Abstract
Background:
Although, patent ductus arteriosus (PDA) is associated with significant morbidity due to hemodynamic instability in preterm infants, the effect of ductus closure on mortality and morbidity is a controversial issue. The aim is to evaluate the efficacy of oral and intravenous (IV) ibuprofen treatment on ductal closure and effects on mortality and bronchoplumonary dysplasia.
Materials and Methods:
The medical records of 292 premature infants treated at Ege University Neonatal Intensive Care Unit were retrospectively evaluated. Patients were classified into 3 groups as; No PDA, hemodynamically insignificant PDA (hiPDA) and hemodynamically significant PDA (hsPDA) according to the presence and hemodynamical significance of PDA by echocardiography. hsPDA group was treated with IV or oral ibuprofen.
Results:
Patent ductus arteriosus was diagnosed by routine echocardiography in 145 patients, of whom 78 (53.7%) had hsPDA. All 65 infants with hiPDA had spontaneous PDA closure. Echocardiographic measurements were similar to those patients treated with oral or IV ibuprofen, as in the response rate to treatment without serious adverse effects. The presence of respiratory distress syndrome, surfactant therapy, late sepsis, bronchopulmonary dysplasia (BPD) and mortality rates were significantly higher in patients with hsPDA. However, with stepwise logistic regression; 5th min Apgar score (odds ratio [OR], 1.321, 95% confidence interval [CI], 1.063-1.641, P = 0.012) and gestational age (OR, 1.422, 95% CI, 1.212-1.662, P < 0.001) were the only significant variables associated with mortality. Gestational age (OR, 0.680, 95% CI, 0.531-0.871, P = 0.002) was the only significant variable associated with BPD shown with logistic regression.
Conclusion:
Ibuprofen treatment is effective for hsPDA closure with minimal side effects. HiPDA can close spontaneously; therefore treatment decision should be individualized. However, medical treatment of PDA does not reduce mortality and BPD.
Keywords: Bronchopulmonary dysplasia, ibuprofen, neonates, patent ductus arteriosus
INTRODUCTION
Ductus arteriosus is the arterial connection between pulmonary artery and aorta that shunts blood away from lungs during fetal life. The constriction of smooth muscle in the ductus structure causes the functional closure of the ductus by luminal constriction in the first 24 h. The permanent closure is the result of degeneration and fibrosis of subintimal layers in a few weeks. In the preterm infant, persistent patency of the arterial duct is usually a result of hypoxia or immaturity, with the ductus usually having a normal structure.[1,2,3]
Incidence of patent ductus arteriosus (PDA) is inversely correlated both with gestational age and birthweight.[1,4,5] The incidence of PDA is 45% in preterm infants under 1750 g and 80% under 1200 g.[6,7,8] In preterm infants, PDA is associated with significant morbidity due to hemodynamic instability. Those patients with significant hemodynamic changes may present congestive heart failure or in the long-term may develop irreversible changes in pulmonary vascularity leading to pulmonary hypertension.[9] There may be significant co-morbidity including pulmonary edema and bleeding, bronchopulmonary dysplasia (BPD), intraventricular hemorrhage (IVH) bleeding, necrotizing enterocolitis (NEC), feeding intolerance and retinopathy of prematurity (ROP).[9] However, in recent articles the effect of ductus closure on mortality and morbidity is a controversial issue.[10,11,12] The objective of this study is to evaluate the efficacy of intravenous (IV) and oral ibuprofen preparations on ductal closure in PDA and to determine whether the presence, hemodynamic significance and particularly treatment of PDA had any effects on mortality and BPD apart from the underlying neonatal characteristics.
MATERIALS AND METHODS
Patient populations
The medical records of 292 premature infants treated at Ege University Neonatal Intensive Care Unit (NICU) between January 1, 2008 and December 31, 2010 were retrospectively evaluated. The inclusion criteria were the following:
Born at the Ege University Hospital or transferred to Ege University NICU within 72 h following the birth,
performance of echocardiography within 24-72 h of life,
gestational age ≤34 weeks,
absence of congenital anomalies, including congenital heart disease other than PDA.
