Abstract
Background:
Primary sarcoma of the prostate is extremely rare and accounts for 0.1% of all prostate cancers. This type of cancer is associated with poor prognosis due to aggressive biological behavior. The World Health Organization histologically classified prostate sarcomas as stromal tumor of unknown malignant potential (STUMP) and stromal sarcoma.
Patients and Methods:
A 39-year-old patient presented with lower urinary tract symptoms over the last few months. On digital rectal examination, the right lobe of the prostate was diffusely hard on palpation. Prostate-specific antigen was 0.5 ng/ml. A biopsy specimen was obtained with the guidance of transrectal ultrasonography. Immunohistochemical examination revealed positive staining for vimentin, actin, and desmin.
Results:
18F-fluorodeoxyglucose-positron-emission tomography/computed tomography scans obtained for staging purposes with the diagnosis of primary spindle cell carcinoma of the prostate revealed widespread lung and liver metastases. A doxorubicin-based systemic chemotherapy (CTx) was initiated.
Conclusion:
Spindle sarcomas of the prostate have quite aggressive nature and they have high potential to metastase. Average life expectancy is <1 year and the prognosis is poor. CTx and radiation therapy can’t yield curative effects due to poor differentiation.
Keywords: Diagnosis, positron-emission tomography/computed tomography, prostat cancer, sarcoma, spindle cell prostatic sarcoma, treatment
INTRODUCTION
Prostate cancer is the most common solid cancer in men. Variant cancers account for 5-10% of all prostate cancers. Prostatic sarcomas (PSs) made 0.1-0.2% of all malignant prostat tumors. Rhabdomyosarcoma is frequent during childhood, whereas leiomyosarcoma is more frequent in adults.[1] In the literature, number of the cases with diagnosed primary PS is 100.[2] In the past, stromal tumors of the prostate were reported using several terms including atypical stromal hyperplasia. Currently, these tumors are classified according to World Health Organization classification as follows: PSs, stromal tumors of unknown malignant potential (STUMP), and stromal PS (high- and low-grade).[3] Pathologically, they are differentiated from other variant tumors by means of immunohistochemical examination. We describe a rare case of low-grade stromal sarcoma (LG-PS) of the specialised prostatic stroma, and we also review the literature concerning these tumours.
CASE REPORT
Clinical features
A 39-year-old patient presented with lower urinary tract symptoms (LUTS). Biochemical analyses were as follows: Glucose, 101 mg/dL; creatinine, 0.6 mg/dL; urea, 32 mg/dL; prostate-specific antigen (PSA), 0.5 ng/mL; aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and gamma glutamyl transferases levels were within normal ranges; white blood cells, 9.98 × 103/μL hemoglobin, 11.2 g/dL; sodium, 134 mmol/L; potassium, 4.1 mmol/L; chlor, 99 mEq/L; and calcium, 8.8 mg/dL. The ultrasonography revealed a prostate volume of 32 mm × 43 mm × 39 mm. In uroflowmetry, maximum flow velocity was 9 mL/s and average flow velocity was 5.5 mL/s. On digital rectal examination, the right lobe of the prostate was diffusely hard on palpation. The prostate tissue was slightly painful. The patient was a heavy smoker. The patient's family history was not remarkable for prostate cancer or other malignancies. Biopsy specimens were obtained from 12 quadrants under the guidance of transrectal UA. 18F-fluorodeoxyglucose (18FDG)-positron-emission tomography/computed tomography (PET/CT) scans were obtained for staging of primary spindle cell sarcoma of the prostate.
Positron-emission tomography/computed tomography features
Late pelvic images of FDG-PET/CT revealed an activity of the primary tumor measuring 32 mm in diameter in the right lobe of the prostate with a standard uptake value (SUVmax) of 12.4 [Figure 1]. Metastatic lesions were observed, measuring 18 mm in the anterobasal lobe of the right lung (SUVmax: 8.1) and 26 mm in the right (SUVmax: 10.3) and 17 mm in the left lobe (SUVmax: 9.6) of the liver [Figure 2].
Figure 1.
