Abstract
Background
Treatment failures in stage IIIC endometrial carcinoma (EC) are predominantly due to occult extrapelvic metastases (EPM). The impact of chemotherapy on occult EPM was investigated according to grade (G), G1/2EC vs G3EC.
Methods
All surgical-stage IIIC EC cases from January 1, 1999, through December 31, 2008, from Mayo Clinic were included. Patient-, disease-, and treatment-specific risk factors were assessed for association with overall survival, cause-specific survival, and extrapelvic disease-free survival (DFS) using Cox proportional hazards regression.
Results
109 cases met criteria, with 92 (84%) having systematic lymphadenectomy (>10 pelvic and >5 paraaortic lymph nodes resected). In patients with documented recurrence sites, occult EPM accounted for 88%. Among G1/2EC cases (n = 48), the sole independent predictor of extrapelvic DFS was grade 2 histology (hazard ratio [HR], 0.28; 95% CI, 0.08–0.91; P = .03) while receipt of adjuvant chemotherapy approached significance (HR 0.13; 95% CI, 0.02, 1.01; P = .0511). The 5-year extrapelvic DFS with and without adjuvant chemotherapy was 93% and 54%, respectively (log-rank, P = .02). Among G3EC (n = 61), the sole independent predictor of extrapelvic DFS was lymphovascular space involvement (HR, 2.63; 95% CI, 1.16–5.97; P = .02). Adjuvant chemotherapy did not affect occult EPM in G3EC; the 5-year extrapelvic DFS for G3EC with and without adjuvant chemotherapy was 43% and 42%, respectively (log-rank, P = .91).
Conclusions
Chemotherapy improves extrapelvic DFS for stage IIIC G1/2EC but not stage IIIC G3EC. Future efforts should focus on prospectively assessing the impact of chemotherapy on DFS in G3EC and developing innovative phase I and II trials of novel systemic therapies for advanced G3EC.
Keywords: Chemotherapy efficacy, Occult extrapelvic metastases, Stage IIIC endometrial cancer, Survival
Introduction
Metastatic involvement of regional or distant lymph nodes is well established as a principal prognostic determinant in solid tumors, including endometrial carcinoma (EC). Nevertheless, the indications for lymphadenectomy (LND) and the merits of this procedure in the overall management of EC continue to be debated [1–8]. Proponents of LND in managing at-risk EC contend that the histologic status of regional lymph nodes facilitates the selection of adjuvant therapy [3,6,9]. Accepting this premise, the therapeutic options available in node-positive patients are radiotherapy or chemotherapy or a combination of these modalities. Nonetheless, contemporary therapeutic algorithms for stage IIIC disease have yielded disparate posttreatment recurrence rates [10–15]. Although multiple studies have demonstrated the efficacy of external-beam radiotherapy (EBRT) in minimizing pelvic relapses, treatment failures at distant sites, including the paraaortic area, are frequently observed [15–19]. Consequently, systemic therapy alone or in combination with EBRT has been advocated, but outcomes are inconsistent [10,11,15,20–22].
Optimal management of stage IIIC EC remains unclear, partly because of inconsistencies in addressing and stratifying relevant disease-specific parameters. Although more than 50% of patients with lymphatic dissemination have paraaortic metastasis [9,23,24], the impact of extended-field radiotherapy on lymphatic recurrences beyond the pelvis remains uncertain [2,4,11,12,25,26]. Similarly, the efficacy of contemporary systemic therapy in effectively managing lymphatic and occult distant metastatic disease in EC has not been elucidated [10,15,16,18,19,21,27–31]. Furthermore, emerging reports suggest marked differences in the therapeutic indices comparing endometrioid to type II EC; the latter shows relative recalcitrance to contemporary chemotherapy [10,15,27,30]. Hence, the objective of this investigation was to critically assess the efficacy of existing therapeutic strategies in controlling regional, but more notably occult, distant lymphatic, hematogenous, and peritoneal disease in stage IIIC EC as a function of uterine histology.
Methods
Study patients
This retrospective outcome analysis was approved by the Mayo Clinic Institutional Review Board. We identified women who presented with EC, were counseled, and elected primary surgical management of their disease at Mayo Clinic (Rochester, Minnesota) from January 1, 1999, through December 31, 2008.
Treatment
The surgical treatment algorithm for EC used at our institution previously has been described in detail [9]. Briefly, in the absence of macroscopic extrauterine disease, hysterectomy with removal of the adnexal structures is performed with immediate frozen-section assessment to determine the need for definitive surgical staging. LND is intentionally omitted in approximately 80% of low-risk patients according to the Mayo algorithm (grade 1 or 2 endometrioid with ≤50% myometrial invasion and tumor diameter≤2 cm, as well as noninvasive endometrioid, regardless of grade). The remaining cohort is considered to have sufficient risk for lymph node metastases and thus requires a pelvic and paraaortic LND; the superior landmark for the latter is the left renal vein. With a diagnosis of uterine serous or clear cell carcinoma, multiple staging biopsies and omentectomy are performed in the absence of detectable macroscopic disease. Cytoreductive surgery is routinely performed in the presence of extrauterine spread, regardless of histology.
In this study, the taxonomy proposed by the World Health Organization was used to designate histologic subtypes [32]. The degree of glandular differentiation and cytologic atypia to determine architectural grade and surgical stage was in accord with International Federation of Gynecology and Obstetrics (FIGO) criteria [33,34]. Pathology review of all cases was conducted by a single gynecologic pathologist (G.L.K.).
For patients electing not to enter available clinical trials, counseling included detailed discussion of the merits and risks of the available adjuvant therapies based on contemporary practice patterns. Apropos to this outcome analysis, consultations regarding radiotherapy focused on local control but acknowledged the risk of distant treatment failures. Radiation-associated untoward sequelae were detailed, including the potential added risks with extended fields. Standard doses of 45.0 to 50.4 Gy to the pelvis, and 45.0 Gy to the paraaortic fields when indicated for lymph node metastases, were recommended. Likewise, the potential merits and associated sequelae of systemic therapy alone or in combination with EBRT were discussed. Platinum-based combination chemotherapy, invariably with paclitaxel or doxorubicin (or both), was the treatment of choice, preferably commencing within 6 weeks postoperatively. Chemoradiation was administered in a serial schema, planning 4 to 6 cycles of systemic therapy before administration of EBRT. When chemotherapy was the sole adjuvant modality, 4 to 6 cycles (more commonly 6 cycles) were recommended and delivered in standard doses. Vaginal brachytherapy was judiciously administered, regardless of the primary adjuvant therapy.
