Abstract
5-Methoxy-N,N-dimethyltryptamine (5-MeO-DMT) and harmaline are serotonin (5-HT) analogs often abused together, which alters thermoregulation that may indicate the severity of serotonin toxicity. Our recent studies have revealed that co-administration of monoamine oxidase inhibitor harmaline leads to greater and prolonged exposure to 5-HT agonist 5-MeO-DMT that might be influenced by cytochrome P450 2D6 (CYP2D6) status. This study was to define the effects of harmaline and 5-MeO-DMT on thermoregulation in wild-type and CYP2D6-humanized (Tg-CYP2D6) mice, as well as the involvement of 5-HT receptors. Animal core body temperatures were monitored noninvasively in the home cages after implantation of telemetry transmitters and administration of drugs. Harmaline (5 and 15 mg/kg, i.p.) alone was shown to induce hypothermia that was significantly affected by CYP2D6 status. In contrast, higher doses of 5-MeO-DMT (10 and 20 mg/kg) alone caused hyperthermia. Co-administration of harmaline (2, 5 or 15 mg/kg) remarkably potentiated the hyperthermia elicited by 5-MeO-DMT (2 or 10 mg/kg), which might be influenced by CYP2D6 status at certain dose combination. Interestingly, harmaline-induced hypothermia was only attenuated by 5-HT1A receptor antagonist WAY-100635, whereas 5-MeO-DMT- and harmaline-5-MeO-DMT-induced hyperthermia could be suppressed by either WAY-100635 or 5-HT2A receptor antagonists (MDL-100907 and ketanserin). Moreover, stress-induced hyperthermia under home cage conditions was not affected by WAY-100635 but surprisingly attenuated by MDL-100907 and ketanserin. Our results indicate that co-administration of monoamine oxidase inhibitor largely potentiates 5-MeO-DMT-induced hyperthermia that involves the activation of both 5-HT1A and 5-HT2A receptors. These findings shall provide insights into development of anxiolytic drugs and new strategies to relieve the lethal hyperthermia in serotonin toxicity.
1. Introduction
Indolealkylamine (IAA) drugs are derivatives of 5-hydroxytryptamine (5-HT or serotonin), a neurotransmitter that modulates attention, behavior and body temperature. Many IAAs are “natural” products, the psychoactive ingredients of a variety of plant, fungus and animal preparations used in medicine, religion and recreation (Bruno et al., 2012; Halberstadt and Geyer, 2011; McIlhenny et al., 2011; McKenna, 2004; Winstock et al., 2014). Acting on the serotonergic system, IAAs are noted as a major class of substances of abuse. Among them, t-HT agonists 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT or by the street name “5-MEO”) and 5-methoxy-N,N-diisopropyltryptamine (5-MeO-DiPT or “Foxy” and “Foxy methoxy”) are relatively newer designer drugs, and they were placed into Schedule I under the Controlled Substances Act in the United States in 2011 and 2004, respectively (DEA, 2010; Drug Enforcement Administration (DEA), 2004). Although the trafficking, distribution and abuse of 5-MeO-DMT are likely underreported because it was not a controlled drug, the System to Retrieve Information from Drug Evidence revealed 23 cases involving 35 drug exhibits identified as 5-MeO-DMT from 1999 to 2009, and the National Forensic Laboratory Information System documented 27 State and local drug cases involving 32 drug exhibits identified as 5-MeO-DMT from 2004 to 2009 (DEA, 2010). With the epidemic of abuse, IAA intoxications have been frequently reported in hospitals in recent years, including a number of confirmed deaths caused by the abuse of 5-MeO-DMT or 5-MeO-DiPT (Bjornstad et al., 2009; Brush et al., 2004; Fuse-Nagase and Nishikawa, 2013; Hill and Thomas, 2011; Long et al., 2003; Muller, 2004; Sklerov et al., 2005; Smolinske et al., 2005; Tanaka et al., 2006).
5-MeO-DMT is mainly inactivated through monoamine oxidase A (MAO-A) mediated deamination. Thus it is often co-abused with MAO-A inhibitor (MAOI) such as harmaline towards an enhanced hallucinogenic effect (Ott, 2001). Recent studies using 5-MeO-DMT and MAOI harmaline as model drugs have demonstrated IAA drug-drug interactions (DDI) as well as metabolic pharmacogenetics (Shen et al., 2010a). Actually, both harmaline and 5-MeO-DMT can be metabolized by cytochrome P450 2D6 (CYP2D6), one of the most important polymorphic phase I drug-metabolizing enzymes (Yu et al., 2003a; Yu et al., 2003b). Harmaline pharmacokinetics is revealed to be determine by CYP2D6 status (Wu et al., 2009), and co-administration of MAOI harmaline results in a sharply increased and prolonged systemic and cerebral exposure to 5-MeO-DMT and the active metabolite bufotenine (Jiang et al., 2013; Shen et al., 2009; Shen et al., 2010b). In addition, harmaline largely elevates the 5-HT levels in vivo (Cheng et al., 2013). Therefore, co-administration of MAOI harmaline leads to a significant alternation in 5-MeO-DMT-induced psychedelic effects (Halberstadt et al., 2008; Halberstadt et al., 2012; Winter et al., 2011).
5-MeO-DMT is a non-selective 5-hydroxytryptamine (5-HT) receptor agonist that acts on 5-HT1A, 5-HT2A and 5-HT2C receptors with moderate to high affinities (Halberstadt and Geyer, 2011; Halberstadt et al., 2011; Riga et al., 2014; Roth et al., 1997; van den Buuse et al., 2011; Winter et al., 2000). Co-administration of 5-HT agonist 5-MeO-DMT and MAOI harmaline causes an excessive activation of serotonergic system, which may cause hyperserotonergic tone or serotonin toxicity/syndrome. Indeed the serotonin syndrome has become more prevalent as a result of increasing use or abuse of serotonergic drugs (Boyer and Shannon, 2005; Haberzettl et al., 2013; Kant and Liebelt, 2012). Serotonin syndrome exhibits a spectrum of characteristic features in patients and animal models, including neuromuscular excitation (e.g., shivering and tremor), autonomic stimulation (e.g., hyperthermia and tachycardia), and change in mental/behavioral status (e.g., confusion and anxiety). Among these symptoms, increase of body temperature is recognized as a critical complication of severe serotonin toxicity that may cause fatality without any intervention. Indeed, a case of harmaline-5-MeO-DMT-induced life threatening serotonin toxicity including hyperthermia (105.2 °F or 40.7 °C), tachycardia and hyperactivity has been reported (Brush et al., 2004).
In this study, we aimed to delineate the impact of harmaline on the thermomodulatory effects of 5-MeO-DMT in mouse models. Wild-type and CYP2D6-humanized (Tg-CYP2D6) mice were used to evaluate possible influence of CYP2D6 status. In addition, 5-HT1A receptor antagonist WAY-100635 and 5-HT2A receptor antagonists MDL-100907 and ketanserin were utilized to define the serotonergic mechanisms underlying thermomodulatory actions of harmaline and 5-MeO-DMT. The results shall improve the understanding of the risks of IAA drug abuse, and offer insights into developing active means to treat drug-induced, severe or lethal hyperthermia.
2. Material and Methods
2.1. Chemicals and materials
5-MeO-DMT oxalate, harmaline hydrochloride dihydrate and WAY-100635 were purchased from Sigma-Aldrich (St. Louis, MO). Ketanserin was bought from Tocris Bioscience (Ellisville, MO). MDL-100907 was generously provided by Sanofi-Aventis (Bridgewater, NJ). Drugs were dissolved in saline and administered according to their free base molecular weights.
2.2. Animals
Age-matched male wild-type FVB/N and Tg-CYP2D6 mice (Corchero et al., 2001) weighing 25–35 g were used in the study. Mice were housed in an animal care facility maintained at 20 ± 2.0°C on a 12-h light/dark cycle, with lights on at 6:00 and off at 18:00. Food and water were provided ad libitum. All animal procedures were approved by the Institutional Animal Care and Use Committee at University at Buffalo, The State University of New York.
