Abstract
Background:
Failure to thrive (FTT) is a common problem of children especially in underdeveloped countries. In addition to its short-term adverse health effects, it is associated with long-term behavioral and cognitive defects. One of the recommended treatment modalities for FTT is using synbiotics. Due to high prevalence of FTT with undefined organic causes and failure of most medications on treatment of this type of FTT, we decided to search the effect of synbiotics on these patients.
Materials and Methods:
A randomized, triple-blinded, placebo-controlled trial study was done from 2011 to 2012. A number of 84 patients were randomly assigned to intervention and control groups. The synbiotics sachets were administered to study group for 6 months. The growth indices were measured at the beginning of the trial after 3 and 6 months, and compared with control.
Results:
Variance analysis of observations showed improvement of growth indices in both groups. The increase in weight was significantly higher in synbiotics group than in controls (P < 0.05). The corresponding figure was not significant for height and head circumference (P > 0.05). At the beginning of the trial, the mean weights were 10.25 ± 0.20 kg and 10.750 ± 0.160 kg in intervention and control groups, respectively, Meanwhile, after 6 months, the mean weights of two groups became 12.280 ± 0.190 and 11.760 ± 0.17 kg in intervention and control groups, respectively. This result has confirmed that the effect of synbiotics is significant on weight gain of our patients.
Conclusion:
Our findings support beneficial effects of synbiotics in weight gain of children with FTT.
Keywords: Children, failure to thrive, growth, synbiotics
INTRODUCTION
The growth indices are a valuable scale for evaluation and monitoring of child health. Failure to thrive (FTT) is defined as a decrease in growth rate according to the reference growth charts.[1,2,3,4]
As a common problem in children, FTT is one of the major health problems especially in underdeveloped and developing countries. It is associated with some defects such as weight, height, cognitive functions, and behavior.[1] About one-third of FTT patients suffer from developmental delay, emotional, and socioeconomic problems.[5] FTT children have long-term behavioral and cognitive defects because these patients are prone to recurrent infections especially in the gastrointestinal and respiratory tracts. Thus, FTT can cause a heavy economic burden for governments as well as for health system at individual and public levels. As the etiology of FTT can be determined in only 20% of cases,[6] the treatment of most cases is a challenging problem for physicians.[6]
One method for improving growth parameters of children is administration of synbiotics, that is, prebiotics and probiotics.[7] Prebiotics are nondigestible food ingredient usually in the form of oligosaccharide (galacto-oligosaccharide and fructo-oligosaccharide). They are used as food for probiotics, and cause their growth.[7] Probiotics are also live, useful microorganisms, and when administered with sufficient dosage (108 -1011 CFU), they colonize the gastrointestinal tract, suppress pathogenic bacteria and may improve health.[8,9]
There are several types of probiotics including Lactobacillus, Bifidobacteria and Sacchaomyces species. Lactobacillus rhamnosus (LGG) is the first probiotic that has been discovered. It is also the most common administered form.[8,9] Several studies on probiotics exist for FTT patients. Saran et al. evaluated the effect of probiotics (Lactobacillus acidophilus) for FTT children in India.[10] They found that probiotics can cause an increment of weight and height in relation of placebo. They also observed lower frequency of diarrhea and fever episodes in this group than in controls.[10]
A study on 402 Chinese FTT children aged 3-5 years showed that probiotics could result in significant improvement of height and weight in these patients.[11] In another 6 months-study on 120 healthy children showed that children treated with fortified formula and LGG had better growth indices than those receiving formula without probiotics.[12] However, a study conducted in Switzerland among healthy term infants showed that the effect of the formula with probiotic and prebiotics was comparable with formula without them, and no difference was seen in anthropometric measures between two groups.[13] Likewise, another study did not confirm any significant effect of probiotics on weight, height and head circumference of 126 infants aged 0-6 months.[14]
The purpose of the current study was to assess the effect of synbiotics on growth indices of a sample of Iranian children with FTT and to compare it with controls. Since this study was not done for Iranian children till now, we have decided to research about this subject.