Data collection and statistical analysis
Patient-, disease-, and treatment-specific risk factors were abstracted from the medical records by a dedicated registered nurse following the American College of Surgeons' National Quality Improvement Program platform [35,36]. When surveillance information from the clinic or tumor registry records was insufficient, rigorous efforts were expended to update patient and disease status, including sending letters to patients or their physicians, conducting telephone interviews, and securing death certificates.
Data were summarized using standard descriptive statistics. Demographic and clinicopathologic characteristics were compared between groups using the 2-sample t test for age and the χ2 test for categorical variables. Duration of follow-up was calculated from the date of surgical treatment to the date of death or last follow-up. Overall survival (OS), cause-specific survival (CSS), and disease-free survival (DFS) were each estimated using the Kaplan–Meier method and compared between groups using the log-rank test. Risk factors were evaluated for an association with DFS based on fitting univariable Cox proportional hazards models. Multivariable models were fit using stepwise and backward variable selection methods considering all variables with a P value < .20 based on univariable analysis. Associations were summarized by calculating hazard ratios (HRs) and corresponding 95% CIs. All calculated P values were 2-sided, and P values < .05 were considered statistically significant. Analyses were performed using the SAS software package, version 9.2 (SAS Institute Inc.).
Results
Patients
During the study period, 1415 women presented with EC, were counseled, and elected primary surgical management of their disease. In accordance with the Minnesota statute for use of medical information in research [37], women who declined consent for use of recorded clinical information for research purposes were excluded from the study population (n = 22). In addition, 79 patients were diagnosed with synchronous cancers and were excluded, rendering an eligible study population of 1314 patients.
Clinicopathologic characteristics
Among the 1314 surgically managed EC patients, 109 received the diagnosis of stage IIIC disease. Forty-eight cases had FIGO grade 1 and 2 endometrioid carcinoma (G1/2EC), and 61 had grade 3 histology (G3EC), including endometrioid, serous, and clear cell carcinomas. Table 1 provides a comparative assessment of the clinical and pathologic characteristics of the 2 cohorts. The mean age of the G1/2EC cohort exceeded the age of the G3EC cohort; this finding was unexpected, but it also was not a statistically significant difference. A systematic LND (defined as removal and histologic assessment of ≥10 pelvic and ≥5 paraaortic nodes) was performed in 85% of G1/2EC and 84% of G3EC patients. The prevalence of stage IIIC2 was independent of grade (P = .21); of the 100 patients with a paraaortic LND, 33.3%, 69.0%, and 55.4% of the patients with grades 1, 2, and 3 had positive paraaortic nodes, respectively. Noteworthy was the absence of lymphovascular space invasion in 73% of the G1/2EC cohort, nearly double that witnessed among the G3EC cases. Overall, 52 (47.7%) patients received adjuvant chemotherapy and, among them, 28 (53.8%) also received EBRT. Among the G1/2EC cohort, 8 received adjuvant chemotherapy and 10 received chemotherapy and EBRT. Among the G3EC cohort, 16 received adjuvant chemotherapy and 18 received chemotherapy and EBRT. Overall, 21 (19.2%) received no adjuvant therapy. Apropos to this report, the dominant adjuvant treatment modality for G1/2EC was EBRT, whereas for G3EC, it was systemic chemotherapy (Table 1). All patients treated with chemotherapy received platinum-based chemotherapy. Only three patients were treated on adjuvant therapy clinical trials. The receipt of chemotherapy was higher among women treated after the results of GOG 122 [27] became available: 41.8% (28/67) of those treated between 1999 and 2005 received chemotherapy compared to 66.7% (24/36) of those treated after 2006 (P = 0.016).
Table 1.
Clinical and pathologic characteristics of grade 1 and 2 endometrioid and Grade 3 endometrioid, serous, and clear cell stage IIIC endometrial cancers.
| Characteristic | G1/2EC (n = 48) |
G3EC (n = 61) |
P value |
|---|---|---|---|
| Age at surgery, mean (SD), y | 66.2 (14.3) | 64.7 (10.4) | .51 |
| FIGO grade, no. (%) | <.001 | ||
| 1 | 15 (31.3) | … | |
| 2 | 33 (68.8) | … | |
| 3 | … | 61 (100.0) | |
| Histology, no. (%) | <.001 | ||
| Endometrioid | 48 (100.0) | 23 (37.7) | |
| Nonendometrioid | … | 38 (62.3) | |
| Lymphovascular space invasion, no. (%) | 13 (27.1) | 36 (59.0) | <.001 |
| Myometrial invasion >50%, no. (%) | 28 (58.3) | 43 (70.5) | .19 |
| Primary tumor diameter >2 cm, no. (%) | 47 (97.9) | 55/59 (93.2) | .25 |
| Cervical stromal invasion, no. (%) | 7 (14.6) | 14 (23.0) | .27 |
| Stage, no. (%)a | .21 | ||
| IIICo | 27 (56.3) | 27 (44.3) | |
| IIICab | 21 (43.8) | 34 (55.7) | |
| Site of node positivity, no. (%) | .90 | ||
| Pelvis only | 23 (47.9) | 30 (49.2) | |
| Paraaortic ± pelvis | 25 (52.1) | 31 (50.8) | |
| Positivity in ≥3 nodes, no. (%) | 21 (43.8) | 30/60 (50.0) | .52 |
| Positive lymph node ratio >0.10, no. (%) | 14 (29.2) | 26/60 (43.3) | .13 |
| Extent of lymphadenectomy, no. (%)b | .80 | ||
| Anything less than systematic | 7 (14.6) | 10 (16.4) | |
| Systematic | 41 (85.4) | 51 (83.6) | |
| Adjuvant chemotherapy, no. (%) | 18/46 (39.1) | 34/57 (59.6) | .04 |
| Adjuvant EBRT, no. (%) | 28/45 (62.2) | 29/58 (50.0) | .22 |
Abbreviations: EBRT, external beam radiotherapy; FIGO, International Federation of Gynecology and Obstetrics; G1/2EC, FIGO grade 1 and 2 endometrioid endometrial carcinoma; G3EC, FIGO grade 3 endometrial carcinoma.