2.3. Surgical preparations
Mice were anaesthetized with isoflurane in oxygen (4%, reduced as necessary). A sterile telemetric transmitter (Physiotel implant TA10TA-F20 system; Data Sciences International, St. Paul, MN) was implanted into the peritoneal cavity through a midline incision, and then the abdominal wall and skin were sutured. All surgery procedures were performed under aseptic conditions. After surgery, animals were singly housed and given subcutaneous (s.c.) or oral (p.o.) doses of 5 mg/kg carprofen once daily for 3 days and were allowed to recover for 2 weeks before body temperature measurement.
2.4. Dosage settings
To investigate the thermoregulatory effects of individual drugs, mice were given an intraperitoneal (i.p.) dose of harmaline (0, 2, 5 or 15 mg/kg) or 5-MeO-DMT (0, 2, 10 or 20 mg/kg). To investigate the impact of harmaline on the thermoregulatory effects of 5-MeO-DMT, mice were administered i.p. with 0, 2, 5 or 15 mg/kg of harmaline (at 0 min) before the treatment with 2 or 10 mg/kg of 5-MeO-DMT (15 min) which is consistent with the dosing regimen in our previous pharmacokinetic studies (Jiang et al., 2013; Shen et al., 2010b).
To define the mechanistic roles of 5-HT1A and 5-HT2A receptors in the thermoregulation by IAA drugs, mice were treated subcutaneously (s.c.) with 5-HT receptor antagonists (1 mg/kg of WAY-100635, MDL-100907, ketanserin or vehicle control) before i.p. administration of IAA drugs (15 mg/kg of harmaline, 20 mg/kg of 5-MeO-DMT, 2 mg/kg of harmaline plus 2 mg/kg of 5-MeO-DMT, or corresponding vehicle control). For the investigations of harmaline induced hypothermia, antagonists were injected at 15 min before harmaline treatment. For the examination of 5-MeO-DMT or harmaline-5-MeO-DMT interaction induced hyperthermia, antagonists were injected at 15 min before 5-MeO-DMT treatment. The dosage of each 5-HT antagonist was selected according to the effective regimen reported previously (Krebs-Thomson et al., 1998; Krebs-Thomson et al., 2006) and our preliminary data to minimize the influence of handling/injection-induced stress on body temperature change.
2.5. Measurement of core body temperature
All experiments were carried out in an isolated and quiet room between 10:30 A.M. and 3:30 P.M. Ambient temperature was maintained at 20 ± 2.0°C throughout the experiment. Animal in its home cage was placed on a configured receiver (Data Sciences International, St. Paul, MN) and the telemetry transmitter was activated at least 12 hr before experiment for overnight stabilization. Before testing, baseline was recorded for 60 min. After drug administrations, core body temperatures were continuously measured every 6 sec, and the values that averaged from every 5 min time interval were used for data analyses.
2.6. Data analyses
Changes in core body temperature (ΔCBT) were calculated as the differences from the baseline level (mean value of the 60 min baseline recordings) and all values are mean ± SD. The maximum change in core body temperature (ΔCBTmax) was defined as the maximal change of core body temperature compared to the baseline CBT. Area under the effect curve (AUEC) values were calculated by trapezoidal rule based on the duration of drug effect and was used for statistical analysis, which was conducted with GraphPad Prism 5 (GraphPad, San Diego, CA). Depending on the number of groups and variances, data were compared using Student’s t-test, one-way ANOVA with Dunnett’s post-hoc comparisons, or two-way ANOVA with Bonferroni’s post-hoc comparisons. Difference was considered statistically significant when P < 0.05.
3. Results
3.1. Harmaline induced a more severe hypothermia in wild-type mice than Tg-CYP2D6 mice
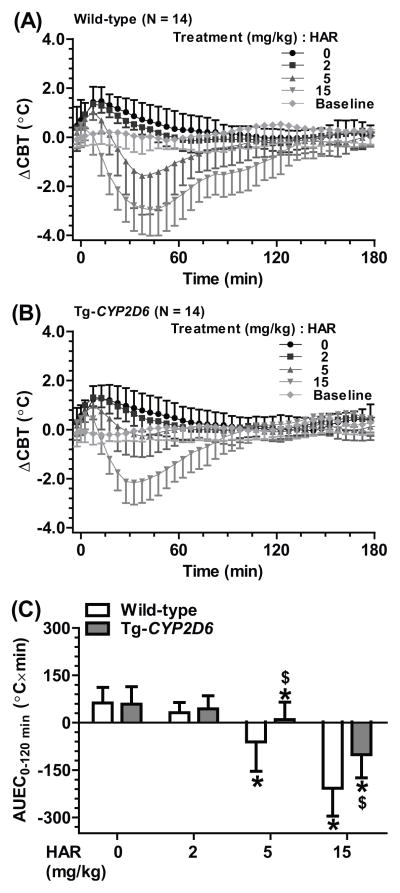
To delineate the impact of harmaline on 5-MeO-DMT-induced thermoregulatory effects, the body temperatures of wild-type and Tg-CYP2D6 mice treated with harmaline alone (0, 2, 5 or 15 mg/kg, i.p.) were studied first. Following vehicle treatment, a transient increase in CBT was observed in both wild-type and Tg-CYP2D6 mice. In contrast, administration of harmaline led to a dose dependent decrease of CBT (Figure 1, Table 1), and the hypothermia was more severe in the wild-type mice. Compared to vehicle control, 2 mg/kg harmaline had no significant influence on the ΔCBTmax in Tg-CYP2D6 mice whereas significantly decreased the ΔCBTmax in wild-type mice. Furthermore, 15 mg/kg harmaline resulted in ΔCBTmax values of −3.19 ± 0.99 °C in wild-type mice and −2.31 ± 0.84 °C in Tg-CYP2D6 mice. The AUEC values after 15 mg/kg harmaline treatment, which were −207 ± 88 °C×min in wild-type mice and −101 ± 74 °C×min in Tg-CYP2D6 mice, also confirmed the more severe hypothermia in wild-type mice.
Figure 1.
Harmaline (HAR) induced a hypothermic effect, which was more severe and prolonged in wild-type (A) mice than Tg-CYP2D6 (B) mice. The severity of hypothermia was also manifested by the AUEC0–120min values (C). Values are mean ± SD (N = 14 in each group). Baseline represents the core body temperature without any interference. Harmaline or vehicle was injected i.p. at 0 min. * P < 0.05, compared to vehicle control within the same genotype of mice; $P < 0.05, compared to wild-type mice under the same treatment.
Table 1.
Harmaline induced hypothermia in a dose-dependent manner, which was more severe in wild-type mice than Tg-CYP2D6 mice. The maximum change of core body temperature (ΔCBTmax) was defined by comparing the maximum change of core body temperature in the animals from 0 to 120 min after harmaline administration to their baseline core body temperature before the treatment. Values represent mean ± SD (N = 14 in each group).
| Dosage regimen (mg/kg, i.p.)
|
ΔCBTmax (°C)
|
|
|---|---|---|
| Harmaline | Wild-type | Tg-CYP2D6 |
| 0 | 1.72 ± 0.51 | 1.44 ± 0.41 |
| 2 | 1.39 ± 0.38* | 1.42 ± 0.39 |
| 5 | −1.72 ± 1.55* | −0.61 ± 0.88*, $ |
| 15 | −3.19 ± 0.99* | −2.31 ± 0.84*, $ |
P < 0.05, compared to the same genotype mice that were treated with drug vehicle (harmaline 0 mg/kg).
P < 0.05, compared to wild-type mice with the same treatment.
