Abstract
Background:
The renin angiotensin aldosterone system (RAAS) plays a vital role in regulating glucose metabolism and blood pressure, electrolyte and fluid homeostasis. The aim of this systematic review is to assess the association of the RAAS genes with diabetes mellitus (DM) and its complications of retinopathy, neuropathy and cardiovascular disease (CVD).
Materials and Methods:
The relevant English-language studies were identified using the key words of DM, type 1 diabetes mellitus (T1DM), T2DM, renin angiotensin aldosterone polymorphisms or genotypes and RAAS from the search engines of MEDLINE/PubMed, and Scopus from January 1, 1995 to July 30, 2014. Inclusion criteria for selecting relevant studies were reporting the role of RAAS gene variants in the pathogenesis of T1DM or T2DM, diabetic retinopathy (DR), diabetic neuropathy and cardiovascular complication of DM.
Results:
The reviewers identified 204 studies of which 73 were eligible for inclusion in the present systematic review. The review indicates the angiotensinogen (AGT) M235T polymorphism might not affect the risk of DM. The role of angiotensin converting enzyme insertion/deletion (ACE I/D) and angiotensin II type 1 receptor gene (AT1R) A1166C polymorphisms in the pathogenesis of DM could not be established. Studies indicate the absence of an association between three polymorphisms of AGT M235T, ACE I/D and AT1R A1166C and DR in DM patients. A protective role for ACE II genotype against diabetic peripheral neuropathy has been suggested. Also, the ACE I/D polymorphism might be associated with the risk of CVD in DM patients.
Conclusion:
More studies with adequate sample size that investigate the influence of all RAAS gene variants together on the risk of DM and its complications are necessary to provide a more clear picture of the RAAS genes polymorphisms involvement in the pathogenesis of DM and its complications.
Keywords: Aldosterone, angiotensin, cardiovascular, diabetes mellitus, diabetic neuropathy, diabetic retinopathy, renin, renin angiotensin aldosterone system genes
INTRODUCTION
Diabetes mellitus (DM) is the main problem of public health with significant economic burden worldwide. It is estimated there will be 552 million people with diabetes until 2030. DM patients have a susceptibility to develop microvascular complications such as retinopathy, and neuropathy and macrovascular complications of cardiovascular disease (CVD), stroke and peripheral arterial disease.[1] Retinopathy is the most common complication of DM. Around 30% of DM patients suffer from some forms of neuropathy.[2] A higher risk of diabetes development in those individuals with a family history of DM, the absence of diabetes micro- and macro-vascular complications in some patients in spite of poor glycemic control and ethnic dependent susceptibility to DM suggest the role of genetics in the etiology of DM.[2] The renin angiotensin aldosterone system (RAAS) is considered as an endocrine system that plays a vital role in regulating blood pressure, electrolyte and fluid homeostasis. The genes of RAAS have important roles in glucose metabolism and regulation of blood pressure. Activation of RAAS leads to elevated levels of the main vasoconstrictor peptide of the angiotensin II (Ang II). Ang II affects glucose homeostasis and is involved in the pathogenesis of DM through inhibition of insulin signal transduction, reduction of glucose uptake, resistance to insulin, and destroying the beta cells of pancreas by inducing oxidative stress.[3] The RAAS genes consisted of renin, angiotensinogen (AGT), angiotensin converting enzyme (ACE), ACE 2, angiotensin II type 1 receptor (AT1R) and AT2R. In some populations, the variants of the RAAS gene have been associated with DM and its complications and inhibition of RAAS has prevented the incidence of DM and its complications.[4,5] The knowledge of functional role of RAAS gene variants in the risk of incidence and progression of DM and inter individuals’ differences in susceptibility to DM and development of its micro- and macro-vascular complications will help to develop personalized medicine in the management of DM and its complications.
The present review will discuss the role of RAAS gene variants in the pathogenesis of DM, diabetic retinopathy (DR), diabetic neuropathy and cardiovascular complication of DM.
MATERIALS AND METHODS
Literature search
The relevant English language publications were identified from the search engines of MEDLINE/PubMed, and Scopus from January 1, 1995 to July 30, 2014. Using the key words of DM, type 1 diabetes mellitus (T1DM), T2DM, renin angiotensin aldosterone polymorphisms or genotypes and RAAS, 204 original, meta-analyses and review articles were identified of which 73 were eligible for inclusion in the present systematic review that includes some complimentary articles of the reference list of the selected articles.
Inclusion and exclusion criteria
Inclusion criteria for selecting relevant studies were reporting the role of RAAS gene variants in the pathogenesis of T1DM or T2DM, DR, diabetic neuropathy and cardiovascular complication of DM. Non-English language studies, unpublished studies and the articles that have reported the influence of RAAS gene variants in the pathogenesis of diabetic nephropathy, end-stage renal disease, renal and cerebrovascular complications of DM were excluded from the study.
Renin angiotensin aldosterone system cascade
Reduction in blood pressure and renal perfusion results in secretion of the renin enzyme from the juxtaglomerular cells of the kidney. The renin, an aspartyl protease, degrades AGT to inactive decapeptide of Ang I. The Ang I is converted to an active octapeptide of Ang II by ACE. Ang II increases the aldosterone secretion, elevates blood pressure and inhibits renin secretion. Further, Ang II is a vasoconstrictor peptide affects the releasing catecholamines from the adrenal medulla and prejunctional nerve endings. The ACE 2 degrades the Ang II to Ang (1-7) [Figure 1]. The Ang (1-7) through binding to its G-protein coupled receptor of the mass exerts vasodilatory, antiproliferative and apoptotic functions.[6]
Figure 1.
