Abstract
Milk thistle has been known for more than 2.000 years as a herbal remedy for a variety of disorders. It has mainly been used to treat liver and gallbladder diseases. Silibum marianum, the Latin term for the plant, and its seeds contain a whole family of natural compounds, called flavonolignans. Silimarin is a dry mixture of these compounds; it is extracted after processing with ethanol, methanol, and acetone. Silimarin contains mainly silibin A, silibin B, taxifolin, isosilibin A, isosilibin B, silichristin A, silidianin, and other compounds in smaller concentrations. Apart from its use in liver and gallbladder disorders, milk thistle has recently gained attention due to its hypoglycemic and hypolipidemic properties. Recently, a substance from milk thistle has been shown to possess peroxisome proliferator-activated receptor γ (PPARγ) agonist properties. PPARγ is the molecular target of thiazolidinediones, which are used clinically as insulin sensitizers to lower blood glucose levels in diabetes type 2 patients. The thiazolidinedione type of PPARγ ligands is an agonist with a very high binding affinity. However, this ligand type demonstrates a range of undesirable side effects, thus necessitating the search for new effective PPARγ agonists. Interestingly, studies indicate that partial agonism of PPARγ induces promising activity patterns by retaining the positive effects attributed to the full agonists, with reduced side effects. In this review, the therapeutic potential of milk thistle in the management of diabetes and its complications are discussed.
Keywords: type 2 diabetes, HbA1c, milk thistle, silibin, anti-diabetic, bioavailability, PPAR gamma
Abbreviations: ACE – angiotensin-converting enzyme; ADMA – asymmetric Ng, Ng-dimethyl-L-arginine; ADP – adenosine diphosphate; ALT – alanine transaminase; aP2 – adipocyte protein 2; ARB – angiotensin receptor blocker; AST – aspartate transaminase; ATP – adenosine triphosphate; CAP – cleavage activating protein; CAT – catalase; CCAAT – cytosine-cytosine-adenosine-adenosine-thymidine; C/EBP – CCAAT/enhancer-binding protein; CYP2E1 – cytochrome P450, family 2, subfamily E, polypeptide 1; DHA – dihydroxyacetone; eGFR – estimated glomerular filtration rate; eNOS – endothelial nitric oxide synthase; GSH – glutathion; GSHPx – glutathione peroxidase; hIAPP – human islet amyloid polypeptide; HbA1c – glycosylated hemoglobin; HCV – hepatitis C virus; HDL – high-density lipoprotein; HOMA-IR – homeostasis model assessment insulin resistance; ICAM-1 – intercellular adhesion molecule 1; Insig-1 – insulin-induced gene 1; ITT – insulin tolerance test; LDL – low-density lipoprotein; LPL – lipoprotein lipase; MDA – malondialdehyde; mRNA – messenger ribonucleic acid; NAD – nicotinamide adenine dinucleotide; NADH – nicotinamide adenine dinucleotide, reduced form; NASH – non-alcoholic steatohepatitis; Nkx6.1 - Nk6 homeobox protein 1; Pdx1 – pancreatic and duodenal homeobox 1; Per os – peroral (by mouth); PK – pyruvate kinase; PPAR – peroxisome proliferator-activated receptor; Pref-1 – preadipocyte factor 1; RT-PCR – reverse transcription polymerase chain reaction; SOD – superoxide dismutase; SREBP1 – sterol regulatory element-binding protein 1; Substance P – neuropeptide powder; STZ – streptozotocin; TNFα – tumor necrosis factor α
1. Introduction
A considerable number of herbs have been studied extensively regarding their hypoglycemic properties which may be useful in the control of diabetes mellitus. Among them is milk thistle (Silibum marianum), which was first considered as a therapeutic agent for liver disorders. S. marianum and its seeds contain a whole family of natural compounds, called flavonolignans. Milk thistle is regarded as a potent agent against insulin resistance and diabetes-induced hyperglycemia (Figure 1) [1-3].
Figure 1.

Milk thistle from the island of Samos, Greece.
In organs that do not depend on insulin for glucose transport, intracellular accumulation of sorbitol takes place under conditions of high glucose concentration. This is aided by the enzyme aldose reductase, with subsequent intracellular water accumulation and tissue damage. Silibin (aka silybin or silibinin) acts as an inhibitor of aldose reductase. Therefore, it is suggested as an attractive candidate for the prevention and treatment of diabetes and its complications. For the preparation of this review, we have searched the Pubmed database using the keywords "thistle" and "diabetes", and we considered articles dating from 1997 to 2014.
2. Taxonomy and chemical structure
During the renaissance, S. marianum was considered as a therapeutic agent for liver disorders. S. marianum and its seeds contain a whole family of natural compounds, called flavonolignans. Silimarin is a dry mixture of these compounds, which are extracted after processing with ethanol, methanol, and acetone. Silimarin contains mainly silibin A, silibin B, taxifolin, isosilibin A, isosilibin B, silichristin A, silidianin, and other compounds in smaller concentrations (Figure 2).