MATERIALS AND METHODS
A randomized, triple-blinded, placebo-controlled trial study was done from 2011 to 2012 in Shahrekord, Iran. The patients, physician, and pharmacy, did not know about sachets content. Herbs and Medicine Research Center of Shahrekord University that made these sachets, have knowledge only about the contents by special coding. The eligibility criteria consisted of being FTT defined as having either a weight for age of <5th percentile or a weight for height of <10th percentile,[4] being aged 12-60 months, having a gestational age of >36 weeks and a birth weight of >2500 g, without congenital anomalies or the other chronic diseases. Those who refused to use synbiotic were excluded from the study. They were visited by one academic pediatrician to rule out organic diseases. For all children, screening was done by complete blood count, electrolytes, sedimentation rate, blood urea nitrogen, creatinine, urinalysis, and stool examination (for occult blood, fat and parasites). All patients had normal laboratory data. By using random table numbers, they were randomly assigned into two groups of 42 patients receiving synbiotics or placebo. Two groups were matched for growth indices and age. In each group, measurement of weight, height, and head circumference were done by the same nurse under standard protocols and by using calibrated instruments. Weight was measured with seca counterweight (Australia, accuracy 0.1 kg), head circumference, length or height by one nonelastic tape measure (somatometer France, accuracy 0.1 cm) at the beginning of the trial, as well as after 3 and 6 months. These data were then compared with indices in the growth chart of each patient. The responsible physician, nurse, parents, and children were not aware of the study group.
The patients in the case and control groups received synbiotics and starch powder in sachet, respectively. Parental consent was given for administration. All sachets were same form and color. Synbiotic sachets were made in form Lactol tablets. The drug is manufactured under license of nature's only company of USA in India. Each tablet contains 100 mg fructo-oligosaccharides and 150 million spore Bacillus coagulans.
Placebo sachets contained 1 g starch powder. Both sachets (placebo and synbiotics) were made by Herbs and Medicine Reseach Center of Shahrekord University as mentioned above. Both synbiotics and placebo sachets had special codes. The patients visited by responsible physician monthly and again received the sachet with the same code. With returning the empty box of previous sachets, we were certain for patients’ compliance. After finishing the study, breaking of codes was done. The average increment of weight, height and head circumference in each group was calculated and analyzed by SPSS 18 software (SPSS Inc., Chicago, Illinois, USA) and with Chi-square, independent t-test examination, and observational variance analysis. For each of the growth indices, a repeated measure analysis of variance was used while three measurements were entered as within-subject variables and the group as between-subject factor. The multivariate F-test of Greenhouse-Geisser was used for within-subject analysis. The interaction factor between within-subject variable and group evaluated to find if the trend of growth parameter during the study vary between two groups or not. Furthermore, the independent t-test and Chi-square test were used to compare each measurement between two groups. P <0.05 was considered as significant.
RESULTS
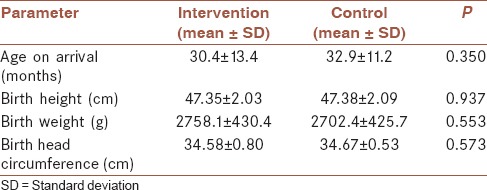
Totally, 84 FTT children (each group 42 children) were admitted to our study, of whom 40 children (47.6%) were boys (P > 0.05). All children were from 12 to 54 months old, the mean age of children at the beginning of the study, and also the mean of their growth parameters at their birth were shown in the Table 1. There was no difference between age of two groups (P > 0.05), also the growth parameters at birth between two groups were not significant (P > 0.05).
Table 1.
Mean of age, height, weight, head circumference at the beginning of the study
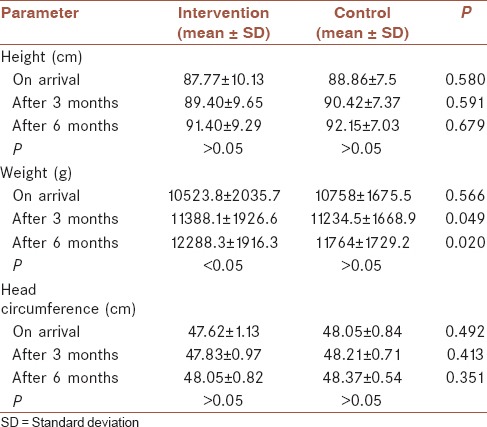
The mean of growth indices, including weight, height, and head circumference during the study, was calculated at the beginning of study and on the 3 and 6 months follow-up, which were shown in the Table 2. There was no difference between height and weight of two groups in the beginning. Nevertheless, head circumference of the control group was significantly higher than the intervention group. However, no significant difference after intervention was seen. After 3 and 6 months, no significant difference of height and weight were seen in the control group. Meanwhile, a significant increment of weight was seen in the intervention group (P < 0.05).