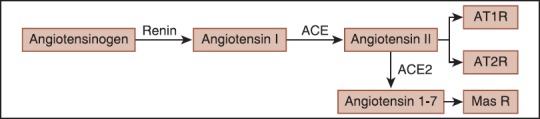
A schematic representation of the angiotensin II and angiotensin (1-7) formation
Local renin angiotensin aldosterone system
All components of the systemic RAAS are present in local or tissue RAAS. Local RAAS is regulated independent of systemic RAAS. The local RAAS has been found in kidneys, heart, blood vessels and many other tissues. It has been suggested that the effect of ACE insertion/deletion (I/D) variants on the progression of renal diseases could be independent of plasma levels of renin and angiotensins that might be attributed to the presence of intrarenal RAAS.[7]
Renin angiotensin aldosterone system, insulin signal transduction and diabetes mellitus
In diabetic patients hyperglycemia increases tissue Ang II that induces oxidative stress, glomerular hyperfiltration, endothelial damage, thrombosis, inflammation and vascular remodelling.[8] Activation of the RAAS and enhanced production of Ang II has an inhibitory effect on insulin signal transduction pathway. The Ang II prevents insulin receptor substrate-1 (IRS-1) phosphorylation with the subsequent decrease in phosphatidylinositol 3 kinase and also it reduces glucose uptake through GLUT4 that resulted in insulin resistance. Further, Ang II increases reactive oxygen species which leads to damaging the pancreatic β-cells and may indirectly impair insulin secretion from the pancreas through vasoconstriction and reduction in islet blood flow. Chronic exposure to high levels of glucose and fat induces oxidative stress, inflammation and apoptosis with participation of Ang II through AT1R in β-cells of pancreas. All these effects resulted in the development of DM.[3] In animal studies chronic infusion of Ang II increases superoxide production through NADH/NADPH oxidase.[9] The other component of the RAAS, aldosterone, decreases the insulin secretion from β-cells in a mechanism involves oxidative stress. Decreased production of Ang II and aldosterone or inhibition of both receptors of AT1R and mineralocorticoid has been improved insulin sensitivity in both in vivo and in vitro studies.[10] It has been demonstrated that the inhibition of RAAS by ACE inhibitors (ACE I) or AT1R blockers prevents the adverse effects of Ang II on glucose metabolism and insulin resistance and reduces the incidence of new-onset T2DM in individuals with hypertension and CVD.[3] The role of RAAS in the pathogenesis of insulin resistance in T2DM has been demonstrated in clinical trial studies using ACE I or Ang II receptor blockers (ARB). In T2DM patients, the benefit effects of ACE I or ARB on the metabolic pathways, cardiovascular and chronic kidney disease have been demonstrated.[11] RAAS blockers prevent insulin resistance in some, but not all T2DM patients indicating inter-individual variability. Results of a meta-analysis indicated that the treatment of nondiabetic individuals with ACE I and ARB decreased the risk of T2DM.[12]
Renin and prorenin
The renin gene is one of the candidate genes for salt-sensitive hypertension in animal studies. This gene locates on chromosome 1q32 contains 10 exons and encodes the inactive precursor of prorenin and also expresses renin.[13] The renin has an important role in the regulation of blood pressure and sodium homeostasis. In DM, elevation of Ang II inhibits renin secretion from juxtaglomerular cells and at the same time it enhances the secretion of prorenin from collecting ducts of the kidney.[14] The increased levels of prorenin and renin have been observed in T1DM patients. Also, in microvascular complications of DM, retinopathy, the plasma level of prorenin is increased.[15]
Angiotensinogen
The rate-limiting step of the RAAS is the enzymatic cleavage of AGT by renin and conversion of AGT to Ang II, which plays a primary role in the regulation of blood pressure. Polymorphisms in the promoter region of AGT are of significance because they may influence the strength of the AGT promoter and consequently, the levels of AGT and Ang II.[16]
Angiotensinogen M235T
The most studied polymorphism of AGT M235T locates on chromosome 1q41-q45 (rs699) and encodes threonine instead of methionine. The presence of AGT 235T allele is associated with increased plasma level of AGT. Although, the AGT M235T polymorphism has been complicated in the pathogenesis of arterial hypertension[17] but it has not been associated with hypertension in T2DM patients.[18] There are controversial reports related to the role of AGT M235T in susceptibility to DM and its complications [Table 1].
Table 1.
Main studies investigating the association between AGT M235T polymorphism and the risk of DM and its complications
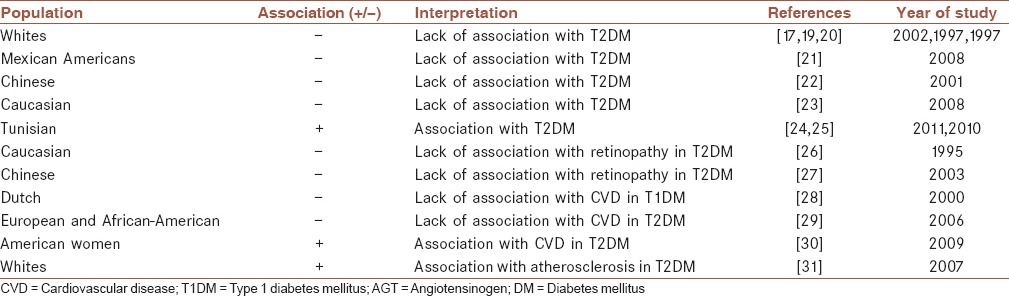
Angiotensinogen M235T and diabetes mellitus
A relationship between the AGT gene, AGT levels, and insulin sensitivity in humans has been suggested with an association between AGT M235T polymorphism and increased insulin resistance.[11]
Lack of association between AGT M235T with T2DM in Caucasian population has been reported.[17,19,20] Also, the AGT M235T polymorphism was not associated with T2DM in Mexican American families[21] and Chinese with T2DM.[22] Further, in a cohort study among Caucasian women without DM this polymorphism was not associated with the occurrence of T2DM.[23] However, among Tunisians the AGT 235T allele was significantly associated with the risk of T2DM.[24,25]
Many reports including large cohort studies in diabetic patients indicate the lack of an association between the AGT M235T polymorphism with the risk of DM.
Angiotensin converting enzyme gene
The ACE is a key RAAS component and plays an important role in blood pressure homeostasis by generating the vasoconstrictor peptide Ang II and by inactivating the vasodilator peptides bradykinin and Ang-(1-7).[32]
Angiotensin converting enzyme insertion/deletion
The ACE gene locates on chromosome 17q23 comprises 26 exons and 25 introns.[33] More than 160 polymorphisms have been known for the ACE gene that the most of them are single nucleotide polymorphisms.[34] The most studied polymorphism of ACE is an I/D polymorphism (rs1799752) consisting of 287-bp within intron 16.[35] This polymorphism was firstly described by Rigat et al.[36] and its presence is associated with higher plasma ACE activity. The presence of D allele is associated with highest ACE activity compared to the presence of I allele with the lowest ACE activity.[36,37] This polymorphism contributes in the pathogenesis of DM and its complications. ACE DD genotype has been associated with lower response to insulin in an oral glucose tolerance test in T2DM patients.[38]
Angiotensin converting enzyme insertion/deletion and susceptibility to diabetes mellitus
The role of ACE I/D polymorphism in the pathogenesis of DM and its complications in various populations are demonstrated in Table 2. In Chinese, Turkish and Malaysian patients the DD genotype of ACE was linked to increased risk of T2DM.[22,39,40] Also, the frequency of ACE D allele was significantly higher in T2DM patients than healthy individuals of Tunisian population.[25] Further, the DD genotype of ACE I/D polymorphism increased the susceptibility to T2DM in two ethnic groups of Arabs and Berbers from Tunisia.[41] In contrast, in another study among Tunisians this polymorphism was not a susceptibility factor for T2DM.[42] The ACE I/D polymorphism was not linked to T2DM in Caucasian Mediterranean population and Mexican American families[20,21] and was not involved in the occurrence of T2DM in Caucasian women.[23] Also, a recent meta-analysis indicated the absence of association between ACE I/D polymorphism and susceptibility to T2DM in Chinese population.[43] Conversely, a study from China reported that the frequency of ACE D allele was lower in T2DM patients, and the ACE II genotype was significantly prevalent in patients compared to controls.[44] The certain role of ACE I/D polymorphism in the pathogenesis of DM could not be confirmed.
