Abstract
Prostaglandins (PGs) increase in bone in response to mechanical loading and stimulate bone formation. Inhibition of cyclooxygenase (COX), the enzyme responsible for PG synthesis, by non-steroidal anti-inflammatory drugs (NSAIDs) impairs the bone formation response to loading in animals when administered before, but not after, loading. The aim was to determine whether the timing of ibuprofen use (400 mg before versus after exercise sessions) is a significant determinant of the adaptive response of BMD to exercise training in older adults. We hypothesized that taking ibuprofen before exercise would attenuate the improvements in total hip and lumbar spine BMD in response to 36 weeks of training when compared with placebo or with ibuprofen use after exercise. Untrained women and men (N = 189) aged 60 to 75 years were randomly assigned to 1 of 3 treatment arms: placebo before and after exercise (PP); ibuprofen before and placebo after exercise (IP); and placebo before and ibuprofen after exercise (PI).
The difference between groups in the change in BMD was not significant when IP was compared with either PP (hip, − 0.5% (− 1.4, 0.4); spine, 0.1% (− 0.9, 1.2)) or PI (hip, 0.3% (− 0.6, 1.2); spine, 0.5% (− 0.5, 1.5)). Ibuprofen use appeared to have more adverse effects on BMD in women than men. The study demonstrated that ibuprofen use did not significantly alter the BMD adaptations to exercise in older adults, but this finding should be interpreted cautiously. It had been expected that the inhibition of bone formation by ibuprofen would be more robust in men than in women, but this did not appear to be the case and may have limited the power to detect the effects of ibuprofen. Further research is needed to understand whether NSAID use counteracts, in part, the beneficial effects of exercise on bone.
Keywords: Exercise training, Bone mineral density, Nonsteroidal anti-inflammatory drugs, Cyclooxygenase, Prostaglandins
Highlights
-
•
The purpose was to determine whether musculoskeletal adaptations to exercise training in older adults are influenced by NSAID use.
-
•
Ibuprofen use did not significantly alter changes in BMD or fat-free mass, but the study may have been inadequately powered.
-
•
Study attrition was significantly lower in the group that took NSAIDs before exercise suggesting improved tolerance of vigorous bone-loading exercise.
-
•
This was the first randomized, double-blinded placebo- controlled study of ibuprofen effects on BMD from exercise in an older population.
1. Introduction
Prostaglandin E2 (PGE2) increases in bone in response to mechanical loading (Chow, 2000, Thorsen et al., 1996) and is an essential signaling factor for the stimulation of bone formation (Chow, 2000, Zaman et al., 1997). Cyclooxygenase (COX) is the key enzyme involved in the production of prostaglandins. There is compelling evidence from basic (i.e., osteoblasts) and preclinical (i.e., animals) studies that inhibition of COX activity by non-steroidal anti-inflammatory drugs (NSAIDs) markedly diminishes the bone formation response to mechanical stress (Chow and Chambers, 1994, Kunnel et al., 2004, Li et al., 2002, Chow et al., 1998, Cheng et al., 1997, Forwood, 1996). The impaired bone formation response has been found to occur when the NSAID is administered before mechanical loading, but not when it is administered after (Chow and Chambers, 1994, Li et al., 2002). A limitation of these preclinical studies was that they evaluated the bone formation response to acute mechanical stress only. One study that utilized a selective COX-2 inhibitor before loading sessions found that bone adaptations were not impaired after 2 weeks of mechanical loading (Sugiyama et al., 2013).
In a proof-of-concept study conducted in young women, we found that the administration of ibuprofen 1 to 2 h before exercise sessions over 36 weeks of exercise training resulted in the least favorable adaptations of bone mineral density (BMD) when compared with taking ibuprofen after exercise or placebo before and after exercise (Kohrt et al., 2010). In contrast, taking ibuprofen after exercise resulted in relatively large gains in BMD. Considering that older adults are at increased risk of osteoporosis and commonly take NSAIDs to treat common pain disorders, it is important to determine whether skeletal adaptations to exercise training in this population are influenced by NSAID use.
Accordingly, the primary aim of this study was to determine whether the timing of ibuprofen use relative to the performance of exercise (before versus after) is a significant determinant of the adaptive BMD response to exercise training in older adults. We hypothesized that taking ibuprofen before exercise sessions would attenuate the improvements in total hip and lumbar spine BMD in response to 36 weeks of exercise training when compared with placebo and with ibuprofen use after exercise (i.e., ibuprofen before exercise < placebo or ibuprofen after exercise). A second aim was to determine whether ibuprofen use before or after exercise influences changes in fat-free mass (FFM) in response to exercise training.
2. Materials and methods
2.1. Study design and participants
This was a randomized, double-blinded, placebo-controlled study of the timing of ibuprofen use relative to exercise on the BMD response to a 36-week exercise training intervention in healthy older adults. Volunteers provided written informed consent to participate in the study, which was approved by the Colorado Multiple Institutional Review Board. The trial was registered under ClinicalTrials.gov: NCT00462722.
The participants were women and men (N = 189), aged 60 to 75 years, who had not been performing regular moderate-to-vigorous weight-bearing or weight-lifting exercise (defined as ≤ 2 days per week during the 6 months prior to study entry) and whose average frequency of NSAID (including low-dose aspirin) or acetaminophen use was < 3 days per month. All women were postmenopausal. The exclusion criteria were: relative or absolute contraindications to regular use of NSAIDs, including history of peptic ulcer or upper gastrointestinal bleeding, anemia, renal impairment (estimated glomerular filtration rate (eGFR) < 45 mL/min/1.73 m2), or chronic hepatobiliary disease; osteoporosis (lumbar spine, total hip, femoral neck, or trochanter T-score ≤− 2.5); diabetes mellitus requiring pharmacologic therapy; congestive heart failure; uncontrolled hypertension (resting systolic pressure > 150 mm Hg or diastolic pressure > 90 mm Hg); indicators of ischemic heart disease or serious arrhythmias at rest or during a graded treadmill exercise test (GXT); thyroid dysfunction (ultrasensitive thyroid stimulating hormone < 0.5 or > 5.0 mU/L); orthopedic problems that limited the ability to perform vigorous exercise; and use of narcotics or medications known to alter bone metabolism in the previous 6 months. Volunteers who met eligibility criteria and were willing to participate in the study were randomly assigned to 1 of 3 drug treatment arms before starting the exercise training: 1) placebo before exercise, placebo after exercise (PP), 2) ibuprofen before exercise, placebo after exercise (IP), and 3) placebo before exercise, ibuprofen after exercise (PI). The randomization was stratified by sex to ensure similar distribution of women and men across treatment arms.
2.2. Drug intervention
Participants were instructed to take one study capsule 1 to 2 h before exercise and one study capsule immediately after on each day of supervised exercise. The capsules were prepared by a local pharmacy (Belmar Pharmacy, Lakewood, CO, USA) and contained either ibuprofen 400 mg or inactive ingredients; ibuprofen content was confirmed by an independent laboratory.
