (Fig. 7C and D) or bracted bristles (Fig. 7A and B). The transformation phenotype was strongly enhanced by mutations in ss (Fig. 8), suggesting that ct and ss cooperate to repress distal leg fates within the developing antenna.
Figure 7.
Loss of cut function transforms distal antenna to distal leg. (A) Low and (B) high magnification views of an antenna carrying a large ct null clone in which the arista is partially transformed toward leg. A bracted bristle characteristic of the medial leg is indicated by the arrows in (A) and (B). The bract is indicated by the arrowhead in (B). (C) Low and (D) high magnification views of an antenna carrying a large ct null clone in which the arista is partially transformed toward leg. A tarsal claw (arrows in C and D) and associated pulvillus (pv, arrowhead in D) characteristic of the distal tip of the leg are apparent. The genotype in both cases is: y, w, ctc145, FRT19a/ M(1), FRT19a; eyFLP. Mutant tissue therefore can be distinguished by the y phenotype in which bristles and cuticle are less pigmented.
Figure 8.
cut and spineless cooperate to specify arista identity. Graph showing the number of arista transformations seen in different genetic backgrounds as a percentage of total antennae assayed. Partial transformations and complete transformants are of the types shown in Figure 7. The sum of these two classes is the total transformant percentage for a given genotype. Using an F test for binomial data, the p values for each of the pairwise combin-ations of phenotypes (moderate vs. moderate transformation; moderate vs. full transformation; full vs. moderate transformation; and full vs. full transformation) range from 4.5 ×10−13 to 3.7 × 10−5. The p value for comparison of total transformation in each genotype is 3.2 × 10−5.
At late third instar, ct expression is restricted to the presumptive proximal antenna. This raises the question of whether ct function in specifying distal antennal fates is cell autonomous. Two lines of evidence lead us to think that ct function in the distal antenna is likely to be cell autonomous. First, ct, like its activator Hth, is expressed in the presumptive distal antenna at second instar.56 Second, the transformed tissue is yellow (Fig. 7). Since the ct null chromosome used to generate the clones is mutant for yellow (y), while the ct+ chromosome carries an y+ allele, this indicates that the transformed tissue is ct−.
ct function in olfaction
ct is expressed weakly in presumptive a3 at late third instar, and the olfactory sensillae of ct null antennae appear normal externally (not shown). Nonetheless, histological sections of adult ct null antennae, revealed striking abnormalities internal to a3. These include the presence of fewer cells and degenerating cells (Fig. 5C and D). This suggests that ct mutants are likely to have olfactory deficits. Olfactory recordings or behavioral assays would be necessary to verify this. To begin to address when ct function is required in a3, we asked when ct is expressed in the presumptive olfactory sensillae. ct is expressed in presumptive a3 during the second instar55 and during pupal stages (not shown), but is not detectably expressed in presumptive a3 at late third instar (Fig. 4A).47
DISCUSSION
How might ct function differently in the antenna than elsewhere?
In Drosophila, ct plays a central role in the development of ES class mechanosensors. In bristles, ct is downstream of the bHLH protein-encoding proneural genes achaete (ac) and scute (sc). The bHLH protein-encoding proneural gene of CHOs is ato. In previously examined mechanosensors, ac, sc and ct expression is mutually exclusive with that of ato.49 In contrast, both ct and ato are expressed in, and required for, normal differentiation of JO. Since ct is not expressed in other CHOs, this function of ct is unique to JO.
What makes the function of ct different in the antennae than elsewhere in the Drosophila body?
Vertebrate CDP/Cux1 has different activities based upon which DNA-binding domains are present in the mature protein. The expression studies we have presented utilize an antibody that is likely to recognize all proteins encoded by the ct locus. We do not know which ct isoforms are expressed in the antenna or whether these differ from those expressed at other times and places. In addition, modifications such as cleavage, phosphorylation, and the tissue specific binding partners likely affect the activity of Drosophila Ct protein. Given the similarity in their antennal phenotypes, an interesting avenue for future research will be testing whether Ct protein can physically interact with Salm or Salr proteins.
Why is ct required for audition?