Table 2.
ACE I/D polymorphism and the risk of DM and its complications in various populations
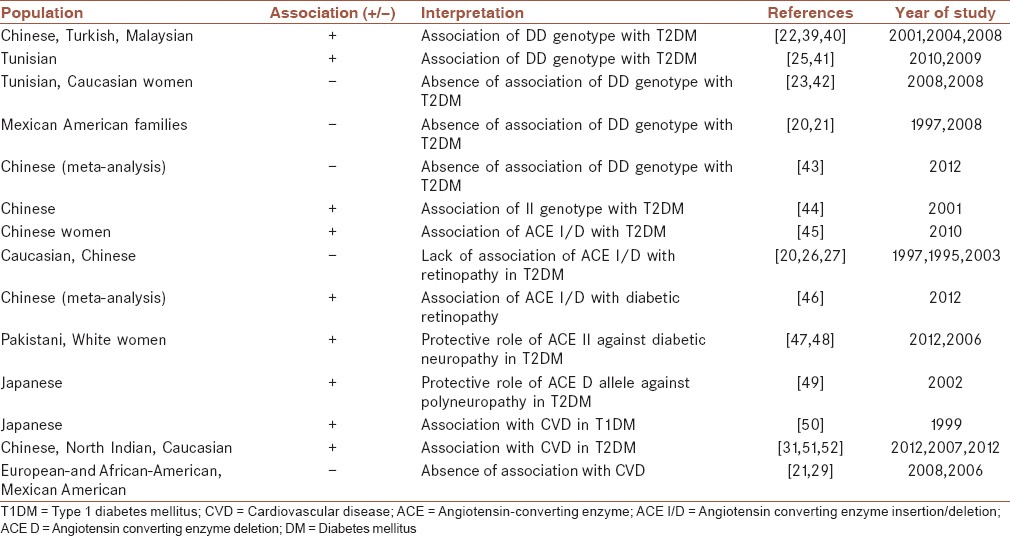
A gender effect of ACE I/D polymorphism on the Ang I level has been reported with a higher level of Ang I in women carriers of DD genotype than men carriers of the same genotype.[53] Also, among Chinese T2DM patients only in females the ACE I/D polymorphism were associated with T2DM.[45]
Angiotensin II type 1 receptor gene
Angiotensin II acts through two major subtypes G-protein coupled receptors of AT1R and AT2R. Both AT1R and AT2R are involved in the control of hypertension. The AT1R gene locates on chromosome 3q21-q25 and consists of five exons, four of which are untranslated and alternatively spliced.[25] The AT1R A1166C polymorphism (rs5186) was described by Bonnardeaux et al.[54] This polymorphism locates in the 3′-untranslated region of AT1R gene and may be involved in posttranscriptional modification of AT1R mRNA.[55]
Table 3 indicates the main studies which examined the role of AT1R A1166C polymorphism in the risk of DM and its complications. Two studies from India reported an association between AT1R 1166 C allele with T2DM.[56,57] Among Tunisians AT1R 1166C allele was associated with a significant risk of T2DM.[25] The lack of association between AT1R A1166C with the risk of DM among Mexican American families[21] and Chinese[22] with T2DM has been reported. Also, in a cohort study of Caucasian women this polymorphism was not associated with the occurrence of T2DM.[23]
Table 3.
Association studies of AT1R A1166C polymorphism with the risk of DM and its complications
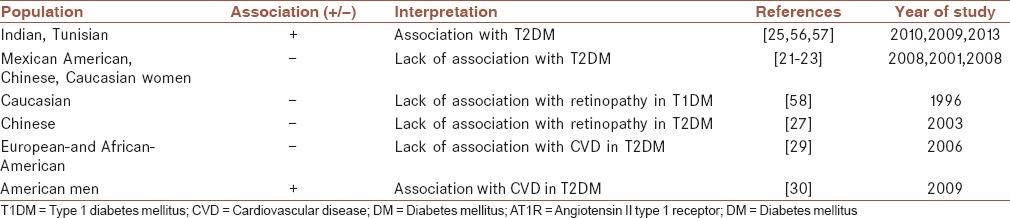
Angiotensin II type 2 receptor gene
The AT2R inhibits cell proliferation, mediates apoptosis and works cardio protectively against AT1R. The activation of AT2R has opposite effects of AT1R and decreases blood pressure through inhibition of renin biosynthesis and secretion from juxtaglomerular cells and causes vasodilation and natriuresis. AT2R is important for hemodynamic control of hypertension by vasodilation.[21] The AT2R gene locates on the chromosome X at the locus Xq23-26 and consists of three exons and two introns. A common AT2R G-1332A (G1675A) polymorphism (rs14035430) locates within intron 1 and is designated as G-1332A.[21,55]
Angiotensin converting enzyme 2 gene
The ACE2 is a new member of RAAS that shares 42% homology with ACE. The ACE2 gene locates on chromosome Xp22 and encodes ACE2 enzyme. ACE2 cleaves Ang I to Ang (1-9) and efficiently degrades Ang II to vasodilator peptide of Ang (1-7). ACE2 might be considered as a compensatory mechanism for hyperglycemia induced RAAS activation. ACE2 overexpression ameliorates the impaired glucose homeostasis by reducing fasting blood glucose and improvement of glucose tolerance in diabetic mice. ACE2 could be a novel target for the prevention of β-cell dysfunction in T2DM.[59]
In human populations, the ACE2 gene is highly polymorphic. The polymorphism of ACE2 G8790A locates in intron 3 and recognizes by Alu I restriction enzyme. In T2DM Chinese patients with and without coronary heart disease, the frequency of alleles and genotypes of G8790A polymorphism was not significantly different.[60]
Aldosterone
Aldosterone is synthesized by the rate-limiting enzyme of aldosterone synthase (CYP 11B2).[61] Aldosterone affects both blood pressure and glucose homeostasis. In patients with DM, the secretion of aldosterone is increased.[62] Aldosterone through down-regulation of IRS-1 in vascular smooth muscle cells impairs insulin signaling.[6]
The CYP11B2 gene locates on chromosome 8q21-q22. The most studied polymorphism of CYP 11B2 is T-344C (rs79998) in the promoter region. In French general population the presence of both polymorphisms of CYP 11B2 T-344C and G3097A was associated with the risk of T2DM in men with a higher risk of T2DM in the presence of TC and CC genotypes and a lower risk in patients’ carriers of CYP 11B2 3097 AA genotype.[63]
Diabetes mellitus complications
Diabetic retinopathy and the renin angiotensin aldosterone system
Diabetic retinopathy is the most common vascular complication of both T1DM and T2DM and its prevalence increases with duration of diabetes. Damage of retinal vasculature by hyperglycemia, hypertension and hyperlipidemia are involved in the pathogenesis of DR. Proliferative retinopathy may develop with new vessel formation threaten the vision.[64] Local accumulation of glucose and its metabolite, succinate, through activation of a G-protein coupled receptor (CPR91) triggers the cell to cell signaling that results in prorenin and renin release from juxtaglomerular cells in early diabetes.[14] It has been suggested that abnormal retinal expression of ACE has adverse effects on the retinal blood flow and vascular structure. Both ACE and Ang II increase the level of vascular endothelial growth factors leading to abnormal retinal angiogenesis with increased risk of retinopathy and its progression. ACE I (lisinopril) and AT1R antagonist (losartan) can inhibit abnormal retinal new vessel formation in retinopathy.[64] Both systemic and local RAAS are implicated in the pathogenesis of retinopathy and all components of the RAAS are expressed in retina with highly elevation of renin, ACE, Ang II and AT1R in patients with DR. Although, the retino protective effect of RAAS inhibition in diabetic patients has been demonstrated but due to the presence of local RAAS and blood-retina barrier in the eye which leads to the lack of influence of RAAS blockers on the local RAAS, these blockers are not completely effective.[65] An association between DR and imbalance in local RAAS with a higher ratio of deleterious axis of ACE/Ang II/AT1R to vasoprotective axis ACE2/Ang (1-7)/Mas has been demonstrated. Increased expression of ACE2/Ang (1-7) conferred protection against DR.[65]