3.2. Higher doses of 5-MeO-DMT elicited a hyperthermia in both Tg-CYP2D6 and wild-type mice
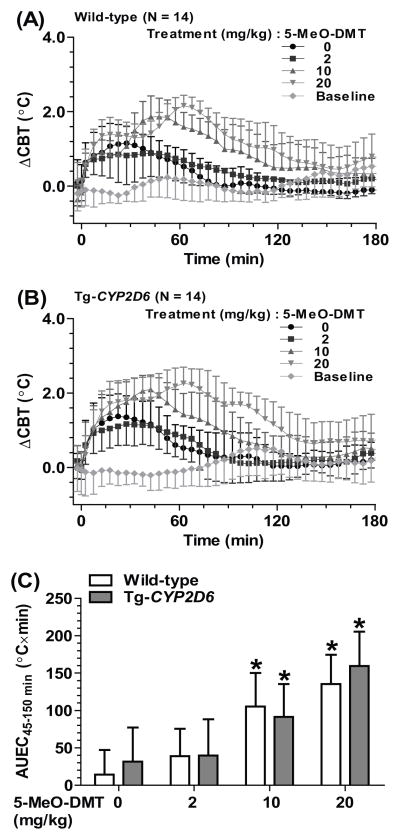
To evaluate the thermomodulatory effects of 5-MeO-DMT in mice and possible influence of CYP2D6 status, ΔCBT was determined following the administration of 5-MeO-DMT (0, 2, 10 or 20 mg/kg, i.p.) in wild-type and Tg-CYP2D6 mice. Handling and vehicle treatment induced an increase of ΔCBT at the early time points (0–45 min), to the same levels in the wild-type (AUEC45-150min, 43.7 ± 20.3 °C×min) and Tg-CYP2D6 (52.6 ± 23.3 °C×min) mice. 5-MeO-DMT-induced effects were readily indicated by the increase of ΔCBT at late time points (45–150 min) (Figure 2 and Table 1). Low dose of 5-MeO-DMT (2 mg/kg) showed no significant effect on thermoregulation. In contract, higher doses of 5-MeO-DMT (10 and 20 mg/kg) led to a remarkable increase of CBT (Table 2 and Figure 2). The ΔCBTmax values in wild-type mice after 10 and 20 mg/kg doses were 1.93 ± 0.45 and 2.20 ± 0.28 °C, respectively, and the AUEC45-150min values were 105 ± 45 and 135 ± 39 °C×min, respectively, which all demonstrated the induction of significant hyperthermia by 5-MeO-DMT. Nevertheless, CYP2D6 did not exhibit any significant influence on 5-MeO-DMT-induced hyperthermia (Table 2 and Figure 2).
Figure 2.
Higher doses of 5-MeO-DMT (10 or 20 mg/kg) induced a hyperthermia in both wild-type (A) and Tg-CYP2D6 (B) mice. Drug-induced hyperthermia was obvious at late phase (AUEC45–150 min) (C), which is different from injection stress-caused hyperthermia at early time points (0–45 min). Values are mean ± SD (N = 14 in each group). Baseline represents the core body temperature without any treatment. 5-MeO-DMT or drug vehicle was injected i.p. at 0 min. *P < 0.05, compared to vehicle control within the same genotype of mice.
Table 2.
5-MeO-DMT induced a hyperthermia dose-dependently, which was similar in wild-type and Tg-CYP2D6 mice. The maximum change of core body temperature (ΔCBTmax) was defined by comparing the maximum change of core body temperature in the animals from 45 to 150 min after 5-MeO-DMT administration to their baseline core body temperature before the treatment. Values represent mean ± SD (N = 14 per group).
| Dosage regimen (mg/kg, i.p.)
|
ΔCBTmax (°C)
|
|
|---|---|---|
| 5-MeO-DMT | Wild-type | Tg-CYP2D6 |
| 0 | 0.85 ± 0.37 | 1.11 ± 0.56 |
| 2 | 1.08 ± 0.48 | 1.14 ± 0.51 |
| 10 | 1.93 ± 0.45* | 1.98 ± 0.46* |
| 20 | 2.20 ± 0.28* | 2.45 ± 0.45* |
P < 0.05, compared to mice of the same genotype that were treated with drug vehicle (5-MeO-DMT 0 mg/kg).
3.3. Harmaline significantly enhanced 5-MeO-DMT-induced hyperthermia that might differ between Tg-CYP2D6 and wild-type mice
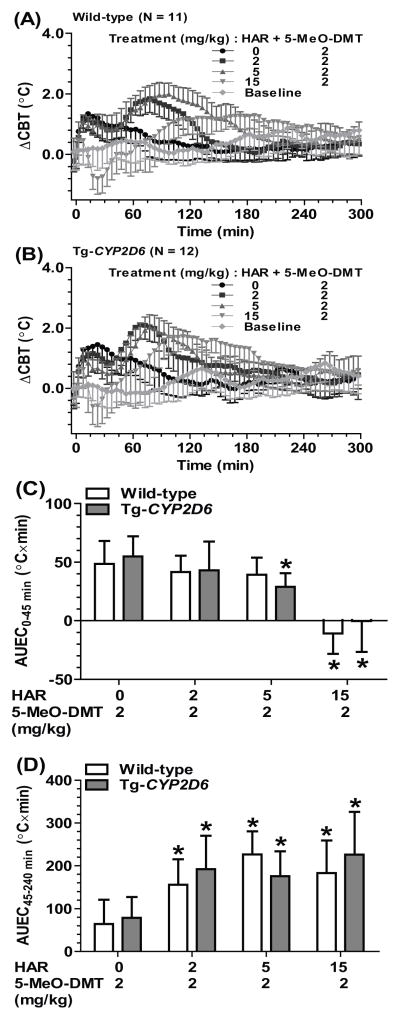
To assess the impact of harmaline on 5-MeO-DMT-induced hyperthermia, different doses of harmaline (0, 2, 5 or 15 mg/kg, i.p.) was administered 15 min prior to the treatment with 5-MeO-DMT (2 or 10 mg/kg, i.p.). The data showed that co-administration of harmaline sharply elevated 5-MeO-DMT-induced late-phase (after 45 min) hyperthermia in a dose dependent manner (Table 3 and Figure 3). For instance, compared to 2 mg/kg 5-MeO-DMT alone, pretreatment with 5 mg/kg harmaline led to a ΔCBTmax about 1.0 °C and AUEC45-240 min around 200 °C×min in both wild-type and Tg-CYP2D6 mice (Figure 3 and Table 3). Furthermore, harmaline-induced early-stage hypothermia retained in mice treated with lower dose (2 mg/kg) of 5-MeO-DMT, which resulted in an overall biphasic effect (Figure 3A–3D). Enhancement of 5-MeO-DMT-induced hyperthermia by harmaline was also observed for a higher dose (10 mg/kg) of 5-MeO-DMT (data not shown). In addition, at certain dose combination such as 15 mg/kg harmaline plus 2 mg/kg 5-MeO-DMT, the ΔCBTmax values were significantly different between wild-type (1.50 ± 0.45 °C) and Tg-CYP2D6 (1.98 ± 0.40 °C) mice (Table 3), suggesting that CYP2D6 status might have a significant influence on the severity of hyperthermia induced by co-administration of harmaline and 5-MeO-DMT.
Table 3.
Harmaline potentiated 5-MeO-DMT-induced hyperthermia. In addition, harmaline-5-MeO-DMT-induced hyperthermia could be affected by CYP2D6 status at specific dose combinations. The maximum change of core body temperature (ΔCBTmax) was defined by comparing the maximum change of core body temperature in the animals from 45 to 240 min after harmaline administration to their baseline core body temperature before the treatment. Harmaline and 5-MeO-DMT were dosed at 0 and 15 min, respectively. Values represent mean ± SD (N = 11 for wild-type mice; N = 12 for Tg-CYP2D6 mice).
| Dosage regimen (mg/kg, i.p.)
|
ΔCBTmax (°C)
|
||
|---|---|---|---|
| Harmaline | 5-MeO-DMT | Wild-type | Tg-CYP2D6 |
| 0 | 0 | 0.92 ± 0.62 | 0.90 ± 0.40 |
|
| |||
| 0 | 2 | 1.05 ± 0.46 | 1.20 ± 0.43 |
| 2 | 1.95 ± 0.44*, # | 2.18 ± 0.44*, # | |
| 5 | 2.12 ± 0.36*, # | 2.10 ± 0.40*, # | |
| 15 | 1.50 ± 0.45*, # | 1.98 ± 0.40*, , #, , $ | |
P < 0.05, compared to mice treated with vehicle only (harmaline 0 mg/kg + 5-MeO-DMT 0 mg/kg).