Figure 2.
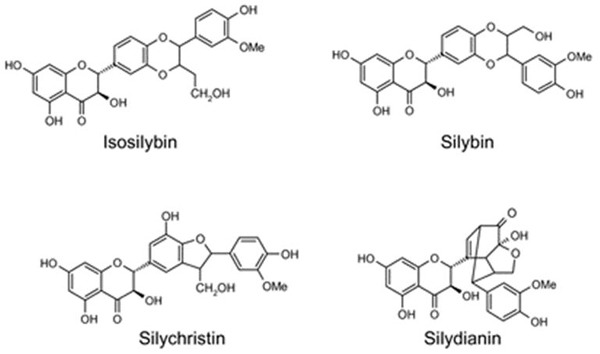
The chemical structure of isosilibin, silibin, silichristin and silidianin compounds.
In 1959, the first member of this family was discovered: silibin, which is the most extensively researched one. Silibin is an equimolar mixture of silibin A (2R,3R)-2-((2R,3R)-2,3-dihydro-3-(4-hydroxy-3-methoxyphenyl)-2-(hydroxymethyl)-1,4-benzodioxin-6-yl)-2,3-dihydro-3,5,7-trihydroxy-4H-1-benzopyran-4-one and silibin B (2R,3R)-2-(2S,3S)-2,3-dihydro-3-(4-hydroxy-3-methoxyphenyl)-2-(hydroxymethyl)-1,4-benzodioxin-6-yl)-2,3-dihydro-3,5,7-trihydroxy-4H-1-benzopyran-4-one. Silibin is a compound that oxidizes swiftly, even in atmospheric oxygen; it has a low solubility in water which increases significantly as pH and temperature rise [4].
3. Silibin's anti-obesity and anti-diabetic properties
3.1 In vitro studies
In organs and biological systems, which do not depend on insulin for glucose transport, such as testis, placenta, peripheral and central nerve system, ophthalmic lens, and pancreatic islets, intracellular accumulation of sorbitol takes place under conditions of high glucose concentration. This event occurs with the help of the enzyme aldose reductase, subsequent intracellular water accumulation, and tissue damage. Silibin acts as an inhibitor of aldose reductase, making it an attractive candidate for prevention and treatment of cataract and diabetic neuropathy. As far as diabetic neuropathy is concerned, it was found that exposure of SY5Y neuroblastoma cells to high glucose concentrations, a typical experimental model of diabetic neuropathy, and subsequent treatment with 10 nM silibin, prevented glucose-induced reduction of mono-ADP-ribosylation and protected the cells' Na-K-ATPase transport activity. This effect could not be observed with equal concentrations of fructose or galactose [5, 6].
In dihydroxyacetone (DHA)-perifused rat hepatocytes and at a concentration of 25 μM (100 μM), silibin decreases DHA gluconeogenesis by 33% (49%) and also decreases glucolysis, as shown by the drop in the rate of lactate plus pyruvate production. Besides, a reduced NADH/NAD redox state and a reduction in the cytosolic ATP/ADP ratio (the latter leading to decreased cellular oxygen consumption) can be attributed to a silibin-induced increase in the lactate:pyruvate ratio. Thus, silibin inhibits gluconeogenesis in perifused hepatocytes independently of the gluconeogenic substrate. Furthermore, silibin inhibits glucose-6-phosphate hydrolysis in a variable manner, independently of the carbohydrate source, but it does not seem to have significant effects in the fructose cycle [7].
In suitably cultured 3T3L-1 cells, silibin prevents the differentiation of pre-adipocytes to adipocytes in a dose-dependent manner, with subsequent reduction in their triglyceride concentration, which is a marker of lipid accumulation and adipocyte hypertrophy. It was shown that silibin plays this role during the first 48 hours of cell treatment, with only minor effects afterwards. The afore-mentioned effects can be attributed to silibin’s variable action on the expression of several genes specific for adipocytes; lower expression of C/EBP, PPARα, aP2, FAS, LPL, and SREBP1c mRNA and higher expression of pref-1, a pre-adipocyte marker, which normally disappears during differentiation of pre-adipocytes to mature adipocytes. Also, silibin's effects are related to increased expression of insig-1 and insig-2. Insig-1 precludes the expression of the adipocyte gene sterol response element binding protein (SREBP) and its subsequent action on gene transcription through binding to cleavage activating protein (CAP). Insig-2 action is similar to the action of insig-1, but its isoform SREBP1c also activates PPARγ, a key adipogenesis transcription factor [8].