Demographic details including maternal problems, gestational age, gender, Apgar scores, early and late-onset sepsis, BPD, mortality and the presence or treatment of PDA were collected. Ethical Committee approval was obtained from Ege University with the Ethical Committee decision number of 11-12.2/4.
Diagnosis of patent ductus arteriosus
As a part of the routine protocol of our unit, echocardiography was performed in the first 48-72 h of life in all preterms admitted to NICU by the same pediatric cardiologist (Z.U.). Hemodynamically significant PDA (hsPDA) was defined with the echocardiographic evidence of ductal lumen over 1.5 mm and left atrium diameter (LA)/aortic root diameter (Ao) ratio over 1.4 and documentation of left to right shunt.[13,14] Patients with hemodynamically insignificant PDA (hiPDA) were not given any medical treatment but were followed up echocardiographically for ductal closure. Infants with no prior evidence of Hs PDA who had developed clinical symptomatology after the first 72 h of life we re-evaluated with echocardiography.
Treatment of patent ductus arteriosus
Medical therapy for hsPDA was given either as IV or oral ibuprofen as a dose of 10 mg/kg/g for the 1st day and 5 mg/kg/g for the second and 3rd day that comprises one course of treatment. All the patients treated for PDA had needed respiratory support. In 2008, IV ibuprofen was quite expensive and difficult to find in markets of Turkey and oral ibuprofen have frequently been used since earlier reports showed successful ductal closure with oral ibuprofen.[15] In the following year both preparations were available, and the choice of medical treatment for PDA was according to individual physician practice. On the 4th day of IV treatment, closure of ductus arteriosus was re-evaluated by echocardiography to decide for the requirement of an additional course of ibuprofen. Patients who failed first oral ibuprofen course therapy was changed to IV ibuprofen up to two courses. The surgical ligation was performed in patients unresponsive to three courses of treatment.
Diagnosis of complications
Clinical sepsis was defined as documentation of infection with a serious systemic illness in which noninfectious explanations for the abnormal pathophysiologic state are excluded or unlikely. By definition, in early clinical sepsis clinical signs appear in the first 5 days and in late sepsis >5 days.[16]
Respiratory distress syndrome (RDS) was diagnosed clinically with early respiratory distress manifested with cyanosis, grunting, retractions and tachypnea. The diagnosis was confirmed with blood gas analysis and chest X-ray with a classical “ground glass” appearance and air bronchograms. Surfactant therapy was administered as described in European Consensus Guidelines and American Academy of Pediatrics.[17,18] Surfactant therapy was applied prophylactically or as rescue therapy to reduce the risk of neonatal mortality.[18] Rescue surfactant was most often administered within the first 12 h after birth when specified threshold criteria for RDS were met.
Bronchopulmonary dysplasia was defined according to universal guidelines in infants with prolonged oxygen requirement and accompanying radiological changes.[19] Infants born before 32 weeks of gestation had standard Vitamin A supplement by intramuscular injection of 5000 IU on 3 days/week for 4 weeks.[20] Caffeine was given prior to extubation to prevent extubation failure.
Statistics
The data were analyzed using SPSS for Windows, Version, 15.0 (Chicago,SPssInc. USA). Data were summarized as percents, means ± standard deviation or median and interquartile ranges (IQRs) where appropriate.
The normal distribution of cases is analyzed by Shapiro-Wilk test. The comparison of categorical data set was performed with Chi-square test. Logistic regression analysis was performed for the analysis of risk factors. In not normally distributed data Mann-Whitney U-test was utilized for comparison. In comparison of more than two groups Kruskal-Wallis test was used. In normally distributed data, two groups were compared with Student's t-test and analysis of variance was used for the comparison of more than two groups. In the analysis of variance, Bonferroni test was used for post-hoc analysis.