P < 0.05, compared to the same genotype mice that were treated with vehicle (harmaline 0 mg/kg) plus the same dose of 5-MeO-DMT.
P < 0.05, compared to wild-type mice with the same treatment.
Figure 3.
Co-administration of harmaline with a low dose of 5-MeO-DMT (2 mg/kg) (A, B, C and D) induced biphasic effects in wild-type (N = 11) (A) and Tg-CYP2D6 (N = 12) (B) mice, a hypothermia (0–45 min) (C) followed by hyperthermia (45–240 min) (D). Values are mean ± SD. Baseline represents the core body temperature without any interference. Harmaline or vehicle was injected i.p. at 0 min, and 5-MeO-DMT was injected i.p. at 15 min. *P < 0.05, compared to corresponding vehicle-5-MeO-DMT treatment within the same genotype of mice.
3.4. Harmaline-induced hypothermia was mediated by 5-HT1A receptor
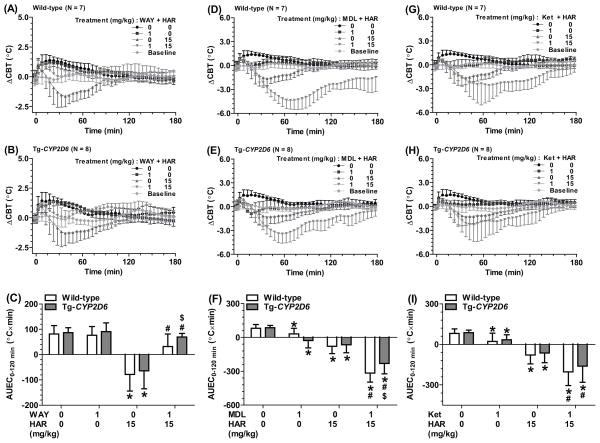
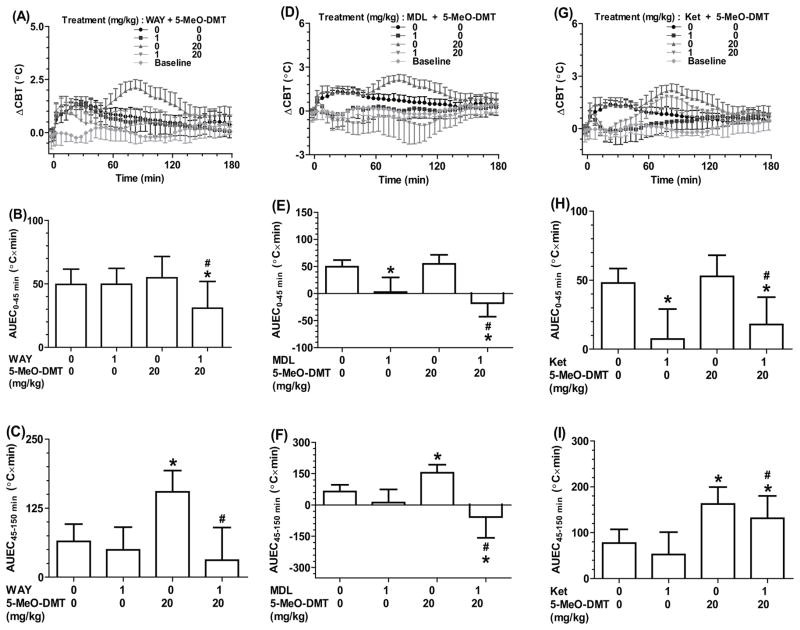
To define the role of 5-HT receptors in harmaline-elicited hypothermia, the effects of 5-HT receptor antagonists were investigated. As shown in Figure 4A and 4B, pretreatment with 5-HT1A receptor antagonist WAY-100635 completely attenuated the hypothermia induced by 15 mg/kg harmaline in both Tg-CYP2D6 and wild-type mice. This was manifested by the AUEC0–120 min values for mice with (−78.5 and −64.1 °C×min in wild-type and Tg-CYP2D6, respectively) and without (31.9 and 69.5°C×min in wild-type and Tg-CYP2D6, respectively) WAY-100635 treatment (Figure 4C). On the contrary, harmaline-induced hypothermia was significantly augmented by 5-HT2A receptor antagonist MDL-100907 (Figure 4D, 4E and 4F) and ketanserin (Figure 4G, 4H and 4I). It is noteworthy that administration of ketanserin or MDL-100907 itself induced hypothermic effects in all mice (Figure 4D–4I). Together, the data support a role for 5-HT1A receptor in harmaline-induced hypothermia.
Figure 4.
WAY-100635 (WAY, a selective 5-HT1A receptor antagonist) attenuated harmaline (HAR)-induced hypothermia (A, B and C) that was more significant in Tg-CYP2D6 mice than that in wild-type mice. In contrast, harmaline-induced hypothermia was enhanced by 5-HT2A receptor antagonists, MDL-100907 (MDL) (D, E and F) and ketanserin (Ket) (G, H and I). Values are mean ± SD (wild-type N = 7; Tg-CYP2D6 N = 8). Baseline represents the core body temperature without any interference. WAY, MDL or Ket (1 mg/kg) or vehicle (0 mg/kg) was injected s.c. at 0 min, and harmaline (15 mg/kg) or vehicle (0 mg/kg) was injected i.p. at 15 min. *P < 0.05, compared to vehicle only treatment within the same genotype of mice; #P < 0.05, compared to vehicle plus harmaline treatment within the same genotype of mice; $P < 0.05, compared to wild-type mice under the same treatment.
3.5. Both 5-HT1A and 5-HT2A receptors are involved in 5-MeO-DMT-induced hyperthermia
5-HT receptor antagonists were also employed to examine the roles of 5-HT1A and 5-HT2A receptors in 5-MeO-DMT-induced hyperthermia. WAY-100635 was revealed to completely attenuated the late-phase hyperthermia (45–150 min) in both wild-type (Figure 5A–C) and Tg-CYP2D6 (data not shown) mice induced by 5-MeO-DMT. For instance, the ΔCBTmax value decreased from 2.2 °C to 1.0 °C and AUEC45–150 min decreased from 160 °C×min to 45 °C×min after the mice were treated with WAY-100635. Interestingly, 5-MeO-DMT-induced late-phase hyperthermia was changed to a mild hypothermia when the mice were treated with MDL-100907, as shown by the ΔCBT profiles and the AUEC values (around −1.2°C and −15°C×min, respectively, Figure 5D–5F). In addition, ketanserin partially suppressed the late-phase hyperthermia induced by 5-MeO-DMT and completely attenuated the early-phase hyperthermia associated with animal handling and injection (Figure 5G–I). Our results indicate that both 5-HT1A and 5-HT2A receptors contribute to the induction of hyperthermia by 5-MeO-DMT.
Figure 5.
5-HT1A receptor antagonist WAY-100635 (WAY) (A, B and C) completely attenuated 5-MeO-DMT-induced late-phase hyperthermia (45–150 min), while partially repressed stress-induced early-phase hyperthermia (0–45 min). 5-HT2A receptor antagonist MDL-100907 (MDL) (D, E and F) not only reduced early-phase hyperthermia but also turned the later-phase hyperthermia into hypothermia. Ketanserin (Ket) (G, H and I) reduced both early-phase and later-phase hyperthermia. Shown are the data from wild-type mice (N = 7). Similar data were obtained from Tg-CYP2D6 (N = 8). Values are mean ± SD. Baseline represents the core body temperature without any interference. WAY, MDL or Ket (1 mg/kg) or vehicle (0 mg/kg) was injected s.c. at 0 min, and 5-MeO-DMT (20 mg/kg) or vehicle (0 mg/kg) was injected i.p. at 15 min. *P < 0.05, compared to vehicle only treatment within the same genotype of mice; #P < 0.05, compared to vehicle plus 5-MeO-DMT treatment within the same genotype of mice.
