Abstract
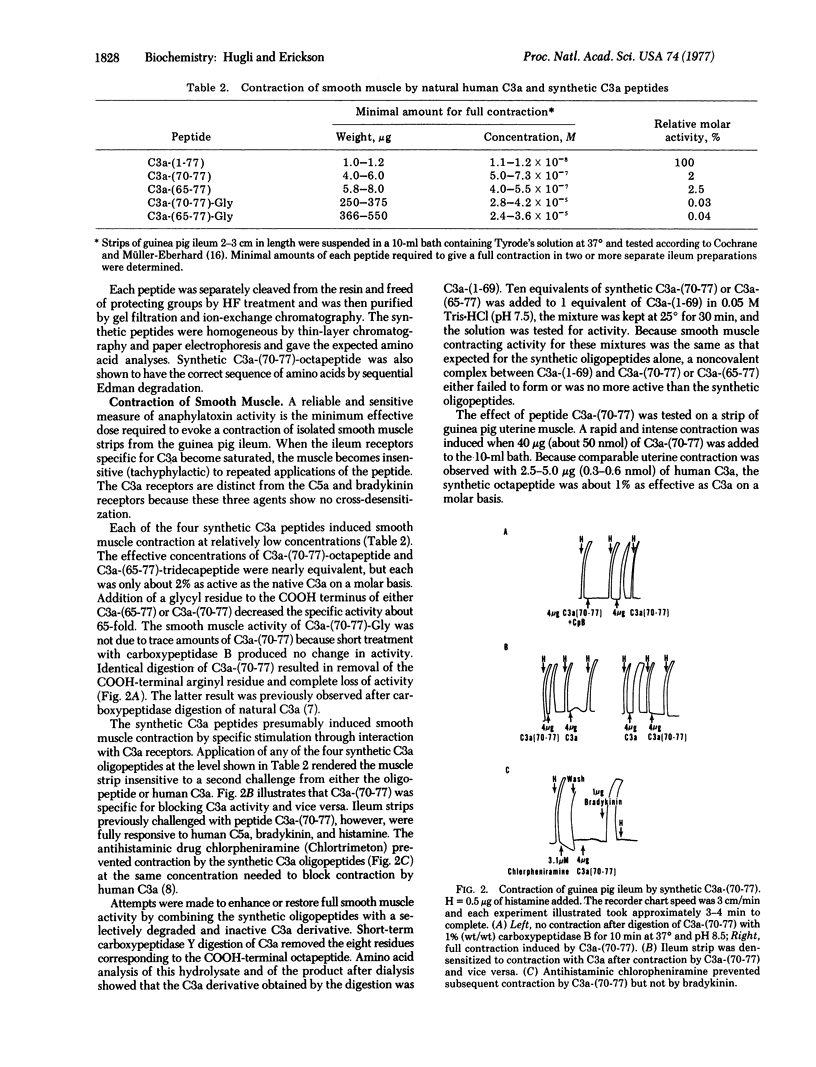
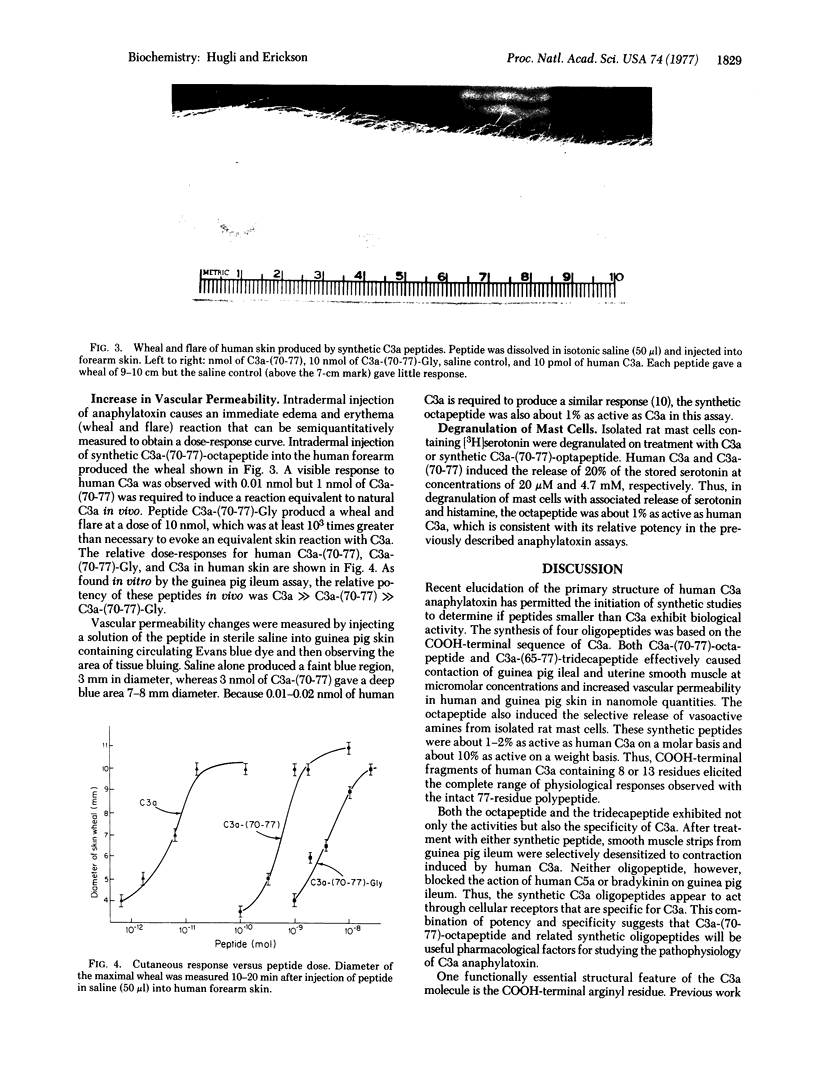
Two peptides identical to the COOH-terminal sequence of human C3a anaphylatoxin and two analogs were synthesized by the solid-phase method and tested for biological activity. The synthetic COOH-terminal octapeptide, C3a-(70-77) or Ala-Ser-His-Leu-Gly-Leu-Ala-Arg, caused contraction of guinea pig ileum and uterus, release of vasoactive amines from rat mast cells, and increased vascular permeability in guinea pig and human skin. On a molar basis, the synthetic octapeptide possessed 1-2% of the biological activities of C3a and specifically desensitized smooth muscle to stimulation by C3a. Like natural C3a, the synthetic C3a=(70-77) was inactivated by digestion with carboxypeptidase B [peptidyl-L-lysine(-L-arginine) hydrolase, EC 3.4.12.3], which removed the essential COOH-terminal arginine. A synthetic nonapeptide [C3a-(70-77)-Gly], containing a glycyl instead of an arginyl COOH terminus, was approximately 1% as active as the octapeptide when assayed with smooth muscle. The COOH-terminal 13-residue peptide of C3a, C3a-(65-77), was equal in activity to C3a=(70-77); similarly, C3a-(65-77)-Gly expressed the same activity as C3a-(70-77)Gly. It is concluded that both the biological specificity and the activity of human C3a anaphylatoxin are determined by eight or fewer residues located at the COOH terminus of the natural protein. However, expression of full activity requires additional groups and the secondary conformational integrity of the C3a molecule.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bokisch V. A., Müller-Eberhard H. J. Anaphylatoxin inactivator of human plasma: its isolation and characterization as a carboxypeptidase. J Clin Invest. 