The absence of a significant difference in the frequency of ACE I/D variants was identified between Caucasians T1DM patients with and without diabetic proliferative retinopathy.[66]
Also, lack of an association between ACE I/D and susceptibility to retinopathy in T2DM patients from Caucasian Mediterranean population has been reported.[20] Further, there was no significant difference between Caucasian T2DM patients and controls related to the frequencies of ACE I/D or AGT M235T variants and also with the risk of retinopathy.[26] Furthermore, in Caucasians with T1DM the AT1R A1166C was not associated with the risk of proliferative retinopathy.[58] No association was detected between ACE I/D, AGT M235T and AT1R A1166C polymorphisms and the risk of retinopathy in Chinese T2DM patients.[27] However, a recent meta-analysis of 17 studies including 1039 cases and 1185 controls revealed an association between the ACE I/D polymorphism with increased risk of proliferative DR among Chinese patients.[46]
Diabetic neuropathy and the renin angiotensin aldosterone system
The damage of peripheral nerve in diabetes could be attributed to polyol accumulation, advanced glycation end-products and oxidative stress. Two mechanisms have been suggested to be involved in the pathogenesis of diabetic neuropathy. The first mechanism is the activation of the RAAS in the presence of hyperglycemia with increased tissue level of Ang II. Ang II stimulates NAD (P) oxidase which enhances oxidative stress and vascular damage and leading to diabetic neuropathy.[67] The other mechanism is disturbance in the metabolism and vasculature of nerve tissue in the presence of excessive uptake of glucose.[47]
In two studies among T2DM patients, the role of ACE I/D polymorphism in the pathogenesis of diabetic peripheral neuropathy have been indicated with a protective role of ACE II genotype against diabetic peripheral neuropathy in both genders[47] (Pakistan) or only in whites women.[48] In contrast, in a small subset of samples from Japanese with T2DM and without macroalbuminuria, the ACE D allele had a protective role on the polyneuropathy.[49]
Infarction of the optic nerve produces a vision loss threatening disease designated anterior ischemic optic neuropathy that has two forms of arteritic and nonarteritic anterior ischemic optic neuropathy (NAION). The AGT M235T and AT1R A1166C polymorphisms were not associated with NAION in a small subset samples. However, ACE I allele was detected as a susceptibility factor for NAION only in young males. Alteration of vascular regulation might be involved in the pathogenesis of disease. The risk of NAION in the presence of ACE I allele might be explained by hypoperfusion and low pressure in the presence of this allele, especially in males.[68]
Cardiovascular complication of diabetes mellitus and the renin angiotensin aldosterone system
Cardiovascular disease is the major complication of DM that comprises 60% of deaths in T2DM patients. The prevalence of CVD is increasing with evidences of oxidative stress in its pathogenesis.[29,69,70,71,72] Hyperglycemia activates the tissue and systemic RAAS that is a major mechanism responsible for the development of atherosclerosis in diabetic patients. Since the activation of RAAS by hyperglycemia is different among patients with T1DM, it has been suggested that genetic variations in the RAAS might cause pathologically activation of the RAAS in response to hyperglycemia and the development of CVD. In a follow-up study of T1DM patients, the presence of coronary artery calcification (CAC) as a marker of subclinical coronary artery disease (CAD) was associated with AGT M235T polymorphism.[73] Also, the risk of CAC progression was significantly higher in the concomitant presence of AGT 235TT and ACE ID genotypes. Further, in those T1DM patients without ACEI/ARB therapy there was an additive effect between AGT TT, ACE DD and AT1R AA/AC genotypes to increase the risk of progression of CAC. These synergistic effects of polymorphisms on the risk of CAC were observed only in the presence of poor metabolic control (HbA1C ≥7%).[73] In Japanese with T1DM, the ACE I/D polymorphism was associated with atherosclerosis family history.[50] However, the single effect of AGT M235T and its interaction with ACE I/D and AT1R A1166C on the risk of CAD in Dutch T1DM patients was not observed.[28] In T2DM patients, some studies indicate the lack of influence of RAAS variants in the risk of CVD. In European American and African American families with T2DM, the ACE I/D, AGT M235T and AT1R A1166C polymorphisms did not affect the risk of subclinical CVD or blood pressure.[29] Further, the ACE I/D polymorphism was not linked to subclinical cardiovascular complication of T2DM in Mexican American families.[21] In contrast, other studies provide an evidence of the association between RAAS gene polymorphisms and the risk of atherosclerosis in T2DM patients. In Chinese T2DM patients, the ACE DD genotype increased the risk of developing CAD. Also, AT2R G1675A polymorphism was associated with the risk of CAD in female patients.[51] Among American men with T2DM the AT1R 1166C allele and among American women the AGT 235T allele was associated with the risk of coronary heart disease.[30] Furthermore, in a cohort study from Netherland the presence of each or three risk variants of ACE I/D, AGT M235T and AT1R C573T in T2DM patients increased the risk of atherosclerosis.[31] Finally, the ACE I/D polymorphism has been associated with multi vessel CAD and myocardial infarction in T2DM patients from India.[52]
It seems that only the ACE I/D polymorphism of the RAAS but not AGT M235 and AT1R A1166C polymorphisms be associated with the risk of CVD in DM patients.