Table 2.
The mean of growth indices (height, weight, and head circumference) at the beginning, after 3 and 6 months
Based on repeated measures analysis of variance, there is obvious increasing trend for all growth parameters during the study. The increasing trend of height and head circumference were statistically equal for both group (P > 0.05), as one could see from the Figures 1 and 2. However, as shown in the Figure 3, the increasing trend of weight was significantly higher in the intervention group (P < 0.05).
Figure 1.
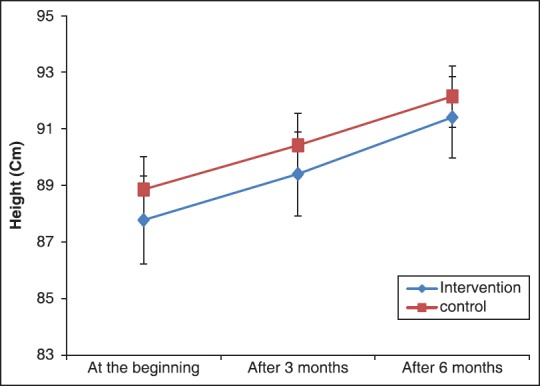
The mean of height at the beginning, after 3 and 6 months
Figure 2.
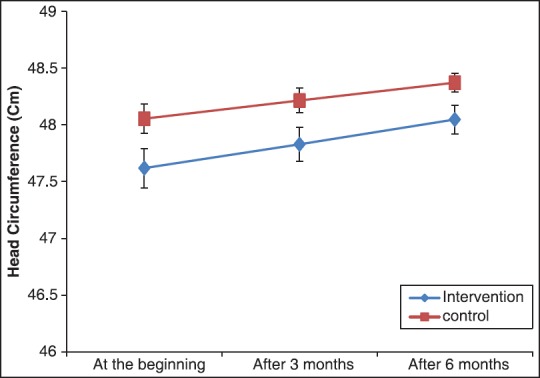
The mean of head circumference at the beginning, after 3 and 6 months
Figure 3.
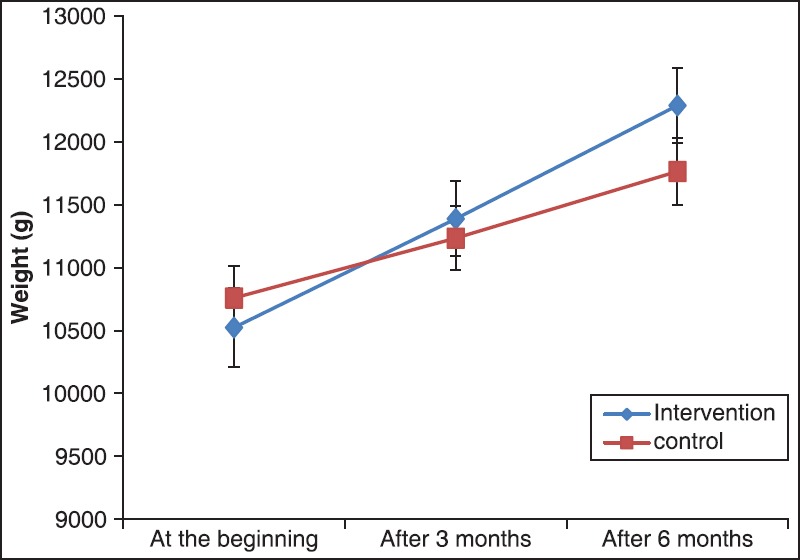
The mean of weight at the beginning, after 3 and 6 months
DISCUSSION
This is a controlled clinical trial, which evaluate the effect of synbiotic medications on improvement of growth indices in children with FTT that no organic cause detected for them. The result of this study showed that ingestion of synbiotics with adequate dose (100 mg fructo-oligosaccharides and 150 million spore B. coagulans) for 6 months caused significant improvement of weight of FTT children, but no effect on height and head circumference.