RESULTS
Incidence of patent ductus arteriosus
Two hundred and ninety-two preterm infants eligible for the study were admitted to our NICU for the 3 years of study period. PDA was diagnosed by echocardiography in 145 patients, of whom 78 (53.7%) had hsPDA [Figure 1]. In whole patients (n = 292), 50 of them were under 28 weeks of gestation and 62% (n = 31) had hsPDA; 120 of them were between 28 and 31 weeks of gestation and 25% (n = 30) had hsPDA and 122 of them >31 weeks of gestation and 13% (n = 17) had hsPDA (P < 0.001).
Figure 1.
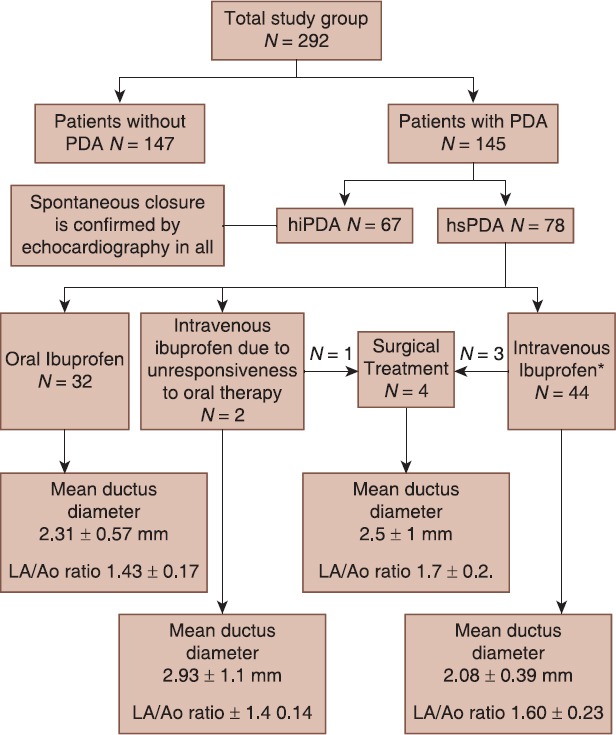
Study diagram showing study subgroups. LA/Ao = Ratio of left atrium diameter to aortic root diameter; hsPDA = Hemodynamically significant patent ductus arteriosus; hiPDA = Hemodynamically insignificant patent ductus arteriosus
Of the 78 of patients with hs PDA; 23 (29.5%) on supplemental oxygen, 13 (16.7%) was on continuous positive airway pressure, 42 (53.8%) was intubated on Synchronized intermittent mandatory ventilation.
The study diagram showing study subgroups and treatment response is given in Figure 1. Clinical variables of subgroups are demonstrated in Table 1. The gender difference was insignificant with respect to hsPDA. Mean birth weight and mean gestational age of patients without PDA were higher than hsPDA and hiPDA subgroups (P < 0.001, P < 0.001) [Table 1].
Table 1.
The clinical variables of patients
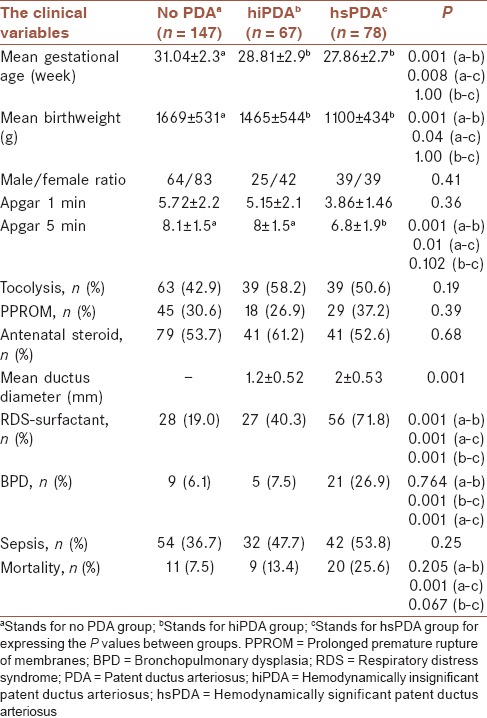
The frequencies of antenatal corticosteroid use, tocolytic therapy and premature rupture of membranes (PROM) were similar (P = 0.68), (P = 0.19), (P = 0.39). 5th min Apgar scores were inversely correlated with the presence of PDA (P < 0.01). Late sepsis, unlike the early sepsis, was significantly correlated with PDA status (P = 0.03) and (P = 0.74).