CONCLUSION
The role of renin gene variants in the pathogenesis of either DM or its complications needs to be clarified in more studies. However, the AGT M235T has been extensively studied, and its contribution to insulin resistance is suggested but this polymorphism might not affect the risk of DM especially in T2DM. Due to the controversy and the lack of sufficient studies, the role of ACE I/D and AT1R A1166C polymorphisms in the pathogenesis of DM could not be established.
An imbalance in the activation of systemic to local RAAS with a higher ratio of ACE to ACE2 has been implicated in the pathogenesis of DR. Bulk of association studies indicates absence of an association between three polymorphisms of AGT M235T, ACE I/D and AT1R A1166C and DR in both T1DM and T2DM patients.
A protective role for ACE II genotype against diabetic peripheral neuropathy has been indicated. The ACE I/D polymorphism might be associated with the risk of CVD in DM patients. However, the role of AGT M235 and AT1R A1166C polymorphisms in the pathogenesis of cardiovascular complication could not be established.
The controversy related to the influence of RAAS gene variants on the development and progression of DM and its micro- and macro-vascular complications could be attributed to the ethnicity, gender, stages of diabetes complications, the presence of intrarenal RAAS, methodological limitations of the association studies in multifactorial diseases, inadequate sample size, genetic heterogeneity, and the lack of studies which involve the variants of all RAAS genes and their interactions. The possible roles of less studied components of the RAAS such as renin, AT2R and aldosterone synthase variants in the pathogenesis of DM and its complications need to be clarified. The evidences of the possible role of the some RAAS gene variants in the pathogenesis of DM and its complications might be considered in the prevention, management and treatment programs of DM patients.
AUTHOR'S CONTRIBUTION
ZR contributed in the conception and design of the work, conducting the study, drafting and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. MM contributed in the conception of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. HN contributed in the conception of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
ACKNOWLEDGMENTS
This work was financially supported by a grant (grant number 92109) from Vice Chancellor for Research of Kermanshah University of Medical Sciences, Kermanshah, Iran.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Khavandi K, Amer H, Ibrahim B, Brownrigg J. Strategies for preventing type 2 diabetes: An update for clinicians. Ther Adv Chronic Dis. 2013;4:242–61. doi: 10.1177/2040622313494986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lardizabal JA, Deedwania PC. The role of renin-angiotensin agents in altering the natural history of type 2 diabetes mellitus. Curr Cardiol Rep. 2010;12:464–71. doi: 10.1007/s11886-010-0138-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou MS, Schulman IH. Prevention of diabetes in hypertensive patients: Results and implications from the VALUE trial. Vasc Health Risk Manag. 2009;5:361–8. doi: 10.2147/vhrm.s4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheen AJ. Renin-angiotensin system inhibition prevents type 2 diabetes mellitus. Diabetes Metab. 2004;30(Part 1):487–96. doi: 10.1016/s1262-3636(07)70146-5. A meta-analysis of randomised clinical trials. [DOI] [PubMed] [Google Scholar]
- 5.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–53. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 6.Hitomi H, Kiyomoto H, Nishiyama A, Hara T, Moriwaki K, Kaifu K, et al. Aldosterone suppresses insulin signaling via the downregulation of insulin receptor substrate-1 in vascular smooth muscle cells. Hypertension. 2007;50:750–5. doi: 10.1161/HYPERTENSIONAHA.107.093955. [DOI] [PubMed] [Google Scholar]
- 7.Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E, et al. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension. 1999;34:1265–74. doi: 10.1161/01.hyp.34.6.1265. [DOI] [PubMed] [Google Scholar]
- 8.Rahimi Z, Mansouri Zaveleh O, Rahimi Z, Abbasi A. AT2R -1332 G:A polymorphism and diabetic nephropathy in type 2 diabetes mellitus patients. J Renal Inj Prev. 2013;2:97–101. doi: 10.12861/jrip.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Buren PN, Toto R. Hypertension in diabetic nephropathy: Epidemiology, mechanisms, and management. Adv Chronic Kidney Dis. 2011;18:28–41. doi: 10.1053/j.ackd.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luther JM, Brown NJ. The renin-angiotensin-aldosterone system and glucose homeostasis. Trends Pharmacol Sci. 2011;32:734–9. doi: 10.1016/j.tips.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Underwood PC, Adler GK. The renin angiotensin aldosterone system and insulin resistance in humans. Curr Hypertens Rep. 2013;15:59–70. doi: 10.1007/s11906-012-0323-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheen AJ. Prevention of type 2 diabetes mellitus through inhibition of the Renin-Angiotensin system. Drugs. 2004;64:2537–65. doi: 10.2165/00003495-200464220-00004. [DOI] [PubMed] [Google Scholar]