A conspicuous defect in ct mutant antennae is a misshapen or absent a2/a3 joint. The joint is a cuticular structure, and ct has been implicated previously in formation of cuticle in the wing.47 In particular, ct mutations lead to loss of the mechano- and chemosensory bristles of the Drosophila wing margin, and to the cuticle-secreting epidermal cells that surround those bristles. This leads to the wing phenotype for which the gene is named. In the antenna, the portions of the cuticle most intimately associated with an individual scolopidium are the attachment sites. If ct were required for the differentiation of these attachment sites, then the scolopidia of ct mutants would lack a substrate and would not be properly extended or arrayed into a larger organ. Such CHOs would appear disorganized, and this is the experimental result in the antenna when ct is removed. The joint cuticle phenotype alone would be sufficient to account for the deafness associated with ct mutations, since movement of the joint is required for audition. However, our phenotypic analysis indicates that there are additional defects in ct mutants.
By analogy to its role in ES differentiation, we anticipated that ct was involved in the differentiation of JO scolopidia. We have shown that some scolopidia form in the absence of ct. While we do not have markers for all scolopidial cell types, we have been able to visualize both scolopale cells and neurons. Both cell types are present and at least partially differentiated in the absence of ct. Specifically, phalloidin stainings indicate that the actin-rich scolopale rods form within ct mutant scolopale cells (Fig. 5). NompA, a component of the extracellular cap, is produced and secreted by ct mutant scolopale cells (not shown). Immunohistochemical staining with both an antibody to the neuronal membranes (anti-HRP; not shown) and an antibody to the neuronal nuclei (anti-Elav; Fig. 5), indicates that scolopidial neurons differentiate in the absence of ct. The presence of differentiated scolopale cells indicates that scolopidia are not transformed toward ES fates in ct mutants and thus that ct function in JO is distinct from ct function in embryonic ES organs. ct mutant scolopidia do not survive into adulthood. Histological analyses of ct mutant adult antennae reveal few scolopidia with degenerating cells. The basis for the degeneration remains unclear. Ct could regulate the expression, directly or indirectly, of trophic factors needed for survival of cells within the scolopidium. Alternatively, ct mutant scolopidia may differentiate incompletely and subsequently activate apoptotic pathways.
Where is ct in the antennal genetic hierarchy?
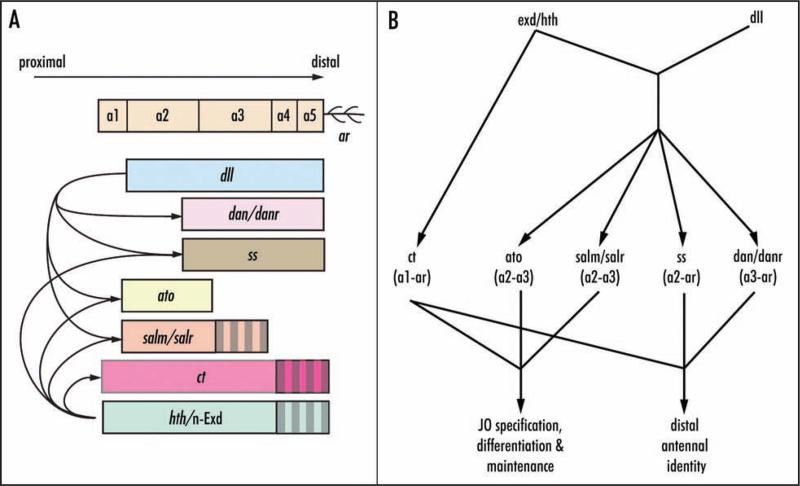
Within the antenna, Ct is activated by Hth42 and Exd (this work) and repressed by Dll,42,46 three factors crucial to proper antennal development (Fig. 9). Ct in turn represses the expression of Dll, dan and danr in the presumptive proximal antenna.42,46 Ct functions in parallel to other genes required for JO development, including salm and ato. It is unknown whether Ct activates genes in the developing JO to promote JO differentiation, whether Ct represses genes not needed in JO (e.g., dan and danr), or both.
Figure 9.