Stage: IIICo, positive node(s) only; IIICab, positive node(s) and adnexal involvement, uterine serosal involvement, vaginal involvement, and/or positive peritoneal cytology.
Systematic was defined as ≥10 pelvic and ≥5 paraaortic lymph nodes removed and histologically assessed.
Table 2 provides a comparative assessment of the clinical and pathologic characteristics of those who received chemotherapy and those who did not receive chemotherapy. Women who received chemotherapy were more likely to be younger and have higher grade, endometrioid, and stage IIICab EC. Additionally, those who received chemotherapy were also more likely to have undergone a systematic lymphadenectomy.
Table 2.
Clinical and pathologic characteristics of patients who received adjuvant chemotherapy and patients who did not receive adjuvant chemotherapy.
| Characteristic | No adjuvant chemotherapy (n = 51) | Adjuvant chemotherapy (n = 52) | P Value |
|---|---|---|---|
| Age at surgery, mean (SD), y | 68.7 (12.9) | 61.5 (10.5) | .004 |
| FIGO grade, no. (%) | .06 | ||
| 1 | 11 (21.6) | 4 (7.7) | |
| 2 | 17 (33.3) | 14 (26.9) | |
| 3 | 23 (45.1) | 34 (65.4) | |
| Histology, no. (%) | .009 | ||
| Endometrioid | 12 (23.5) | 25 (48.1) | |
| Nonendometrioid | 39 (76.5) | 27 (51.9) | |
| Lymphovascular space invasion, no. (%) | 23 (45.1) | 24 (46.2) | .91 |
| Myometrial invasion >50%, no. (%) | 32 (62.7) | 34 (65.4) | .78 |
| Primary tumor diameter >2 cm, no. (%) | 46/50 (92.0) | 50/51 (98.0) | .16 |
| Cervical stromal invasion, no. (%) | 8 (15.7) | 12 (23.1) | .34 |
| Stage, no. (%)a | .002 | ||
| IIICo | 33 (64.7) | 18 (34.6) | |
| IIICab | 18 (35.3) | 34 (65.4) | |
| Site of node positivity, no. (%) | .14 | ||
| Pelvis only | 29 (56.9) | 22 (42.3) | |
| Paraaortic ± pelvis | 22 (43.1) | 30 (57.7) | |
| Positivity in ≥3 nodes, no. (%) | 22 (43.1) | 25/51 (49.0) | .55 |
| Positive lymph node ratio >0.10, no. (%) | 17 (33.3) | 21/51 (41.2) | .41 |
| Extent of lymphadenectomy, no. (%)b | .03 | ||
| Anything less than systematic | 12 (23.5) | 4 (7.7) | |
| Systematic | 39 (76.5) | 48 (92.3) | |
| Adjuvant EBRT, no. (%) | 29 (58.0) | 28 (53.8) | .67 |
Abbreviations: EBRT, external beam radiotherapy; FIGO, International Federation of Gynecology and Obstetrics.
Stage: IIICo, positive node(s) only; IIICab, positive node(s) and adnexal involvement, uterine serosal involvement, vaginal involvement, and/or positive peritoneal cytology.
Systematic was defined as ≥10 pelvic and ≥5 paraaortic lymph nodes removed and histologically assessed.
Comparative assessment of oncologic survival outcomes
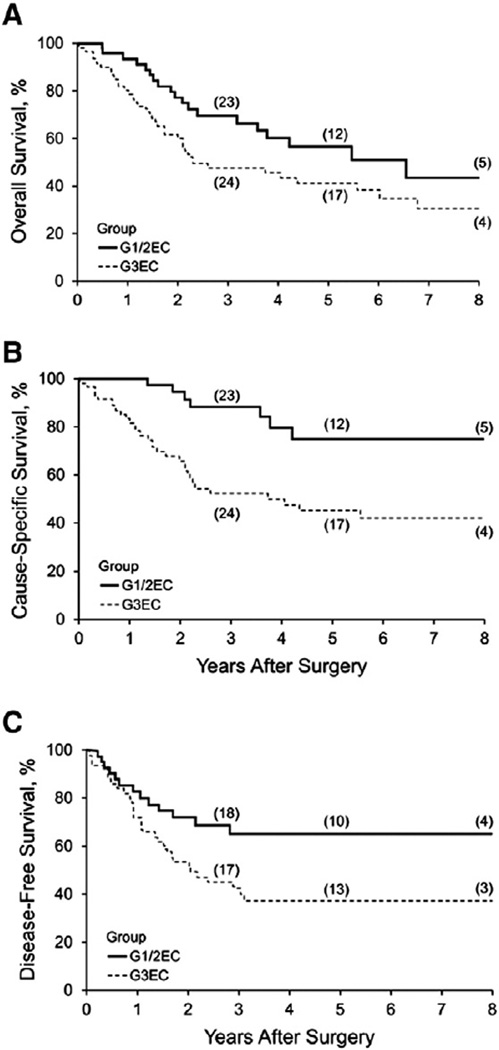
Among the 109 patients, 60 deaths (22 in G1/2EC and 38 in G3EC) were documented, of which 38 were due to cancer (7 in G1/2EC and 31 in G3EC). Among the patients alive at the last follow-up, the median duration of follow-up was 4.4 years (interquartile range, 2.5–6.1 years). Overall survival at 3 years was 56.9% and overall survival at 5 years was 47.6%. As shown in Fig. 1A, the comparative OS estimates for stage IIIC G1/2EC (n = 48) vs grade 3 endometrioid and nonendometrioid carcinomas (n = 61) appeared to favor G1/2EC, but the difference was not statistically significant (log-rank test, P = .06). Specifically, OS estimates for grade 3 endometrioid (n = 23) and grade 3 nonendometrioid (n = 38) EC did not differ statistically (log-rank test, P = .53).
Fig. 1.
Kaplan–Meier survival estimates for stage IIIC EC. A, Overall survival (P = .06). B, Cause-specific survival (P < .001). C, Disease-free survival (P = .03). EC denotes endometrial carcinoma; G1/2EC, grade 1 and 2 endometrioid EC; G3EC, grade 3 endometrioid and nonendometrioid EC.