- 13.Sun B, Williams JS, Pojoga L, Chamarthi B, Lasky-Su J, Raby BA, et al. Renin gene polymorphism: Its relationship to hypertension, renin levels and vascular responses. J Renin Angiotensin Aldosterone Syst. 2011;12:564–71. doi: 10.1177/1470320311405873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peti-Peterdi J, Kang JJ, Toma I. Activation of the renal renin-angiotensin system in diabetes — new concepts. Nephrol Dial Transplant. 2008;23:3047–9. doi: 10.1093/ndt/gfn377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deinum J, Tarnow L, van Gool JM, de Bruin RA, Derkx FH, Schalekamp MA, et al. Plasma renin and prorenin and renin gene variation in patients with insulin-dependent diabetes mellitus and nephropathy. Nephrol Dial Transplant. 1999;14:1904–11. doi: 10.1093/ndt/14.8.1904. [DOI] [PubMed] [Google Scholar]
- 16.Shahvaisizadeh F, Movafagh A, Omrani MD, Vaisi-Raygani A, Rahimi Z, Rahimi Z. Synergistic effects of angiotensinogen -217 G:A and T704C (M235T) variants on the risk of severe preeclampsia. J Renin Angiotensin Aldosterone Syst. 2012;15:156–61. doi: 10.1177/1470320312467555. [DOI] [PubMed] [Google Scholar]
- 17.Fradin S, Goulet-Salmon B, Chantepie M, Grandhomme F, Morello R, Jauzac P, et al. Relationship between polymorphisms in the renin-angiotensin system and nephropathy in type 2 diabetic patients. Diabetes Metab. 2002;28:27–32. [PubMed] [Google Scholar]
- 18.Bengtsson K, Orho-Melander M, Lindblad U, Melander O, Bøg-Hansen E, Ranstam J, et al. Polymorphism in the angiotensin converting enzyme but not in the angiotensinogen gene is associated with hypertension and type 2 diabetes: The Skaraborg Hypertension and diabetes project. J Hypertens. 1999;17:1569–75. doi: 10.1097/00004872-199917110-00010. [DOI] [PubMed] [Google Scholar]
- 19.Ringel J, Beige J, Kunz R, Distler A, Sharma AM. Genetic variants of the renin-angiotensin system, diabetic nephropathy and hypertension. Diabetologia. 1997;40:193–9. doi: 10.1007/s001250050662. [DOI] [PubMed] [Google Scholar]
- 20.Gutierrez C, Vendrell J, Pastor R, Lior C, Aguilar C, Broch M, et al. Angiotensin I-converting enzyme and angiotensinogen gene polymorphisms in non-insulin-dependent diabetes mellitus. Lack of relationship with diabetic nephropathy and retinopathy in Caucasian Mediterranean population. Metabolism. 1997;46:976–80. doi: 10.1016/s0026-0495(97)90090-1. [DOI] [PubMed] [Google Scholar]
- 21.Thameem F, Puppala S, Arar N, Blangero J, Stern MP, Duggirala R, et al. Genetic polymorphisms in the renin-angiotensin system (RAS) genes and their association analysis with type 2 diabetes and related traits in Mexican Americans. Diabetes Res Clin Pract. 2008;79:e14–6. doi: 10.1016/j.diabres.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 22.Thomas GN, Tomlinson B, Chan JC, Sanderson JE, Cockram CS, Critchley JA. Renin-angiotensin system gene polymorphisms, blood pressure, dyslipidemia, and diabetes in Hong Kong Chinese: A significant association of tne ACE insertion/deletion polymorphism with type 2 diabetes. Diabetes Care. 2001;24:356–61. doi: 10.2337/diacare.24.2.356. [DOI] [PubMed] [Google Scholar]
- 23.Conen D, Glynn RJ, Buring JE, Ridker PM, Zee RY. Association of renin-angiotensin and endothelial nitric oxide synthase gene polymorphisms with blood pressure progression and incident hypertension: Prospective cohort study. J Hypertens. 2008;26:1780–6. doi: 10.1097/HJH.0b013e3283077eef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mtiraoui N, Ezzidi I, Turki A, Chaieb M, Mahjoub T, Almawi WY. Renin-angiotensin-aldosterone system genotypes and haplotypes affect the susceptibility to nephropathy in type 2 diabetes patients. J Renin Angiotensin Aldosterone Syst. 2011;12:572–80. doi: 10.1177/1470320310396542. [DOI] [PubMed] [Google Scholar]
- 25.Mehri S, Koubaa N, Hammami S, Mahjoub S, Chaaba R, Nakbi A, et al. Genotypic interactions of renin-angiotensin system genes with diabetes type 2 in a Tunisian population. Life Sci. 2010;87:49–54. doi: 10.1016/j.lfs.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Dudley CR, Keavney B, Stratton IM, Turner RC, Ratcliffe PJ. U.K. Prospective Diabetes Study. XV: Relationship of renin-angiotensin system gene polymorphisms with microalbuminuria in NIDDM. Kidney Int. 1995;48:1907–11. doi: 10.1038/ki.1995.490. [DOI] [PubMed] [Google Scholar]
- 27.Thomas GN, Critchley JA, Tomlinson B, Yeung VT, Lam D, Cockram CS, et al. Renin-angiotensin system gene polymorphisms and retinopathy in chinese patients with type 2 diabetes. Diabetes Care. 2003;26:1643–4. doi: 10.2337/diacare.26.5.1643. [DOI] [PubMed] [Google Scholar]
- 28.van Ittersum FJ, de Man AM, Thijssen S, de Knijff P, Slagboom E, Smulders Y, et al. Genetic polymorphisms of the renin-angiotensin system and complications of insulin-dependent diabetes mellitus. Nephrol Dial Transplant. 2000;15:1000–7. doi: 10.1093/ndt/15.7.1000. [DOI] [PubMed] [Google Scholar]
- 29.Burdon KP, Langefeld CD, Wagenknecht LE, Carr JJ, Freedman BI, Herrington D, et al. Association analysis of genes in the renin-angiotensin system with subclinical cardiovascular disease in families with Type 2 diabetes mellitus: The Diabetes Heart Study. Diabet Med. 2006;23:228–34. doi: 10.1111/j.1464-5491.2005.01777.x. [DOI] [PubMed] [Google Scholar]
- 30.Lin J, Hu FB, Qi L, Curhan GC. Genetic polymorphisms of angiotensin-2 type 1 receptor and angiotensinogen and risk of renal dysfunction and coronary heart disease in type 2 diabetes mellitus. BMC Nephrol. 2009;10:9. doi: 10.1186/1471-2369-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yazdanpanah M, Aulchenko YS, Hofman A, Janssen JA, Sayed-Tabatabaei FA, van Schaik RH, et al. Effects of the renin-angiotensin system genes and salt sensitivity genes on blood pressure and atherosclerosis in the total population and patients with type 2 diabetes. Diabetes. 2007;56:1905–12. doi: 10.2337/db06-1127. [DOI] [PubMed] [Google Scholar]