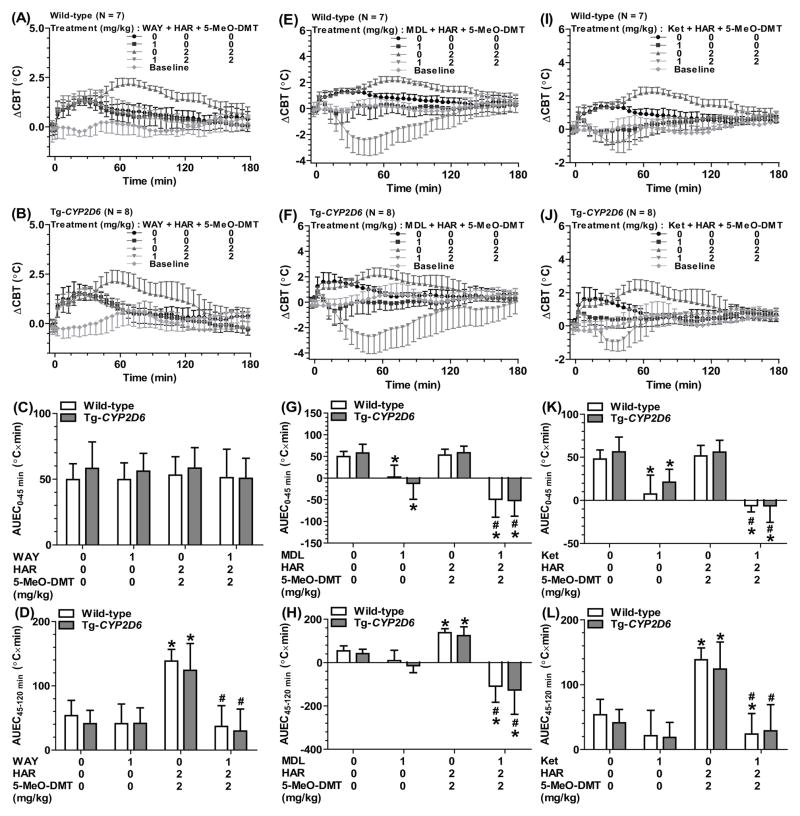
3.6. 5-HT1A receptor antagonist completely attenuated harmaline-5-MeO-DMT-induced hyperthermia, whereas 5-HT2A receptor antagonist converted it into hypothermia
Given the clear late-phase hyperthermic effects induced by 2 mg/kg of harmaline plus 2 mg/kg of 5-MeO-DMT, as manifested by the ΔCBTmax and AUEC45-120 min values in both Tg-CYP2D6 and wild-type mice (Table 3, Figure 3 and Figure 6), this dose combination was used to investigate the involvement of 5-HT1A and 5-HT2A receptors in harmaline-5-MeO-DMT-induced hyperthermia. Our data showed that WAY-100635 completely attenuated the late-phase hyperthermia induced by the drugs while it had no effect on the early-phase hyperthermia caused by animal handling/injection (Figure 6A–D). In contrast, ketanserin completely blocked the late-phase hyperthermia, following the conversion of early-phase hyperthermia into hypothermia (Figure 6I–L). Furthermore, MDL-100907 strikingly turned both the early- and late-phase hyperthermia to significant hypothermia (Figure 6E–H).
Figure 6.
WAY-100635 (WAY) lessened the late-phase hyperthermia (45–120 min) induced by harmaline-5-MeO-DMT interactions (DDI) (A, B, C and D). In contrast, MDL-100907 (MDL) (E, F, G and H) or ketanserin (Ket) (I, J, K and L) sharply suppressed the late-phase hyperthermia or even turned it into a significant hypothermia, in addition to the conversion of early-phase hyperthermia into hypothermia. Values are mean ± SD (wild-type N = 7; Tg-CYP2D6 N = 8). Baseline represents the core body temperature without any treatment. WAY, MDL or Ket (1 mg/kg) or vehicle was injected s.c. at 0 min. Harmaline (2 mg/kg) and 5-MeO-DMT (2 mg/kg) or drug vehicle were injected i.p. at 0 and 15 min, respectively. *P < 0.05, compared to vehicle only treatment within the same genotype of mice; #P < 0.05, compared to vehicle plus harmaline-5-MeO-DMT treatment within the same genotype of mice.
4. Discussion and Conclusion
In the present study, we demonstrated that co-administration of MAOI harmaline significantly potentiated 5-MeO-DMT-induced hyperthermia. The severity of hyperthermia might be influenced by CYP2D6 status at particular dose combination, due to the impact of CYP2D6 on harmaline pharmacokinetics (Jiang et al., 2013; Wu et al., 2009). Most importantly, we showed that the thermomodulatory effects of harmaline and 5-MeO-DMT were attributed to the activation of both 5-HT1A and 5-HT2A receptors.
Our recent pharmacokinetic studies have revealed that elimination of harmaline is significantly faster in Tg-CYP2D6 mice with additional CYP2D6 activity compared to wild-type mice (Jiang et al., 2013; Wu et al., 2009). Consistently, results from the present study showed that harmaline pharmacodynamics, as indicated by the hypothermic effect, was significantly affected by CYP2D6 status (Figure 1 and Table 1). However, the present study did not distinguish the thermoregulatory effect of low dose 5-MeO-DMT (2 mg/kg) from handling/injection in mice (Figure 2 and Table 2), which is different from the findings in rats (Gudelsky et al., 1986). In addition to the difference in animal models, the CBT profiles in the present study were acquired noninvasively from animals kept in their home cages. Higher doses of 5-MeO-DMT (10 and 20 mg/kg) indeed elicited a clear hyperthermia (Figure 2 and Table 2), which agrees with previous observation (Gudelsky et al., 1986). The severity of such late-phase hyperthermia was similar between wild-type and Tg-CYP2D6 mice, which is in accordance with our recent findings on a similar non-linear pharmacokinetic property for 5-MeO-DMT in wild-type and Tg-CYP2D6 mice (Shen et al., 2011; Shen et al., 2010b). Nevertheless, because of the significant impact of CYP2D6 on harmaline pharmacokinetics (Jiang et al., 2013), CYP2D6 status might affect the severity of hyperthermia induced by harmaline-5-MeO-DMT at a particular dose combination (Figure 3 and Table 3).
Co-administration of harmaline (2, 5 or 15 mg/kg) with 5-MeO-DMT (2 mg/kg) caused a dose-dependent increase in CBT in both wild-type and Tg-CYP2D6 mice at late stage (Figure 3A–3D and Table 3), despite that lower dose of 5-MeO-DMT (2 mg/kg) showed no significant influence on thermoregulation (Figure 2 and Table 2). The initial hypothermic effects in mice treated with harmaline plus 2 mg/kg 5-MeO-DMT was mostly due to harmaline administered at 15 min before 5-MeO-DMT treatment (Figure 3 and Figure 1). Since our results (Figure 1 and Table 1) and previous studies (Abdel-Fattah et al., 1997; Wu et al., 2009) consistently demonstrated that harmaline alone induced only hypothermia in rodents, the enhanced hyperthermia under the DDI condition indicates a significant potentiation of 5-MeO-DMT-induced hyperthermia by harmaline. Indeed, harmaline also enhanced the late-phase hyperthermia induced by 10 mg/kg 5-MeO-DMT, whereas the early-phase hypothermia induced by co-administered harmaline was interestingly overtaken by 10 mg/kg 5-MeO-DMT (data not shown). It is also noticeable that the hyperthermic effects were prolonged to a greater degree with the increase of harmaline dose (Figure 3), which may be explained by the prolonged 5-MeO-DMT exposure (Jiang et al., 2013). In addition, high dose combinations of harmaline (5 or 15 mg/kg) and 5-MeO-DMT (10 mg/kg) obviously induced serotonin toxicity (CBT > 39 °C) in mouse models (Isbister and Buckley, 2005). Harmaline not only increases 5-HT levels through inhibition of MAO-A activity (Cheng et al., 2013; Kim et al., 1997) but also binds to multiple neurotransmitter receptors such as 5-HT, norepinephrine, dopamine D2/D3 and N-methyl-D-aspartate (NMDA) glutamate receptors (Airaksinen et al., 1987; Iseri et al., 2011; Miralles et al., 2005; Ossowska et al., 2014; Paterson et al., 2009) that may all affect thermoregulation. Therefore, the potentiation of hyperthermia and induction of serotonin toxicity by co-administration of harmaline with 5-MeO-DMT could be mechanistically due to the alteration of 5-MeO-DMT pharmacokinetics (Jiang et al., 2013), inhibition of MAO-A and binding of various neurotransmitter receptors. Rather, the DDI between harmaline and 5-MeO-DMT occurring at both pharmacokinetic/toxicokinetic and pharmacodynamic/toxicodynamic levels warrants further investigation using other indicators and mathematical models.