We recognized the prevalence of medical comorbidities that are characteristically associated with G1/2EC, and we therefore conducted CSS analyses to account for non-disease-related deaths. As shown in Fig. 1B, the Kaplan–Meier CSS estimates for G1/2EC were significantly more favorable (log-rank test, P < .001) compared with G3EC; CSS estimates comparing G3 endometrioid and nonendometrioid were similar (log-rank test, P = .87). However, the marked disparity between OS and CSS in G1/2EC relative to G3EC was intriguing and prompted further comparative assessment of treatment failures. Recurrences were documented in 14 G1/2EC cases (30%) and 36 G3EC cases (60%), with 7 (50%) and 29 (81%) of those patients, respectively, dying of disease. Among patients for whom the precise time of recurrence was documented, the disease-free attrition rate, as illustrated in Fig. 1C, was relatively rapid during the initial 12 to 18 months across both cohorts, with nearly all recurrences evident within 36 months (93%). DFS for the G1/2EC cohort was more favorable compared with the G3EC cohort (log-rank test, P = .03); histology within the latter cohort was not discriminating (log-rank test, P = .78).
Efficacy of managing regional disease
Although the diagnostic value of LND in identifying patients at risk for lymphatic failure is evident, its role in removing occult regional lymphatic disease in concert with adjuvant radiotherapy or chemotherapy or both would presuppose control of regional disease. Within the G1/2EC cohort, no patient with positive pelvic or paraaortic nodes managed with pelvic or extended-field radiation or chemotherapy or a combination of these adjuvant modalities had recurrence in the treated regional node-bearing compartments. After excluding 2 of 29 G3EC patients with unknown disease status or site of progression, regional control in the 27 patients receiving adjuvant EBRT with (n = 18) or without (n = 9) chemotherapy was achieved in 23 of 24 patients with positive pelvic nodes and in 8 of 10 patients with positive paraaortic nodes. Similarly, 12 of 15 G3EC patients (1 excluded for unknown site of recurrence) treated with LND and adjuvant chemotherapy alone had control of disease in the pelvic and paraaortic node-bearing regions.
Efficacy of systemic chemotherapy in managing distant disease
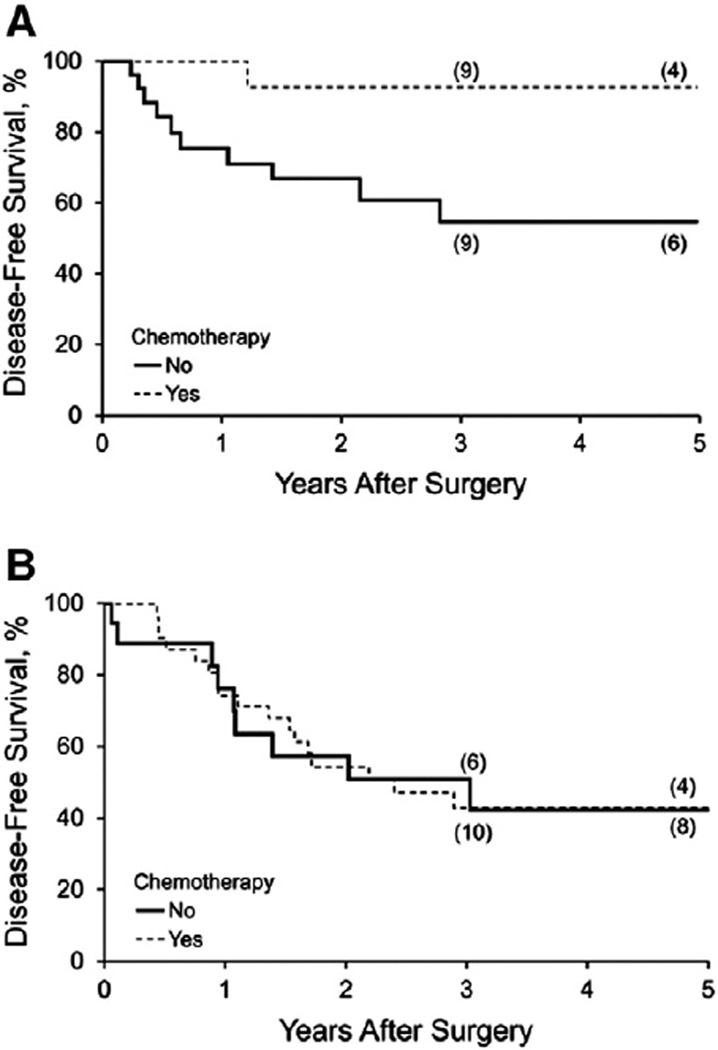
Considering that treatment failures in stage IIIC EC are predominantly due to occult extrapelvic metastases, the principal tenet in the administration of adjuvant chemotherapy is to treat occult disease and thereby reduce extrapelvic recurrences. Therefore, extrapelvic DFS was deemed to be the most appropriate metric for assessing the efficacy of adjuvant systemic treatment. The impact of chemotherapy on hematogenous, intraperitoneal, and lymphatic (beyond the radiotherapy fields) DFS was assessed in both the G1/2EC and G3EC cohorts. To assess risk factors that affected extrapelvic DFS in stage IIIC disease, all patient-, pathologic-, and treatment-specific variables with a P value < .20 on univariable analysis (Table 1) were allowed to compete in multivariable modeling. Of the 14 patients with G1/2EC who had recurrence, 12 recurrences (86%) occurred at distant sites. The 5-year extrapelvic DFS with and without adjuvant chemotherapy for patients with G1/2EC was 93% and 54%, respectively (log-rank test, P = .02; Fig. 2A). Among G1/2EC (n = 48), the sole independent predictor of extrapelvic DFS was grade 2 histology (P = .02) (Table 3). However, there was a trend toward decreased extrapelvic DFS associated with receipt of chemotherapy (P = .12) when stage IIIC substages were included in the analyses. When not considering stage IIIC substages, FIGO grade 2 (HR (95% CI) 0.28 (0.08, 0.91); P = .03) remained an independent predictor of improved extrapelvic DFS and the impact of adjuvant chemotherapy receipt (HR (95% CI) 0.13 (0.02, 1.01); P = .0511) approached significance.
Fig. 2.