In the vast majority of patients with type 2 diabetes, the formation of human islet amyloid polypeptide deposits in pancreatic beta-cells plays a crucial role in the decreasing capacity to produce insulin. Silibin seems to be capable of increasing the viability of pancreatic beta-cells by inhibiting fibrillation, improving oligomerization, and decreasing beta-cell cytotoxicity of human islet amyloid polypeptide (hIAPP) in a dose-dependent manner [9]. Isosylibin A was recently identified as the first PPARγ flavonoglycan agonist. At a concentration of 30 μg/ml, it demonstrated a 19% activation in a PPARγ-driven luciferase reporter gene assay, while the rest of silimarin's compounds (silibin A, silibin B, isosilibin B, silichristin, silidianin and taxifolin) did not produce an activation [10].
3.2 Animal ex vivo studies
It was shown that silibin decreases hepatic glucolysis through the inhibition of pyruvate kinase (PK) and reduction of DHA phosphorylation, which can be attributed to a decrease in ATP content. Even at a low dose of 10 μM, silibin inhibits ROS production due to DHA metabolism; the full antioxidant potential is unfolded at concentrations between 25 μM and 100 μM [11]. Although silibin does not affect blood glucose levels, it is able to prevent excessive protein mono-ADP-ribosylation and the decline of substance P-like immune-reactivity axonal transport in the sciatic nerve of alloxan-induced diabetic rats at a concentration of 1 μM [12]. This capability puts it on the list of possible candidate compounds for treatment of diabetic neuropathy.
Another study demonstrated better glycemic control after 60 days of silimarin administation in rats with STZ/nicotinamide-induced diabetes. In this study silimarin treatment was linked with beneficial effects on kidney function, which were based on a significant decrease in serum creatinine, urine volume, and urine albumin level. This effect on renal function has been confirmed by renal biopsies of diabetic rats treated with low-dose silimarin, where regenerated tubular epithelium and moderate intertubular hemorrhage was noted. In the high-dose group, normal tubules, intact epithelium, and scarce presence of erythrocytes were seen [13].
3.3 Animal in vivo studies
In alloxan-induced diabetic rats, silibin reduced serum glucose levels and protected the pancreas of rats from alloxan-induced lipid peroxidation by maintaining levels of malondialdehyde (MDA) and glutathione lower [14]. In silimarin-treated diabetic rats, normalization of glucose levels began after the first week of administration and reached the values of controls nine weeks afterwards, while insulin levels normalized after 7 days of treatment. RT-PCR amplification for insulin and Pdx1 (a critical regulator of insulin promoter activity) genes yielded similar mRNA content in silimarin-treated and control groups, followed by restoration of Langerhans islet morphology in the former group [15].
In partially pancreatomized rats, silibin (at a dose of 200 mg/kg per os) continued to demonstrate a significant reduction in serum glucose levels compared with control and beta-cell neogenesis two months after the procedure [16]. Neogenesis could be attributed to an increase in gene expression of Nkx6.1 (a factor playing a crucial role in differ-rentiation, maintenance and beta-cell neogenesis) and insulin [16].
Asymmetric Ng, Ng-dimethyl-L-arginine (ADMA), inhibits the production of endothelial nitric oxide synthase (eNOS), which plays a central role in endothelial dysfunction related to diabetes mellitus. Silibin decreases ADMA levels in db/db mice to a level that is lower than in their heterozygous lean counterparts (db/m), which served as controls [17]. In experimentally induced diabetic retinopathy, silibin seems to have a favorable effect as vascular leukostasis and levels of retinal ICAM-1 are significantly reduced [18].
Use of silibin nanoparticles greatly enhances its hypoglycemic activity in STZ-induced diabetic mice compared with its conventional variant. Also, the levels of HbA1c, insulin, cholesterol, triglyceride, liver enzyme levels, and antioxidant status parameters (including superoxide dismutase (SOD), catalase, and glutathion (GSH)) are close to that of healthy controls when compared with animals treated with conventional silibin. In another study, silimarin succeeded in restoring SOD, glutathione peroxidase (GSHPx), and catalase (CAT) values to approximately those of the control group, providing another strong indication that it is a free-radical scavenger, able to protect the pancreas from further alloxan-induced damage [19, 20].