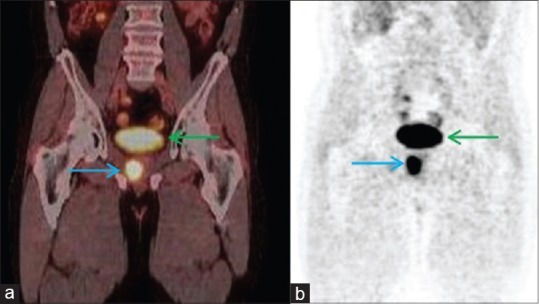
(a) The coronal 18F-fluorodeoxyglucose (18FDG)-positronemission tomography/computed tomography scans show, (blue arrow: Primary cancer, green arrow: Bladder FDG accumulation), SUVmax: 12.4, (b) maximum intensity projection images, (blue arrow: Primary cancer, green arrow: Bladder)
Figure 2.
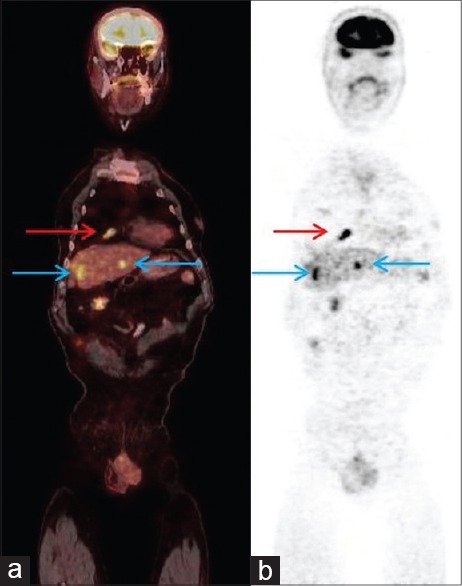
(a) The coronal 18F-fluorodeoxyglucose-positron-emission tomography/computed tomography scans show, red arrow: Lung metastasis, SUVmax: 10.9. Blue arrows: Liver metastases SUVmax: 10.3 and 9.6, (b) maximum intensity projection images, (red arrow: Lung metastasis, blue arrows: Liver metastases)
Immunohistopathological findings
The pathological diagnosis and grade of the tumor was evaluated according to the classification of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group. The histological grade was scored based on the level of differentiation, and the presence of mitosis and necrosis in each high power field.[4] Immunohistochemical examination revealed positive staining for smooth muscle actin, vimentin, and desmin and negative staining for CD34, S100 and progesterone receptors [Figure 3]. Ki-67 proliferation index was 2%.
Figure 3.
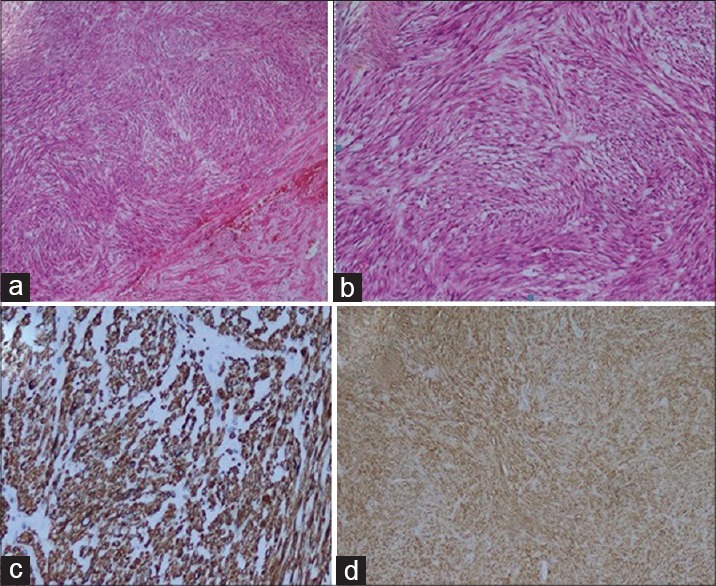
(a) H and E, demonstrating, tumor composed of spindle cells (H and E, ×40), (b) H and E, demonstrating, tumor composed of spindle cells (H and E, ×100), (c) immunohistochemistry showing, desmin (×200), (d) immunohistochemistry showing, actin (×40)
Treatment and survival
The staging of the tumor was based on a system developed by the American Joint Cancer Committee-2013 on the staging of soft tissue sarcomas. A doxorubicin-based chemotherapy (CTx) was initiated due to the presence of Stage IV metastatic disease.