In other parts of the world, few studies were done in which oral administration of prebiotic + probiotic Lactobacillus acidiphillus to FTT children for 6 months causes improvement of weight gain and height.[10,11] He et al. resulted significant increase in weight and height of children receiving probiotic.[11] In our study, only increment of weight occurred but no effect on height and head circumference were observed. This study and previous works show a significant increase in weight, which may be due to stimulation of damaged gastrointestinal mucosa by these agents.[10,11] Inattention to many clinical advantages of probiotics, several factors including bacterial type, dosage, route of administration, and some factors such as age and dietary regimen probably are responsible for different effects of these drugs. For FTT, the first decreasing factor is weight. Decreasing in height, and then head circumference gradually will happen through progression of malnutrition. With the improvement of FTT, weight is the first factor that will rise and then height, and head circumference subsequently will increase with time. It should be noted that FTT children in our study mostly had not appeared short stature. Since there is the benefit of synbiotics on stimulation of mucosal immunity and improvement of intestinal absorption as well as these effects need sufficient time, this is another reason that also shows possibility of height velocity increment needs more prolonged use of synbiotic (>6 months).
In the opposite to the mentioned studies, Kerac et al. in Malavia reported probiotic administration for children with acute FTT, which had no effect on weight and height.[15] This situation may be due to the difference in statistical community or route and dose of probiotic use. In Kerac et al. study, there were some HIV-positive patients who had received co-trimoxazole that could decrease intestinal probiotic colonization.[15] For the existence of the ideal response to probiotics, they must pass from upper gastrointestinal tract to a small intestine along with colon in appropriate condition. Because of killing of some probiotics during this transmission, it seems that 1 bolus dose has better therapeutic effect than divided dose that used in Kerac et al. study.[15] Kerac et al. administered probiotics milk for which dose of ingested probiotics was not precise.[15]
For previous studies, the effect of probiotics on head circumference did not show a positive response.[10,11,15] Our study confirms the same results. The maximum increase of head circumference and brain size after birth is in the 6 months of the 1st year, which is equal to remaining life. In the second half of the 1st year, the rate of increment in head circumference reaches 0.5 cm/month. The average age of children in our study is 12-60 months children. Thus, we must not expect a significant increase in head circumference of patients that received probiotics. If the study performs on earlier ages, the effect of probiotics on head circumference, cognition, performance of the nervous system, also could be evaluated.
The kind of probiotic administered also could be effective because different kinds of probiotics can have different effects on patients with different clinical conditions. Hence, different results may also be found in part due to different types of probiotics.[16]
Another cause for a positive effect of probiotics on weight gain of children can be due to decrease episodes of infections including pneumonia and gastroenteritis.[17,18] There are some evidences that Lactobacil bacteria and Bifidobacteria interact directly with viruses and pathogens in food and water as well as toxin-producing microbes.[19] Considerable researches have, therefore, been carried out regarding the treatment and prevention of diarrhea.[20,21,22,23,24] Some investigations showed that L. rhamnosus (Lcr35) can control bacterial, viral and bacterial respiratory and gastrointestinal disease significantly. Long-term ingestion of Lcr35 decreased the incidence of bacterial infections.[17] In our study, episodes of acute gastroenteritis and febrile illness decreased significantly among children that received probiotic (P < 0.05).
One problem with administration of synbiotics is safety of these agents. Probiotics are live microorganisms and may cause infectious diseases in the young infant and immune deficient children. Meanwhile, several investigations demonstrated that infection rates with Lactobacillus or Bifidobacteria are rare, and ingestion of these bacteria is safe.[14,20] All patients entered in our investigation tolerated synbiotics well without any adverse effect.
The route of administration of synbiotics can also be influenced on results. Synbiotics ingestion in the form of medication is more effective than synbiotics supplement in milk or yogurt because sufficient and precise dose administered with a bolus dose in the form of drug, than uncertain dose in the latter form.
More studies with more cases of FTT children are need for supporting these results. It is better to do such studies with more prolong time to evaluate the effect of synbiotics on height and head circumference also.