- 32.Rahimi Z, Rahimi Z, Mozafari H, Parsian A. Preeclampsia and angiotensin converting enzyme (ACE) I/D and angiotensin II type-1 receptor (AT1R) A1166C polymorphisms: Association with ACE I/D polymorphism. J Renin Angiotensin Aldosterone Syst. 2013;14:174–80. doi: 10.1177/1470320312448950. [DOI] [PubMed] [Google Scholar]
- 33.Rahimi Z, Felehgari V, Rahimi M, Mozafari H, Yari K, Vaisi-Raygani A, et al. The frequency of factor V Leiden mutation, ACE gene polymorphism, serum ACE activity and response to ACE inhibitor and angiotensin II receptor antagonist drugs in Iranians type II diabetic patients with microalbuminuria. Mol Biol Rep. 2011;38:2117–23. doi: 10.1007/s11033-010-0338-1. [DOI] [PubMed] [Google Scholar]
- 34.Rahimi Z. ACE insertion/deletion (I/D) polymorphism and diabetic nephropathy. J Nephropathol. 2012;1:143–51. doi: 10.5812/nephropathol.8109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Felehgari V, Rahimi Z, Mozafari H, Vaisi-Raygani A. ACE gene polymorphism and serum ACE activity in Iranians type II diabetic patients with macroalbuminuria. Mol Cell Biochem. 2011;346:23–30. doi: 10.1007/s11010-010-0587-2. [DOI] [PubMed] [Google Scholar]
- 36.Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86:1343–6. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rahimi Z, Hasanvand A, Felehgari V. Interaction of MTHFR 1298C with ACE D allele augments the risk of diabetic nephropathy in Western Iran. DNA Cell Biol. 2012;31:553–9. doi: 10.1089/dna.2011.1364. [DOI] [PubMed] [Google Scholar]
- 38.Cong ND, Hamaguchi K, Saikawa T, Hara M, Sakata T. The I/D polymorphism of angiotensin-converting enzyme gene but not the angiotensinogen gene is associated with insulin response to oral glucose in Japanese. Proc Soc Exp Biol Med. 1999;220:46–51. doi: 10.1046/j.1525-1373.1999.d01-7.x. [DOI] [PubMed] [Google Scholar]
- 39.Arzu Ergen H, Hatemi H, Agachan B, Camlica H, Isbir T. Angiotensin-I converting enzyme gene polymorphism in Turkish type 2 diabetic patients. Exp Mol Med. 2004;36:345–50. doi: 10.1038/emm.2004.45. [DOI] [PubMed] [Google Scholar]
- 40.Ramachandran V, Ismail P, Stanslas J, Shamsudin N, Moin S, Mohd Jas R. Association of insertion/deletion polymorphism of angiotensin-converting enzyme gene with essential hypertension and type 2 diabetes mellitus in Malaysian subjects. J Renin Angiotensin Aldosterone Syst. 2008;9:208–14. doi: 10.1177/1470320308097499. [DOI] [PubMed] [Google Scholar]
- 41.Baroudi T, Bouhaha R, Moran-Moguel C, Sanchez-Corona J, Ben Maiz H, Kammoun Abid H, et al. Association of the insertion/deletion polymorphism of the angiotensin-converting enzyme gene with type 2 diabetes in two ethnic groups of Jerba Island in Tunisia. J Renin Angiotensin Aldosterone Syst. 2009;10:35–40. doi: 10.1177/1470320309102314. [DOI] [PubMed] [Google Scholar]
- 42.Arfa I, Abid A, Nouira S, Elloumi-Zghal H, Malouche D, Mannai I, et al. Lack of association between the angiotensin-converting enzyme gene (I/D) polymorphism and diabetic nephropathy in Tunisian type 2 diabetic patients. J Renin Angiotensin Aldosterone Syst. 2008;9:32–6. doi: 10.3317/jraas.2008.002. [DOI] [PubMed] [Google Scholar]
- 43.Zhou D, Ruiter R, Zhang J, Zhou M, Liu H, Liu W, et al. Angiotensin-converting enzyme I/D polymorphism is not associated with type 2 diabetes in a Chinese population. J Renin Angiotensin Aldosterone Syst. 2012;13:372–8. doi: 10.1177/1470320311435535. [DOI] [PubMed] [Google Scholar]
- 44.Thomas GN, Critchley JA, Tomlinson B, Lee ZS, Young RP, Cockran CS, et al. Albuminuria and the renin-angiotensin system gene polymorphisms in type-2-diabetic and in normoglycemic hypertensive Chinese. Clin Nephrol. 2001;55:7–15. [PubMed] [Google Scholar]
- 45.Yang JK, Zhou JB, Xin Z, Zhao L, Yu M, Feng JP, et al. Interactions among related genes of renin-angiotensin system associated with type 2 diabetes. Diabetes Care. 2010;33:2271–3. doi: 10.2337/dc10-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu Y, Ge Y, Hu Q, Shi Y, Xue C, Shi Y, et al. Association between angiotensin-converting enzyme gene polymorphism and diabetic retinopathy in the Chinese population. J Renin Angiotensin Aldosterone Syst. 2012;13:289–95. doi: 10.1177/1470320311432187. [DOI] [PubMed] [Google Scholar]
- 47.Mansoor Q, Javaid A, Bilal N, Ismail M. Angiotensin-converting enzyme (ACE) gene II genotype protects against the development of diabetic peripheral neuropathy in type 2 diabetes mellitus. J Diabetes. 2012;4:257–61. doi: 10.1111/j.1753-0407.2012.00205.x. [DOI] [PubMed] [Google Scholar]
- 48.Stephens JW, Dhamrait SS, Acharya J, Humphries SE, Hurel SJ. A common variant in the ACE gene is associated with peripheral neuropathy in women with type 2 diabetes mellitus. J Diabetes Complications. 2006;20:317–21. doi: 10.1016/j.jdiacomp.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 49.Ito H, Tsukui S, Kanda T, Utsugi T, Ohno T, Kurabayashi M. Angiotensin-converting enzyme insertion/deletion polymorphism and polyneuropathy in type 2 diabetes without macroalbuminuria. J Int Med Res. 2002;30:476–82. doi: 10.1177/147323000203000502. [DOI] [PubMed] [Google Scholar]
- 50.Miura J, Uchigata Y, Yokoyama H, Omori Y, Iwamoto Y. Genetic polymorphism of renin-angiotensin system is not associated with diabetic vascular complications in Japanese subjects with long-term insulin dependent diabetes mellitus. Diabetes Res Clin Pract. 1999;45:41–9. doi: 10.1016/s0168-8227(99)00059-5. [DOI] [PubMed] [Google Scholar]
- 51.Lei HP, Chen HM, Zhong SL, Yao QZ, Tan HH, Yang M, et al. Association between polymorphisms of the renin-angiotensin system and coronary artery disease in Chinese patients with type 2 diabetes. J Renin Angiotensin Aldosterone Syst. 2012;13:305–13. doi: 10.1177/1470320311435533. [DOI] [PubMed] [Google Scholar]