The highly selective 5-HT2A receptor antagonist MDL-100907 completely attenuated the 5-MeO-DMT-induced hyperthermia (AUEC45–150min) in all genotyped mice, suggesting that activation of 5-HT2A receptor was essential for 5-MeO-DMT-induced hyperthermia (Figure 5F). Another 5-HT2A receptor antagonist ketanserin partially attenuated the hyperthermia induced by the same dose of 5-MeO-DMT (Figure 5I), suggesting that ketanserin might be less effective than MDL-100907 in antagonizing 5-HT2A receptor-mediated pharmacological and toxicological effects. The difference in attenuation of 5-MeO-DMT-induced hyperthermia between MDL-100907 and ketanserin might be attributed to their differing pharmacodynamic profiles because ketanserin also binds to some other targets such as noradrenaline and histamine receptors and vesicular monoamine transporter-2 (Gopalakrishnan et al., 2007; Larrauri and Levin, 2010; Marin et al., 1990). In addition, both MDL-100907 and ketanserin attenuated harmaline-5-MeO-DMT-induced hyperthermia, and converted it to significant hypothermia (Figure 6), indicating 5-HT2A receptor played a predominant role in harmaline-5-MeO-DMT-induced hyperthermic effect.
It has been reported that activations of 5-HT1A and 5-HT2A receptors lead to opposite effects on thermoregulation (Capuano et al., 2010; Salmi and Ahlenius, 1998). Thus 5-HT1A receptor antagonist WAY-100635 was expected to enhance the hyperthermia elicited by 5-MeO-DMT. However, our data showed that pretreatment with WAY-100635 actually attenuated the late-phase hyperthermia induced by 5-MeO-DMT alone or harmaline-5-MeO-DMT combination (Figure 5 and Figure 6). It is possible that WAY-100635 interacts with other neurotransmitter receptors (Chemel et al., 2006) and then modulates serotonergic system to influence the body temperature. Thermoregulation is indeed a complex process. Activation of 5-HT2A receptor in hypothalamus induces a series of signal transduction events from brain to spinal cord and eventually causes non-shivering thermogenesis in the peripheral brown adipose tissue (Morrison et al., 2008). Indeed there is a renaissance for the role of brown adipose in energy homeostasis and thermogenesis in adult humans (Lee et al., 2013; van Marken Lichtenbelt et al., 2009). Interestingly, 5-HT1A and 5-HT7 receptors in spinal cord may stimulate thermogenesis, which is opposite to their role in provoking heat loss in brain (Morrison et al., 2008; Naumenko et al., 2011). Spinal microinjection of 5-HT or 8-hydroxy-N,N-dipropyl-2-aminotetralin (a 5-HT1A receptor full agonist) increased thermogenesis in brown adipose and sympathetic nerve activity, which was totally attenuated by WAY-100635 (Madden and Morrison, 2006, 2008; Morrison et al., 2008). Nevertheless, our findings suggest that, besides the use of 5-HT2A receptor antagonists which is a standard therapeutic strategy for the treatment of serotonin syndrome (Cooper and Sejnowski, 2013; Haberzettl et al., 2013; Iqbal et al., 2012), antagonism of 5-HT1A receptor may also help to relieve harmaline-5-MeO-DMT-caused hyperthermia and serotonin toxicity.
Finally, the early-phase hyperthermia that occurred in all animals (Figure 1–6) was mainly due to handling/injection-induced stress. Stress-induced hyperthermia (SIH) is obvious in animals and may complicate data interpretation when ignored. SIH animal models have been developed for the evaluation of anxiolytic drugs (O’Connor et al., 2013; Sweeney et al., 2014; Vinkers et al., 2010). In our studies, SIH was more transient and the onset of SIH was much faster than 5-MeO-DMT-induced hyperthermia, as indicated by the ΔCBT profiles (Figure 2 and Figure 3). Unlike 5-MeO-DMT-induced, late-phase hyperthermia that was completely suppressed by both 5-HT1A (WAY-100635) and 5-HT2A (ketanserin and MDL-100907) antagonists (Figure 5 and 6), the SIH was not affected by 5-HT1A antagonist WAY-100635 but selectively attenuated by 5-HT2A antagonists (Figure 4–6). Our findings suggested that the SIH caused by the handling/injection of drug vehicle in the present study might be attributed to a mechanism that is dependent on 5-HT2A receptor rather than 5-HT1A receptor. This finding was different from previous observations (Vinkers et al., 2010; Wieronska et al., 2012), and might be due to the differences in the methods used for developing SIH animal models and measuring animal core body temperatures.
In summary, our data demonstrated that harmaline alone induced hypothermia through activation of 5-HT1A receptor, and the severity of hypothermia was influenced by the status of CYP2D6 that determines harmaline pharmacokinetics. 5-MeO-DMT alone provoked hyperthermia that involved the activations of both 5-HT1A and 5-HT2A receptors. Co-administration of harmaline largely enhanced 5-MeO-DMT-induced hyperthermic effects, which might be different in subjects with distinct CYP2D6 activities at a given dose combination. Thermomodulation by co-administered harmaline and 5-MeO-DMT involved the actions of both 5-HT1A and 5-HT2A receptors. These findings indicated that severe hyperthermia caused by indolealkylamine drugs might be rescued by appropriate treatments with 5-HT1A and 5-HT2A receptor antagonists.
Highlights.
MAOI harmaline induces hypothermia that is mediated by 5-HT1A receptor.
CYP2D6 status has a significant influence on harmaline-induced hypothermia.
5-MeO-DMT-induced hyperthermia is potentiated by MAOI harmaline.
5-MeO-DMT-induced hyperthermia is relieved by 5-HT1A and 5-HT2A antagonists.
Stress-induced hyperthermia is dependent on 5-HT2A receptor.
Acknowledgments
This project was supported by Award Number R01DA021172 from the National Institute on Drug Abuse, National Institutes of Health (NIH). XLJ was supported by a Pfizer fellowship.
