Abstract
Background:
The success of treatment of chronic hepatitis C (CHC) with pegylated interferon-α (PEG-IFN-α) and ribavirin (RBV) is affected by several host, viral, and treatment factors. This study was designed to describe the association of interleukin (IL) 28B genotypes for rs12979860 with sustained virologic response (SVR) in patients with genotype 1 CHC infection treated with PEG-IFN α-2 and RBV.
Materials and Methods:
Interleukin-28B genotype in 100 studied patients was detected by tagman real-time polymerase chain reaction. Before treatment blood samples were obtained, then patients were treated for 48-week with a combination therapy using of the PEG-IFN α-2 and RBV. SVR evaluated 6 months after stopping therapy, and was defined as undetectable plasma hepatitis C virus-RNA.
Results:
Among studied patients, 65% were IL-28B CT, 27% CC, and 8% TT. In all studied patients, SVR was 58.3%, relapse 15.6%, and null virological response 26.1%. SVR rates were 76.9% in IL-28B-CC, 56.4% in IL-28B-CT, and 12.5% in IL-28B-TT patients. Relapse rates were 7.7% in IL-28B-CC, 12.9% in IL-28B-CT, and 62.5% in IL-28B-TT patients. There was a significant difference between response to treatment in patients IL-28B-CC, CT, and TT (P = 0.003). IL-28B genotype CC, (odds ratio = 0.053, 95% confidence interval; 0.005-0.54, P = 0.03), was the independent predicting factor.
Conclusion:
Interleukin-28B was an important predictor of CHC treatment outcome with Peg-IFN-α and RBV. IL-28B-CC seems to be more important than IL-28B-CT/TT in predicting positive treatment response.
Keywords: Chronic hepatitis C, interleukin-28B, pegylated interferon-α, ribavirin
INTRODUCTION
Most infections with hepatitis C virus (HCV) do not resolve spontaneously. Chronic hepatitis C (CHC) virus is the most common cause of chronic liver disease.[1,2] It affects a significant proportion of the world, accounting for 3% of the total population.[3] Furthermore, it is estimated that affects approximately 130-170 million people worldwide.[4,5] WHO reported that every year >350,000 people die from liver disease caused by CHC and 3-4 million new infections occur every year.[6,7]
Pegylated interferon-α (PEG-IFN α-2) with ribavirin (RBV) is the standard treatment for patients with CHC. HCV genotype is a factor that can influence treatment decision and clinical outcomes. Among HCV genotypes, genotype 1, 2, and 3 are the most common HCV genotype in the world. However, in approximately half of the treated patients with HCV genotype 1 or 4, this therapy does not eradicate HCV-infected and is associated with high failure rates due to its limited efficacy and significant adverse effects.[8,9] Sustained virologic response (SVR) rates of 47-54% are reported for this treatment for previously untreated patients.[10] In Iran, the rate of SVR was reported from 48% to 54%,[11,12,13] and the United States and Europe patients with CHC genotype 1, achieved an SVR 42-52%.[14]
It is clarified that the success of treatment is affected by several host, viral, and treatment factors. A reduced risk of long-term complications, such as cirrhosis and hepatocellular carcinoma, obesity, ethnicity, gender, and age can influence SVR.[15,16,17]
A host-specific genetic-based HCV response to treatment was described during 2009-2010.[18,19,20] In a study of 1600 CHC patients identified a single nucleotide polymorphism (SNP) located on chromosome 19, who were homozygous for the major C allele at the rs12979860 SNP in proximity to interleukin (IL) 28B rs12979860 which was strongly associated with SVR. It was also shown that the CC genotype of IL-28B to be associated with a >2 fold increase in SVR as compared with the CT and TT genotypes.[18,19,20]
The study of genetic association in different worldwide population required to replicate. In the general population of Iran, the prevalence of HCV infection is <0.5%.[21] The most prevalent HCV genotype in Iran is genotype 1, and the number of nonresponders to PEG-IFN and RBV increases daily.[22] In a study, on 921 Iranian patients with CHC, the frequency of IL-28B rs12979860 CC, CT, and TT genotypes was reported 38%, 48.8%, and 13.2%, respectively.[22] Studies on relation between homozygous genotypes and SVR rates in Iranian CHC patients are limited, hence, the present study was designed to describe the frequency of the IL-28B genotypes for rs12979860 in a cohort of Iranian patients with genotype 1 CHC infections treated with PEG-IFN and RBV, and also evaluated the association of IL-28B genotypes for rs12979860 with SVR in this population and investigated whether the determination of IL-28B genotypes for rs12979860 would contribute to the prediction of SVR in these patients.