Non-alcoholic steatohepatitis (NASH) is a common finding in obese patients with type 2 diabetes. A 5-week regimen with 200 mg/kg silibin demonstrated a beneficial effect on rats with NASH. Firstly, a decrease in liver weight and liver index compared to the NASH group was noted in the silibin-treated group. Secondly, although glucose levels were not reduced significantly, insulin and homeostasis model assessment of insulin resistance (HOMA-IR) values were decreased as well as total cholesterol and triglyceride levels. Thirdly, grade 0 or 1 steatosis was observed in over 60% of the rats in the silibin-treated group, compared to the NASH group, which included only animals with grade 2 and 3 steatosis. Also, silibin reduced oxidative stress demonstrated by lower levels of MDA and GSH compared to the non-silibin treated NASH group. Furthermore, silibin significantly reduced tumor necrosis factor α (TNFα) levels, which are elevated under conditions of obesity-related inflammation, leading ultimately to insulin resistance. However, silibin in this study failed to reduce liver enzymes significantly and to reverse mitochondrial dysfunction, and it failed to prevent both increased expression of cytochrome P450 2E1 (CYP2E1) and decreased expression of PPARα [21]. Nevertheless, although liver enzymes were not significantly reduced, this was not the case in another study, conducted under similar conditions, where a statistically significant reduction of alanine transaminase (ALT) and aspartate transaminase (AST) was noted [22].
Given the fact that visceral obesity is a risk factor in the development of insulin resistance and type 2 diabetes, animals on a high fat diet treated with silibin demonstrate a significantly lower body weight, with lower visceral and subcutaneous fat, decreased visceral fat to total body weight ratio, and reduced insulin resistance, demonstrated by a decrease in HOMA-IR and increased insulin tolerance test (ITT) slope, compared with the control group [23]. Despite the fact that 20 mg/kg/day dose of silimarin produced a significant increase in body weight after 15 days of treatment, from the second day of administration onwards, blood glucose levels of rats with STZ-induced diabetes were reduced [23]. This effect was sustained for two weeks, with even some events of hypoglycemia occurring in both the control and the diabetic group [23]. In contrast to another in vitro study, no differences in basal insulin levels were seen. These observations may lead to the hypothesis that silimarin's hypoglycemic effect is mainly due to the inhibition of hepatic glucose production and/or increased glucose utilization by muscle and adipose tissue [24]. However, an in vitro study did not support this hypothesis [25]. Other hypotheses include restoration of normal insulin sensitivity or inhibition of tubular renal glucose re-absorption, but they have not yet been verified.
In Psammomys obesus, a sand rat of the gerbil family and an animal model of human obesity and diabetes, 100 mg/g/day silibin per os for 7 weeks reversed high triglyceride levels, reduced insulin resistance, restored antioxidant status, and partially reversed liver steatosis [26]. In rats with diabetic neuropathy, formalin-induced hyperalgesia was significantly reduced, thermal hyperalgesia restored, and motor nerve conduction deficit reversed in the treatment group [27].
3.4 Human studies
In patients with diabetes and alcoholic liver cirrhosis, a silimarin daily dose of 600 mg for 6 months produced a significant reduction in fasting blood glucose and mean daily glucose levels from the second month of treatment onwards, without any increase in episodes of hypoglycemia, compared with the period before silimarin treatment. Insulin requirement was also decreased by 20%, suggesting an alleviation of insulin resistance due to silimarin treatment. A significant decrease of 0.5% in HbA1c levels and a decrease in levels of MDA were also noted after 6 months of treatment, with a patient satisfaction rate of 100% [28].
Diabetic patients with end-stage renal disease received a 350 mg intravenous bolus dose of silibin over 24 hours. In the treated patients, the cellular surface thiol status in peripheral blood lymphocytes was restored. The same phenomenon occurred at the intracellular level 48 hours later, with subsequent improvement in T cell activation and reduction of TNFα levels and inflammation. These effects can be attributed to the activation of γ-glutamyl transferase in the lymphocyte's membrane and to functional improvement of intracellular γ-glutamyl-cysteine synthetase and/or glutathione synthetase that regulates GSH synthesis [29].
A randomized, controlled study consisted of a sample of 60 patients with type 2 diabetes who presented at baseline with diabetic macroalbuminuria (urinary albumin excretion > 300 mg/24h in two occasions), estimated glomerular filtration rate (eGFR) > 30 ml/min/1.73 m², HbA1c < 10%, and blood pressure < 160 / 100 mmHg [30]. The patients were on maximum doses of angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) for at least 6 months. A dose of 140 mg of silimarin three times a day led to a 50% reduction in urinary albumin excretion, urinary TNFα levels, and urinary and serum MDA levels in almost half of the patients in the treatment group [30].
In a sample of 25 patients, treated for four months with 200 mg silimarin three times a day before meals, there was a significant reduction in blood glucose levels (from 156 ± 46 mg/dl to 133 ± 39 mg/dl), compared to an increase in the placebo-treated group. In the same period, HbA1c levels fell from 7.82 ± 2.01% to 6.78 ± 1.05%. This result confirms the aforementioned hypothesis concerning the in vivo animal study that silimarin reduces blood glucose levels via mechanisms that are independent of insulin production. Also, total cholesterol, HDL cholesterol, LDL cholesterol, triglyceride, AST, and ALT levels were significantly reduced [31]. Finally, silimarin demonstrated a synergistic hypoglycemic effect when administered as a fixed combination with an alkaloid derived from Berberis aristata in patients with type 2 diabetes [32].