All capsules were identical in appearance, with the exception that capsules taken before exercise sessions were green and those taken after were red. Participants recorded the time that the pre-exercise capsule was taken when they arrived at the laboratory for an exercise session. The post-exercise capsule was taken immediately upon completion of the exercise session and the time was recorded. A research pharmacist managed the randomization process, maintained all drug intervention records, and prepared and dispensed study drug packets.
2.3. Exercise intervention
All participants engaged in a 36-week supervised progressive exercise program. They were asked to complete a minimum of 3 sessions per week. The goal of the training was to increase BMD using high-intensity resistance exercise and other weight-bearing exercises that generated relatively high bone-loading forces (e.g., floor jumps, stair climbing/descending). Each exercise session included 3 sets each of 7 resistance exercises, 2 sets of floor jumping activities, and 1 set of stair climbing/descending. The resistance exercises were initially performed at a moderate intensity (60% to 70% of 1-repetition maximum (1RM); 3 sets of 8 to 12 repetitions). Resistance was progressively increased to the goal of 80% of 1RM (2 sets of 5 to 8 repetitions after 1 warm-up set at a lower intensity). Two resistance exercise plans were performed on alternating days (plan A: lateral pull down, bench press, hip abduction and adduction, biceps curls, seated row, and assisted chin-ups; plan B: overhead press, leg press, tricep extension, knee extension and flexion, heel raise, and shoulder external rotation). The floor jumping sets included jumping jacks and multidirectional 4-square jumps with two-footed landings. Participants completed patterns of forward, backward, lateral, and diagonal movements on a 4-square diagram on the floor. Stair climbing/descending took place in a stairwell with a handrail. The number of jumps and stair flights was increased approximately every two weeks, beginning conservatively with 2 sets of 6 jumps and 4 stair flights (12 steps/flight) and progressing to 2 sets of 30 jumps and 15 stair flights. Each exercise session began and ended with a 5-minute treadmill walk. The total exercise time was approximately 60 min per session, excluding the warm-up and cool-down.
2.4. Dietary supplements
Combination calcium citrate (630 mg/day) and vitamin D (400 IU/day) supplements were provided to all participants. They were instructed to take one tablet (315 mg/200 units) twice daily throughout the 36-week intervention. If a participant was already taking a calcium supplement, the home supplement was discontinued.
2.5. BMD and body composition
At baseline and after 36 weeks of training, BMD of the proximal femur (total and neck, trochanter, and subtrochanteric regions) and lumbar spine (L1–4), fat-free mass (FFM), and fat mass were measured by dual-energy X-ray absorptiometry (DXA) using a Hologic Discovery W instrument (version 12.6; Hologic, Inc., Bedford, MA). In our laboratory, the coefficients of variation (CVs) for lumbar spine, total hip, femoral neck, trochanter, and subtrochanter BMD are (mean (SD)) 1.2% (0.8%), 0.8% (0.6%), 1.9% (0.9%), 1.5% (1.0%), and 1.1% (0.6%). CVs for FFM and fat mass are 1.2% (0.8%) and 1.8% (0.9%), respectively. Hip and spine images that included metal implants were excluded from the BMD analyses. DXA scan reports were reviewed by two investigators for quality assurance.
2.6. Safety monitoring
A monthly health status survey was administered that included questions about recent illnesses, visits to a clinician, hospitalizations, changes in over-the-counter and prescription drugs, and perception of overall health. Blood samples were drawn every 3 months to monitor study-specific adverse events, defined as: 1) decrease in hemoglobin (Hb) to below the sex-specific lower limit of normal or a decrease of > 3 g/dL from baseline; 2) impaired liver function, defined as an increase in AST or ALT to more than 1.5 times the upper limit of normal; 3) decrease in renal function, defined as an increase in serum potassium to > 5.5 mmol/L, increase in resting blood pressure to > 180 mm Hg systolic or > 100 mm Hg diastolic, or decrease in eGFR to < 40 mL/min/1.73 m2 or a decrease of 25% or more from baseline.
At every exercise session, participants recorded any use of pain relieving medications since the last exercise session in their exercise log. For the relief of discomfort or pain, subjects were instructed to use ice, heat, or over-the-counter topical creams containing only menthol, capsaicin, or salicylates with very low systemic absorption (e.g., trolamine salicylate) as first line therapy. If ineffective, second line therapy was methyl salicylate creams or acetaminophen, and third line therapy was oral NSAIDs.
2.7. Statistical analyses
We hypothesized that the timing of ibuprofen use relative to exercise would be a significant determinant of the changes in BMD (IP < PP or PI). The primary outcomes were the 36-week changes in total hip and lumbar spine BMD. Sample size requirements for the primary outcomes were estimated from a pilot study in which young women underwent 36 weeks of exercise training and were treated with IBUP 400 mg before or after exercise sessions (IP vs PI; n = 10 per group). It was estimated that 40 evaluable subjects per group would provide 90% power to detect a difference between any two treatment groups of 1.14% for lumbar spine BMD and 1.44% for total hip BMD, based on a 2-sided test at an alpha level of 0.05 or less. To allow for attrition, the plan was to randomize 150 participants (50 per group). Although the pilot study on which statistical power was based included only women, we expected that the inclusion of men would magnify the effect of ibuprofen before exercise to inhibit an increase in BMD. This was based on the observation that use of COX-2 selective inhibitors had a more harmful effect on BMD in men than in postmenopausal women (Richards et al., 2006).
Participant characteristics at baseline were compared across groups by one-way ANOVA for continuous measures or chi-squared tests of equal proportions for categorical variables. The effects of timing of ibuprofen relative to exercise were evaluated by analysis of covariance (ANCOVA) regressing the percent change from baseline to 36 weeks in the outcome measure on the baseline value, sex (because it was a stratification variable), and an indicator for treatment group. The primary intention-to-treat (ITT) analyses compared the differences in the changes in outcome measures between 1) IP and PP; and 2) IP and PI. The analyses were repeated for subgroups of participants who were compliant to exercise (attended at least 80% of exercise sessions) and drug (reported taking at least 80% of prescribed doses). Comparisons were considered significant at p = 0.05 or less. Post-hoc analyses of the percent changes from baseline to 36 weeks in total hip, hip regions, and lumbar spine within each treatment group were performed using one sample t-tests. Because these were not primary outcomes of the trial, changes are presented with raw p values adjusted for multiple comparisons using Benjamini and Hochberg's false discovery rate, (FDR) (Benjamini and Hocberg, 1995). Data are presented as mean (SD), unless otherwise specified.