MATERIALS AND METHODS
This cross-sectional study is in compliance with the Helsinki Declaration, and the study protocol was approved by the institutional review boards of Isfahan University of medical science, Isfahan, Iran. After a full explanation of the study, written informed consent was obtained from all the participants of this study.
Between 2012 and 2013, one hundred patients with HCV genotype 1 infection that referred to hepatitis outpatient clinic of the Seyed Al-Shohadh Hospital of Isfahan University of Medical Sciences were enrolled in this cohort study. Patients of both sex, over or at age of 18 years old who had been detected of anti-HCV and HCV RNA within the serum were eligible if they had hemoglobin >10 g/dL, neutrophils >1500 cells/mm3, platelets >7500 cells/mm3, and no renal dysfunction (serum creatinine >1.5 mg/dL). Other inclusion criteria include no history of liver-function disorder, heart failure, uncontrollable diabetes, obstructive pulmonary disease, thyroid dysfunction, and no history of autoimmune hepatitis and pregnancy. Furthermore, patients with psychiatric disorders that may affect treatment adherence, those with end-stage kidney disease, suspected hepatocellular carcinoma, a serious high blood-pressure, a serious coronary artery disease, a serious psychological disease and who were co-infected with either hepatitis B virus or HIV or who had undergone liver transplantation did not enter to the study. Patients who did not receive the full course of the planned treatment, or those for whom treatment response information was not available were excluded from the analysis.
Interleukin-28B genotype of SNPs rs12979860 was detected by tagman real-time polymerase chain reaction, and allele discrimination was achieved by detecting fluorescence using System SDS software (Applied Biosystems, Foster City, CA, USA).[19,23] The rs12979860 polymorphism detection results were designated in three genotype; CC, CT and TT.
Selected patients treated with a combination therapy using of the standard doses PEG-IFN α-2 (PEG-INTRON, Behestan Darou, Iran) and a weight-based dose of RBV (Rebetol), 1000 mg/day for <75 kg and 1200 mg/day for ≥75 kg, with the duration of 48-week.[24] Demographic, clinical, and laboratory data were collected in all patients. Before treatment, blood was collected in ethylenediaminetetraacetic acid tubes using standard procedures. Genomic DNA was extracted from 200 μL of the cell suspension with pure gene blood core c kit (Quagen, American) according to the manufacturer's instructions.
The scheduled visits were at baseline (variables included age, sex, body mass index (BMI), alanine aminotransferase (ALT), and HCV-RNA) and end of treatment. To evaluate SVR, a visit 6 months after stopping therapy was also conducted. Null virological response (NVR) was defined as detectable plasma HCV RNA at the end of treatment, and SVR was defined as undetectable plasma HCV RNA 6 months after the completion of therapy, and undetectable HCV RNA 6 months after the end of therapy without achieving SVR was considered as relapse.[25]
The collected data were analyzed statistically with SPSS software version 20 (SPSS Inc.; Chicago, IL, USA). Normality of data was assessed by Shapiro-Wilk normality test. All data were normally distributed (P > 0.05). Continuous variables were summarized as mean ± standard deviation and categorical variables as frequency and percentage. Individual characteristics among groups were compared using the one-way ANOVA (using Bonferroni as Post-hoc test) and independent sample t-test for continuous variables, and the Chi-squared test for categorical data. Multivariate logistic regression analysis was used to identify factors significantly associated response to treatment and SVR 6 months after the end of treatment. P < 0.05 was considered significant.
RESULTS
Figure 1 shows the study flow chart, of 100 enrolled patients the most prevalent IL-28B genotype was CT (65%), followed by CC (27%) and TT (8%). During studied period, one patient with IL-28B-CC and three patients with IL-28B-CT were dropped out due to side effects (Fever, myalgia, fatigue, and general weakness). 6 months followed-up after completion of treatment, SVR rates were 77% in IL-28B-CC, 56% in IL-28B-CT, and 12.5% in IL-28B-TT.
Figure 1.