Kaplan–Meier estimates of hematologic, peritoneal, and lymphatic recurrences beyond the radiation field. A, Improved disease-free survival was observed with receipt of chemotherapy in stage IIIC, grade 1/2 endometrioid endometrial carcinoma (P = .02). B, Disease-free survival was unchanged after adjuvant chemotherapy in stage IIIC grade 3 endometrioid and type II endometrial carcinoma combined (P = .91).
Table 3.
Multivariable analysis as a function of extrapelvic (hematologic, peritoneal, and lymphatic recurrences beyond the radiation field) disease-free survival in patients with grade 1 and 2 endometrioid endometrial cancera.
| Variable | No. of patients | Univariable analysisHR (95% CI) | Multivariable analysisHR (95% CI) | P value |
|---|---|---|---|---|
| FIGO grade | .02 | |||
| 1 | 15 | Reference | Reference | |
| 2 | 33 | 0.25 (0.08–0.80) | 0.25 (0.08–0.80) | |
| Stage b | ||||
| IIICo | 27 | Reference | ||
| IIICab | 21 | 0.41 (0.11–1.54) | ||
| Adjuvant chemotherapy | ||||
| No | 28 | Reference | ||
| Yes | 18 | 0.12 (0.02–0.94) |
Abbreviations: FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio.
Only clinicopathologic variables with P < .20 on univariable analysis were considered.
Stage: IIICo, positive node(s) only; IIICab, positive node(s) and adnexal involvement, uterine serosal.
Of the 36 documented recurrences in the G3EC cohort, the site was known for 29, and 26 of 29 patients (90%) had treatment failure at 1 or more sites beyond the pelvis or extended radiotherapy field. Adjuvant chemotherapy did not affect occult extrapelvic metastasis in G3EC; the 5-year extrapelvic DFS for G3EC with and without adjuvant chemotherapy was 43% and 42%, respectively (log-rank test, P = .91; Fig. 2B). Within the G3EC cohort, the sole independent predictor of DFS was lymphovascular space involvement (P = .04) (Table 4).
Table 4.
Multivariable analysis as a function of extrapelvic (hematologic, peritoneal, and lymphatic recurrences beyond the radiation field) disease-free survival in patients with grade 3 endometrioid, serous, and clear cell endometrial cancera.
| Variable | No. of patients | Univariable analysisHR (95% CI) | Multivariable analysisHR (95% CI) | P value |
|---|---|---|---|---|
| Lymphovascular space invasion | .04 | |||
| No | 25 | Reference | Reference | |
| Yes | 36 | 2.53 (1.05–6.10) | 2.53 (1.05–6.10) | |
| Cervical stromal invasion | ||||
| No | 47 | Reference | ||
| Yes | 14 | 1.91 (0.80–4.58) | ||
| Site of node positivity | ||||
| Pelvis only | 30 | Reference | ||
| Paraaortic ± pelvis | 31 | 2.03 (0.93–4.44) | ||
| Node positivity | ||||
| <3 | 30 | Reference | ||
| ≥3 | 30 | 1.68 (0.77–3.63) |
Abbreviation: HR, hazard ratio.
Only clinicopathologic variables with P < .20 on univariable analysis were considered.
Discussion
Multiple descriptive studies and prospective randomized trials, including Gynecologic Oncology Group (GOG) protocol 99 and the Post Operative Radiation Therapy in Endometrial Carcinoma (PORTEC) trials, have validated the effectiveness of pelvic irradiation for control of local and regional occult disease in women with EC [38–42]. These reports also indicate that treatment failures in these at-risk EC cases are predominantly due to occult EPM. Accordingly, in the past 2 decades, therapeutic algorithms for intermediate and high-risk disease have dramatically increased the incorporation of adjuvant systemic cytotoxic agents [15]. Exemplary of this paradigm shift is the transition to systemic chemotherapy alone or in combination with EBRT in the management of stage IIIC EC, despite inconsistent outcomes [10,11,15,20–22]. Considering that surgical and adjuvant therapy options (including the choices of cytotoxic agents) are limited for managing stage IIIC disease, these inconsistent outcomes may be attributable to phenotypic or genotypic heterogeneity or both.
Our findings demonstrate significant variance in DFS and a more striking difference in CSS between stage IIIC G1/2EC and the grade 3 endometrial subtypes combined (G3EC). Nonetheless, hematogenous, intraperitoneal, and distant lymphatic recurrences were the foremost sites of treatment failures in both cohorts, accounting for 86% of recurrences in the G1/2EC and 90% in the G3EC cohorts with known sites of recurrence. The sole independent risk factor influencing extrapelvic DFS in the G1/2EC cohort was FIGO grade 2 histology. However, the impact of adjuvant chemotherapy for G1/2EC approached significance in reducing extrapelvic DFS when controlling for grade. Most notably, the impact of adjuvant chemotherapy was pronounced compared with the corresponding treatment strategies without chemotherapy in G1/2EC (log-rank test, P = .02). Conversely, lymphovascular space invasion was the only independent predictor of extrapelvic DFS in the G3EC cohort. This was not surprising as previous investigations have shown a substantial proportion of G3EC to have LVSI [43] and the presence of LVSI is associated with worse survival [10,12,24]. Histologic subtype and adjuvant therapy did not appear to affect extrapelvic DFS in the G3EC cohort. Within the G3EC cohort, similar extrapelvic DFS was witnessed when comparing patients receiving chemotherapy (with or without radiotherapy) to those who either did not receive adjuvant therapy or were treated with radiotherapy only. Considering that the greater majority of treatment failures were extrapelvic, the very low salvage rate in grade 3 patients with failed primary treatment further suggests that contemporary chemotherapy is relatively ineffective in managing high-grade EC.