2.8. Drug dispensation error
In the third year of the trial, a drug dispensation error occurred that resulted in ibuprofen being dispensed in place of placebo for the before-exercise dosing for some study participants. It was subsequently determined by weighing capsules that the product used for placebo capsules was less dense than ibuprofen, resulting in placebo capsules weighing less than ibuprofen capsules in a consistent manner. All participants had returned unused capsules from study drug dispensations, so these capsules were weighed to determine who had received correct or incorrect study drugs and at what points in the intervention. A total of 39 participants were affected by the dispensing error and could be categorized into 3 groups: 1) 25 received inconsistent dispensations during the intervention (excluded from the primary analyses); 2) 3 received consistent dispensations that were different from their randomized assignment but were 1 of the 3 treatment arms (included in the primary analyses based upon their reassigned arm); and 3) 11 received the incorrect treatment during the first dispensation period (e.g., weeks 1–12) and were invited to extend the intervention by 12 weeks so that they would undergo 36 weeks of training with the correct treatment (6 did so and were included in the primary analyses). Double-blinding was maintained during the time of the drug dispensation error.
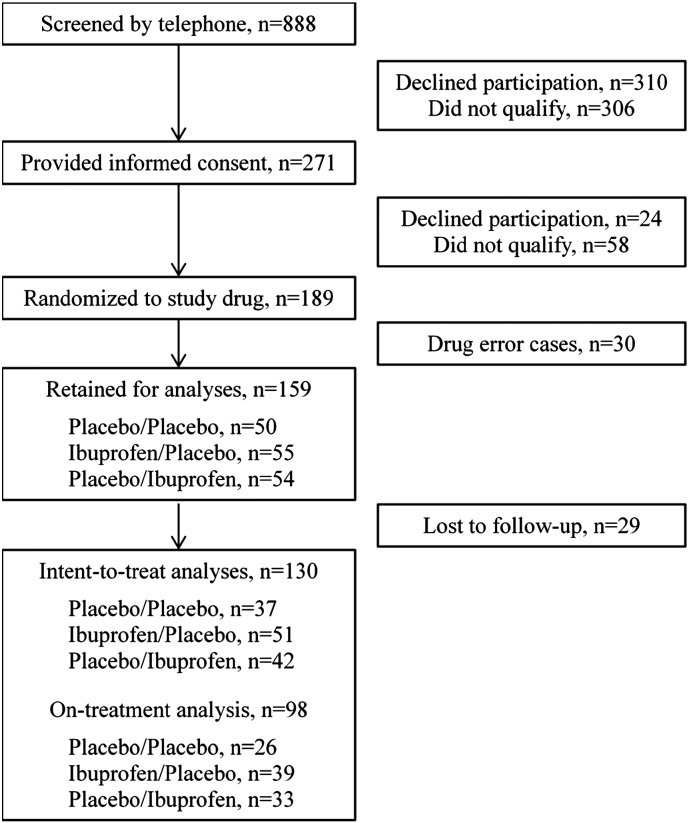
Because of the drug dispensation error, 39 new participants (total of 189) were randomized to treatment (Fig. 1). The drug dispensation error resulted in 30 cases being excluded from further analyses. Of the 159 remaining participants, 29 were lost to follow-up. When compared to those lost to follow-up (7 men, 22 women), the finishers (48 men, 82 women) had lower fat mass (28.0 (8.0) vs 31.6 (8.7) kg; p = 0.030) and were taller (170 (10) vs 160 (10) cm; p = 0.033). The baseline characteristics of participants included in the ITT analyses were well matched across groups (Table 2), although there was a difference in the proportion of Caucasians. Study attrition differed among the groups (p = 0.020), with the IP group having a higher proportion of finishers than the other groups (IP, 93%; PP, 74%; PI 78%).
Fig. 1.
Recruitment, enrollment, and retention of study volunteers.
Table 2.
Average exercise training volume after familiarization to the equipment (weeks 5 to 8) and before final testing (weeks 29 to 32).
| Weeks 5 to 8 |
Weeks 29 to 32 |
Increase, % |
|||
|---|---|---|---|---|---|
| Repetitions per set | Weight per session, lb | Repetitions per set | Weight per session, lb | Weight per session, lb | |
| Resistance exercises | |||||
| Leg extension | 8 (2) | 1182 (598) | 8 (2) | 1498 (673)a | 32 (30) |
| Leg flexion | 9 (2) | 1520 (734) | 8 (2) | 1873 (751)a | 29 (29) |
| Leg press | 9 (2) | 4183 (1851) | 9 (2) | 5264 (2112)a | 26 (23)b |
| Hip abduction | 9 (2) | 1874 (773) | 8 (1) | 2263 (729)a | 23 (22) |
| Hip adduction | 9 (2) | 2061 (913) | 8 (2) | 2679 (1066)a | 39 (89) |
| Lateral pulldown | 8 (2) | 2272 (961) | 8 (2) | 2820 (1103)a | 24 (24) |
| Bench press | 8 (1) | 1236 (803) | 8 (2) | 1589 (938)a | 32 (34) |
| Overhead press | 8 (1) | 987 (530) | 7 (2) | 1215 (550)a | 26 (25) |
| Biceps curlc | 8 (2) | 632 (265) | 8 (2) | 789 (333)a | 26 (33) |
| Tricep extensionc | 9 (2) | 386 (205) | 8 (2) | 607 (254)a | 67 (48) |
| Seated row | 8 (2) | 1481 (668) | 8 (1) | 1837 (735)a | 25 (23) |
| Jumps/session | 36 (17) | 65 (30) | 101 (77) | ||
| Stair flights/session | 7 (3) | 15 (6) | 141 (80) | ||
Values are mean (SD).
Increase from weeks 5 to 8, p < 0.001.
One outlier was removed from the analysis; if included, the increase was 47 (227)%.
Performed unilaterally.
3. Results
Participants who finished the intervention (48 men, 82 women) had lower fat mass (28.0 (8.0) vs 31.6 (8.7) kg; p = 0.030) and were taller (170 (10) vs 160 (10) cm; p = 0.033) when compared to those lost to follow-up (7 men, 22 women). The baseline characteristics of participants included in the ITT analyses were well matched across groups (Table 1). Study attrition differed among the groups (p = 0.020), with the IP group having a higher proportion of finishers than the other groups (IP, 93%; PP, 74%; PI 78%).
Table 1.
Baseline characteristics of participants.
| Placebo/placebo (n = 37)a | Ibuprofen/placebo (n = 51)a | Placebo/ibuprofen (n = 42) | p value | |
|---|---|---|---|---|
| Females | 23 (62) | 32 (63) | 27 (64) | 0.979 |
| Caucasian | 33 (89) | 47 (92) | 35 (83) | 0.418 |
| Hispanic | 2 (5) | 2 (4) | 1 (2) | 0.779 |
| Age, years | 64 (4) | 64 (4) | 66 (4) | 0.079 |
| BMI, kg/m2 | 28 (5) | 27 (5) | 27 (4) | 0.586 |
| Height, m | 1.7 (0.1) | 1.7 (0.1) | 1.7 (0.1) | 0.726 |
| Weight, kg | 79.1 (16.3) | 79.0 (17.0) | 77.7 (14.8) | 0.901 |
| Fat mass, kg | 28.4 (8.4) | 28.2 (8.6) | 27.3 (6.9) | 0.822 |
| Fat-free mass, kg | 50.8 (12.5) | 50.8 (12.0) | 50.3 (11.7) | 0.980 |
| BMD, g/cm2 | ||||
| Lumbar spine | 1.021 (0.139) | 1.035 (0.248) | 1.023 (0.176) | 0.938 |
| Total hip | 0.934 (0.125) | 0.904 (0.132) | 0.942 (0.157) | 0.377 |
| Femoral neck | 0.752 (0.107) | 0.728 (0.094) | 0.784 (0.142) | 0.075 |
| Trochanter | 0.709 (0.103) | 0.691 (0.110) | 0.718 (0.148) | 0.556 |
| Subtrochanter | 1.107 (0.151) | 1.078 (0.161) | 1.118 (0.172) | 0.468 |
Values are number of subjects (%) or mean (SD).