Echocardiographic findings
The mean ductus diameter was significantly higher in hsPDA group than hiPDA group as expected (P < 0.05) [Table 1]. The ductus diameters were not significantly different between oral and IV ibuprofen groups (P = 0.081) [Table 2].
Table 2.
Clinical features of hsPDA patients who had intravenous or oral ibuprofen treatment
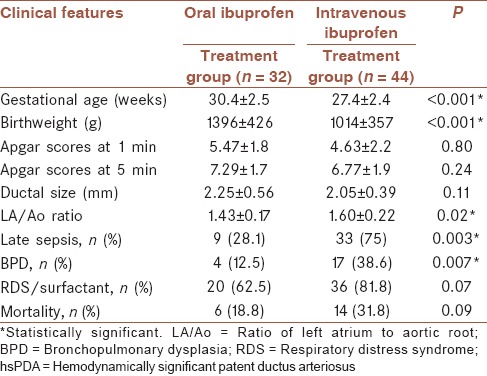
Treatment with surfactant
Prophylactic or rescue surfactant was given to 91 patients with the diagnosis of RDS, of whom 56 (73.7%) had hsPDA. The presence of RDS, surfactant therapy, BPD development and mortality rates were significantly higher in patients with hsPDA (P < 0.001) for all [Table 1].
Treatment with ibuprofen
Thirty-two patients received oral ibuprofen and 44 patients had IV ibuprofen. The response rates were 93.7% and 91.3% to oral ibuprofen and IV ibuprofen treatments, respectively. One of the two preterm infants who were unresponsive to oral ibuprofen therapy was successfully treated with IV ibuprofen therapy. The median postnatal age at the start of the first treatment was 2 days (min = 1, max = 14, IQR = 1).
The other patient did not respond to treatment well and had surgical ductal ligation procedure. Three other patients who were unresponsive to repeated IV ibuprofen treatment underwent surgical ductal ligation. These four infants who required surgical closure had ductus diameters of 2, 2, 2, 4 mm; were 880, 1090, 800, 1200 g and 27, 27, 25 and 30 gestational weeks. Three infants who had surgical ductal closure were discharged from hospital, but one infant died during the operation [Figure 1]. All 65 infants with hiPDA findings on echocardiography had spontaneous PDA closure. There were no serious adverse effects of ibuprofen treatment necessitating interruption of therapy. The minor side-effects were feeding intolerance in 7 cases, mild thrombocytopenia in 4 cases and mild creatinine elevation in 8 cases.
Patent ductus arteriosus was diagnosed after the 7th day in 7 patients, all of whom were in the late sepsis episodes. Four other preterms who were previously treated for PDA had late sepsis associated with PDA reopening and all responded again to ibuprofen treatment.