- 52.Narne P, Ponnaluri KC, Singh S, Siraj M, Ishaq M. Relationship between angiotensin-converting enzyme gene insertion/deletion polymorphism, angiographically defined coronary artery disease and myocardial infarction in patients with type 2 diabetes mellitus. J Renin Angiotensin Aldosterone Syst. 2012;13:478–86. doi: 10.1177/1470320312448947. [DOI] [PubMed] [Google Scholar]
- 53.Reyes-Engel A, Morcillo L, Aranda FJ, Ruiz M, Gaitan MJ, Mayor-Olea A, et al. Influence of gender and genetic variability on plasma angiotensin peptides. J Renin Angiotensin Aldosterone Syst. 2006;7:92–7. doi: 10.3317/jraas.2006.015. [DOI] [PubMed] [Google Scholar]
- 54.Bonnardeaux A, Davies E, Jeunemaitre X, Féry I, Charru A, Clauser E, et al. Angiotensin II type 1 receptor gene polymorphisms in human essential hypertension. Hypertension. 1994;24:63–9. doi: 10.1161/01.hyp.24.1.63. [DOI] [PubMed] [Google Scholar]
- 55.Rahimi Z, Rahimi Z, Aghaei A, Vaisi-Raygani A. AT2R -1332 G:A polymorphism and its interaction with AT1R 1166 A:C, ACE I/D and MMP-9 -1562 C:T polymorphisms: Risk factors for susceptibility to preeclampsia. Gene. 2014;538:176–81. doi: 10.1016/j.gene.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 56.Ahluwalia TS, Ahuja M, Rai TS, Kohli HS, Bhansali A, Sud K, et al. ACE variants interact with the RAS pathway to confer risk and protection against type 2 diabetic nephropathy. DNA Cell Biol. 2009;28:141–50. doi: 10.1089/dna.2008.0810. [DOI] [PubMed] [Google Scholar]
- 57.Shah VN, Cheema BS, Sharma R, Khullar M, Kohli HS, Ahluwalia TS, et al. ACACß gene (rs2268388) and AGTR1 gene (rs5186) polymorphism and the risk of nephropathy in Asian Indian patients with type 2 diabetes. Mol Cell Biochem. 2013;372:191–8. doi: 10.1007/s11010-012-1460-2. [DOI] [PubMed] [Google Scholar]
- 58.Tarnow L, Cambien F, Rossing P, Nielsen FS, Hansen BV, Ricard S, et al. Angiotensin-II type 1 receptor gene polymorphism and diabetic microangiopathy. Nephrol Dial Transplant. 1996;11:1019–23. [PubMed] [Google Scholar]
- 59.Bindom SM, Hans CP, Xia H, Boulares AH, Lazartigues E. Angiotensin I-converting enzyme type 2 (ACE2) gene therapy improves glycemic control in diabetic mice. Diabetes. 2010;59:2540–8. doi: 10.2337/db09-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chaoxin J, Daili S, Yanxin H, Ruwei G, Chenlong W, Yaobin T. The influence of angiotensin-converting enzyme 2 gene polymorphisms on type 2 diabetes mellitus and coronary heart disease. Eur Rev Med Pharmacol Sci. 2013;17:2654–9. [PubMed] [Google Scholar]
- 61.Saha S, Bornstein SR, Graessler J, Kopprasch S. Very-low-density lipoprotein mediates transcriptional regulation of aldosterone synthase in human adrenocortical cells through multiple signaling pathways. Cell Tissue Res. 2012;348:71–80. doi: 10.1007/s00441-012-1346-3. [DOI] [PubMed] [Google Scholar]
- 62.Hollenberg NK, Stevanovic R, Agarwal A, Lansang MC, Price DA, Laffel LM, et al. Plasma aldosterone concentration in the patient with diabetes mellitus. Kidney Int. 2004;65:1435–9. doi: 10.1111/j.1523-1755.2004.00524.x. [DOI] [PubMed] [Google Scholar]
- 63.Bellili NM, Foucan L, Fumeron F, Mohammedi K, Travert F, Roussel R, et al. Associations of the -344 T > C and the 3097 G > A polymorphisms of CYP11B2 gene with hypertension, type 2 diabetes, and metabolic syndrome in a French population. Am J Hypertens. 2010;23:660–7. doi: 10.1038/ajh.2010.44. [DOI] [PubMed] [Google Scholar]
- 64.Shah CA. Diabetic retinopathy: A comprehensive review. Indian J Med Sci. 2008;62:500–19. [PubMed] [Google Scholar]
- 65.Verma A, Shan Z, Lei B, Yuan L, Liu X, Nakagawa T, et al. ACE2 and Ang-(1-7) confer protection against development of diabetic retinopathy. Mol Ther. 2012;20:28–36. doi: 10.1038/mt.2011.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tarnow L, Cambien F, Rossing P, Nielsen FS, Hansen BV, Lecerf L, et al. Lack of relationship between an insertion/deletion polymorphism in the angiotensin I-converting enzyme gene and diabetic nephropathy and proliferative retinopathy in IDDM patients. Diabetes. 1995;44:489–94. doi: 10.2337/diab.44.5.489. [DOI] [PubMed] [Google Scholar]
- 67.Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: Upstream mediators. Circ Res. 2002;91:406–13. doi: 10.1161/01.res.0000033523.08033.16. [DOI] [PubMed] [Google Scholar]
- 68.Markoula S, Giannopoulos S, Asproudis I, Kostoulas C, Nikas A, Bagli E, et al. Renin-angiotensin-aldosterone system genes and nonarteritic anterior ischemic optic neuropathy. Mol Vis. 2011;17:1254–60. [PMC free article] [PubMed] [Google Scholar]
- 69.Nasri H. Elevated serum parathyroid hormone is a heart risk factor in hemodialysis patients. J Parathyroid Dis. 2013;1:13–4. [Google Scholar]
- 70.Nasri H, Rafieian-Kopaei M. Protective effects of herbal antioxidants on diabetic kidney disease. J Res Med Sci. 2014;19:82–3. [PMC free article] [PubMed] [Google Scholar]
- 71.Baradaran A. Lipoprotein(a), type 2 diabetes and nephropathy; the mystery continues. J Nephropathol. 2012;1:126–9. doi: 10.5812/nephropathol.8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tavafi M. Diabetic nephropathy and antioxidants. J Nephropathol. 2013;2:20–7. doi: 10.5812/nephropathol.9093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kretowski A, McFann K, Hokanson JE, Maahs D, Kinney G, Snell-Bergeon JK, et al. Polymorphisms of the renin-angiotensin system genes predict progression of subclinical coronary atherosclerosis. Diabetes. 2007;56:863–71. doi: 10.2337/db06-1321. [DOI] [PubMed] [Google Scholar]