References
- Abdel-Fattah AF, Matsumoto K, Murakami Y, Adel-Khalek Gammaz H, Mohamed MF, Watanabe H. Central serotonin level-dependent changes in body temperature following administration of tryptophan to pargyline- and harmaline-pretreated rats. Gen Pharmacol. 1997;28:405–409. doi: 10.1016/s0306-3623(96)00300-x. [DOI] [PubMed] [Google Scholar]
- Airaksinen MM, Lecklin A, Saano V, Tuomisto L, Gynther J. Tremorigenic effect and inhibition of tryptamine and serotonin receptor binding by beta-carbolines. Pharmacol Toxicol. 1987;60:5–8. doi: 10.1111/j.1600-0773.1987.tb01711.x. [DOI] [PubMed] [Google Scholar]
- Bjornstad K, Hulten P, Beck O, Helander A. Bioanalytical and clinical evaluation of 103 suspected cases of intoxications with psychoactive plant materials. Clinical toxicology. 2009;47:566–572. doi: 10.1080/15563650903037181. [DOI] [PubMed] [Google Scholar]
- Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352:1112–1120. doi: 10.1056/NEJMra041867. [DOI] [PubMed] [Google Scholar]
- Bruno R, Matthews AJ, Dunn M, Alati R, McIlwraith F, Hickey S, Burns L, Sindicich N. Emerging psychoactive substance use among regular ecstasy users in Australia. Drug Alcohol Depend. 2012;124:19–25. doi: 10.1016/j.drugalcdep.2011.11.020. [DOI] [PubMed] [Google Scholar]
- Brush DE, Bird SB, Boyer EW. Monoamine oxidase inhibitor poisoning resulting from Internet misinformation on illicit substances. J Toxicol Clin Toxicol. 2004;42:191–195. doi: 10.1081/clt-120030949. [DOI] [PubMed] [Google Scholar]
- Capuano B, Crosby IT, McRobb FM, Taylor DA, Vom A, Blessing WW. JL13 has clozapine-like actions on thermoregulatory cutaneous blood flow in rats: Involvement of serotonin 5-HT1A and 5-HT2A receptor mechanisms. Progress in neuro-psychopharmacology & biological psychiatry. 2010;34:136–142. doi: 10.1016/j.pnpbp.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Chemel BR, Roth BL, Armbruster B, Watts VJ, Nichols DE. WAY-100635 is a potent dopamine D4 receptor agonist. Psychopharmacology (Berl) 2006;188:244–251. doi: 10.1007/s00213-006-0490-4. [DOI] [PubMed] [Google Scholar]
- Cheng J, Zhen Y, Miksys S, Beyoglu D, Krausz KW, Tyndale RF, Yu A, Idle JR, Gonzalez FJ. Potential role of CYP2D6 in the central nervous system. Xenobiotica. 2013 doi: 10.3109/00498254.2013.791410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper BE, Sejnowski CA. Serotonin syndrome: recognition and treatment. AACN Adv Crit Care. 2013;24:15–20. doi: 10.1097/NCI.0b013e31827eecc6. quiz 21–12. [DOI] [PubMed] [Google Scholar]
- Corchero J, Granvil CP, Akiyama TE, Hayhurst GP, Pimprale S, Feigenbaum L, Idle JR, Gonzalez FJ. The CYP2D6 humanized mouse: effect of the human CYP2D6 transgene and HNF4alpha on the disposition of debrisoquine in the mouse. Molecular pharmacology. 2001;60:1260–1267. doi: 10.1124/mol.60.6.1260. [DOI] [PubMed] [Google Scholar]
- DEA DoJ. Schedules of controlled substances: placement of 5-methoxy-N,N-dimethyltryptamine into Schedule I of the Controlled Substances Act. Final rule. Federal register. 2010;75:79296–79300. [PubMed] [Google Scholar]
- Drug Enforcement Administration (DEA) D. o. J. Schedules of controlled substances: placement of alpha-methyltryptamine and 5-methoxy-N,N-diisopropyltryptamine into schedule I of the Controlled Substances Act. Final rule. Federal register. 2004;69:58950–58953. [PubMed] [Google Scholar]
- Fuse-Nagase Y, Nishikawa T. Prolonged delusional state triggered by repeated ingestion of aromatic liquid in a past 5-methoxy-N, N-diisopropyltryptamine abuser. Addict Sci Clin Pract. 2013;8:9. doi: 10.1186/1940-0640-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan A, Sievert M, Ruoho AE. Identification of the substrate binding region of vesicular monoamine transporter-2 (VMAT-2) using iodoaminoflisopolol as a novel photoprobe. Molecular pharmacology. 2007;72:1567–1575. doi: 10.1124/mol.107.034439. [DOI] [PubMed] [Google Scholar]
- Gudelsky GA, Koenig JI, Meltzer HY. Thermoregulatory responses to serotonin (5-HT) receptor stimulation in the rat. Evidence for opposing roles of 5-HT2 and 5-HT1A receptors. Neuropharmacology. 1986;25:1307–1313. doi: 10.1016/0028-3908(86)90101-2. [DOI] [PubMed] [Google Scholar]
- Haberzettl R, Bert B, Fink H, Fox MA. Animal models of the serotonin syndrome: A systematic review. Behav Brain Res. 2013;256C:328–345. doi: 10.1016/j.bbr.2013.08.045. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, Buell MR, Masten VL, Risbrough VB, Geyer MA. Modification of the effects of 5-methoxy-N,N-dimethyltryptamine on exploratory behavior in rats by monoamine oxidase inhibitors. Psychopharmacology (Berl) 2008;201:55–66. doi: 10.1007/s00213-008-1247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA. Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology. 2011;61:364–381. doi: 10.1016/j.neuropharm.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Koedood L, Powell SB, Geyer MA. Differential contributions of serotonin receptors to the behavioral effects of indoleamine hallucinogens in mice. J Psychopharmacol. 2011;25:1548–1561. doi: 10.1177/0269881110388326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Nichols DE, Geyer MA. Behavioral effects of alpha,alpha,beta,beta-tetradeutero-5-MeO-DMT in rats: comparison with 5-MeO-DMT administered in combination with a monoamine oxidase inhibitor. Psychopharmacology (Berl) 2012;221:709–718. doi: 10.1007/s00213-011-2616-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SL, Thomas SH. Clinical toxicology of newer recreational drugs. Clinical toxicology. 2011;49:705–719. doi: 10.3109/15563650.2011.615318. [DOI] [PubMed] [Google Scholar]
- Iqbal MM, Basil MJ, Kaplan J, Iqbal MT. Overview of serotonin syndrome. Annals of clinical psychiatry : official journal of the American Academy of Clinical Psychiatrists. 2012;24:310–318. [PubMed] [Google Scholar]
- Isbister GK, Buckley NA. The pathophysiology of serotonin toxicity in animals and humans: implications for diagnosis and treatment. Clin Neuropharmacol. 2005;28:205–214. doi: 10.1097/01.wnf.0000177642.89888.85. [DOI] [PubMed] [Google Scholar]
- Iseri PK, Karson A, Gullu KM, Akman O, Kokturk S, Yardymoglu M, Erturk S, Ates N. The effect of memantine in harmaline-induced tremor and neurodegeneration. Neuropharmacology. 2011;61:715–723. doi: 10.1016/j.neuropharm.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Jiang XL, Shen HW, Mager DE, Yu AM. Pharmacokinetic interactions between monoamine oxidase A inhibitor harmaline and 5-methoxy-N,N-dimethyltryptamine, and the impact of CYP2D6 status. Drug metabolism and disposition: the biological fate of chemicals. 2013;41:975–986. doi: 10.1124/dmd.112.050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant S, Liebelt E. Recognizing serotonin toxicity in the pediatric emergency department. Pediatric emergency care. 2012;28:817–821. doi: 10.1097/PEC.0b013e31826289d9. quiz 822–814. [DOI] [PubMed] [Google Scholar]
- Kim H, Sablin SO, Ramsay RR. Inhibition of monoamine oxidase A by beta-carboline derivatives. Arch Biochem Biophys. 1997;337:137–142. doi: 10.1006/abbi.1996.9771. [DOI] [PubMed] [Google Scholar]