Mortality and morbidity
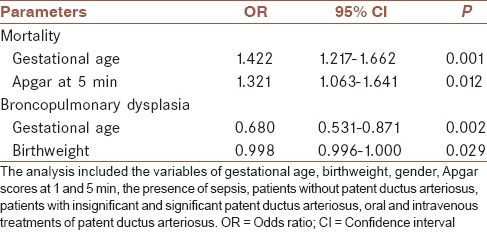
Hemodynamically significant PDA was related to significantly higher mortality rate (25.6%) compared with hiPDA (13.9%) and patients without PDA (7.5%) (P = 0.001). However; when individual effects of gestational age, birthweight, gender, sepsis, Apgar scores, no PDA, hiPDA, hsPDA, oral and IV therapies were analyzed with stepwise logistic regression. With stepwise forward logistic regression; 5th min Apgar score (odds ratio [OR], 1.321, 95% confidence interval [CI], 1.063-1.641, P = 0.012) and gestational age (OR, 1.422, 95% CI, 1.212-1.662, P < 0.001) were the only significant variables associated with mortality. Gestational age (OR, 0.680, 95% CI, 0.531-0.871, P = 0.002) was the only significant variable associated with BPD shown with logistic regression [Table 3].
Table 3.
The results of forward stepwise logistic regression analysis for mortality and bronchopulmonary dysplasia
DISCUSSION
Ductus arteriosus, which has a very important role in fetal circulation generally, closes in the first few days of life in term neonates. However in preterm infants functional closure of the ductus arteriosus is more difficult due to oxygen insensitivity of immature ductal tissues.[2,3,6] The presence of PDA is negatively correlated with gestational age and birthweight.[1,4,5] In this study, PDA was present in 145 out of 292 preterm infants (48.4%); of whom 78 had hsPDA. Mean birth weight and mean gestational age were negatively correlated with the presence of PDA and hsPDA (P < 0.001).
Respiratory distress syndrome, perinatal asphyxia and excessive fluid replacement in first few days of life increase the risk of hsPDA in preterm infants. Infection and inflammatory cytokines delay ductus closure and/or causing re-opening of ductus arteriosus in later stages.[21] The use of surfactant in RDS improves pulmonary disease, decreases pulmonary vascular resistance; however, increases the risk of left-to-right shunt. Kumar et al.[22] showed that newborn infants treated with surfactant were more likely to have hsPDA, larger transductal diameters and increased frequency of therapeutic interventions to close the ductus. The administration of antenatal steroids decreases the frequency of RDS and symptomatic PDA both.[21] However; Kesiak et al.[23] did not find such relation. In our study group, antenatal steroid use was not related to the incidence of PDA and patients with hsPDA had higher rates of low Apgar scores at 5 min, RDS and surfactant therapy [Table 1]. Therefore, we conclude that asphyxia, RDS and surfactant treatment increase the risk of hsPDA. We also showed that sepsis specifically late sepsis was concerned with the hsPDA. Four infants had re-opening of ductus arteriosus and three other infants without previous PDA, developed PDA in the course of late sepsis attacks. Both situations may be explained by the effects of cytokine release during sepsis. However, hsPDA frequency was not related to PROM or early sepsis.
Echocardiographic criteria for hsPDA are ductus diameter over 1.5 mm (1.5-2.0 mm), LA/Ao ratio over 1.4 (1.5-1.3) the presence of left-to-right shunt.[5,13,14,24] In the present study, the mean PDA diameter is 2.2 ± 0.5 and LA/Ao ratio is 1.5 ± 0.2 in preterms who received medical treatment due to hsPDA. The mean ductus diameter was higher in this group compared with hiPDA group who had spontaneous ductal closure (P < 0.001).
After the diagnosis of PDA the treatment alternatives are conservative management, pharmacological therapy and surgical ligation. Medical closure of PDA is often performed for very low birth weight infants with hsPDA with the hope of decreasing mortality and morbidity.[25]
Oral administration of ibuprofen in very preterm infants is associated with excellent absorption, and it is easy to administer and inexpensive and may be an alternative for IV administration.[15,26] In some studies, IV ibuprofen is shown to be effective when there is no response to oral ibuprofen.[27] Cherif et al.[28] compared oral and IV routes of ibuprofen and declared similar rates of ductal closure with oral ibuprofen, however oral route was associated with fewer adverse effects. Gokmen et al.[29] claimed that oral ibuprofen was more effective than IV ibuprofen for ductal closure in infants with very low birth weight. In a large scale study of Olukman et al.[30] oral ibuprofen therapy was found as efficacious as IV ibuprofen with some concerns about increased sepsis and BPD incidence. On the other hand, IV ibuprofen was used in smaller infants in this study, and increased frequency of hypernatremia was observed.