Expression domains (A) and interactions (B) of genes required for antenna patterning and Johnston's organ specification, differentiation and maintenance. (A) dll is expressed in the presumptive antenna from a2-arista36,37 where it is required for activation of ss,62 dan/darn,45,46 salm/salr,41 and ato,42 and for the repression of ct.42,46 hth and n-Exd are expressed at early third instar throughout the antenna disc. Strong expression (solid fill) is restricted to presumptive head capsule, a1 and a2 by late third instar.32 hth41,42 and n-Exd (this work) cooperate with dll to activate ss, salm/salr and ato. hth42 and n-Exd (this work) activate ct. (B) Summary of regulatory relationships among and functions of antennal genes. ato is required for specification of JO precursors.43 salm/salr are required for differentiation and maintenance of JO scolopidial subunits.44 ct (this work) is required for maintenance of scolopidial fates and for distal antenna identity. ss62 and dan/darn45,46 also are required for distal antenna identity.
Evolution of ct function
The myriad roles of ct during development include ES specification, regulation of da neuron dendritic arborization, Malphigian tubule differentiation, and wing margin ES organ and epidermal differentiation.11,14,20,47 We speculate that ct might originally have been deployed in a neural capacity because many of the identified roles for ct include neural tissues or their associated cuticular structures. Consistent with this, mouse Cux2 is highly expressed in the nervous system and has been implicated in the differentiation of subsets of telencephalic interneurons and projection neurons.57 Vertebrate CDP/Cux1 is essential for normal lung and hair follicle development and for male fertility.58-60 CDP/Cux1 has been implicated in kidney development,61,62 raising the possibility that ct and its homologs are involved in the development of some types of primary cilia, a structure shared between CHOs and vertebrate kidneys. Central to the question of making a comparison between the ancestral roles of vertebrate and invertebrate ct is a better understanding of the particulars of how these genes are working in the tissues in which they are required.
Acknowledgements
We would like to thank the following individuals for their generous gifts of reagents and input into this work: Dr. Hugo Bellen for the guinea pig Ato antibody; Dr. Seth Blair for the ctC145 fly stock; and Drs. Reinhard Schuh and Bertrand Mollereau for rabbit Sal antibody. The images shown here were collected on a Zeiss confocal microscope in the Department of Medical Microbiology and Immunology that is supported by NIH RR12294. The authors thank Drs. Margaret McFall-Ngai and Ned Ruby for access to this microscope. We also thank our two anonymous reviewers for helpful comments. The monoclonal Ct and Dac antibodies used in these studies were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242. Dominic J.S. Ebacher is a trainee of Genetics Training Grant T32 GM07133. Sokol V. Todi was supported by American Heart Association fellowship AHA0410077Z. Grace E. Boekhoff-Falk was the recipient of a Vilas Life Cycle grant. This work also was supported by NIH grants DC04848 (Daniel F. Eberl) and GM59871 (Grace E. Boekhoff-Falk).
Abbreviations and Acronyms
Gene names:
- ac
achaete
- ato
atonal
- bab
bric a brac
- bft
bereft
- Cux1
murinect homolog 1
- ct
cut
- da
daughterless
- dac
dachshund
- dan
distal antenna
- danr
distal antenna related
- dll
distal-less
- elav
embryonic lethal, abnormal vision
- exd
extradenticle
- ey
eyeless
- hth
homothorax
- poxn
pox neuro
- salm
spalt major
- salr
spalt related
- sc
scute
- sens
senseless
- ss
spineless
- CDP
CAAT-displacement protein
- CHO
chordotonal organ
- da
dendritic arborization (neuron)
- ES
external sense (organ)
- FLP
flippase
- FRT
FLP recombination target
- GFP