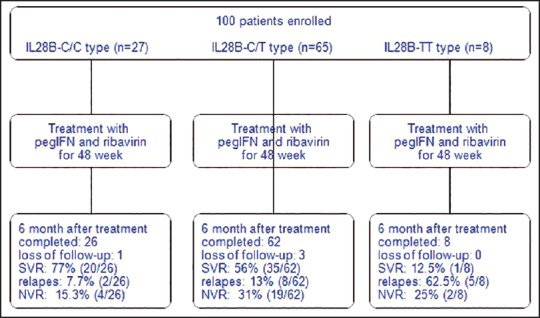
Study flowchart - NVR = Null virological response; SVR = Sustained virologic response
The mean age of studied patients was 35.5 ± 12.5 years old. Eighty-one of patients (84%) were male, and 15 patients (16%) were female. Demographic and biochemical features of patients by IL-28B genotype are reported in Table 1. There were no significant differences between patients IL-28B genotype CC, CT, and TT for age, gender combination, level of ALT and HCV-RNA (P > 0.05). The mean of BMI was significantly lower in patients with IL-28B-TT than others (P = 0.001).
Table 1.
Demographic and biochemical features of patients by IL-28B genotype

Table 2 shows the association between IL-28B genotypes with response to PEG-IFN-α/RBV treatment 6 months after completion of treatment. Relapse was observed in 15 (15.6%) of 96 patients, and the rates of SVR and NVR were 58.3% (56/96) and 20.1% (25/96) in studied patients. Response to treatment was significantly different among IL-28B-CC, CT, and TT genotypes (P = 0.003). Most of the patients with SVR had IL-28B-CC, most of the patients with NVR had IL-28B-CT and relapse was observed in most of patients with IL-28B-TT.
Table 2.
The frequency of IL-28B genotype with response to PEG-IFN-α/RBV treatment

Characteristics and biochemical features of patients in regard to response to treatment 6 months after completion of treatment were reported in Table 3. There were no significant differences between treatment responses for mean of age, gender combination, BMI, and ALT (P > 0.05). Most of the patients (65%) with relapse or NVR had HCV-RNA lower than 106, whereas most of the patients with SVR (79%) had HCV-RNA than 106 (P = 0.001).
Table 3.
Demographic and biochemical features of patients by SVR and relapse

Results on factors associated with SVR 6 months after treatment by multivariate logistic regression are shown in Table 4. On multivariate analysis, at the end of treatment, only IL-28B CC (odds ratio [OR] = 0.053, 95% confidence interval [CI]; 0.005-0.54, P = 0.013), was independent predicting factor for response to treatment. And other factors such as age, gender, ALT, and IL-28B CT were not significant predicting factors (P > 0.05).
Table 4.
Factors associated with SVR by multivariate logistic regression

DISCUSSION
Interleukin-28B variations were shown to be the strongest pretreatment predictor of virological response in HCV genotype 1 patients.[26] In the present study, we validated the clinical significance of the IL-28B polymorphisms on treatment outcome in patients with CHC genotypes 1, and our results showed that the prevalence of IL-28B genotypes was 65%, 27%, and 8% for CT, CC, and TT, respectively. 6 months after the end of the treatment, relapse and NVR rate was 41.7%, and the rate of SVR was 58.3%. SVR rate in patients with IL-28B-CC was 76.9%, in patients with IL-28B-CT was 56.4%, and in patients with IL-28B-TT was 12.5%. Among IL-28B genotypes, IL-28B CC with OR = 3.180, 95% CI; 1.109-9.117, was the only significant independent predicting factor.
Previous studies reported varies rate of SVR. In a retrospective study reported, 81.2% in all genotypes and 75.5% in genotype 1.[27] Kang et al., reported 69.5% in genotype 1,[28] and Lee et al., reported the rate of 68.4% in genotype 1.[29] Furthermore, SVR rates have been reported as 42-51% in genotype 1 in Western countries.[14,30,31] In the present study, 6 months after the end of 48-week treatment, the rate of SVR was 58.3%, which was lower to some previous reports[27,28,29] and was higher other reports in Western countries, African-Americans, and Asians patients.[30,31,32,33] Some reasons might be explained the discrepancy among studies, such as the high compliance percentage of patients available for follow-up observation after treatment type in studies with high SVR rates. A difference in race in studied population is another probable factor to explain the different in SVR rates. The IL-28B gene allele frequency was reported to vary according to ethnicity. It is indicated that a large proportion of the Asian population in some studies from different parts of Asia is of IL-28B genotype CC,[20,33] which explains the high SVR rates in patients in this area, whereas IL-28B TT genotype is reported more common in African-American patients with CHC who typically do not respond to PEG-IFN α-2 and RBV treatment.