3 participants had uninterpretable hip scans (1 placebo/placebo, 2 ibuprofen/placebo) and 1 had an uninterpretable spine scan (ibuprofen/placebo).
3.1. Exercise intervention
There were no significant differences (all p > 0.300) among the groups in the amount of exercise performed during the intervention (i.e., weight lifted, jumps and stair flights completed; data not shown). In training weeks 5 to 8 (after familiarization with the equipment), the number of sessions completed per week was (mean (SD)) 3.3 (0.8), 3.5 (0.7), and 3.5 (0.7) in the IP, PP, and PI groups, respectively (p = 0.255). In training weeks 29 to 32 (before final testing), the number of sessions completed per week was 2.9 (0.8), 2.8 (0.8), and 2.7 (0.8) in the IP, PP, and PI groups, respectively (p = 0.523). The amount of weight lifted and number of jumps and stair flights completed increased from weeks 5–8 to weeks 29–32 (Table 2; all p < 0.001).
Exercise sessions lasted approximately 1 h. The time between the before- and after-exercise doses of study drug averaged 2.9 (0.7) h, indicating that participants were taking the before-exercise dose approximately 2 h before exercise, as instructed.
3.2. Intention-to-treat analysis
3.2.1. Changes in BMD (Fig. 2, Fig. 3, Table 3)
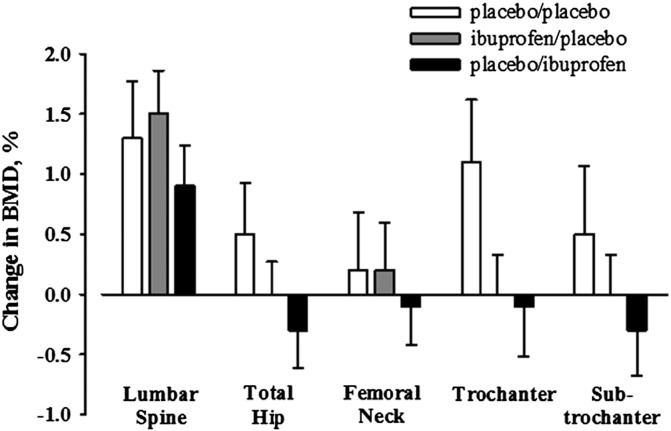
Fig. 2.
Relative changes in BMD, adjusted for baseline BMD, in response to 36 weeks of exercise training in older adults who took ibuprofen 400 mg before (ibuprofen/placebo) or after (placebo/ibuprofen) exercise sessions or placebo at both time points (placebo/placebo). The error bars represent SD.
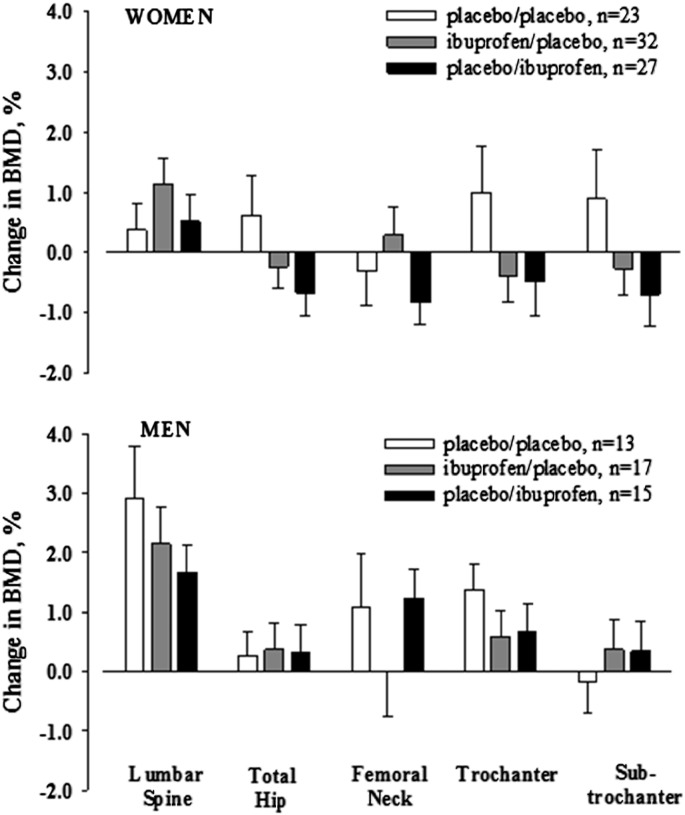
Fig. 3.
Relative changes in BMD, adjusted for baseline BMD, in response to 36 weeks of exercise training in women (top panel) and men (bottom panel) who took ibuprofen 400 mg before (ibuprofen/placebo) or after (placebo/ibuprofen) exercise sessions or placebo at both time points (placebo/placebo). The error bars represent SD.
Table 3.
Relative changes (%) in bone mineral density within groups and the between-group differences in the responses adjusted for baseline values and sex.
| PPa |
IPa |
PI |
IP–PP |
IP–PI |
|
|---|---|---|---|---|---|
| Mean (SD) | Mean (95% CI) | ||||
| Lumbar spine | 1.3 (2.8) | 1.5 (2.5) | 0.9 (2.2) | 0.1 (− 0.9, 1.2) | 0.5 (− 0.5, 1.5) |
| Total hip | 0.5 (2.6) | 0.0 (1.9) | − 0.3 (2.0) | − 0.5 (− 1.4, 0.4) | 0.3 (− 0.6, 1.2) |
| Femoral neck | 0.2 (2.9) | 0.2 (2.8) | − 0.1 (2.1) | 0.0 (− 1.2, 1.1) | 0.2 (− 0.9, 1.3) |
| Trochanter | 1.1 (3.1) | 0.0 (2.3) | − 0.1 (2.7) | − 1.1 (− 2.3, 0.0) | 0.1 (− 1.0, 1.2) |
| Sub-trochanter | 0.5 (3.4) | 0.0 (2.3) | − 0.3 (2.5) | − 0.6 (− 1.8, 0.6) | 0.2 (− 0.9, 1.4) |
PP, placebo before and placebo after exercise; IP, ibuprofen before and placebo after exercise; PI, placebo before and ibuprofen after exercise.