green fluorescent protein
- JO
Johnston's organ
- HRP
horse radish peroxidase
- lch5
larval pentascolopidial chordotonal organ
- SOP
sense organ precursor
- UAS
upstream activation sequence
References
- 1.Jarman AP. Studies of mechanosensation using the fly. Human Molecular Genetics. 2002;11:1215–8. doi: 10.1093/hmg/11.10.1215. [DOI] [PubMed] [Google Scholar]
- 2.Brewster R, Bodmer R. Origin and specification of type II sensory neurons in Drosophila. Development. 1995;121:2923–36. doi: 10.1242/dev.121.9.2923. [DOI] [PubMed] [Google Scholar]
- 3.Todi S, Sharma Y, Eberl D. Anatomical and molecular design of the Drosophila antenna as a flagellar auditory organ. Microscopy Research Techniques. 2004;63:388–99. doi: 10.1002/jemt.20053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caldwell J, Eberl D. Towards a molecular understanding of Drosophila hearing. Journal of Neurobiology. 2002;53:172–89. doi: 10.1002/neu.10126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brewster R, Bodmer R. Cell lineage analysis of the Drosophila peripheral nervous system. Developmental Genetics. 1996;18:50–63. doi: 10.1002/(SICI)1520-6408(1996)18:1<50::AID-DVG6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Göpfert M, Robert D. Turning the key on Drosophila audition. Nature. 2001;411:908. doi: 10.1038/35082144. [DOI] [PubMed] [Google Scholar]
- 7.Boekhoff-Falk G. Hearing in Drosophila: Development of Johnston's organ and emerging parallels to vertebrate ear development. Dev Dyn. 2005;232:550–8. doi: 10.1002/dvdy.20207. [DOI] [PubMed] [Google Scholar]
- 8.Burglin T, Cassata G. Loss and gain of domains during evolution of cut superclass homeobox genes. International Journal of Developmental Biology. 2002;46:115–23. [PubMed] [Google Scholar]
- 9.Blochlinger K, Jan L, Jan Y. Transformation of sensory organ identity by ectopic expression of Cut in Drosophila. Genes and Development. 1991;5:1124–35. doi: 10.1101/gad.5.7.1124. [DOI] [PubMed] [Google Scholar]
- 10.Ludlow C, Choy R, Blochlinger K. Functional analysis of Drosophila and mammalian cut proteins in files. Developmental Biology. 1996;178:149–59. doi: 10.1006/dbio.1996.0205. [DOI] [PubMed] [Google Scholar]
- 11.Bodmer R, Barbel S, Shepherd S, Jack JW, Jan LY, Jan YN. Transformation of sensory organs by mutations of the cut locus of D. melanogaster. Cell. 1987;51:293–307. doi: 10.1016/0092-8674(87)90156-5. [DOI] [PubMed] [Google Scholar]
- 12.Blochlinger K, Bodmer R, Jan L, Jan Y. Patterns of expression of cut, a protein required for external sensory organ development in wild-type and cut mutant Drosophila embryos. Genes and Development. 1990;4:1322–31. doi: 10.1101/gad.4.8.1322. [DOI] [PubMed] [Google Scholar]
- 13.Merritt D. Transformation of external sensilla to chordotonal sensilla in the cut mutant of Drosophila assessed by singel-cell marking in the embryo and larva. Microscopy Research and Technique. 1997;39:492–505. doi: 10.1002/(SICI)1097-0029(19971215)39:6<492::AID-JEMT4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 14.Grueber W, Jan L, Jan Y. Different levels of the homeodomain protein cut regulate distinct dendrite branching patterns of Drosophila. Cell. 2003;112:805–18. doi: 10.1016/s0092-8674(03)00160-0. [DOI] [PubMed] [Google Scholar]
- 15.Liu S, Jack J. Regulatory interactions and role in cell type specification of the Malpighian tubules by the cut, Kruppel, and caudal genes of Drosophila. Developmental Biology. 1992;150:133–43. doi: 10.1016/0012-1606(92)90013-7. [DOI] [PubMed] [Google Scholar]
- 16.Nepveu A. Role of the multifunctional CDP/Cut/Cux homeodomain transcription factor in regulating differentiation, cell growth and development. Gene. 2001;270:1–15. doi: 10.1016/s0378-1119(01)00485-1. [DOI] [PubMed] [Google Scholar]