In the present study, the IL-28B CT genotype, followed by the CC genotype, was the most prevalent. SVR rate was 76.9%, 56.4%, and 12.5%, in patients with IL-28B-CC, IL-28B-CT, and IL-28B-TT, respectively. In a multicenter German study, HCV genotype-1 patients were enrolled and treated with Peg-IFN and RBV, SVR was achieved in 85%, 58%, and 46% of patients with CC, CT, and TT IL-28B genotypes, respectively.[34] As shown, SVR rate in patients with IL-28B-CC and IL-28B-CT is similar in these two studies but in German TT IL-28B genotypes SVR is reported higher than our results. Our findings agreed with another study that reported 79% of SVR in patients with IL-28B-CC and 24% of those with IL-28B-TT,[35] and agreed well with the observations by Ge et al., who reported 81% SVR in IL-28B-CC and 33% in IL-28B-TT genotypes.[19] Another study by Thompson et al., showed that in patients with the IL-28B-CC genotype, the SVR was 69%, compared to 33% in those with the IL-28B-CT genotype and 27% in those with the IL-28B-TT genotype.[36] In the same study by Stättermayer et al., the SVR rate was 79.1% in the CC genotype vs. 43.2% in patients with TT genotype.[37] Similar results were obtained in the group of patients in the study by Sporea et al., which reported 73.1% SVR rate in patients with IL-28B-CC genotype, 40.9% in patients with IL-28B-CT genotype, and 57.1% in patients with IL-28B-TT genotype.[38] These results investigated that in CHC infected patients, the IL-28B genotype is a potent predictor of response therapy. Thus, for genotype1-infected patients, tests for IL-28B rs12979860 polymorphisms may help guide the physician in treatment regimen and design and/or the patient's decision to undergo therapy.
The present study has a limitation by selection bias because this was conducted on patients in a single center. Furthermore, only selected patients who agreed to human gene sequencing of IL-28B and only one IL-28B genotyping of rs12979860 was assessed in the present study. Another limitation is the lack of data on biopsies findings because most of the studied patients refused biopsy, therefore, supplementary studies are suggested to assess the relation between genotyping, treatment response, and different alleles based on biopsies findings.
In summary, our results have shown that IL-28B (rs12979860) was an important predictor of CHC treatment outcome with PEG-IFN α-2 and RBV in patients with HCV genotype I infection in Isfahan, Iran. Patients with the IL-28B-CC genotype had higher rates of SVR than those with the IL-28B CT or TT genotype, and IL-28B-CC seems to be more important than IL-28B-CT/TT in predicting positive treatment response. However, to clarify the mechanism of IL-28B genotyping and its impact on treatment response, further studies are needed to be done.
AUTHOR'S CONTRIBUTION
HK carried out the design and coordinated the study, participated in most of the experiments and prepared the manuscript. TT provided assistance in the design of the study, coordinated and carried out all the experiments and participated in manuscript preparation. BB and ShH participated in most of the experiments and prepared the manuscript. All authors have read and approved the content of the manuscript.
ACKNOWLEDGMENT
This study was supported by a grant from Isfahan University of Medical Sciences, Isfahan, Iran, (No, 393290).