3.5 Safety
Oral administration of 140, 280, 560, and 700 mg of silimarin every 8 hours for 7 days in non-cirrhotic patients with chronic hepatitis C did not produce any adverse reactions or abnormal findings from follow-up, which included physical examination, biochemical tests, and electrocardiogram. Two reported events of nausea and headache were unrelated to silimarin administration [33].
In another study, patients with chronic hepatitis C received 600 mg and 1200 mg/day silimarin treatment 20% (n = 5) for 12 weeks. The patients reported symptoms from the gastrointestinal tract; two patients reported headache/dizziness, and one pruritus [34]. Similarly, in patients with primary sclerosing cholangiitis, a silimarin dose of 140 mg three times a day for 7 to 221 months caused dyspepsia in some patients, with one reporting diarrhea, which disappeared after silimarin's discontinuation [35]. Silimarin seems to reverse increased AST levels induced by maca, such that it can be safely co-administered with the latter for 90 days in patients with the metabolic syndrome [36]. Furthermore, 420 mg/day of micronized silimarin (BIO-C) was administered to 25 healthy lactating mothers for 63 days and did not cause dropout from the study as compliance and tolerability were described as "very good" [37]. Also, no side-effects were reported after local application of a silimarin-based cream for melasma treatment [38]. Finally, patients with hepatitis C virus (HCV) infection awaiting transplantation showed similar side-effects as those mentioned in the above studies after intravenous silibin treatment for 21 days. Also, a transient increase in bilirubin levels was documented, which at the end of the study returned to levels similar to the placebo group [39].
Generally, silimarin is considered safe, even in doses as high as 13 g/day, with no interactions at low concentrations (10 μM) with chemotherapeutic agents like vincristine or L-asparaginase. However, in higher concentrations (30 μM) a synergistic effect with the latter was noted. At doses as high as 20 g/day, asymptomatic liver toxicity was documented [40].
3.6 Pharmacokinetics and bioavailability
Silibin is rapidly absorbed from the stomach, but absorption is low due to low water solubility. The use of labeled silibin in the rat has enabled to show that the intestinal absorption of 20 mg/kg amounts to approximately 35%. Peak radioactivity is found in the plasma 30 minutes after ingestion [41]. Bioavailability is also low due to high reactivity with phase II conjugation in the liver. Approximately 90% of the circulating silibin is conjugated with sulfates and glucuronides.
Because of the potential inactivation of CYP3A4 and CYP2C9, silibin should be carefully co-administered with drugs (e.g. nifedipine, metronidazole, irinotecan, indinavir), and should be cleared by drugs, especially in high doses. Elimination of conjugated and unconjugated forms is equally fast with a mean elimination half-life of 6.32 hours. Also, silibin's excretion in the urine ranges from 1 to 7% [42]. Bioavailability of silibin can be enhanced up to three fold eight hours after consumption of a phytosome form, i.e. a complex of a natural active ingredient and a phospholipid, in healthy individuals [43]. Parenteral administration of silibin seems to be better when using a phosphatidyl choline-bile salt-mixed micelles formulation.
Silibin B and silichristin demonstrate non-linear pharmacokinetics compared to the rest of silimarin's compounds. This is attributable to the saturation of conjugating enzymes and delayed elimination as a result of the extensive entero-hepatic cycling of the aforementioned conjugates of the silibin compounds. Peak concentrations were achieved for all doses after 2 hours and silimarin compounds' half-life ranged from 0.8 to 2.4 hours. The highest bioavailability was documented for silibin A at the 700 mg dose [44].
Comparison of 3 silibin-containing preparations (liverman capsule, legalon capsule, and silimarin tablet) revealed best absorption and bioavailability by the liverman capsule [45].
4. Conclusions
Milk thistle contains flavonolignans like silibin A. An isoform of silibin, isoform 3, has been demonstrated to possess partial PPARγ agonist effects. Being a newly discovered PPARγ activator, isoform 3 may serve as a prototype for future development of new PPARγ agonists. It cannot be terminally answered, whether PPARγ activation by milk thistle compounds is clinically relevant and suggests the use of silimarin as an herbal remedy. The answer needs further investigation.
Silibin has also demonstrated beneficial effects on several diabetic complications, including diabetic neuropathy, diabetic nephropathy, and non-alcoholic steatohepatitis, mainly by means of its anti-oxidant properties. Future research should focus on the potential of this component of milk thistle to control diabetes and its complications, and on improving the bioavailability of the plants compounds.
Disclosures: The authors report no conflict of interests.