3 participants had uninterpretable hip scans (1 placebo/placebo, 2 ibuprofen/placebo) and 2 participants had uninterpretable spine scans (2 ibuprofen/placebo).
We hypothesized that changes in lumbar spine and hip BMD would be reduced in the IP group when compared with both the PP and PI groups, but this was not supported (Fig. 2, Table 3). The PP group had the most favorable adaptations in hip BMD, but differences between the groups did not reach statistical significance. None of the within-group changes was statistically significant after adjusting for multiple comparisons (Benjamini and Hocberg, 1995). In the PP group, trochanter BMD increased 1.2% (3.2%) (unadjusted p = 0.037; adjusted p = 0.262). In the IP group, lumbar spine BMD increased 1.4% (5.1%) (unadjusted p = 0.05; adjusted p = 0.262). In the PI group, lumbar spine BMD increased 2.0% (4.3%) (unadjusted p = 0.008; adjusted p = 0.128).
Although the study was not powered to detect sex differences, we evaluated changes in BMD in women and men separately in secondary analyses (Fig. 3). Within each sex, there were no significant differences among the groups in the change in BMD at any skeletal region. Ibuprofen use either before or after exercise appeared to have a more deleterious effect (albeit nonsignificant) on hip, trochanter, and subtrochanter BMD in women than in men.
3.2.2. Changes in body composition (Table 4)
Table 4.
Changes in body composition (kg) in response to exercise training conditioned on baseline values and sex.
| PP (n = 37) | IP (n = 51) | PI (n = 42) | IP–PP | IP–PI | |
|---|---|---|---|---|---|
| Weight | − 0.5 (1.1) | − 0.9 (4.2) | − 0.3 (3.1) | − 0.4 (− 1.7, 1.0) | − 0.5 (− 1.8, 0.8) |
| Fat-free mass | 0.6 (1.1) | 0.4 (1.6) | 0.6 (1.4) | − 0.1 (− 0.7, 0.5) | − 0.1 (− 0.7, 0.4) |
| Fat mass | − 1.1 (1.5) | − 1.3 (3.3) | − 0.9 (2.6) | − 0.3 (− 1.4, 0.7) | − 0.4 (− 1.4, 0.7) |
Values are mean (SD or 95% CI).
PP, placebo before and placebo after exercise; IP, ibuprofen before and placebo after exercise; PI, placebo before and ibuprofen after exercise.
The average increases in FFM in response to exercise training were 0.4 to 0.6 kg across groups and the average decreases in fat mass were − 0.9 to − 1.1 kg. There were no significant effects of ibuprofen use before or after exercise on the changes in body composition in response to exercise training.
3.3. Adherence analyses
Adherence to the study drugs averaged 89% (18%) for the pre-exercise study doses and 96% (15%) for the post-exercise doses. The subgroup that was adherent to drug included 34 participants in the PP group, 38 in the IP group, and 35 in the PI group (p = 0.092). The subgroup that was adherent to exercise included 36 in the PP group, 46 in the IP group, and 38 in the PI group (p = 0.389). Secondary analyses were conducted in the subgroups that were adherent to drug and/or exercise. The results of these analyses (data not shown) were similar to those of the ITT analyses.
3.4. Adverse events
There were no adverse events based on the criteria for changes in hemoglobin, liver function, serum potassium, blood pressure or self-reported symptoms. One participant had a decrease in eGFR of greater than 25% that triggered the discontinuation of study drug. However, on average, there were decreases in eGFR in all groups that were small in magnitude and similar in placebo- and ibuprofen-treated participants.
4. Discussion
The intent of this study was to determine whether the use of ibuprofen before exercise diminished improvements in BMD in response to exercise training. The mechanistic basis for the study was the evidence in animals that the bone formation response to mechanical loading is markedly impaired when NSAIDs are present before loading, but not when NSAIDs are introduced after loading (Chow and Chambers, 1994, Li et al., 2002). Accordingly, the hypothesis was that increases in BMD would be lessened by the use of ibuprofen before exercise sessions when compared with either no ibuprofen use or with ibuprofen use after exercise sessions (i.e., IP < PP, PI). The most favorable changes in BMD tended to occur in the PP group, but there were no significant differences among the groups in the BMD responses to exercise training. However, because this was the first trial of the effects of NSAID use on skeletal adaptations to exercise in older adults, the results should be interpreted cautiously.
4.1. Preclinical evidence for effects of NSAIDs on bone formation
To our knowledge, Chow and Chambers were the first to observe that the timing of NSAID administration relative to mechanical loading influenced the bone formation response (Chow and Chambers, 1994). In that study, a single dose of indomethacin administered to 3-month-old female rats 3 h before loading reduced the bone formation response by 65% when compared with vehicle treatment, but only 20% when administered 6 h after loading. In a similar experiment (Li et al., 2002), the loading-stimulated bone formation rate in 7-month-old female rats was significantly reduced when a COX-2 selective inhibitor was injected 180 min (− 70%) or 30 min (− 45%) before loading, but not when given 30 min after (− 20%) loading. We are not aware of any studies that have evaluated sex differences in these responses or whether responses are different in young adult versus aged animals.
A limitation of the studies of animals (Chow and Chambers, 1994, Li et al., 2002) was that they evaluated bone responses to only a single loading event. Because prostaglandins can stimulate both bone resorption and formation, the net effects of NSAIDs on bone adaptations to repeated mechanical loading could be beneficial, detrimental, or neutral (Blackwell et al., 2010). We are aware of only one published study of the influence of NSAIDs on adaptations of bone to repeated episodes of mechanical loading (Sugiyama et al., 2013). In 19-week-old female mice, a COX-2 selective inhibitor administered 3 h before loading sessions did not attenuate the gains in trabecular or cortical bone induced by 2 weeks of mechanical loading. The relevance of this finding to the current study is uncertain for the following reasons. First, the study was conducted in young adult female mice and responses may be influenced by age and/or sex. Second, the effects of a COX-2 inhibitor on skeletal adaptations to loading may be different from effects of non-selective inhibitors, such as ibuprofen. Although it is COX-2 that is believed to mediate the bone formation response to loading, mice with a null mutation of the COX-2 gene had a normal response to loading that appeared to be mediated by an up-regulation of COX-1 (Alam et al., 2005). Finally, it is not clear whether external mechanical loading mimics the pro-inflammatory effects of vigorous exercise (Gleeson, 2007). If it does not, there could be differential effects of NSAIDs on skeletal adaptations to exercise (humans) versus external mechanical loading (animals) because of the pro-resorption effects of inflammatory cytokines (Redlich and Smolen, 2012). Alternatively, if the external mechanical loading induced inflammation in mice, the COX-2 inhibitor may have reduced inflammation and attenuated joint damage (Poulet et al., 2011, Ko et al., 2013), leading to greater cage activity and thereby negating the detrimental effects of NSAIDs on bone formation.