In the present study, Apgar scores at the first and 5th min and ductal diameters were similar between IV and oral treatment groups. However, the patients in the IV ibuprofen group had significantly higher sepsis and BPD rates indicating that clinicians tend to choose IV treatment for the sicker infants [Table 2]. However, the response rates were similar to findings those of 93.7% for oral ibuprofen and 91.3% for IV ibuprofen. No serious side-effects that might cause the cessation of treatment occurred during the courses of two types of medical therapies.
Although both indomethacin and ibuprofen are demonstrated to be effective for PDA closure, the issue of the requirement for ductal closure is controversial. In a recent Cochrane meta-analysis, the ductal closure has been shown to have no beneficial effect on NEC, ROP, BPD, IVH, periventricular leukomalacia and mortality comparing placebo and treatment groups.[31] Mortality and morbidity in premature infants are not well explained by PDA itself, treatment of PDA or prematurity.[32] In the present study, mortality and BPD incidence were significantly higher in premature infants with hsPDA despite closure with medical or surgical treatment [Table 1]. On the other hand, logistic regression analysis showed that it was not hsPDA and treatment choices, which were effective in mortality. Moreover, BPD is significantly associated with lower gestational age, but not with hsPDA and treatment choices on logistic regression analysis [Table 3].
These findings point out that even with successful closure of PDA, poor outcomes related to asphyxia, prematurity, low birth weight and sepsis may not be prevented.
CONCLUSION
Hemodynamically significant PDA is encountered more frequently in very low birth weight infants, who also have RDS and need surfactant therapy. Both oral and IV preparations of ibuprofen are effective with minimal side-effects while hiPDA close spontaneously; therefore treatment decision should be individualized. However, to introduce a medical treatment for PDA is controversial because of inconclusive effects of treatment on mortality and morbidity.
AUTHOR'S CONTRIBUTION
All authors have contributed in designing and conducting the study. All authors have assisted in preparation of the first draft of the manuscript or revising it critically for important intellectual content. All authors have read and approved the content of the manuscript and confirmed the accuracy or integrity of any part of the work.
ACKNOWLEDGMENT
We would like to acknowledge Professor Mehmet Orman, from the Department of Biostatistics, Ege University Faculty of Medicine, for his assistance with the statistical analysis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Clyman RI. Mechanisms regulating the ductus arteriosus. Biol Neonate. 2006;89:330–5. doi: 10.1159/000092870. [DOI] [PubMed] [Google Scholar]
- 2.Antonucci R, Bassareo P, Zaffanello M, Pusceddu M, Fanos V. Patent ductus arteriosus in the preterm infant: New insights into pathogenesis and clinical management. J Matern Fetal Neonatal Med. 2010;23(Suppl 3):34–7. doi: 10.3109/14767058.2010.509920. [DOI] [PubMed] [Google Scholar]
- 3.Sankar MN, Clyman RI. Pharmacological closure of patent ductus arteriosus in the neonate. Neoreviews. 2003;8:215–21. [Google Scholar]