References
- 1.Shojaii A, Dabaghian FH, Goushegir A, Fard MA. Antidiabetic plants of Iran. Acta Med Iran. 2011;49(10):637–642. [PubMed] [Google Scholar]
- 2.McCarty MF. Potential utility of natural polyphenols for reversing fat-induced insulin resistance. Med Hypotheses. 2005;64(3):628–635. doi: 10.1016/j.mehy.2003.11.042. [DOI] [PubMed] [Google Scholar]
- 3.Wei F, Liu SK, Liu XY, Li ZJ, Li B, Zhou YL, Zhang HY, Li YW. Meta-analysis: silymarin and its combination therapy for the treatment of chronic hepatitis B. Eur J Clin Microbiol Infect Dis. 2013;32(5):657–669. doi: 10.1007/s10096-012-1789-1. [DOI] [PubMed] [Google Scholar]
- 4.Biedermann D, Vavrikova E, Cvak L, Kren V. Chemistry of silybin. Nat Prod Rep. 2014;31(9):1138–1157. doi: 10.1039/c3np70122k. [DOI] [PubMed] [Google Scholar]
- 5.Santos J, Mira LB, Freire AM, Azevedo M, Manso C. Placental aldose reductase inhibition by Silybin. Acta Medica Port. 1984;5(4-5):115–117. [PubMed] [Google Scholar]
- 6.Di Giulio AM, Lesma E, Germani E, Gorio A. Inhibition of high glucose-induced protein mono-ADP-ribosylation restores neuritogenesis and sodium-pump activity in SY5Y neuroblastoma cells. J Neurosci Res. 1999;46(5):565–571. [PubMed] [Google Scholar]
- 7.Guigas B, Naboulsi R, Villanueva GR, Taleux N, Lopez-Novoa JM, Leverve XM, El-Mir LY. The flavonoid silibinin decreases glucose-6-phosphate hydrolysis in perifused rat hepatocytes by an inhibitory effect on glucose-6-phosphatase. Cell Physiol Biochem. 2007;20(6):925–934. doi: 10.1159/000110453. [DOI] [PubMed] [Google Scholar]
- 8.Ka SO, Kim KA, Kwon KB, Park JW, Park BH. Silibinin attenuates adipogenesis in 3T3-L1 preadipocytes through a potential upregulation of the insig pathway. Int J Mol Med. 2009;23(5):633–637. doi: 10.3892/ijmm_00000174. [DOI] [PubMed] [Google Scholar]
- 9.Cheng B, Gong H, Li X, Sun Y, Zhang X, Chen H, Liu X, Zheng L, Huang Kl. Silibinin inhibits the toxic aggregation of human islet amyloid polypeptide. Biochem Biophys Res Commun. 2012;419(3):495–499. doi: 10.1016/j.bbrc.2012.02.042. [DOI] [PubMed] [Google Scholar]
- 10.Pferschy-Wenzig EM, Atanasov AG, Malainer C, Noha SM, Kunert O, Schuster D, Heiss EH, Oberlies NH, Wagner H, Bauer R. et al. Identification of Isosilybin A from Milk Thistle Seeds as an Agonist of Peroxisome Proliferator-Activated Receptor Gamma. J Nat Prod. 2014;77(4):842–847. doi: 10.1021/np400943b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Detaille D, Sanchez C, Sanz N, Lopez-Novoa JM, Leverve X, El-Mir MY. Interrelation between the inhibition of glycolytic flux by silibinin and the lowering of mitochondrial ROS production in perifused rat hepatocytes. Life Sci. 2008;82(21-22):1070–1076. doi: 10.1016/j.lfs.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Di Giulio AM. Alterations of protein mono-ADP-ribosylation and diabetic neuropathy: a novel pharmacological approach. Eur J Pharmacol. 1996;311(1):21–28. doi: 10.1016/0014-2999(96)00351-2. [DOI] [PubMed] [Google Scholar]
- 13.Nasri H. Effect of silymarin on streptozotocin-nicotinamide-induced type 2 diabetic nephropathy in rats. Iran J Kidn Dis. 2013;7(5):414–415. [PubMed] [Google Scholar]
- 14.Soto CP, Perez BL, Favari LP, Reyes JL. Prevention of alloxan-induced diabetes mellitus in the rat by silymarin. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1998;119(2):125–129. doi: 10.1016/s0742-8413(97)00198-9. [DOI] [PubMed] [Google Scholar]
- 15.Soto C, Mena R, Luna J, Cerbon M, Larrieta E, Vital P, Uria E, Sanchez M, Recoba R, Barron H. et al. Silymarin induces recovery of pancreatic function after alloxan damage in rats. Life Sci. 2004;75(18):2167–2180. doi: 10.1016/j.lfs.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 16.Soto C, Raya L, Perez J, Gonzalez I, Perez S. Silymarin induces expression of pancreatic Nkx6.1 transcription factor and beta-cell neogenesis in a pancreatectomy model. Molecules. 2014;19(4):4654–4668. doi: 10.3390/molecules19044654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volti GL, Salomone S, Sorrenti V, Mangiameli A, Urso V, Siarkos I. Effect of silibinin on endothelial dysfunction and ADMA levels in obese diabetic mice. Cardiovasc Diabetol. 2011;10:62. doi: 10.1186/1475-2840-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang HT, Shi K, Baskota A, Zhou FL, Chen YX, Tian HM. Silybin reduces obliterated retinal capillaries in experimental diabetic retinopathy in rats. Eur J Pharmacol. 2014;740:233–239. doi: 10.1016/j.ejphar.2014.07.033. [DOI] [PubMed] [Google Scholar]