DISCUSSION
Spindle cell lesions of the prostate are included in a broad spectrum covering both benignant and malignant processes. In the differential diagnosis one should consider many benignant and malignant tumors such as some leimyomas and leimyosarcomas, rhabromyosarcoma, inflammatory myofibroblastic tumor, solitary fibrous tumors, and phylloides tumor.[5] Spindle cell sarcoma of the prostate is one of the rare, insidiously progressing, aggressive variant tumors with high potential to make metastasis. More than half of the patients diagnosed as having these tumors consist of the cases diagnosed as having prostatic adenocarcinoma and showing sarcomatoid differentiation as a consequence of the treatments. This differentiation occurs over a long period ranging from 6 months to 16 years. In the literature, average time of differentiation is 7-year.[6]
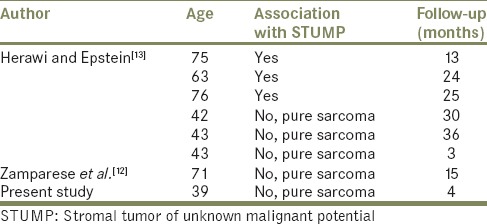
Histopathogenesis of the spindle cell cancer of the prostate is not exactly known. Proposed mechanisms include transformation of the epithelial structures to sarcomatous components and two-way differentiation of the epithelial stem cells to both malignant components.[7] According to these theories, the disease process may occur through two separate differentiation pathways. The more commonly accepted theory, however, is that the disease arises from one origin, and creates a different form with sarcomatoid differentiation. Operation materials and specimens of these patients obtained during diagnostic procedures for sarcomatoid carcinoma also indicate that the simultaneous carcinoma contain high-grade epithelial components.[8] Thus, sarcomatoid differentiation is more common in the presence of high-grade tumor. Receiving therapies such as hormonal therapy and/or radiation therapy is considered to trigger sarcomatoid differentiation.[8] The stromal tumors of the prostate are rare tumors arising from the stroma of the prostate exhibiting different histological features. Now these lesions have been classified as lesions of uncertain malignant potential (STUMP) and stromal sarcoma (low-grade and high-grade).[9] The currently used classification of STUMP was first proposed in 1998 by Gaudin et al.[10] High-grade prostate sarcoma and low-grade prostate sarcoma have the potential to metastasize. In contrast to stromal sarcoma, the neoplastic nature of STUMPs is controversial. STUMPs may be considered neoplastic based on the observation that they may diffusely infiltrate the prostate gland and extend into adjacent tissues.[11] In the past, the histologically pattern of STUMP was often reported as benign prostatic hyperplasia (BPH). This pathological complexity may complicate the differentiation of stromal sarcoma. Although STUMP can be histologically misdiagnosed as BPH, it is important to recognize that these are neoplasms with unique local morbidity and malignant potential. In contrast to BPH, STUMP can recur frequently and occur at younger men.[12] Sometimes high- and low-grade prostatic stromal sarcoma was associated with STUMP. Herawi and Epstein, in fact, found that seven (14%) of the 50 stromal tumors of the prostate that they analyzed were STUMPs associated with sarcoma (four high-grade stromal sarcoma, three LG-PS).[13] The primary PS are really rare neoplasms. The characteristics of low-grade prostate sarcomas reported in the literature are summarized in Table 1.
Table 1.
Review of the literature of low-grade prostatic sarcoma
Having the knowledge of prognosis in cancers implies that the behavior of cancer in terms of diagnosis, treatment, recurrence, metastasis, response to therapy, and survival is known to a large extent, and these data are invaluable for the physicians, patients, as well as in regard to public health. The knowledge of the prognosis is critical in determining the individuals that are at risk, predicting survival, and establishing future strategies based on the incidence and prevalence rates. The biological behavior of prostate sarcomas is not predictable, as is the case with other sarcomas of the soft tissue; however, the disease is associated with poor prognosis and high metastatic potential. The lack of a laboratory parameter that would not be used to monitor the disease course in prostate sarcoma represents another reason for continuous patient follow-up.