- 17.Mailly F, Berube G, Harada R, Mao P, Phillips S, Nepveu A. The human cut homeodomain protein can repress gene expression by two distinct mechanisms: Active repression and competition for binding site occupancy. Molecular and Cellular Biology. 1996;16:5346–57. doi: 10.1128/mcb.16.10.5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li S, Moy L, Pittman N, Shue G, Aufiero B, Neufeld E, LeLeiko N, Walsh M. Transcriptional repression of the cystic fibrosis transmembrane conductance regulator gene, mediated by CCAAT displacement protein/cut homolog, is associated with histone deacetylation. Journal of Biological Chemistry. 1999;274:7803–15. doi: 10.1074/jbc.274.12.7803. [DOI] [PubMed] [Google Scholar]
- 19.Barberis A, Superti-Furga G, Busslinger M. Mutually exclusive interaction of the CCAAT-binding factor and of a displacement protein with overlapping sequences of a histone gene promoter. Cell. 1987;50:347–59. doi: 10.1016/0092-8674(87)90489-2. [DOI] [PubMed] [Google Scholar]
- 20.Luo W, Skalnik D. CCAAT displacement protein competes with multiple transcriptional activators for binding to four sites in the proximal gp91phox promoter. Journal of Biological Chemistry. 1996;271:18203–10. doi: 10.1074/jbc.271.30.18203. [DOI] [PubMed] [Google Scholar]
- 21.Skalnik D, Strauss E, Orkin S. CCAAT displacement protein as a repressor of the myelomonocytic-specific gp91-phox gene promoter. Journal of Biological Chemistry. 1991;266:16736–44. [PubMed] [Google Scholar]
- 22.Lievens P, Donady J, Tufarelli C, Neufeld E. Repressor activity of CCAAT displacement protein in HL-60 myeloid leukemia cells. Journal of Biological Chemistry. 1995;270:12745–50. doi: 10.1074/jbc.270.21.12745. [DOI] [PubMed] [Google Scholar]
- 23.Coqueret O, Berube G, Nepveu A. The mammalian Cut homeodomain protein functions as a cell-cycle-dependent transcriptional repressor which downmodulates p21WAF1/CIP1/SDI1 in S phase. EMBO Journal. 1998;17:4680–94. doi: 10.1093/emboj/17.16.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moon N, Premdas P, Truscott M, Leduy L, Berube G, Nepveu A. S phase-specific proteolytic cleavage is required to activate stable DNA binding by the CDP/Cut homeodomain protein. Molecular and Cellular Biology. 2001:6332–45. doi: 10.1128/MCB.21.18.6332-6345.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goulet B, Baruch A, Moon N, Poirier M, Sansregret L, Erickson A, Bogyo M, Nepveu A. A cathepsin L isoform that is devoid of a signal peptide localizes to the nucleus in S phase and processes the CDP/Cux transcription factor. Molecular Cell. 2004;14:207–19. doi: 10.1016/s1097-2765(04)00209-6. [DOI] [PubMed] [Google Scholar]
- 26.Hardiman K, Brewster R, Khan S, Deo M, Bodmer R. The bereft gene, a potential target of the neural selector gene cut, contributes to bristle morphogenesis. Genetics. 2002;161:231–47. doi: 10.1093/genetics/161.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang A, Rubin G. A misexpression screen identifies genes that can modulate RAS1 pathway signaling in Drosophila melanogaster. Genetics. 2000;156:1219–30. doi: 10.1093/genetics/156.3.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levis R. Genes bft and MESK1 are probably allelic. FlyBase. 2003 [Google Scholar]
- 29.Sato T. A new homeotic mutation affecting antenna and legs. Drosophila Information Service. 1984;60:180–2. [Google Scholar]
- 30.Sunkel CE, Whittle JRS. Brista: A gene involved in the specification and differentiation of distal cephalic and thoracic structures in Drosophila melanogaster. Wilhelm Roux’s Archives of Developmental Biology. 1987;196:124–32. doi: 10.1007/BF00402034. [DOI] [PubMed] [Google Scholar]
- 31.Cohen SM, Jurgens G. Proximal-distal pattern formation in Drosophila: Cell autonomous requirement for Distal-less gene activity in limb development. EMBO Journal. 1989;8:2045–55. doi: 10.1002/j.1460-2075.1989.tb03613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casares F, Mann RS. Control of antennal versus leg development in Drosophila. Nature. 1998;392:723–6. doi: 10.1038/33706. [DOI] [PubMed] [Google Scholar]