- 19.Soto C, Recoba R, Barron H, Alvarez C, Favari L. Silymarin increases antioxidant enzymes in alloxan-induced diabetes in rat pancreas. Comp Biochem Physiol Part C Toxicol Pharmacol. 2003;136(3):205–212. doi: 10.1016/s1532-0456(03)00214-x. [DOI] [PubMed] [Google Scholar]
- 20.Das S, Roy P, Pal R, Auddy RG, Chakraborti AS, Mukherjee A. Engineered silybin nanoparticles educe efficient control in experimental diabetes. Plos One. 2014;9(7):e101818. doi: 10.1371/journal.pone.0101818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haddad Y, Vallerand D, Brault A, Haddad PS. Antioxidant and hepatoprotective effects of silibinin in a rat model of nonalcoholic steatohepatitis. Evid Based Complement Alternat Med. 2011;2011:1–10. doi: 10.1093/ecam/nep164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao J, Zhi M, Gao X, Hu P, Li C, Yang X. Effect and the probable mechanisms of silibinin in regulating insulin resistance in the liver of rats with non-alcoholic fatty liver. Braz J Med Biol Res. 2013;46(3):270–277. doi: 10.1590/1414-431X20122551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Von Shonfled, Weisbrod B, Muller MK. Silibinin, a plant extract with anti-oxidant and membrane stabilizing properties, protects exocrine pancreas form cyclosporine A toxicity. Cell Mol Life Sci. 1997;53(11-12):917–920. doi: 10.1007/s000180050111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nomura M, Takahashi T, Nagata N, Tsutsumi K, Kobayashi S, Akiba T, Yokogawa K, Moritani S, Miyamoto K. Inhibitory mechanisms of flavonoids on insulin-stimulated glucose uptake in MC3T3-G2/PA6 adipose cells. Biol Pharm Bull. 2008;31(7):1403–1409. doi: 10.1248/bpb.31.1403. [DOI] [PubMed] [Google Scholar]
- 25.Maghrani M, Zeggwagh NA, Lemhadri A, El Amraoui M, Michel JB, Eddouks M. Study of the hypoglycaemic activity of Fraxinus excelsior and Silybum marianum in an animal model of type 1 diabetes mellitus. J Ethnopharmacol. 2004;91(2-3):309–316. doi: 10.1016/j.jep.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Bouderba S, Sanchez-Martin C, Villanueva GR, Detaille D, Koceir EA. Beneficial effects of silibinin against the progression of metabolic syndrome, increased oxidative stress, and liver steatosis in Psammomys obesus, a relevant animal model of human obesity and diabetes. J Diabetes. 2014;6(2):184–192. doi: 10.1111/1753-0407.12083. [DOI] [PubMed] [Google Scholar]
- 27.Baluchnejadmojarad T, Roghani M, Khastehkhodaie Z. Chronic treatment of silymarin improves hyperalgesia and motor nerve conduction velocity in diabetic neuropathic rat. Phytother Res. 2010;24(8):1120–1125. doi: 10.1002/ptr.3078. [DOI] [PubMed] [Google Scholar]
- 28.Velussi M, Cernigoi AM, Viezzoli L, Dapas F, Caffau C, Zilli M. Silymarin reduces hyperinsulinemia, malondialdehyde levels, and daily insulin need in cirrhotic diabetic patients. Curr Therap Res. 1993;53(5):533–545. [Google Scholar]
- 29.Dietzmann J, Thiel U, Ansorge S, Neumann KH, Tüger M. Thiol-inducing and immunoregulatory effects of flavonoids in peripheral blood mononuclear cells from patients with end-stage diabetic nephropathy. Free Radic Biol Med. 2002;33(10):1347–1354. doi: 10.1016/s0891-5849(02)01043-2. [DOI] [PubMed] [Google Scholar]