4.2. NSAID use and BMD in humans
Observational studies suggest that NSAID use can have beneficial effects on BMD in older adults (Morton et al., 1998, Bauer et al., 1996, Carbone et al., 2003, Vestergaard et al., 2012). In these studies, BMD tended to be similar or higher in women (Morton et al., 1998, Bauer et al., 1996, Carbone et al., 2003, Vestergaard et al., 2012) and men (Carbone et al., 2003) who reported using NSAIDs when compared with those who did not. One study that included both women and men evaluated associations of BMD with use of COX-1 or COX-2 selective inhibitors, with or without aspirin use (Carbone et al., 2003). The results were not presented separately for women and men, but sex was included as a covariate. The major finding was that hip BMD was higher in older adults who used both a COX-2 inhibitor and aspirin than in nonusers (4.6%). Only those who used a COX-2 inhibitor alone had a lower BMD than nonusers (− 1.6%), but the difference was not statistically significant.
The Canadian Multicentre Osteoporosis Study also evaluated the associations of BMD with use of COX-2 inhibitors, and reported results separately for men, women on menopausal hormone therapy (HT), and women not on HT (Richards et al., 2006). Daily use of COX-2 inhibitors was associated with lower proximal femur and spine BMD in men (ranging from − 5% to − 2% across regions), but greater proximal femur BMD in postmenopausal women not on HT (ranging from 1% to 6% across regions). Use of COX-2 inhibitors was associated with greater spine, but not hip, BMD in postmenopausal women on HT. The authors suggested that the net effect of COX-2 inhibitors on BMD is likely determined by the dominant actions of NSAIDs to 1) reduce inflammation-mediated bone loss (i.e., anti-resorption effect), and/or 2) block mechanical load-related bone gain (i.e., anti-formation effect). They speculated that the lower BMD levels in men using COX-2 inhibitors reflected an inhibition of the skeletal benefits of mechanical loading, whereas the higher BMD in women not on HT reflected an attenuation of inflammation-mediated resorption. This interpretation was consistent with the finding in our proof-of-concept study in premenopausal women (Kohrt et al., 2010) that use of NSAIDs after exercise seemed to amplify the increase in BMD. We speculated that use of NSAIDS after exercise would 1) allow a normal bone formation response, because introducing NSAIDs before, but not after, loading impairs bone formation in animals (Chow and Chambers, 1994, Li et al., 2002) and 2) attenuate inflammation-induced bone resorption, thereby 3) influencing the balance between formation and resorption in a manner that favors formation.
Based on the findings from the Canadian Multicentre Osteoporosis Study (Carbone et al., 2003) and our proof-of-concept study of NSAID use and exercise in young women (Kohrt et al., 2010), we had expected that the benefit of NSAID use after exercise to increase BMD would be even greater in older adults than in young women because of the greater potential to reduce inflammation-related bone resorption in the former. We had also expected that the potential effect of NSAID use before exercise to attenuate increases in BMD by inhibiting bone formation would be more pronounced in men than in women because of greater anabolic potential in men. Neither of these expectations was realized. Although differences did not reach statistical significance, there was no signal for a benefit of NSAID use after exercise to enhance increases in BMD, as we had observed previously in young women (Kohrt et al., 2010). Further research will be needed to determine whether NSAID use either before or after exercise impairs the BMD adaptations in some regions of the proximal femur (Fig. 2), particularly in women (Fig. 3). To our knowledge, whether age and/or sex influence the effects of NSAIDs on bone adaptations to loading has not been investigated in animal models.
Importantly, study attrition was significantly lower in the group that took NSAIDs before exercise (7%) than in the other two groups (22%, 26%). This suggests that taking NSAIDs before exercise improves the ability to tolerate the type of vigorous exercise that can increase bone mass and strength. In animal models, repetitive intermittent compressive bone loading over several weeks induced articular cartilage damage characteristic of osteoarthritis (Poulet et al., 2011, Ko et al., 2013, Warner et al., 2002). It is possible that NSAID use before exercise alleviates joint pain in older adults and contributes to better exercise tolerance. Because exercise is recommended for the prevention of osteoporosis, it is important to understand whether the benefits are blocked by NSAID use. Further research will be necessary to determine if the effects of NSAIDs to impair or augment skeletal adaptations to exercise are influenced by such factors as age, sex, and the type and dose of NSAID.
4.3. NSAID use and fat-free mass
There is a paucity of research on the effects of NSAIDs on skeletal muscle metabolism. At the time the current trial was initiated, it had been demonstrated that ibuprofen reduced the increase in fractional muscle protein synthesis following high-intensity resistance exercise by more than 50% (Trappe et al., 2002). Expression of prostaglandin E2 and F2α in muscle tissue was also blunted in ibuprofen-treated men when compared with placebo-treated controls (Trappe et al., 2001). This led us to hypothesize that the increase in fat-free mass would be reduced in the group that took NSAIDs before each exercise session when compared with the placebo group. However, this did not occur, as the increases in fat-free mass were not significantly different among the 3 treatment groups. The same investigators who found that ibuprofen impaired acute muscle responses to exercise in young men (Trappe et al., 2001, Trappe et al., 2002) subsequently reported that ibuprofen augmented the gains in muscle volume and strength after 12 weeks of resistance exercise training in older women and men (Trappe et al., 2011). Because prostaglandins are involved in the regulation of both anabolic and catabolic processes in skeletal muscle (Rodemann and Goldberg, 1982), the investigators speculated that protein breakdown was attenuated by ibuprofen to a greater extent than protein synthesis, thereby augmenting protein accretion.
The failure of NSAIDs in the current study to augment gains in muscle mass and strength as observed by others (Trappe et al., 2011) was not related to the age or sex of the participants; both studies included older women and men. However, a notable difference between the studies was the dosing of NSAIDs. In the previous study ibuprofen was taken daily at a dose of 1200 mg, whereas in the current study it was taken only on exercise days at a dose of 400 mg. If ibuprofen augments the exercise-induced gain in muscle mass by suppressing chronic inflammation-related muscle catabolism, it is possible that the low dosing used in the current study was not sufficient to suppress muscle protein breakdown.
4.4. Safety
Potential adverse events related to NSAID use were monitored through health status questionnaires administered every 4 weeks and blood tests obtained every 12 weeks. The only adverse event that triggered the criterion for stopping drug in an individual was a decrease in eGFR, which occurred in the IP group. However, on average eGFR declined similarly during the intervention in all three treatment groups, so it was not clear whether the event was related to ibuprofen use.
4.5. Limitations
The study did not include a no-exercise control group. However, in the absence of intervention, the trajectory of change in hip BMD in older adults is downward; small increases in spine BMD can occur as the result of compression fractures or extravertebral ossification. In our previous study of placebo versus dehydroepiandrosterone therapy in women and men 60 to 80 years of age (Jankowski et al., 2006), the average changes in lumbar spine and hip BMD in the placebo group were + 0.4% and − 0.4%, respectively, versus + 1.3% and + 0.5%, respectively, in the current study. This supports the idea that the observed changes were attributable to the exercise.