- 33.Pai CY, Kuo T, Jaw T, Kurant E, Chen C, Bessarab D, Salzberg A, Sun Y. The Homothorax homeoprotein activates the nuclear localization of another homeoprotein, Extradenticle, and suppresses eye development in Drosophila. Genes and Development. 1998;12:435–6. doi: 10.1101/gad.12.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalez-Crespo S, Morata G. Control of Drosophila adult pattern by extradenticle. Development. 1995;121:2117–25. doi: 10.1242/dev.121.7.2117. [DOI] [PubMed] [Google Scholar]
- 35.Rauskolb C, Smith KM, Peifer M, Wieschaus E. Extradenticle determines segmental identities throughout Drosophila development. Development. 1995;121:3663–73. doi: 10.1242/dev.121.11.3663. [DOI] [PubMed] [Google Scholar]
- 36.Diaz-Benjumea F, Cohen B, Cohen SM. Cell interaction between compartments establishes the proximal-distal axis of Drosophila legs. Nature. 1994;372:175–8. doi: 10.1038/372175a0. [DOI] [PubMed] [Google Scholar]
- 37.Panganiban G, Nagy L, Carroll SB. The role of the Distal-less gene in the development and evolution of insect limbs. Current Biology. 1994;4:671–5. doi: 10.1016/s0960-9822(00)00151-2. [DOI] [PubMed] [Google Scholar]
- 38.Rieckhof GE, Casares F, Ryoo HD, Abu-Shaar M, Mann RS. Nuclear translocation of Extradenticle requires homothorax, which encodes an Extradenticle-related homeodomain protein. Cell. 1997;91:171–83. doi: 10.1016/s0092-8674(00)80400-6. [DOI] [PubMed] [Google Scholar]
- 39.Campbell G, Tomlinson A. The roles of the homeobox genes aristaless and Distal-less in patterning the legs and wings of Drosophila. Development. 1998;125:4483–93. doi: 10.1242/dev.125.22.4483. [DOI] [PubMed] [Google Scholar]
- 40.Gorfinkiel N, Morata G, Guerrero I. The homeobox gene Distal-less induces ventral appendage development in Drosophila. Genes and Development. 1997;11:2259–71. doi: 10.1101/gad.11.17.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dong PDS, Chu J, Panganiban G. Coexpression of the homeobox genes Distal-less and homothorax determines Drosophila antennal identity. Development. 2000;127:209–16. doi: 10.1242/dev.127.2.209. [DOI] [PubMed] [Google Scholar]
- 42.Dong PDS, Dicks JS, Panganiban G. Distal-less and homothorax regulate multiple targets to pattern the Drosophila antenna. Development. 2002;129:1967–74. doi: 10.1242/dev.129.8.1967. [DOI] [PubMed] [Google Scholar]
- 43.Jarman AP, Sun Y, Jan LY, Jan YN. Role of the proneural gene, atonal, in formation of Drosophila chordotonal organs and photoreceptors. Development. 1995:2019–30. doi: 10.1242/dev.121.7.2019. [DOI] [PubMed] [Google Scholar]
- 44.Dong PDS, Todi SV, Eberl DF, Boekhoff-Falk G. Drosophila spalt/spalt-related mutants exhibit Townes-Brocks’ Syndrome phenotypes. Proceedings of the National Academy of Sciences USA. 2003;100:10293–8. doi: 10.1073/pnas.1836391100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suzanne M, Estella C, Calleja M, Sanchez-Herrero E. The hernandez and fernandez genes of Drosophila specify eye and antenna. Developmental Biology. 2003;260:465–83. doi: 10.1016/s0012-1606(03)00249-5. [DOI] [PubMed] [Google Scholar]
- 46.Emerald B, Curtiss J, Mlodzik M, Cohen S. Distal antenna and distal antenna relatedencode nuclear proteins containing pipsqueak motifs involved in antenna development in Drosophila. Development. 2003;130:1171–80. doi: 10.1242/dev.00323. [DOI] [PubMed] [Google Scholar]
- 47.Blochlinger K, Jan LY, Jan YN. Postembryonic patterns of expression of cut, a locus regulating sensory organ identity in Drosophila. Development. 1993;117:441–50. doi: 10.1242/dev.117.2.441. [DOI] [PubMed] [Google Scholar]
- 48.Jarman AP, Grau Y, Jan LY, Jan YN. Atonal is a proneural gene that directs chordotonal organ formation in the Drosophila peripheral nervous system. Cell. 1993;73:1307–21. doi: 10.1016/0092-8674(93)90358-w. [DOI] [PubMed] [Google Scholar]