- 30.Fallahzadeh MK, Dormanesh B, Sagheb MM, Roozbeh J, Vessal G, Pakfetrat M, Daneshbod Y, Kamali-Sarvestani E, Lankarani KB. Effect of addition of silymarin to renin-angiotensin system inhibitors on proteinuria in type 2 diabetic patients with overt nephropathy: a randomized, double-blind, placebo-controlled trial. Am J Kidney Dis. 2012;60(6):896–903. doi: 10.1053/j.ajkd.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Huseini HF, Larijani B, Heshmat R, Fakhrzadeh H, Radjabipour B, Toliat T, Raza M. The efficacy of Silybum marianum (L.) Gaertn. (silymarin) in the treatment of type II diabetes: a randomized, double-blind, placebo-controlled, clinical trial. Phytother Res. 2006;20(12):1036–1039. doi: 10.1002/ptr.1988. [DOI] [PubMed] [Google Scholar]
- 32.Di Pierro F, Putignano P, Montesi L, Moscatiello S, Marchesini Reggiani G, Villanova N. Preliminary study about the possible glycemic clinical advantage in using a fixed combination of Berberis aristata and Silybum marianum standardized extracts versus only Berberis aristata in patients with type 2 diabetes. Clin Pharmacol. 2013;5:167–174. doi: 10.2147/CPAA.S54308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hawke RL, Schrieber SJ, Soule TA, Wen Z, Smith PC, Reddy KR, Hawed AS, Belle SH, Afdhal NH, Navarro NJ. et al. Silymarin ascending multiple oral dosing phase I study in noncirrhotic patients with chronic hepatitis C. J Clin Pharmacol. 2010;50(4):434–449. doi: 10.1177/0091270009347475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gordon A, Hobbs DA, Bowden DS, Bailey MJ, Mitchell J, Francis AJ, Roberts SK. Effects of Silybum marianum on serum hepatitis C virus RNA, alanine aminotransferase levels and well-being in patients with chronic hepatitis C. J Gastroenterol Hepatol. 2006;21(1):275–280. doi: 10.1111/j.1440-1746.2006.04138.x. [DOI] [PubMed] [Google Scholar]
- 35.Angulo P, Jorgensen RA, Kowdley KV, Lindor KD. Silymarin in the treatment of patients with primary sclerosing cholangitis: an open-label pilot study. Dig Dis Sci. 2008;53(6):1716–1720. doi: 10.1007/s10620-007-0052-6. [DOI] [PubMed] [Google Scholar]
- 36.Valentova K, Stejskal D, Bartek J, Dvorackova S, Kren V, Ulrichova J, Simanek V. Maca (Lepidium meyenii) and yacon (Smallanthus sonchifolius) in combination with silymarin as food supplements: in vivo safety assessment. Food Chem Toxicol. 2008;46(3):1006–1013. doi: 10.1016/j.fct.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 37.Di Pierro F, Callegari A, Carotenuto D, Tapia MM. Clinical efficacy, safety and tolerability of BIO-C (micronized Silymarin) as a galactagogue. Acta Biomed. 2008;79(3):205–210. [PubMed] [Google Scholar]
- 38.Altaei T. The treatment of melasma by silymarin cream. BMC Dermatol. 2012;12(1):18. doi: 10.1186/1471-5945-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferenci P, Beinhardt S. Silibinin: an old drug in the high tech era of liver transplantation. J Hepatol. 2013;58(3):409–411. doi: 10.1016/j.jhep.2012.11.030. [DOI] [PubMed] [Google Scholar]
- 40.Lecomte J. Les proprietes pharmacologiques de la silybine et de la silymarine. Rev Med Liege. 1975;30:110–114. [PubMed] [Google Scholar]
- 41.Tamayo C, Diamond S. Review of clinical trials evaluating safety and efficacy of milk thistle (Silybum marianum (L.) Gaertn) Integr Cancer Ther. 2007;6(2):146–157. doi: 10.1177/1534735407301942. [DOI] [PubMed] [Google Scholar]
- 42.Wu JW, Lin LC, Tsai TH. Drug-drug interactions of silymarin on the perspective of pharmacokinetics. J Ethnopharmacol. 2009;121(2):185–193. doi: 10.1016/j.jep.2008.10.036. [DOI] [PubMed] [Google Scholar]
- 43.Kidd PM. Bioavailability and activity of phytosome complexes from botanical polyphenols: the silymarin, curcumin, green tea, and grape seed extracts. Altern Med Rev. 2009;14(3):226–246. [PubMed] [Google Scholar]
- 44.Duan R, Sun X, Liu J, Gong T, Zhang Z. Mixed micelles loaded with silybin-polyene phosphatidylcholine complex improve drug solubility. Acta Pharmacol Sin. 2010;32(1):108–115. doi: 10.1038/aps.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim YC, Kim EJ, Lee ED, Kim JH, Jang SW, Kim YG, Kwon JW, Kim WB, Lee MG. Comparative bioavailability of silibinin in healthy male volunteers. Int J Clin Pharmacol Ther. 2003;41(12):593–596. doi: 10.5414/cpp41593. [DOI] [PubMed] [Google Scholar]