Two previous randomized prospective trials have reported disparate outcomes for endometrial histologic subtypes treated with contemporary chemotherapy, although this was not the intended primary focus of either trial. In GOG 122, the combination of doxorubicin and cisplatin provided superior progression-free and OS compared with whole-abdomen irradiation in advanced EC [27]. However, contrasting HRs for progression-free survival were observed for endometrioid and serous cell types (0.687 and 0.909, respectively), as well as for OS (0.482 and 1.025, respectively). Using multivariable regression analysis, a significant HR of 2.24 was generated when comparing grade 3 to grade 1 lesions; the corresponding HR for grade 2 vs grade 1was not significant. Similarly, Hogberg et al. [30] randomized 140 early stage serous and clear cell EC cases to radiation with or without chemotherapy. The corresponding progression-free survival, OS, and CSS did not differ significantly (P values were .59, .88, and .49, respectively) suggesting the absence of benefit from the addition of chemotherapy in treating these high-risk subtypes. By contrast, the addition of chemotherapy to the radiotherapy regimen in treating endometrioid EC significantly improved clinical outcomes, as demonstrated by the corresponding HRs for progression-free survival and CSS (0.50 and 0.42, respectively; P = .03 for both). Additionally, improvement in OS approached statistical significance (0.55; P = .08). The trend only in OS was likewise observed in our stage IIIC endometrioid cohort reflecting death from other causes. Furthermore, the recent multi-institutional report by Secord et al. [15] analyzing 203 stage IIIC patients managed with either chemotherapy (n = 46) or radiotherapy (n = 45) or a combination of these modalities (n = 161) showed extrapelvic recurrence rates of 17%, 18%, and 22%, respectively; in addition, 74% of all treatment failures were extrapelvic. The recurrence-free survival HR was 2.2 for chemotherapy only compared with the combination of chemotherapy and radiation (P = .02); the latter was equivalent to radiotherapy alone (P = .92). Furthermore, the HR for grade 3 vs grades 1 and 2 was 2.9 (P < .001), suggesting a relative recalcitrance to chemotherapy for grade 3 tumors.
Limitations of this current study include the non-randomized use of adjuvant therapies. In the absence of a clinical trial, patients were managed according to contemporary clinical practice patterns and adjuvant therapy approaches were heterogenous. Nevertheless, as the primary outcome of this current study was presumed occult EPM at the time of initial diagnosis as measured by extrapelvic DFS, receipt of systemic cytotoxic therapy, albeit heterogeneous in regimens, was considered as a single variable. Notwithstanding the current treatment strategies to control local and regional diseases, future therapeutic success in stage IIIC EC mandates effective management of occult EPM. The current collective evidence suggests that adjuvant therapy with contemporary systemic chemotherapy, with or without radiotherapy, should be offered to all patients with stage IIIC FIGO grade 1 and 2 EC. Similar to findings in stage I/II grade 3 EC [10], the evidence continues to strengthen for the lack of efficacy of current chemotherapy strategies in treating occult EPM in FIGO grade 3 EC (regardless of histology). The results of ongoing GOG protocol 258 should shed additional light on the impact of chemotherapy, with or without EBRT, in advanced stage EC. However, separate subgroup analyses of G1/2EC and G3EC may be underpowered. The lack of efficacy of contemporary chemotherapy in high-grade EC merits readdressing the current standard of care for these apparent treatment-refractory histologies. Development of novel innovative phase I and II clinical trials for these histologies is imperative.
HIGHLIGHTS.
Occult extra-pelvic metastases account for greater than 85% of recurrences in stage IIIC endometrial cancer.
Contemporary adjuvant chemotherapy substantially attenuates extra-pelvic recurrences in stage IIIC grade 1 and 2 endometrioid carcinomas.
Chemotherapy does not impact extra-pelvic recurrences in stage IIIC grade 3 endometrioid, serous and clear cell carcinomas.
Acknowledgments
Funding
None.
Abbreviations
- CI
confidence interval
- CSS
cause-specific survival
- DFS
disease-free survival
- EBRT
external-beam radiotherapy
- EC
endometrial carcinoma
- FIGO
International Federation of Gynecology and Obstetrics
- G1/2EC
FIGO grade 1 and 2 endometrioid endometrial carcinoma
- G3EC
FIGO grade 3 endometrial carcinoma
- GOG
Gynecologic Oncology Group
- HR
hazard ratio
- LND
lymphadenectomy
- OS
overall survival
- PORTEC
Post Operative Radiation Therapy in Endometrial Carcinoma
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- 1.Kilgore LC, Partridge EE, Alvarez RD, Austin JM, Shingleton HM, Noojin F, 3rd, et al. Adenocarcinoma of the endometrium: survival comparisons of patients with and without pelvic node sampling. Gynecol Oncol. 1995 Jan;56(1):29–33. doi: 10.1006/gyno.1995.1005. [DOI] [PubMed] [Google Scholar]
- 2.Mariani A, Webb MJ, Galli L, Podratz KC. Potential therapeutic role of para-aortic lymphadenectomy in node-positive endometrial cancer. Gynecol Oncol. 2000 Mar;76(3):348–356. doi: 10.1006/gyno.1999.5688. [DOI] [PubMed] [Google Scholar]
- 3.Havrilesky LJ, Cragun JM, Calingaert B, Synan I, Secord AA, Soper JT, et al. Resection of lymph node metastases influences survival in stage IIIC endometrial cancer. Gynecol Oncol. 2005 Dec;99(3):689–695. doi: 10.1016/j.ygyno.2005.07.014. [Epub 2005 Aug 29]. [DOI] [PubMed] [Google Scholar]