The variability of the BMD responses to NSAIDs and exercise was greater in older adults than in our previous study of young women; data from that study were used to calculate statistical power. Using the SD of the percent change in hip BMD from the current study (2.15%) a between-group difference of 1% would have been significant at the p = 0.05 level with 40 subjects per group; the largest observed difference was 0.5%. Similarly, using the SD of the change in FFM for the current study (1.39 kg) a between-group difference of 0.9 kg would have been significant at the 0.05 level; the largest observed difference was 0.1 kg. It was assumed that men would respond similarly based on the observation that BMD is low in men who use COX-2 inhibitors and that the mechanism of action is likely to be an impaired response to mechanical stimulation (Carbone et al., 2003). Although secondary analyses of sex differences did not yield significant differences, the results suggested that sex differences should be more rigorously evaluated (Fig. 3). The variability in responses between women and men was greater than expected and this may have limited the ability to detect significant effects of NSAIDs.
The results of the study may be specific to ibuprofen and not to other NSAIDs. Factors that could be important to consider for future research include the relative COX selectivity and the half-life of the NSAID. Ibuprofen was selected for this trial because it 1) is widely used, 2) inhibits both COX-1 and COX-2 and at the dose used should inhibit the activity of both isozymes by 80% (Capone et al., 2007); this may be important if there is a compensatory increase in COX-1 when COX-2 is inhibited (Alam et al., 2005), and 3) has a short half-life (approximately 2 h) (Davies, 1998), which reduced the likelihood that dosing after an exercise session would have residual effects on exercise performed the following day.
4.6. Summary
In older adults, taking NSAIDs either 2 h before or immediately after exercise sessions did not significantly attenuate or augment the effects of a 36-week exercise training program on BMD or fat-free mass. Because this was the first randomized controlled trial to investigate whether NSAIDs influence musculoskeletal adaptations to exercise, the negative results should be interpreted cautiously. The patterns of change in BMD did not convincingly demonstrate that NSAIDs do not diminish the skeletal benefits of exercise in older adults. It has been estimated that more than 29 million adults in the U.S. were regular users of NSAIDs in 2010, which was 41% higher than in 2005 (Zhou et al., 2013) and, although data are sparse, the prevalence of use is likely to be very high in people who exercise regularly (Warner et al., 2002, Gorski et al., 2011, Holmes et al., 2013). The widespread use of NSAIDs, coupled with the essential role of exercise in maintaining bone health, underscore the need for further research to understand whether NSAIDs diminish the beneficial effects of exercise on bone.
Acknowledgments
This research was supported by National Institutes of Health Awards R01 AG018857, UL1 TR001082 (Colorado Clinical and Translational Sciences Institute), and P30 DK048520 (Colorado Nutrition and Obesity Research Center).
Author roles:
Study design: CJ, PW, JK, RS, WK; study conduct: CJ, KS, DB, SL, JK, WK; data analysis: PW, JK; data interpretation: CJ, PW, WK; drafting manuscript: CJ, KS, PW, SL, WK.
Disclosures
The authors have no conflicts of interest to report.
Edited by Peter Ebeling
References
- Alam I., Warden S.J., Robling A.G., Turner C.H. Mechanotransduction in bone does not require a functional cyclooxygenase-2 (COX-2) gene. J. Bone Miner. Res. 2005;20:438–446. doi: 10.1359/JBMR.041124. [DOI] [PubMed] [Google Scholar]
- Bauer D.C., Orwoll E.S., Fox K.M., Vogt T.M., Lane N.E., Hochberg M.C., Stone K., Nevitt M.C. Aspirin and NSAID use in older women: effect on bone mineral density and fracture risk. Study of Osteoporotic Fractures Research Group. J. Bone Miner. Res. 1996;11:29–35. doi: 10.1002/jbmr.5650110106. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hocberg Y. Controlling the false discovery rate: a new and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B. 1995:1289–1300. [Google Scholar]
- Blackwell K.A., Raisz L.G., Pilbeam C.C. Prostaglandins in bone: bad cop, good cop? Trends Endocrinol. Metab. 2010;21:294–301. doi: 10.1016/j.tem.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capone M.L., Tacconelli S., Di Francesco L., Sacchetti A., Sciulli M.G., Patrignani P. Pharmacodynamic of cyclooxygenase inhibitors in humans. Prostaglandins Other Lipid Mediat. 2007;82:85–94. doi: 10.1016/j.prostaglandins.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Carbone L.D., Tylavsky F.A., Cauley J.A., Harris T.B., Lang T.F., Bauer D.C., Barrow K.D., Kritchevsky S.B. Association between bone mineral density and the use of nonsteroidal anti-inflammatory drugs and aspirin: impact of cyclooxygenase selectivity. J. Bone Miner. Res. 2003;18:1795–1802. doi: 10.1359/jbmr.2003.18.10.1795. [DOI] [PubMed] [Google Scholar]
- Cheng M.Z., Zaman G., Rawlinson S.C., Pitsillides A.A., Suswillo R.F., Lanyon L.E. Enhancement by sex hormones of the osteoregulatory effects of mechanical loading and prostaglandins in explants of rat ulnae. J. Bone Miner. Res. 1997;12:1424–1430. doi: 10.1359/jbmr.1997.12.9.1424. [DOI] [PubMed] [Google Scholar]
- Chow J.W. Role of nitric oxide and prostaglandins in the bone formation response to mechanical loading. Exerc. Sport Sci. Rev. 2000;28:185–188. [PubMed] [Google Scholar]
- Chow J.W., Chambers T.J. Indomethacin has distinct early and late actions on bone formation induced by mechanical stimulation. Am. J. Physiol. 1994;267:E287–E292. doi: 10.1152/ajpendo.1994.267.2.E287. [DOI] [PubMed] [Google Scholar]
- Chow J.W., Fox S.W., Lean J.M., Chambers T.J. Role of nitric oxide and prostaglandins in mechanically induced bone formation. J. Bone Miner. Res. 1998;13:1039–1044. doi: 10.1359/jbmr.1998.13.6.1039. [DOI] [PubMed] [Google Scholar]
- Davies N.M. Clinical pharmacokinetics of ibuprofen. The first 30 years. Clin. Pharmacokinet. 1998;34:101–154. doi: 10.2165/00003088-199834020-00002. [DOI] [PubMed] [Google Scholar]