1970 Dec;49(12):2427–2436. doi: 10.1172/JCI106462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane C. G., Müller-Eberhard H. J. The derivation of two distinct anaphylatoxin activities from the third and fifth components of human complement. J Exp Med. 1968 Feb 1;127(2):371–386. doi: 10.1084/jem.127.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin N. C., Hugli T. E. The primary structure of porcine C3a anaphylatoxin. J Immunol. 1976 Sep;117(3):990–995. [PubMed] [Google Scholar]
- Erickson B. W., Merrifield R. B. Use of chlorinated benzyloxycarbonyl protecting groups to eliminate N -branching at lysine during solid-phase peptide synthesis. J Am Chem Soc. 1973 May 30;95(11):3757–3763. doi: 10.1021/ja00792a047. [DOI] [PubMed] [Google Scholar]
- Fernandez H. N., Hugli T. E. Partial characterization of human C5a anaphylatoxin. I. Chemical description of the carbohydrate and polypeptide prtions of human C5a. J Immunol. 1976 Nov;117(5 Pt 1):1688–1694. [PubMed] [Google Scholar]
- Hugli T. E. Human anaphylatoxin (C3a) from the third component of complement. Primary structure. J Biol Chem. 1975 Nov 10;250(21):8293–8301. [PubMed] [Google Scholar]
- Hugli T. E., Morgan W. T., Müller-Eberhard H. J. Circular dichroism of C3a anaphylatoxin. Effects of pH, heat, guanidinium chloride, and mercaptoethanol on conformation and function. J Biol Chem. 1975 Feb 25;250(4):1479–1483. [PubMed] [Google Scholar]
- Hugli T. E., Vallota E. H., Müller-Eberhard H. J. Purification and partial characterization of human and porcine C3a anaphylatoxin. J Biol Chem. 1975 Feb 25;250(4):1472–1478. [PubMed] [Google Scholar]
- Müller-Eberhard H. J. Complement. Annu Rev Biochem. 1975;44:697–724. doi: 10.1146/annurev.bi.44.070175.003405. [DOI] [PubMed] [Google Scholar]
- Wuepper K. D., Bokisch V. A., Müller-Eberhard H. J., Stoughton R. B. Cutaneous responses to human C 3 anaphylatoxin in man. Clin Exp Immunol. 1972 May;11(1):13–20. [PMC free article] [PubMed] [Google Scholar]