CONCLUSION
Due to high prevalence of FTT in children and its complications on cognitive function and behavior, it seems necessary to find the appropriate method for treatment of FTT. It may be suggested that synbiotics as adjunct therapy for FTT children can improve weight gain and decrease complications through considering results of this and some previous studies. Although more studies are needed to more clearly determine its effects.
AUTHOR'S CONTRIBUTION
FF Principal investigator (PI) Supervisor and corresponding author, contributed in the conception of the work, conducting the study, drafting and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. AK contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. AG Resident of Pediatrics, contributed in the conception of the work, conducting the study, revising the draft, and agreed for all aspects of the work. SK Statistics consultant, contributed in the conception of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. HS Co-PI of the study, contributed in the conception of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. RK Co-PI of the study, contributed in the design of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
ACKNOWLEDGMENT
We would like to thank Herbs and Medicine Research Center of Shahrekord University and IRCT, because this report would not have been possible without the valuable information (Project number 938).
Footnotes
Source of Support: Nil
Conflict of Interest: We would like that this study will also be done with more patients and for longer duration to show the effect of synbiotics on height and head circumference.
REFERENCES
- 1.Black MM, Dubowitz H, Krishnakumar A, Starr RH., Jr Early intervention and recovery among children with failure to thrive: Follow-up at age 8. Pediatrics. 2007;120:59–69. doi: 10.1542/peds.2006-1657. [DOI] [PubMed] [Google Scholar]
- 2.Olsen EM. Failure to thrive: Still a problem of definition. Clin Pediatr (Phila) 2006;45:1–6. doi: 10.1177/000992280604500101. [DOI] [PubMed] [Google Scholar]
- 3.Olsen EM, Petersen J, Skovgaard AM, Weile B, Jørgensen T, Wright CM. Failure to thrive: The prevalence and concurrence of anthropometric criteria in a general infant population. Arch Dis Child. 2007;92:109–14. doi: 10.1136/adc.2005.080333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mei Z, Grummer-Strawn LM, Thompson D, Dietz WH. Shifts in percentiles of growth during early childhood: Analysis of longitudinal data from the California Child Health and Development Study. Pediatrics. 2004;113:e617–27. doi: 10.1542/peds.113.6.e617. [DOI] [PubMed] [Google Scholar]
- 5.McLean HS, Price DT. Failure to thrive. In: Kliegman RM, Behrman RE, Jenson HB, Stanton BF, editors. Nelson Textbook of Pediatrics. 19th ed. Philadelphia, PA: Saunders Elsevier; 2011. pp. 147–9. [Google Scholar]
- 6.Gahagan S. Failure to thrive: A consequence of undernutrition. Pediatr Rev. 2006;27:e1–11. doi: 10.1542/pir.27-1-e1. [DOI] [PubMed] [Google Scholar]
- 7.Firmansyah A, Dwipoerwantoro PG, Kadim M, Alatas S, Conus N, Lestarina L, et al. Improved growth of toddlers fed a milk containing synbiotics. Asia Pac J Clin Nutr. 2011;20:69–76. [PubMed] [Google Scholar]
- 8.Parvez S, Malik KA, Ah Kang S, Kim HY. Probiotics and their fermented food products are beneficial for health. J Appl Microbiol. 2006;100:1171–85. doi: 10.1111/j.1365-2672.2006.02963.x. [DOI] [PubMed] [Google Scholar]
- 9.Gupta V, Garg R. Probiotics. Indian J Med Microbiol. 2009;27:202–9. doi: 10.4103/0255-0857.53201. [DOI] [PubMed] [Google Scholar]
- 10.Saran S, Gopalan S, Krishna TP. Use of fermented foods to combat stunting and failure to thrive. Nutrition. 2002;18:393–6. doi: 10.1016/s0899-9007(01)00790-0. [DOI] [PubMed] [Google Scholar]