REFERENCES
- 1.Alter MJ. Epidemiology of hepatitis C virus infection. World J Gastroenterol. 2007;13:2436–41. doi: 10.3748/wjg.v13.i17.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ray Kim W. Global epidemiology and burden of hepatitis C. Microbes Infect. 2002;4:1219–25. doi: 10.1016/s1286-4579(02)01649-0. [DOI] [PubMed] [Google Scholar]
- 3.Munir S, Saleem S, Idrees M, Tariq A, Butt S, Rauff B, et al. Hepatitis C treatment: Current and future perspectives. Virol J. 2010;7:296. doi: 10.1186/1743-422X-7-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alavian SM. We need a new national approach to control hepatitis C: It is becoming too late. Hepat Mon. 2008;8:165–9. [Google Scholar]
- 5.Brown RS, Jr, Gaglio PJ. Scope of worldwide hepatitis C problem. Liver Transpl. 2003;9:S10–3. doi: 10.1053/jlts.2003.50244. [DOI] [PubMed] [Google Scholar]
- 6.WHO-World Health Organization. Hepatitis C virus. 2014. [cited 2014 July 10]. Available from: http://www.who.int/mediacentre/factsheets/fs164/en/
- 7.Chen SL, Morgan TR. The natural history of hepatitis C virus (HCV) infection. Int J Med Sci. 2006;3:47–52. doi: 10.7150/ijms.3.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsubota A, Fujise K, Namiki Y, Tada N. Peginterferon and ribavirin treatment for hepatitis C virus infection. World J Gastroenterol. 2011;17:419–32. doi: 10.3748/wjg.v17.i4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fried MW, Hadziyannis SJ. Treatment of chronic hepatitis C infection with peginterferons plus ribavirin. Semin Liver Dis. 2004;24(Suppl 2):47–54. doi: 10.1055/s-2004-832928. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi N, Takehara T. Antiviral therapy for chronic hepatitis C: Past, present, and future. J Gastroenterol. 2006;41:17–27. doi: 10.1007/s00535-005-1740-7. [DOI] [PubMed] [Google Scholar]
- 11.Alavian SM, Hajarizadeh B, Hajibeigi B, Doroudi T, Hamadanizadeh AK, Abar K. Efficacy and safety of pegylated interferon alfa-2a plus ribavirin for treatment of chronic hepatitis C and cirrhosis in Iranian. Hepat Mon. 2004;4:53–8. [Google Scholar]
- 12.Bafandeh Y, Saberi Firouzi M, Bagheri Lankarani K. Evaluation of combination therapy with interferon and ribavirin in patients with chronic hepatitis C: A genotype based study. J Mazandaran Univ Med Sci. 2007;17:9–16. [Google Scholar]
- 13.Namazee N, Sali S, Asadi S, Shafiei M, Behnava B, Alavian SM. Real response to therapy in chronic hepatitis C virus patients: A study from Iran. Hepat Mon. 2012;12:e6151. doi: 10.5812/hepatmon.6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Jr, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–82. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 15.Neumann-Haefelin C, Spangenberg HC, Blum HE, Thimme R. Host and viral factors contributing to CD8+ T cell failure in hepatitis C virus infection. World J Gastroenterol. 2007;13:4839–47. doi: 10.3748/wjg.v13.i36.4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen TY, Hsieh YS, Wu TT, Yang SF, Wu CJ, Tsay GJ, et al. Impact of serum levels and gene polymorphism of cytokines on chronic hepatitis C infection. Transl Res. 2007;150:116–21. doi: 10.1016/j.trsl.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Younossi ZM, McCullough AJ. Metabolic syndrome, non-alcoholic fatty liver disease and hepatitis C virus: Impact on disease progression and treatment response. Liver Int. 2009;29(Suppl 2):3–12. doi: 10.1111/j.1478-3231.2008.01949.x. [DOI] [PubMed] [Google Scholar]
- 18.Suppiah V, Moldovan M, Ahlenstiel G, Berg T, Weltman M, Abate ML, et al. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet. 2009;41:1100–4. doi: 10.1038/ng.447. [DOI] [PubMed] [Google Scholar]
- 19.Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N, et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41:1105–9. doi: 10.1038/ng.449. [DOI] [PubMed] [Google Scholar]
- 21.Merat S, Rezvan H, Nouraie M, Jafari E, Abolghasemi H, Radmard AR, et al. Seroprevalence of hepatitis C virus: The first population-based study from Iran. Int J Infect Dis. 2010;14(Suppl 3):e113–6. doi: 10.1016/j.ijid.2009.11.032. [DOI] [PubMed] [Google Scholar]