- 4.Gomelia TL, Cunningham MD, editors. Cardiac abnormalities. 4th ed. Chapter 62. New York: Appleton and Lange; 1999. Section IV. Diseases and disorders; pp. 335–52. Neonatology: Management, Procedures, On-Call Problems, Diseases, and Drugs. [Google Scholar]
- 5.Sekar KC, Corff KE. Treatment of patent ductus arteriosus: Indomethacin or ibuprofen? J Perinatol. 2008;28(Suppl 1):S60–2. doi: 10.1038/jp.2008.52. [DOI] [PubMed] [Google Scholar]
- 6.Park MK, Troxler RG. Manifestation of cardiac problems in newborns. In: Par MK, editor. Pediatric Cardiology for Practitioners. St. Louis: Mosby; 2002. pp. 386–8. [Google Scholar]
- 7.Giliberti P, De Leonibus C, Giordano L, Giliberti P. The physiopathology of the patent ductus arteriosus. J Matern Fetal Neonatal Med. 2009;22(Suppl 3):6–9. doi: 10.1080/14767050903198215. [DOI] [PubMed] [Google Scholar]
- 8.Reller MD, Rice MJ, McDonald RW. Review of studies evaluating ductal patency in the premature infant. J Pediatr. 1993;122:S59–62. doi: 10.1016/s0022-3476(09)90044-0. [DOI] [PubMed] [Google Scholar]
- 9.Cambonie G, Dupuy AM, Combes C, Vincenti M, Mesnage R, Cristol JP. Can a clinical decision rule help ductus arteriosus management in preterm neonates? Acta Paediatr. 2012;101:e213–8. doi: 10.1111/j.1651-2227.2011.02469.x. [DOI] [PubMed] [Google Scholar]
- 10.Noori S. Patent ductus arteriosus in the preterm infant: To treat or not to treat? J Perinatol. 2010;30(Suppl):S31–7. doi: 10.1038/jp.2010.97. [DOI] [PubMed] [Google Scholar]
- 11.Clyman RI, Couto J, Murphy GM. Patent ductus arteriosus: Are current neonatal treatment options better or worse than no treatment at all? Semin Perinatol. 2012;36:123–9. doi: 10.1053/j.semperi.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans N. Preterm patent ductus arteriosus: Should we treat it? J Paediatr Child Health. 2012;48:753–8. doi: 10.1111/j.1440-1754.2012.02542.x. [DOI] [PubMed] [Google Scholar]
- 13.Akısu M, Ozyurek AR, Dorak C, Parlar A, Kultursay N. The efficacy and safety of enteral ibuprofen and indomethacin in the treatment of premature infants with patent ductus arteriosus. Turkish Pediatric Journal. 2001;44:56–60. [Google Scholar]
- 14.Zonnenberg I, de Waal K. The definition of a haemodynamic significant duct in randomized controlled trials: A systematic literature review. Acta Paediatr. 2012;101:247–51. doi: 10.1111/j.1651-2227.2011.02468.x. [DOI] [PubMed] [Google Scholar]
- 15.Heyman E, Morag I, Batash D, Keidar R, Baram S, Berkovitch M. Closure of patent ductus arteriosus with oral ibuprofen suspension in premature newborns: A pilot study. Pediatrics. 2003;112:e354. doi: 10.1542/peds.112.5.e354. [DOI] [PubMed] [Google Scholar]
- 16.Chiesa C, Panero A, Osborn JF, Simonetti AF, Pacifico L. Diagnosis of neonatal sepsis: A clinical and laboratory challenge. Clin Chem. 2004;50:279–87. doi: 10.1373/clinchem.2003.025171. [DOI] [PubMed] [Google Scholar]
- 17.Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, et al. European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants - 2010 update. Neonatology. 2010;97:402–17. doi: 10.1159/000297773. [DOI] [PubMed] [Google Scholar]
- 18.Engle WA. American Academy of Pediatrics Committee on Fetus and Newborn. Surfactant-replacement therapy for respiratory distress in the preterm and term neonate. Pediatrics. 2008;121:419–32. doi: 10.1542/peds.2007-3283. [DOI] [PubMed] [Google Scholar]
