Abstract
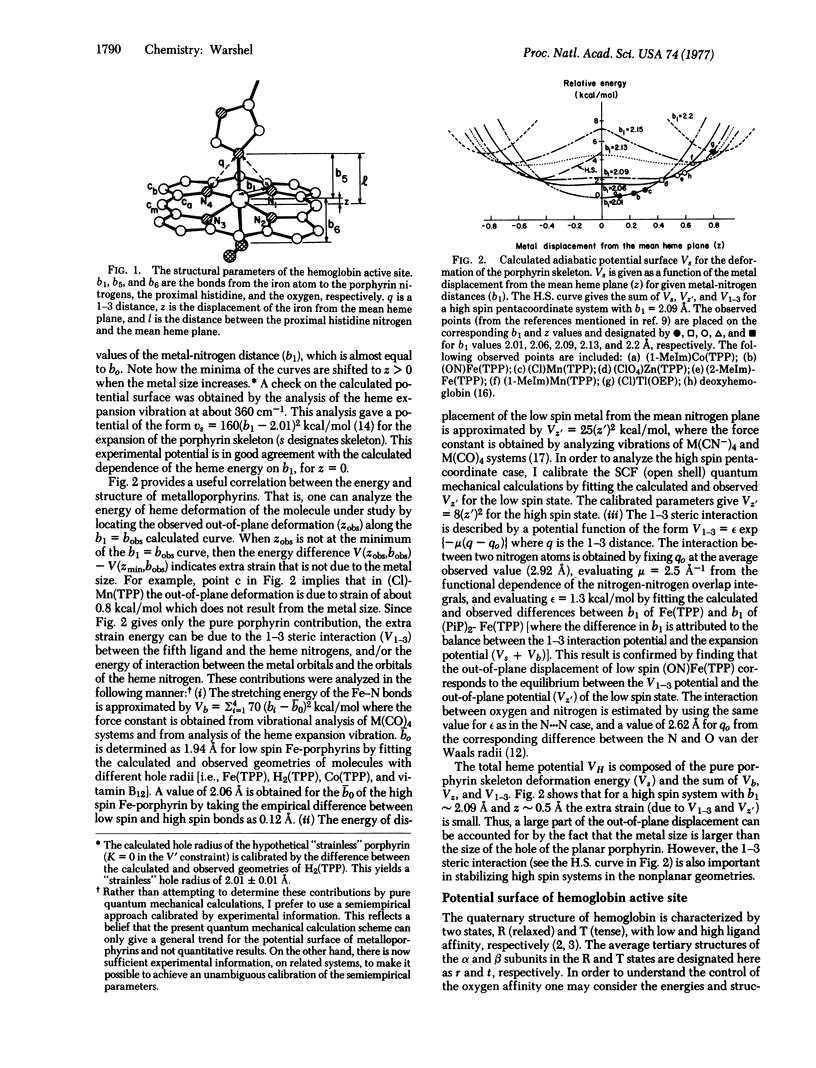
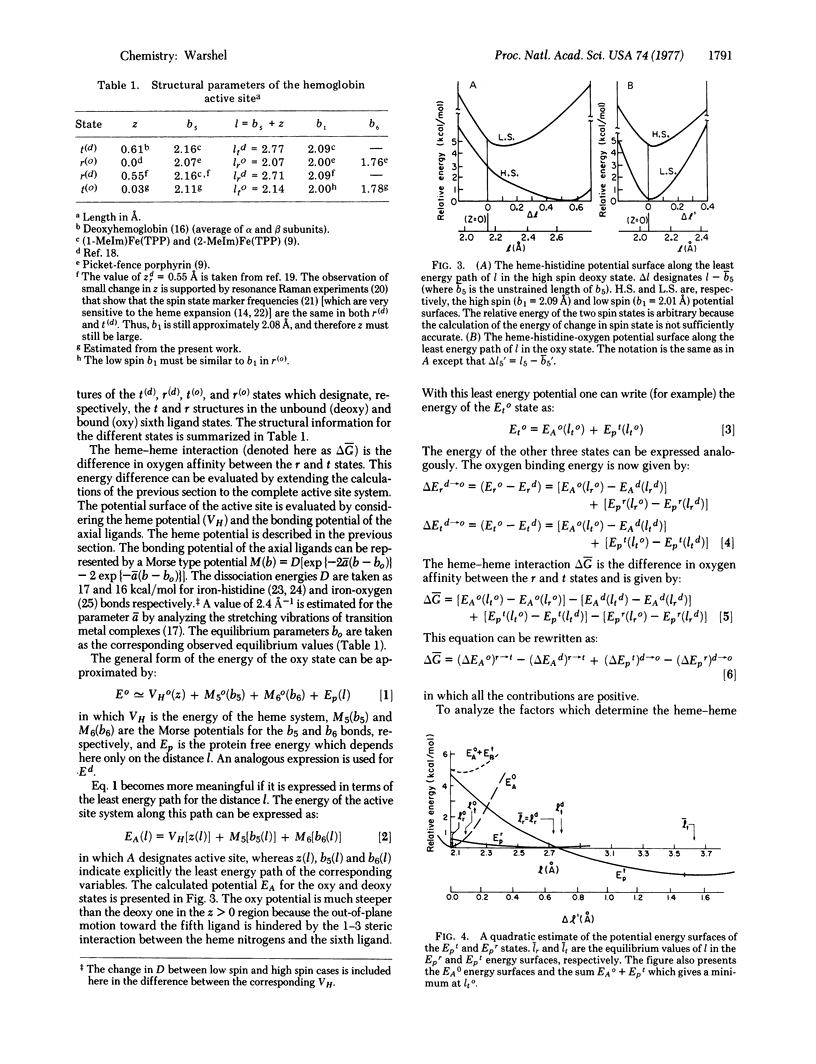
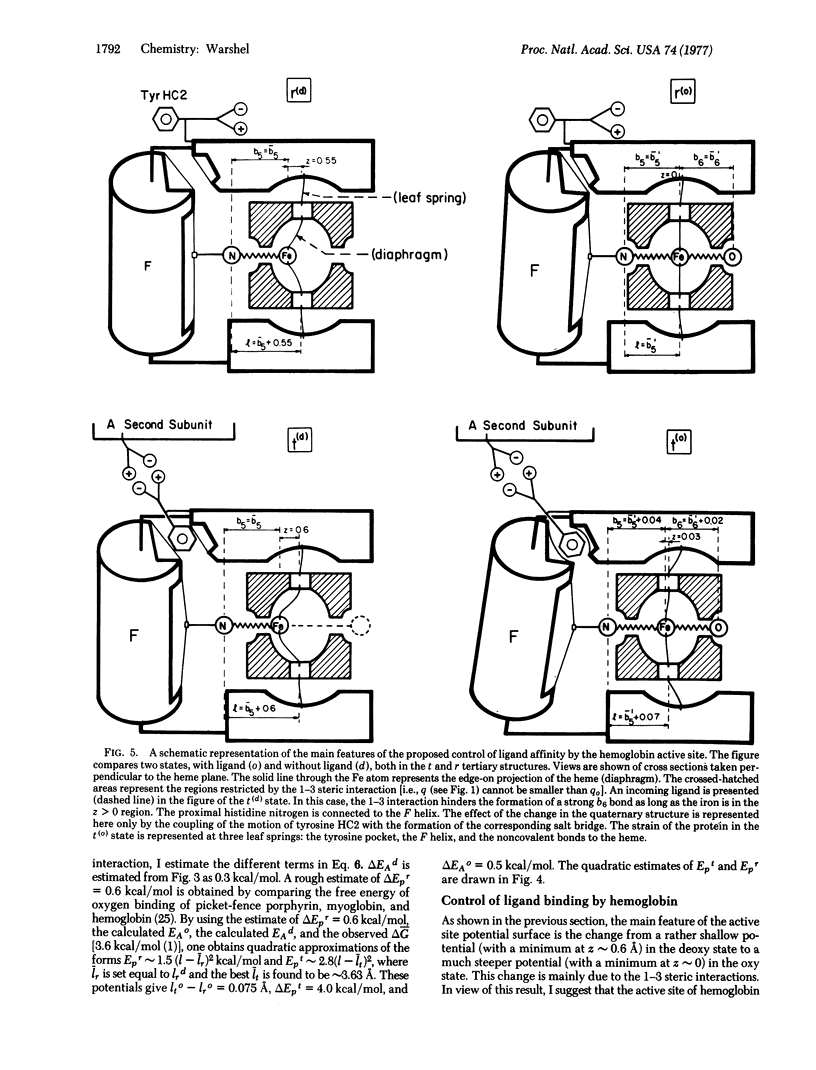
The contribution of the porphyrin skeleton to the potential energy surface metalloporphyrins is calculated by the semiempirical method of quantum mechanical extension of the consistent force field to eta electron molecules. This calculation makes it possible to correlate the observed structure of metalloporphyrins with the strain energy of the porphyrin skeleton. It is found that the out-of-plane metal displacement in pentacoordinate heme systems is due to both the restricted size of the porphyrin hole and the "1-3" steric interaction between the axial ligand and the heme nitrogens. The main components of the active site of hemoglobin are simulated by a histidine-heme-oxygen system. The energy surface of this system provides a quantitative explanation for the control of ligand binding by hemoglobin. It is shown that the heme acts as a diaphragm, designed to provide simultaneous binding to the histidine and the sixth ligand under the steric requirements of the 1-3 interactions. The dependence of the hemoglobin potential surface on the distance between the proximal histidine and the heme plane is evaluated for the R and T states, using the calculated heme potential and the observed energy of heme-heme interaction.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin J. M. Structure and function of haemoglobin. Prog Biophys Mol Biol. 1975;29(3):225–320. doi: 10.1016/0079-6107(76)90024-9. [DOI] [PubMed] [Google Scholar]