- 4.Mariani A, Dowdy SC, Cliby WA, Haddock MG, Keeney GL, Lesnick TG, et al. Efficacy of systematic lymphadenectomy and adjuvant radiotherapy in node-positive endometrial cancer patients. Gynecol Oncol. 2008 May;101(2) doi: 10.1016/j.ygyno.2006.01.032. [Epub 2006 Feb 28]. [DOI] [PubMed] [Google Scholar]
- 5.Chan JK, Kapp DS, Cheung MK, Osann K, Shin JY, Cohn D, et al. The impact of the absolute number and ratio of positive lymph nodes on survival of endometrioid uterine cancer patients. Br J Cancer. 2007 Sep 3;97(5):605–611. doi: 10.1038/sj.bjc.6603898. [Epub 2007 Jul 31]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aalders JG, Thomas G. Endometrial cancer: revisiting the importance of pelvic and para aortic lymph nodes. Gynecol Oncol. 2007 Jan;104(1):222–231. doi: 10.1016/j.ygyno.2006.10.013. [Epub 2006 Nov 28]. [DOI] [PubMed] [Google Scholar]
- 7.Benedetti Panici P, Basile S, Maneschi F, Alberto Lissoni A, Signorelli M, Scambia G, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008 Dec 3;100(23):1707–1716. doi: 10.1093/jnci/djn397. [Epub 2008 Nov 25]. [DOI] [PubMed] [Google Scholar]
- 8.Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK ASTEC study group. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009 Jan 10;373(9658):125–136. doi: 10.1016/S0140-6736(08)61766-3. Epub 2008 Dec 16. Erratum in Lancet Jan 10 2009;373(9677):1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mariani A, Dowdy SC, Cliby WA, Gostout BS, Jones MB, Wilson TO, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008 Apr;109(1):11–18. doi: 10.1016/j.ygyno.2008.01.023. [Epub 2008 Mar 4]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ayeni TA, Bakkum-Gamez JN, Mariani A, McGree ME, Weaver AL, Haddock MG, et al. Comparative outcomes assessment of uterine grade 3 endometrioid, serous, and clear cell carcinomas. Gynecol Oncol. 2013 Jun;129(3):478–485. doi: 10.1016/j.ygyno.2013.03.011. [Epub 2013 Mar 25]. [DOI] [PubMed] [Google Scholar]
- 11.Onda T, Yoshikawa H, Mizutani K, Mishima M, Yokota H, Nagano H, et al. Treatment of node-positive endometrial cancer with complete node dissection, chemotherapy and radiation therapy. Br J Cancer. 1997;75(12):1836–1841. doi: 10.1038/bjc.1997.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson G, Randall M, Sutton G, Moore D, Hurteau J, Look K. FIGO stage IIIC endometrial carcinoma with metastases confined to pelvic lymph nodes: analysis of treatment outcomes, prognostic variables, and failure patterns following adjuvant radiation therapy. Gynecol Oncol. 1999 Nov;75(2):211–214. doi: 10.1006/gyno.1999.5569. [DOI] [PubMed] [Google Scholar]
- 13.Mundt AJ, Murphy KT, Rotmensch J, Waggoner SE, Yamada SD, Connell PP. Surgery and postoperative radiation therapy in FIGO Stage IIIC endometrial carcinoma. Int J Radiat Oncol Biol Phys. 2001 Aug 1;50(5):1154–1160. doi: 10.1016/s0360-3016(01)01590-5. [DOI] [PubMed] [Google Scholar]
- 14.Gadducci A, Cosio S, Fabrini MG, Guerrieri ME, Greco C, Genazzani AR. Analysis of failures in patients with FIGO stage IIIc1–IIIc2 endometrial cancer. Anticancer Res. 2012 Jan;32(1):201–205. [PubMed] [Google Scholar]
- 15.Secord AA, Geller MA, Broadwater G, Holloway R, Shuler K, Dao NY, et al. A multicenter evaluation of adjuvant therapy in women with optimally resected stage IIIC endometrial cancer. Gynecol Oncol. 2013 Jan;128(1):65–70. doi: 10.1016/j.ygyno.2012.10.010. [Epub 2012 Oct 17]. [DOI] [PubMed] [Google Scholar]
- 16.Alvarez Secord A, Havrilesky LJ, Bae-Jump V, Chin J, Calingaert B, Bland A, et al. The role of multi-modality adjuvant chemotherapy and radiation in women with advanced stage endometrial cancer. Gynecol Oncol. 2007 Nov;107(2):285–291. doi: 10.1016/j.ygyno.2007.06.014. [Epub 2007 Aug 6]. [DOI] [PubMed] [Google Scholar]
- 17.Hoekstra AV, Kim RJ, Small W, Jr, Rademaker AW, Helenowski IB, Singh DK, et al. FIGO stage IIIC endometrial carcinoma: prognostic factors and outcomes. Gynecol Oncol. 2009 Aug;114(2):273–278. doi: 10.1016/j.ygyno.2009.04.013. [Epub 2009 May 9]. [DOI] [PubMed] [Google Scholar]
- 18.Klopp AH, Jhingran A, Ramondetta L, Lu K, Gershenson DM, Eifel PJ. Node-positive adenocarcinoma of the endometrium: outcome and patterns of recurrence with and without external beam irradiation. Gynecol Oncol. 2009 Oct;115(1):6–11. doi: 10.1016/j.ygyno.2009.06.035. [Epub 2009 Jul 25]. [DOI] [PubMed] [Google Scholar]
- 19.Lee LJ, Viswanathan AN. Combined chemotherapy and radiation improves survival for node-positive endometrial cancer. Gynecol Oncol. 2012 Oct;127(1):32–37. doi: 10.1016/j.ygyno.2012.06.026. [Epub 2012 Jun 24]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeshima N, Umayahara K, Fujiwara K, Hirai Y, Takizawa K, Hasumi K. Effectiveness of postoperative chemotherapy for para-aortic lymph node metastasis of endometrial cancer. Gynecol Oncol. 2006 Aug;102(2):214–217. doi: 10.1016/j.ygyno.2005.12.010. [Epub 2006 Feb 7]. [DOI] [PubMed] [Google Scholar]
- 21.Hiura M, Nogawa T, Matsumoto T, Yokoyama T, Shiroyama Y, Wroblewski J. Long-term survival in patients with para-aortic lymph node metastasis with systematic retroperitoneal lymphadenectomy followed by adjuvant chemotherapy in endometrial carcinoma. Int J Gynecol Cancer. 2010 Aug;20(6):1000–1005. doi: 10.1111/IGC.0b013e3181d80aff. [DOI] [PubMed] [Google Scholar]
- 22.Scribner DR, Jr, Puls LE, Gold MA. A phase II evaluation of docetaxel and carboplatin followed by tumor volume directed pelvic plus or minus paraaortic irradiation for stage III endometrial cancer. Gynecol Oncol. 2012 May;125(2):388–393. doi: 10.1016/j.ygyno.2012.02.003. [Epub 2012 Feb 10]. [DOI] [PubMed] [Google Scholar]