Among all urogenital sarcomas, 5-year mean survival is 82% for retroperitoneal sarcomas, 73% for bladder sarcomas, 44% for prostate sarcomas, and 39% for kidney sarcomas.[14] 1-year mortality of the disease is 20%.[8] Average survival is 9.5-14 months. Survival is directly related especially to stage of the tumor and presence of metastasis. Presence of necrosis, grade of the tumor, and the presence of adenocancer component are not parameters affecting survival directly. Difficulty of diagnosis and treatment of this disease is related to the fact that these malignant tumors don’t produce PSA and no other biochemical parameter exists indicative of them. Furthermore, a short time to progress and the LUTS are only seen in the advanced cases causes’ difficulty in diagnosis. The fact that they are included in the rare causes of the LUTS prolongs the time to diagnosis and diagnosis is possible only by means of biopsy. In addition, the fact that the disease begins as adenocarcinoma and inability to detect the variant components in the first biopsy are other difficulties in the diagnosis. The fact that serial PSA measurement is not conclusive in the patients receiving androgen blocking treatment with a diagnosis of prostatic adenocarcinoma is also an important issue. In the patient presented here, the disease process began primarily without adenocarcinoma component and STUMP in contrast to the majority of the cases in the literature and at the time of diagnosis the patient had widespread metastases in the lungs and liver, which are not primary metastatic site for the prostatic carcinoma. Treatment of the local disease is with curative methods such as radical prostatectomy. However, the disease has high potential to generate locally advanced disease and metastases. Chemotherapies used in the treatment of the sarcomas are almost the first option and may be combined with radiation therapy. The present case was placed on a doxorubicin-based CTx program. The local stage of the tumor is the basic factor for surgical resection in prostate sarcoma. Surgical control of the tumor is a good prognostic factor.
The presence of metastasis at diagnosis is one of the most important predictors of prognosis. The mean survival is shorter in patients with metastatic disease at diagnosis. In the study by Lee et al., the presence of metastasis at diagnosis was found to be associated with poor survival in both univariate and multivariate analyses.[14]
The presence of lung metastasis from sarcomas and the presence of resectable lung metastasis can be associated with long-term disease-free survival in selected patients. However, the importance of the role played by other factors remains unknown, such as the slow growth of the tumor. The value of resecting liver metastases, in terms of survival, is unknown.[15] In their study, Putnam and Roth suggested that limited resection of lung metastases was associated with the long-term disease-free survival; however, the contribution of low tumor load, slow tumor growth, and long disease-free interval remains unknown.[16] The sarcomas sensitive to CTx are regarded to be associated with a better prognosis. In the literature, overexpression of P16 and P53 tumor suppressor proteins has been found in sarcomas. This might be a prognostic indicator in prostate sarcoma.[17] The overexpression of MED12 gene mutation in sarcomas can be an indicator of poor prognosis.[18] Furthermore, the replication of the DNA profiles obtained from sarcomas revealed the presence of repetitive genomic changes. Among these genomic changes, 17q duplication was found to be associated with long-term disease-free survival and low risk of metastasis. Zhao et al., increased PRUNE2 (prune homolog 2, Drosophila) protein expression was found to be associated with a good prognosis in patients with sarcoma. The present study revealed that increased PRUNE2 protein expression was an independent prognostic factor for overall survival in patients with sarcomas.[19]
CONCLUSION