- Brault D., Rougee M. Binding of imidazole and 2-methylimidazole by hemes in organic solvents. Evidence for five-coordination. Biochem Biophys Res Commun. 1974 Apr 8;57(3):654–659. doi: 10.1016/0006-291x(74)90596-8. [DOI] [PubMed] [Google Scholar]
- Brault D., Rougee M. Ferrous porphyrins in organic solvents. I. Preparation and coordinating properties. Biochemistry. 1974 Oct 22;13(22):4591–4597. doi: 10.1021/bi00719a019. [DOI] [PubMed] [Google Scholar]
- Collman J. P., Brauman J. I., Suslick K. S. Letter: Oxygen binding to iron porphyrins. J Am Chem Soc. 1975 Nov 26;97(24):7185–7186. doi: 10.1021/ja00857a050. [DOI] [PubMed] [Google Scholar]
- Eisenberger P., Shulman R. G., Brown G. S., Ogawa S. Structure-function relations in hemoglobin as determined by x-ray absorption spectroscopy. Proc Natl Acad Sci U S A. 1976 Feb;73(2):491–495. doi: 10.1073/pnas.73.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fermi G. Three-dimensional fourier synthesis of human deoxyhaemoglobin at 2-5 A resolution: refinement of the atomic model. J Mol Biol. 1975 Sep 15;97(2):237–256. doi: 10.1016/s0022-2836(75)80037-4. [DOI] [PubMed] [Google Scholar]
- Heidner E. J., Ladner R. C., Perutz M. F. Structure of horse carbonmonoxyhaemoglobin. J Mol Biol. 1976 Jul 5;104(3):707–722. doi: 10.1016/0022-2836(76)90130-3. [DOI] [PubMed] [Google Scholar]
- Hopfield J. J. Relation between structure, co-operativity and spectra in a model of hemoglobin action. J Mol Biol. 1973 Jun 25;77(2):207–222. doi: 10.1016/0022-2836(73)90332-x. [DOI] [PubMed] [Google Scholar]
- Ogawa S., Shulman R. G. High resolution nuclear magnetic resonance spectra of hemoglobin. 3. The half-ligated state and allosteric interactions. J Mol Biol. 1972 Sep 28;70(2):315–336. doi: 10.1016/0022-2836(72)90542-6. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Heidner E. J., Ladner J. E., Beetlestone J. G., Ho C., Slade E. F. Influence of globin structure on the state of the heme. 3. Changes in heme spectra accompanying allosteric transitions in methemoglobin and their implications for heme-heme interaction. Biochemistry. 1974 May 7;13(10):2187–2200. doi: 10.1021/bi00707a028. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Ladner J. E., Simon S. R., Ho C. Influence of globin structure on the state of the heme. I. Human deoxyhemoglobin. Biochemistry. 1974 May 7;13(10):2163–2173. doi: 10.1021/bi00707a026. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Nature of haem-haem interaction. Nature. 1972 Jun 30;237(5357):495–499. doi: 10.1038/237495a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970 Nov 21;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- Spiro T. G., Strekas T. C. Resonance Raman spectra of heme proteins. Effects of oxidation and spin state. J Am Chem Soc. 1974 Jan 23;96(2):338–345. doi: 10.1021/ja00809a004. [DOI] [PubMed] [Google Scholar]
- Sussner H., Mayer A., Brunner H., Fasold H. Raman study on the two quaternary states of unligated hemoglobin. Eur J Biochem. 1974 Feb 1;41(3):465–469. doi: 10.1111/j.1432-1033.1974.tb03288.x. [DOI] [PubMed] [Google Scholar]
- Warshel A. Interpretation of resonance Raman spectra of biological molecules. Annu Rev Biophys Bioeng. 1977;6:273–300. doi: 10.1146/annurev.bb.06.060177.001421. [DOI] [PubMed] [Google Scholar]
- Warshel A., Karplus M. Calculation of pi-pi excited state conformations and vibronic structure of retinal and related molecules. J Am Chem Soc. 1974 Sep 4;96(18):5677–5689. doi: 10.1021/ja00825a001. [DOI] [PubMed] [Google Scholar]