- 49.Jarman A, Ahmed I. The specificity of proneural genes in determining Drosophila sense organ identity. Mechanisms of Development. 1998;76:117–25. doi: 10.1016/s0925-4773(98)00116-6. [DOI] [PubMed] [Google Scholar]
- 50.Göpfert M, Robert D. The mechanical basis of Drosophila audition. Journal of Experimental Biology. 2002;205:1199–208. doi: 10.1242/jeb.205.9.1199. [DOI] [PubMed] [Google Scholar]
- 51.Eberl D, Hardy R, Kernan M. Genetically similar transduction mechanisms for touch and hearing in Drosophila. The Journal of Neuroscience. 2000;20:5981–8. doi: 10.1523/JNEUROSCI.20-16-05981.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Göpfert MC, Stocker H, Robert D. atonal is required for exoskeletal joint formation in the Drosophila auditory system. Developmental Dynamics. 2002;225:106–9. doi: 10.1002/dvdy.10136. [DOI] [PubMed] [Google Scholar]
- 53.Newsome TP, Asling B, Dickson BJ. Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development. 2000;127:851–60. doi: 10.1242/dev.127.4.851. [DOI] [PubMed] [Google Scholar]
- 54.Xu T, Rubin G. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–37. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- 55.Tauber E, Eberl D. Acoustic communication in Drosophila. Behavioral Processes. 2003;64:197–210. [Google Scholar]
- 56.Kenyon K, Ranade S, Curtiss J, Mlodzik M, Pignoni F. Coordinating proliferation and tissue specification to promote regional identity in the Drosophila head. Developmental Cell. 2003;5:403–14. doi: 10.1016/s1534-5807(03)00243-0. [DOI] [PubMed] [Google Scholar]
- 57.Zimmer C, Tiveron M, Bodmer R, Cremer H. Dynamics of Cux2 expression suggests that an early pool of SVZ precursors is fated to become upper cortical layer neurons. Cerebral Cortex. 2004;14:1408–20. doi: 10.1093/cercor/bhh102. [DOI] [PubMed] [Google Scholar]
- 58.Ellis T, Gambardella L, Horcher M, Tschanz S, Capol J, Bertram P, Jochum W, Barrandon Y, Busslinger M. The transcriptional repressor CDP (Cutl1) is essential for epithelial cell differentiation of the lung and the hair follicle. Genes and Development. 2001;15:2307–19. doi: 10.1101/gad.200101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tufarelli C, Fujiwara Y, Zappulla D, Neufeld E. Hair defects and pup loss in mice with targeted deletion of the first cut repeat domain of the Cux/CDP homeoprotein gene. Developmental Biology. 1998;200:69–81. doi: 10.1006/dbio.1998.8950. [DOI] [PubMed] [Google Scholar]
- 60.Luong M, van der Meijden C, Xing D, Hesselton R, Monuki E, Jones S, Lian J, Stein J, Stein G, Neufeld E, van Wijnen A. Genetic ablation of the CDP/Cux protein C terminus results in hair cycle defects and reduced male fertility. Molecular and Cellular Biology. 2002;22:14242–1437. doi: 10.1128/mcb.22.5.1424-1437.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Quaggin S, Yeger H, Igarashi P. Antisense oligonucleotides to Cux-1, a Cut-related homeobox gene, cause increased apoptosis in mouse embryonic kidney cultures. Journal of Clinical Investigation. 1997;99:718–24. doi: 10.1172/JCI119216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ledford A, Brantley J, Kemeny G, Foreman T, Quaggin S, Igarashi P, Oberhaus S, Rodova M, Calvet J, Vanden Heuvel G. Deregulated expression of the homeobox gene Cux-1in transgenic mice results in downregulation of p27(kip1) expression during nephrogenesis, glomerular abnormalities, and multiorgan hyperplasia. Developmental Biology. 2002;245:157–71. doi: 10.1006/dbio.2002.0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duncan DM, Burgess E, Duncan I. Control of distal antennal identity and tarsal development in Drosophila by spineless-aristapedia, a homolog of the mammalian dioxin receptor. Genes and Development. 1998;12:1290–303. doi: 10.1101/gad.12.9.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]