- 11.He M, Yang YX, Han H, Men JH, Bian LH, Wang GD. Effects of yogurt supplementation on the growth of preschool children in Beijing suburbs. Biomed Environ Sci. 2005;18:192–7. [PubMed] [Google Scholar]
- 12.Vendt N, Grünberg H, Tuure T, Malminiemi O, Wuolijoki E, Tillmann V, et al. Growth during the first 6 months of life in infants using formula enriched with Lactobacillus rhamnosus GG: Double-blind, randomized trial. J Hum Nutr Diet. 2006;19:51–8. doi: 10.1111/j.1365-277X.2006.00660.x. [DOI] [PubMed] [Google Scholar]
- 13.Chouraqui JP, Grathwohl D, Labaune JM, Hascoet JM, de Montgolfier I, Leclaire M, et al. Assessment of the safety, tolerance, and protective effect against diarrhea of infant formulas containing mixtures of probiotics or probiotics and prebiotics in a randomized controlled trial. Am J Clin Nutr. 2008;87:1365–73. doi: 10.1093/ajcn/87.5.1365. [DOI] [PubMed] [Google Scholar]
- 14.Vlieger AM, Robroch A, van Buuren S, Kiers J, Rijkers G, Benninga MA, et al. Tolerance and safety of Lactobacillus paracasei ssp. paracasei in combination with Bifidobacterium animalis ssp. lactis in a prebiotic-containing infant formula: A randomised controlled trial. Br J Nutr. 2009;102:869–75. doi: 10.1017/S0007114509289069. [DOI] [PubMed] [Google Scholar]
- 15.Kerac M, Bunn J, Seal A, Thindwa M, Tomkins A, Sadler K, et al. Probiotics and prebiotics for severe acute malnutrition (PRONUT study): A double-blind efficacy randomised controlled trial in Malawi. Lancet. 2009;374:136–44. doi: 10.1016/S0140-6736(09)60884-9. [DOI] [PubMed] [Google Scholar]
- 16.Saneian H, Tavakkol K, Adhamian P, Gholamrezaei A. Comparison of Lactobacillus Sporogenes plus mineral oil and mineral oil alone in the treatment of childhood functional constipation. J Res Med Sci. 2013;18:85–8. [PMC free article] [PubMed] [Google Scholar]
- 17.Safavi M, Farajian S, Kelishadi R, Mirlohi M, Hashemipour M. The effects of synbiotic supplementation on some cardio-metabolic risk factors in overweight and obese children: A randomized triple-masked controlled trial. Int J Food Sci Nutr. 2013;64:687–93. doi: 10.3109/09637486.2013.775224. [DOI] [PubMed] [Google Scholar]
- 18.Weizman Z, Alsheikh A. Safety and tolerance of a probiotic formula in early infancy comparing two probiotic agents: A pilot study. J Am Coll Nutr. 2006;25:415–9. doi: 10.1080/07315724.2006.10719554. [DOI] [PubMed] [Google Scholar]
- 19.Lin JS, Chiu YH, Lin NT, Chu CH, Huang KC, Liao KW, et al. Different effects of probiotic species/strains on infections in preschool children: A double-blind, randomized, controlled study. Vaccine. 2009;27:1073–9. doi: 10.1016/j.vaccine.2008.11.114. [DOI] [PubMed] [Google Scholar]
- 20.Liu KX, Zhu YG, Zhang J, Tao LL, Lee JW, Wang XD, et al. Probiotics’ effects on the incidence of nosocomial pneumonia in critically ill patients: A systematic review and meta-analysis. Crit Care. 2012;16:R109. doi: 10.1186/cc11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rautava S, Salminen S, Isolauri E. Specific probiotics in reducing the risk of acute infections in infancy — a randomised, double-blind, placebo-controlled study. Br J Nutr. 2009;101:1722–6. doi: 10.1017/S0007114508116282. [DOI] [PubMed] [Google Scholar]
- 22.Ouwehand AC. The reduction of incidence and duration of disease by probiotics — A brief overview of prophylactic studies. US Paediatr. 2008;4:54–6. [Google Scholar]
- 23.Samaržija D, Tudor M, Prtilo T, Dolenčić Špehar I, Zamberlin Š, Havranek J. Probiotic bacteria in prevention and treatment of diarrhea. Mljekarstvo. 2009;59:28–32. [Google Scholar]
- 24.Ouwehand A, Vesterlund S. Health aspects of probiotics. IDrugs. 2003;6:573–80. [PubMed] [Google Scholar]