- 23.McMeekin DS, Lashbrook D, Gold M, Johnson G, Walker JL, Mannel R. Analysis of FIGO stage IIIc endometrial cancer patients. Gynecol Oncol. 2001 May;81(2):273–278. doi: 10.1006/gyno.2001.6157. [DOI] [PubMed] [Google Scholar]
- 24.Fotopoulou C, Savvatis K, Kraetschell R, Schefold JC, Lichtenegger W, Sehouli J. Systematic pelvic and aortic lymphadenectomy in intermediate and high-risk endometrial cancer: lymph-node mapping and identification of predictive factors for lymph-node status. Eur J Obstet Gynecol Reprod Biol. 2010 Apr;149(2):199–203. doi: 10.1016/j.ejogrb.2009.12.021. [Epub 2010 Jan 22]. [DOI] [PubMed] [Google Scholar]
- 25.Rose PG, Cha SD, Tak WK, Fitzgerald T, Reale F, Hunter RE. Radiation therapy for surgically proven para-aortic node metastasis in endometrial carcinoma. Int J Radiat Oncol Biol Phys. 1992;24(2):229–233. doi: 10.1016/0360-3016(92)90676-9. [DOI] [PubMed] [Google Scholar]
- 26.McMeekin DS, Lashbrook D, Gold M, Scribner DR, Kamelle S, Tillmanns TD, et al. Nodal distribution and its significance in FIGO stage IIIc endometrial cancer. Gynecol Oncol. 2001 Aug;82(2):375–379. doi: 10.1006/gyno.2001.6278. [DOI] [PubMed] [Google Scholar]
- 27.Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowler J, et al. Gynecologic Oncology Group study. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2006 Jan 1;24(1):36–44. doi: 10.1200/JCO.2004.00.7617. [Epub 2005 Dec 5]. [DOI] [PubMed] [Google Scholar]
- 28.Maggi R, Lissoni A, Spina F, Melpignano M, Zola P, Favalli G, et al. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial. Br J Cancer. 2006 Aug 7;95(3):266–271. doi: 10.1038/sj.bjc.6603279. [Epub 2006 Jul 25]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Susumu N, Sagae S, Udagawa Y, Niwa K, Kuramoto H, Satoh S, et al. Japanese Gynecologic Oncology Group. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: a Japanese Gynecologic Oncology Group study. Gynecol Oncol. 2008 Jan;108(1):226–233. doi: 10.1016/j.ygyno.2007.09.029. [Epub 2007 Nov 9]. [DOI] [PubMed] [Google Scholar]
- 30.Hogberg T, Signorelli M, de Oliveira CF, Fossati R, Lissoni AA, Sorbe B, et al. Sequential adjuvant chemotherapy and radiotherapy in endometrial cancer: results from two randomised studies. Eur J Cancer. 2010 Sep;46(13):2422–2431. doi: 10.1016/j.ejca.2010.06.002. [Epub 2010 Jul 7]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah PH, Kudrimoti M, Feddock J, Randall M. Adjuvant treatment for stage IIIC endometrial cancer: options and controversies. Gynecol Oncol. 2011 Sep;122(3):675–683. doi: 10.1016/j.ygyno.2011.05.018. [Epub 2011 Jun 12]. [DOI] [PubMed] [Google Scholar]
- 32.Scully RE, Bonfiglio TA, Kurman RJ, Silverberg SG, Wilkinson EJ. Histological typing of female genital tract tumours. 2nd ed. New York (NY): Springer-Verlag; 1994. pp. 13–18. [Google Scholar]
- 33.FIGO stages: 1988 revision. Definition of the clinical stages in carcinoma of the vulva. Gynecol Oncol. 1989;35:125–127. [Google Scholar]
- 34.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009 May;105(2):103–104. doi: 10.1016/j.ijgo.2009.02.012. Erratum in Int J Gynaecol Obstet May 2009;108(2):176. [DOI] [PubMed] [Google Scholar]
- 35.Khuri SF, Daley J, Henderson WG. The comparative assessment and improvement of quality of surgical care in the Department of Veterans Affairs. Arch Surg. 2002 Jan;137(1):20–27. doi: 10.1001/archsurg.137.1.20. [DOI] [PubMed] [Google Scholar]
- 36.Hall BL, Hamilton BH, Richards K, Bilimoria KY, Cohen ME, Ko CY. Does surgical quality improve in the American College of Surgeons National Surgical Quality Improvement Program: an evaluation of all participating hospitals. Ann Surg. 2009 Sep;250(3):363–376. doi: 10.1097/SLA.0b013e3181b4148f. [DOI] [PubMed] [Google Scholar]
- 37.Disclosure of health records for external research. Minn Rev Stat. 2012;144:295. [Google Scholar]
- 38.Aalders J, Abeler V, Kolstad P, Onsrud M. Postoperative external irradiation and prognostic parameters in stage I endometrial carcinoma: clinical and histopathologic study of 540 patients. Obstet Gynecol. 1980 Oct;56(4):419–427. [PubMed] [Google Scholar]
- 39.Creutzberg CL, van Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Warlam-Rodenhuis CC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicenter randomised trial. Post operative radiation therapy in endometrial carcinoma. Lancet. 2000 Apr 22;355(9213):1404–1411. doi: 10.1016/s0140-6736(00)02139-5. [DOI] [PubMed] [Google Scholar]
- 40.Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, Bloss JD, et al. Gynecologic Oncology Group. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004 Mar;92(3):744–751. doi: 10.1016/j.ygyno.2003.11.048. Erratum in Gynecol Oncol Mar 2004;94(1):241–2. [DOI] [PubMed] [Google Scholar]
- 41.Scholten AN, van Putten WL, Beerman H, Smit VT, Koper PC, Lybeert ML, et al. PORTEC Study Group. Postoperative radiotherapy for Stage 1 endometrial carcinoma: long-term outcome of the randomized PORTEC trial with central pathology review. Int J Radiat Oncol Biol Phys. 2005 Nov 1;63(3):834–838. doi: 10.1016/j.ijrobp.2005.03.007. [Epub 2005 May 31]. [DOI] [PubMed] [Google Scholar]
- 42.Blake P, Swart AM, Orton J, Kitchener H, Whelan T, Lukka H, et al. ASTEC/EN.5 study group. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): pooled trial results, systematic review, and meta-analysis. Lancet. 2009 Jan 10;373(9658):137–146. doi: 10.1016/S0140-6736(08)61767-5. [Epub 2008 Dec 16]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rasool N, Fader AN, Seamon L, Neubauer NL, Shahin FA, Alexander HA, et al. Stage I, grade 3 endometrioid adenocarcinoma of the endometrium: an analysis of clinical outcomes and patterns of recurrence. Gynecol Oncol. 2010 Jan;116(1):10–14. doi: 10.1016/j.ygyno.2009.10.043. [Epub 2009 Oct 28]. [DOI] [PubMed] [Google Scholar]