- 22.Sharafi H, Pouryasin A, Alavian SM, Behnava B, Keshvari M, Salimi S, et al. Distribution of IL28B genotypes in Iranian patients with chronic hepatitis C and healthy individuals. Hepat Mon. 2012;12:e8387. doi: 10.5812/hepatmon.8387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balagopal A, Thomas DL, Thio CL. IL28B and the control of hepatitis C virus infection. Gastroenterology. 2010;139:1865–76. doi: 10.1053/j.gastro.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gheorghe L, Iacob S, Sporea I, Grigorescu M, Sirli R, Damian D, et al. Efficacy, tolerability and predictive factors for early and sustained virologic response in patients treated with weight-based dosing regimen of PegIFN alpha-2b ribavirin in real-life healthcare setting. J Gastrointestin Liver Dis. 2007;16:23–9. doi: 10.1007/s11749-007-0047-9. [DOI] [PubMed] [Google Scholar]
- 25.Toyoda H, Kumada T, Tada T, Kawaguchi T, Murakami Y, Matsuda F. Impact of genetic polymorphisms near the IL28B gene and amino acid substitutions in the hepatitis C virus core region on interferon sensitivity/resistance in patients with chronic hepatitis C. J Med Virol. 2011;83:1203–11. doi: 10.1002/jmv.22092. [DOI] [PubMed] [Google Scholar]
- 26.Lange CM, Zeuzem S. IL28B single nucleotide polymorphisms in the treatment of hepatitis C. J Hepatol. 2011;55:692–701. doi: 10.1016/j.jhep.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Jeong SH, Jung YK, Yang JW, Park SJ, Kim JW, Kwon OS, et al. Efficacy of peginterferon and ribavirin is associated with the IL28B gene in Korean patients with chronic hepatitis C. Clin Mol Hepatol. 2012;18:360–7. doi: 10.3350/cmh.2012.18.4.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang MJ, Jung EU, Park SW, Choi P, Kim JH, Park SJ, et al. Effects of pegylated interferon and ribavirin in Korean patients with chronic hepatitis C virus infection. Korean J Hepatol. 2008;14:318–30. doi: 10.3350/kjhep.2008.14.3.318. [DOI] [PubMed] [Google Scholar]
- 29.Lee HJ, Eun JR, Choi JW, Kim KO, Moon HJ. Comparison of therapeutic results between combination therapy of peginterferon alpha-2a plus ribavirin and interferon alpha-2b plus ribavirin according to treatment duration in patients with chronic hepatitis C. Korean J Hepatol. 2008;14:46–57. doi: 10.3350/kjhep.2008.14.1.46. [DOI] [PubMed] [Google Scholar]
- 30.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: A randomised trial. Lancet. 2001;358:958–65. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 31.Hadziyannis SJ, Sette H, Jr, Morgan TR, Balan V, Diago M, Marcellin P, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: A randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346–55. doi: 10.7326/0003-4819-140-5-200403020-00010. [DOI] [PubMed] [Google Scholar]
- 32.Rangnekar AS, Fontana RJ. Meta-analysis: IL-28B genotype and sustained viral clearance in HCV genotype 1 patients. Aliment Pharmacol Ther. 2012;36:104–14. doi: 10.1111/j.1365-2036.2012.05145.x. [DOI] [PubMed] [Google Scholar]
- 33.Liu CH, Liu CJ, Lin CL, Liang CC, Hsu SJ, Yang SS, et al. Pegylated interferon-alpha-2a plus ribavirin for treatment-naive Asian patients with hepatitis C virus genotype 1 infection: A multicenter, randomized controlled trial. Clin Infect Dis. 2008;47:1260–9. doi: 10.1086/592579. [DOI] [PubMed] [Google Scholar]
- 34.Sarrazin C, Schwendy S, Moeller B, Dikopoulos N, Buggisch P, Encke J, et al. Completely individualized treatment durations with peginterferon-alfa-2b and ribavirin in HCV genotype 1-infected patients and importance of IL28B genotype. Hepatology. 2010;52:384. [Google Scholar]
- 35.Lindh M, Lagging M, Arnholm B, Eilard A, Nilsson S, Norkrans G, et al. IL28B polymorphisms determine early viral kinetics and treatment outcome in patients receiving peginterferon/ribavirin for chronic hepatitis C genotype 1. J Viral Hepat. 2011;18:e325–31. doi: 10.1111/j.1365-2893.2010.01425.x. [DOI] [PubMed] [Google Scholar]
- 36.Thompson AJ, Muir AJ, Sulkowski MS, Ge D, Fellay J, Shianna KV, et al. Interleukin-28B polymorphism improves viral kinetics and is the strongest pretreatment predictor of sustained virologic response in genotype 1 hepatitis C virus. Gastroenterology. 2010;139:120–9. doi: 10.1053/j.gastro.2010.04.013. e18. [DOI] [PubMed] [Google Scholar]
- 37.Stättermayer AF, Stauber R, Hofer H, Rutter K, Beinhardt S, Scherzer TM, et al. Impact of IL28B genotype on the early and sustained virologic response in treatment-naïve patients with chronic hepatitis C. Clin Gastroenterol Hepatol. 2011;9:344–350. doi: 10.1016/j.cgh.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 38.Sporea I, Popescu A, Curescu M, Sirli R, Dan I, Goldis A, et al. The Correlation of Il28B genotype with sustained virologic response in romanian patients with chronic hepatitis C. Hepat Mon. 2011;11:975–9. doi: 10.5812/kowsar.1735143x.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]