- Forwood M.R. Inducible cyclo-oxygenase (COX-2) mediates the induction of bone formation by mechanical loading in vivo. J. Bone Miner. Res. 1996;11:1688–1693. doi: 10.1002/jbmr.5650111112. [DOI] [PubMed] [Google Scholar]
- Gleeson M. Immune function in sport and exercise. J. Appl. Physiol. 2007;103:693–699. doi: 10.1152/japplphysiol.00008.2007. ((1985)) [DOI] [PubMed] [Google Scholar]
- Gorski T., Cadore E.L., Pinto S.S., da Silva E.M., Correa C.S., Beltrami F.G., Kruel L.F. Use of NSAIDs in triathletes: prevalence, level of awareness and reasons for use. Br. J. Sports Med. 2011;45:85–90. doi: 10.1136/bjsm.2009.062166. [DOI] [PubMed] [Google Scholar]
- Holmes N., Cronholm P.F., Duffy A.J., III, Webner D. Nonsteroidal anti-inflammatory drug use in collegiate football players. Clin. J. Sport Med. 2013;23:283–286. doi: 10.1097/JSM.0b013e318286d0fa. [DOI] [PubMed] [Google Scholar]
- Jankowski C.M., Gozansky W.S., Schwartz R.S., Dahl D.J., Kittelson J.M., Scott S.M., Van Pelt R.E., Kohrt W.M. Effects of dehydroepiandrosterone replacement therapy on bone mineral density in older adults: a randomized, controlled trial. J. Clin. Endocrinol. Metab. 2006;91:2986–2993. doi: 10.1210/jc.2005-2484. [DOI] [PubMed] [Google Scholar]
- Ko F.C., Dragomir C., Plumb D.A., Goldring S.R., Wright T.M., Goldring M.B., van der Meulen M.C. In vivo cyclic compression causes cartilage degeneration and subchondral bone changes in mouse tibiae. Arthritis Rheum. 2013;65:1569–1578. doi: 10.1002/art.37906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohrt W.M., Barry D.W., Van Pelt R.E., Jankowski C.M., Wolfe P., Schwartz R.S. Timing of ibuprofen use and bone mineral density adaptations to exercise training. J. Bone Miner. Res. 2010;25:1415–1422. doi: 10.1002/jbmr.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunnel J.G., Igarashi K., Gilbert J.L., Stern P.H. Bone anabolic responses to mechanical load in vitro involve COX-2 and constitutive NOS. Connect. Tissue Res. 2004;45:40–49. doi: 10.1080/03008200490278133. [DOI] [PubMed] [Google Scholar]
- Li J., Burr D.B., Turner C.H. Suppression of prostaglandin synthesis with NS-398 has different effects on endocortical and periosteal bone formation induced by mechanical loading. Calcif. Tissue Int. 2002;70:320–329. doi: 10.1007/s00223-001-1025-y. [DOI] [PubMed] [Google Scholar]
- Morton D.J., Barrett-Connor E., Schneider D.L. Nonsteroidal anti-inflammatory drugs and bone mineral density in older women: the Rancho Bernardo study. J. Bone Miner. Res. 1998;13:1924–1931. doi: 10.1359/jbmr.1998.13.12.1924. [DOI] [PubMed] [Google Scholar]
- Poulet B., Hamilton R.W., Shefelbine S., Pitsillides A.A. Characterizing a novel and adjustable noninvasive murine joint loading model. Arthritis Rheum. 2011;63:137–147. doi: 10.1002/art.27765. [DOI] [PubMed] [Google Scholar]
- Redlich K., Smolen J.S. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat. Rev. Drug Discov. 2012;11:234–250. doi: 10.1038/nrd3669. [DOI] [PubMed] [Google Scholar]
- Richards J.B., Joseph L., Schwartzman K., Kreiger N., Tenenhouse A., Goltzman D. The effect of cyclooxygenase-2 inhibitors on bone mineral density: results from the Canadian Multicentre Osteoporosis Study. Osteoporos. Int. 2006;17:1410–1419. doi: 10.1007/s00198-006-0142-x. [DOI] [PubMed] [Google Scholar]
- Rodemann H.P., Goldberg A.L. Arachidonic acid, prostaglandin E2 and F2 alpha influence rates of protein turnover in skeletal and cardiac muscle. J. Biol. Chem. 1982;257:1632–1638. [PubMed] [Google Scholar]
- Sugiyama T., Meakin L.B., Galea G.L., Lanyon L.E., Price J.S. The cyclooxygenase-2 selective inhibitor NS-398 does not influence trabecular or cortical bone gain resulting from repeated mechanical loading in female mice. Osteoporos. Int. 2013;24:383–388. doi: 10.1007/s00198-012-1922-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsen K., Kristoffersson A.O., Lerner U.H., Lorentzon R.P. In situ microdialysis in bone tissue. Stimulation of prostaglandin E2 release by weight-bearing mechanical loading. J. Clin. Investig. 1996;98:2446–2449. doi: 10.1172/JCI119061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trappe T.A., Fluckey J.D., White F., Lambert C.P., Evans W.J. Skeletal muscle PGF(2)(alpha) and PGE(2) in response to eccentric resistance exercise: influence of ibuprofen acetaminophen. J. Clin. Endocrinol. Metab. 2001;86:5067–5070. doi: 10.1210/jcem.86.10.7928. [DOI] [PubMed] [Google Scholar]
- Trappe T.A., White F., Lambert C.P., Cesar D., Hellerstein M., Evans W.J. Effect of ibuprofen and acetaminophen on postexercise muscle protein synthesis. Am. J. Physiol. 2002;282:E551–E556. doi: 10.1152/ajpendo.00352.2001. [DOI] [PubMed] [Google Scholar]
- Trappe T.A., Carroll C.C., Dickinson J.M., LeMoine J.K., Haus J.M., Sullivan B.E., Lee J.D., Jemiolo B., Weinheimer E.M., Hollon C.J. Influence of acetaminophen and ibuprofen on skeletal muscle adaptations to resistance exercise in older adults. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;300:R655–R662. doi: 10.1152/ajpregu.00611.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard P., Hermann P., Jensen J.E., Eiken P., Mosekilde L. Effects of paracetamol, non-steroidal anti-inflammatory drugs, acetylsalicylic acid, and opioids on bone mineral density and risk of fracture: results of the Danish Osteoporosis Prevention Study (DOPS) Osteoporos. Int. 2012;23:1255–1265. doi: 10.1007/s00198-011-1692-0. [DOI] [PubMed] [Google Scholar]
- Warner D.C., Schnepf G., Barrett M.S., Dian D., Swigonski N.L. Prevalence, attitudes, and behaviors related to the use of nonsteroidal anti-inflammatory drugs (NSAIDs) in student athletes. J. Adolesc. Health. 2002;30:150–153. doi: 10.1016/s1054-139x(01)00325-1. [DOI] [PubMed] [Google Scholar]
- Zaman G., Suswillo R.F., Cheng M.Z., Tavares I.A., Lanyon L.E. Early responses to dynamic strain change and prostaglandins in bone-derived cells in culture. J. Bone Miner. Res. 1997;12:769–777. doi: 10.1359/jbmr.1997.12.5.769. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Boudreau D.M., Freedman A.N. Trends in the use of aspirin and nonsteroidal anti-inflammatory drugs in the general U.S. population. Pharmacoepidemiol. Drug Saf. 2013;23:43–50. doi: 10.1002/pds.3463. [DOI] [PubMed] [Google Scholar]