- 19.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–9. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 20.Ambalavanan N, Wu TJ, Tyson JE, Kennedy KA, Roane C, Carlo WA. A comparison of three vitamin A dosing regimens in extremely-low-birth-weight infants. J Pediatr. 2003;142:656–61. doi: 10.1067/mpd.2003.214. [DOI] [PubMed] [Google Scholar]
- 21.Bancalari E, Claure N, Gonzalez A. Patent ductus arteriosus and respiratory outcome in premature infants. Biol Neonate. 2005;88:192–201. doi: 10.1159/000087582. [DOI] [PubMed] [Google Scholar]
- 22.Kumar A, Lakkundi A, McNamara PJ, Sehgal A. Surfactant and patent ductus arteriosus. Indian J Pediatr. 2010;77:51–5. doi: 10.1007/s12098-009-0299-3. [DOI] [PubMed] [Google Scholar]
- 23.Kesiak M, Nowiczewski M, Gulczynska E, Kasprzak E, Binikowska J, Gadzinowski J. Can we expect decreasing the incidence of patent ductus arteriosus (PDA) in the population of premature neonates who had received antenatal steroid therapy? Ginekol Pol. 2005;76:812–8. [PubMed] [Google Scholar]
- 24.Desandes R, Jellimann JM, Rouabah M, Haddad F, Desandes E, Boubred F, et al. Echocardiography as a guide for patent ductus arteriosus ibuprofen treatment and efficacy prediction. Pediatr Crit Care Med. 2012;13:324–7. doi: 10.1097/PCC.0b013e31822882b5. [DOI] [PubMed] [Google Scholar]
- 25.Jhaveri N, Moon-Grady A, Clyman RI. Early surgical ligation versus a conservative approach for management of patent ductus arteriosus that fails to close after indomethacin treatment. J Pediatr. 2010;157:381–7.e1. doi: 10.1016/j.jpeds.2010.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barzilay B, Youngster I, Batash D, Keidar R, Baram S, Goldman M, et al. Pharmacokinetics of oral ibuprofen for patent ductus arteriosus closure in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2012;97:F116–9. doi: 10.1136/adc.2011.215160. [DOI] [PubMed] [Google Scholar]
- 27.Yalaz M, Calkavur S, Cetinkaya B, Peker E, Levent E, Akisu M, et al. Intravenous ibuprofen treatment in a premature infant unresponsive to two different treatment modalities. The Bulletin of Ege Pediatrics. 2004;11:209–13. [Google Scholar]
- 28.Cherif A, Khrouf N, Jabnoun S, Mokrani C, Amara MB, Guellouze N, et al. Randomized pilot study comparing oral ibuprofen with intravenous ibuprofen in very low birth weight infants with patent ductus arteriosus. Pediatrics. 2008;122:e1256–61. doi: 10.1542/peds.2008-1780. [DOI] [PubMed] [Google Scholar]
- 29.Gokmen T, Erdeve O, Altug N, Oguz SS, Uras N, Dilmen U. Efficacy and safety of oral versus intravenous ibuprofen in very low birth weight preterm infants with patent ductus arteriosus. J Pediatr. 2011;158:549–54.e1. doi: 10.1016/j.jpeds.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Olukman O, Calkavur S, Ercan G, Atlihan F, Oner T, Tavli V, et al. Comparison of oral and intravenous Ibuprofen for medical closure of patent ductus arteriosus: Which one is better? Congenit Heart Dis. 2012;7:534–43. doi: 10.1111/j.1747-0803.2012.00668.x. [DOI] [PubMed] [Google Scholar]
- 31.Ohlsson A, Walia R, Shah SS. Ibuprofen for the treatment of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2013;4:CD003481. doi: 10.1002/14651858.CD003481.pub5. [DOI] [PubMed] [Google Scholar]
- 32.Herrman K, Bose C, Lewis K, Laughon M. Spontaneous closure of the patent ductus arteriosus in very low birth weight infants following discharge from the neonatal unit. Arch Dis Child Fetal Neonatal Ed. 2009;94:F48–50. doi: 10.1136/adc.2007.129270. [DOI] [PubMed] [Google Scholar]