Spindle cell sarcoma of the prostate is a disease very hard to diagnose and treat. Multiplicity of the metastatic cases at the time of diagnosis limits therapeutic options. Long-term follow-up is usually impossible due to poor prognosis and potential to make metastasis. Impact of the adjuvant or neo-adjuvant CTx and the radiation therapy remains limited because of poor differentiation of the tumor. Option of surgical treatment is possible only for local disease. The elucidation of the so-called “chaotic” genetic and molecular basis of the tumor will help to predict the prognosis.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Cheville JC, Dundore PA, Nascimento AG, Meneses M, Kleer E, Farrow GM, et al. Leiomyosarcoma of the prostate. Report of 23 cases. Cancer. 1995;76:1422–7. doi: 10.1002/1097-0142(19951015)76:8<1422::aid-cncr2820760819>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 2.Zizi-Sermpetzoglou A, Savvaidou V, Tepelenis N, Galariotis N, Olympitis M, Stamatiou K. Sarcomatoid carcinoma of the prostate: A case report. Int J Clin Exp Pathol. 2010;3:319–22. [PMC free article] [PubMed] [Google Scholar]
- 3.Eble JN, Sauter G, Ebstein JI, Sesterhenn IA. Lyon: IARC Press; 2004. Pathology and Genetics of Tumors of the Urinary System and Male Genital Organs. [Google Scholar]
- 4.Coindre JM, Terrier P, Bui NB, Bonichon F, Collin F, Le Doussal V, et al. Prognostic factors in adult patients with locally controlled soft tissue sarcoma. A study of 546 patients from the French Federation of Cancer Centers Sarcoma Group. J Clin Oncol. 1996;14:869–77. doi: 10.1200/JCO.1996.14.3.869. [DOI] [PubMed] [Google Scholar]
- 5.Zhou M, Netto GJ, Ebstein JI. Philadelphia: Saunders; 2012. Uropathology. [Google Scholar]
- 6.Humphrey PA. Histological variants of prostatic carcinoma and their significance. Histopathology. 2012;60:59–74. doi: 10.1111/j.1365-2559.2011.04039.x. [DOI] [PubMed] [Google Scholar]
- 7.Rogers CG, Parwani A, Tekes A, Schoenberg MP, Epstein JI. Carcinosarcoma of the prostate with urothelial and squamous components. J Urol. 2005;173:439–40. doi: 10.1097/01.ju.0000149969.76999.7c. [DOI] [PubMed] [Google Scholar]
- 8.Hansel DE, Epstein JI. Sarcomatoid carcinoma of the prostate: A study of 42 cases. Am J Surg Pathol. 2006;30:1316–21. doi: 10.1097/01.pas.0000209838.92842.bf. [DOI] [PubMed] [Google Scholar]
- 9.Fraggetta F, Pepe P, Giunta ML, Aragona F. Primary high grade sarcoma of the specialised prostatic stroma: A case report with clinico-pathological considerations. Pathologica. 2008;100:482–4. [PubMed] [Google Scholar]
- 10.Gaudin PB, Rosai J, Epstein JI. Sarcomas and related proliferative lesions of specialized prostatic stroma: A clinicopathologic study of 22 cases. Am J Surg Pathol. 1998;22:148–62. doi: 10.1097/00000478-199802000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Fukuhara S, Matsuoka Y, Hanafusa T, Nakayama M, Takayama H, Tsujihata M, et al. A case report of prostatic stromal tumor of uncertain malignant potential (STUMP) Acta Urol Jpn. 2008;54:377–81. [PubMed] [Google Scholar]
- 12.Zamparese R, Corini F, Braccischi A, D’Angelo A, Diamanti L, Del Vecchio M, et al. Primary sarcoma of the specialised prostatic stroma: A case report and review of the literature. Case Rep Pathol 2011. 2011:252805. doi: 10.1155/2011/252805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herawi M, Epstein JI. Specialized stromal tumors of the prostate: A clinicopathologic study of 50 cases. Am J Surg Pathol. 2006;30:694–704. doi: 10.1097/00000478-200606000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Lee G, Lee SY, Seo S, Jeon S, Lee H, Choi H, et al. Prognostic factors and clinical outcomes of urological soft tissue sarcomas. Korean J Urol. 2011;52:669–73. doi: 10.4111/kju.2011.52.10.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Geel AN, Pastorino U, Jauch KW, Judson IR, van Coevorden F, Buesa JM, et al. Surgical treatment of lung metastases: The european organization for research and treatment of cancer-soft tissue and bone sarcoma group study of 255 patients. Cancer. 1996;77:675–82. doi: 10.1002/(sici)1097-0142(19960215)77:4<675::aid-cncr13>3.3.co;2-h. [DOI] [PubMed] [Google Scholar]
- 16.Putnam JB, Jr, Roth JA. Surgical treatment for pulmonary metastases from sarcoma. Hematol Oncol Clin North Am. 1995;9:869–87. [PubMed] [Google Scholar]
- 17.Hakverdi S, Güngören A, Yaldiz M, Hakverdi AU, Toprak S. Immunohistochemical analysis of p16 expression in uterine smooth muscle tumors. Eur J Gynaecol Oncol. 2011;32:513–5. [PubMed] [Google Scholar]
- 18.Schwetye KE, Pfeifer JD, Duncavage EJ. MED12 exon 2 mutations in uterine and extrauterine smooth muscle tumors. Hum Pathol. 2014;45:65–70. doi: 10.1016/j.humpath.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Zhao LR, Tian W, Wang GW, Chen KX, Yang JL. The prognostic role of PRUNE2 in leiomyosarcoma. Chin J Cancer. 2013;32:648–52. doi: 10.5732/cjc.013.10069. [DOI] [PMC free article] [PubMed] [Google Scholar]