- Krebs-Thomson K, Lehmann-Masten V, Naiem S, Paulus MP, Geyer MA. Modulation of phencyclidine-induced changes in locomotor activity and patterns in rats by serotonin. Eur J Pharmacol. 1998;343:135–143. doi: 10.1016/s0014-2999(97)01557-4. [DOI] [PubMed] [Google Scholar]
- Krebs-Thomson K, Ruiz EM, Masten V, Buell M, Geyer MA. The roles of 5-HT1A and 5-HT2 receptors in the effects of 5-MeO-DMT on locomotor activity and prepulse inhibition in rats. Psychopharmacology (Berl) 2006;189:319–329. doi: 10.1007/s00213-006-0566-1. [DOI] [PubMed] [Google Scholar]
- Larrauri JA, Levin ED. PPI deficit induced by amphetamine is attenuated by the histamine H1 antagonist pyrilamine, but is exacerbated by the serotonin 5-HT2 antagonist ketanserin. Psychopharmacology (Berl) 2010;212:551–558. doi: 10.1007/s00213-010-2005-6. [DOI] [PubMed] [Google Scholar]
- Lee P, Swarbrick MM, Ho KK. Brown adipose tissue in adult humans: a metabolic renaissance. Endocr Rev. 2013;34:413–438. doi: 10.1210/er.2012-1081. [DOI] [PubMed] [Google Scholar]
- Long H, Nelson LS, Hoffman RS. Alpha-methyltryptamine revisited via easy Internet access. Vet Hum Toxicol. 2003;45:149. [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Serotonin potentiates sympathetic responses evoked by spinal NMDA. J Physiol. 2006;577:525–537. doi: 10.1113/jphysiol.2006.116574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Brown adipose tissue sympathetic nerve activity is potentiated by activation of 5-hydroxytryptamine (5-HT)1A/5-HT7 receptors in the rat spinal cord. Neuropharmacology. 2008;54:487–496. doi: 10.1016/j.neuropharm.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin J, Reviriego J, Fernandez-Alfonso MS. Ability of ketanserin to block different receptors in human placental vessels. J Pharm Pharmacol. 1990;42:217–220. doi: 10.1111/j.2042-7158.1990.tb05394.x. [DOI] [PubMed] [Google Scholar]
- McIlhenny EH, Riba J, Barbanoj MJ, Strassman R, Barker SA. Methodology for and the determination of the major constituents and metabolites of the Amazonian botanical medicine ayahuasca in human urine. Biomedical chromatography : BMC. 2011;25:970–984. doi: 10.1002/bmc.1551. [DOI] [PubMed] [Google Scholar]
- McKenna DJ. Clinical investigations of the therapeutic potential of ayahuasca: rationale and regulatory challenges. Pharmacol Ther. 2004;102:111–129. doi: 10.1016/j.pharmthera.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Miralles A, Esteban S, Sastre-Coll A, Moranta D, Asensio VJ, Garcia-Sevilla JA. High-affinity binding of beta-carbolines to imidazoline I2B receptors and MAO-A in rat tissues: norharman blocks the effect of morphine withdrawal on DOPA/noradrenaline synthesis in the brain. European journal of pharmacology. 2005;518:234–242. doi: 10.1016/j.ejphar.2005.06.023. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp Physiol. 2008;93:773–797. doi: 10.1113/expphysiol.2007.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller AA. New drugs of abuse update: Foxy Methoxy. J Emerg Nurs. 2004;30:507–508. doi: 10.1016/j.jen.2004.07.037. [DOI] [PubMed] [Google Scholar]
- Naumenko VS, Kondaurova EM, Popova NK. On the role of brain 5-HT7 receptor in the mechanism of hypothermia: comparison with hypothermia mediated via 5-HT1A and 5-HT3 receptor. Neuropharmacology. 2011;61:1360–1365. doi: 10.1016/j.neuropharm.2011.08.022. [DOI] [PubMed] [Google Scholar]
- O’Connor RM, Thakker DR, Schmutz M, van der Putten H, Hoyer D, Flor PJ, Cryan JF. Adult siRNA-induced knockdown of mGlu7 receptors reduces anxiety in the mouse. Neuropharmacology. 2013;72:66–73. doi: 10.1016/j.neuropharm.2013.03.028. [DOI] [PubMed] [Google Scholar]
- Ossowska K, Wardas J, Berghauzen-Maciejewska K, Glowacka U, Kuter K, Pilc A, Zorn SH, Doller D. Lu AF21934, a positive allosteric modulator of mGlu4 receptors, reduces the harmaline-induced hyperactivity but not tremor in rats. Neuropharmacology. 2014;83:28–35. doi: 10.1016/j.neuropharm.2014.03.018. [DOI] [PubMed] [Google Scholar]
- Ott J. Pharmepena-Psychonautics: Human intranasal, sublingual and oral pharmacology of 5-methoxy-N,N-dimethyl-tryptamine. J Psychoactive Drugs. 2001;33:403–407. doi: 10.1080/02791072.2001.10399925. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Malekiani SA, Foreman MM, Olivier B, Hanania T. Pharmacological characterization of harmaline-induced tremor activity in mice. Eur J Pharmacol. 2009;616:73–80. doi: 10.1016/j.ejphar.2009.05.031. [DOI] [PubMed] [Google Scholar]
- Riga MS, Soria G, Tudela R, Artigas F, Celada P. The natural hallucinogen 5-MeO-DMT, component of Ayahuasca, disrupts cortical function in rats: reversal by antipsychotic drugs. Int J Neuropsychopharmacol. 2014;17:1269–1282. doi: 10.1017/S1461145714000261. [DOI] [PubMed] [Google Scholar]
- Roth BL, Choudhary MS, Khan N, Uluer AZ. High-affinity agonist binding is not sufficient for agonist efficacy at 5-hydroxytryptamine2A receptors: evidence in favor of a modified ternary complex model. The Journal of pharmacology and experimental therapeutics. 1997;280:576–583. [PubMed] [Google Scholar]
- Salmi P, Ahlenius S. Evidence for functional interactions between 5-HT1A and 5-HT2A receptors in rat thermoregulatory mechanisms. Pharmacol Toxicol. 1998;82:122–127. doi: 10.1111/j.1600-0773.1998.tb01410.x. [DOI] [PubMed] [Google Scholar]
- Shen HW, Jiang XL, Winter JC, Yu AM. Psychedelic 5-methoxy-N,N-dimethyltryptamine: metabolism, pharmacokinetics, drug interactions, and pharmacological actions. Current drug metabolism. 2010a;11:659–666. doi: 10.2174/138920010794233495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HW, Jiang XL, Yu AM. Development of a LC-MS/MS method to analyze 5-methoxy-N,N-dimethyltryptamine and bufotenine, and application to pharmacokinetic study. Bioanalysis. 2009;1:87–95. doi: 10.4155/bio.09.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HW, Jiang XL, Yu AM. Nonlinear pharmacokinetics of 5-methoxy-N,N-dimethyltryptamine in mice. Drug metabolism and disposition: the biological fate of chemicals. 2011;39:1227–1234. doi: 10.1124/dmd.111.039107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HW, Wu C, Jiang XL, Yu AM. Effects of monoamine oxidase inhibitor and cytochrome P450 2D6 status on 5-methoxy-N,N-dimethyltryptamine metabolism and pharmacokinetics. Biochemical pharmacology. 2010b;80:122–128. doi: 10.1016/j.bcp.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklerov J, Levine B, Moore KA, King T, Fowler D. A fatal intoxication following the ingestion of 5-methoxy-N,N-dimethyltryptamine in an ayahuasca preparation. Journal of analytical toxicology. 2005;29:838–841. doi: 10.1093/jat/29.8.838. [DOI] [PubMed] [Google Scholar]
- Smolinske SC, Rastogi R, Schenkel S. Foxy methoxy: a new drug of abuse. Journal of medical toxicology : official journal of the American College of Medical Toxicology. 2005;1:22–25. [PubMed] [Google Scholar]
- Sweeney FF, O’Leary OF, Cryan JF. Activation but not blockade of GABAB receptors during early-life alters anxiety in adulthood in BALB/c mice. Neuropharmacology. 2014;81:303–310. doi: 10.1016/j.neuropharm.2013.08.039. [DOI] [PubMed] [Google Scholar]
- Tanaka E, Kamata T, Katagi M, Tsuchihashi H, Honda K. A fatal poisoning with 5-methoxy-N,N-diisopropyltryptamine, Foxy. Forensic science international. 2006;163:152–154. doi: 10.1016/j.forsciint.2005.11.026. [DOI] [PubMed] [Google Scholar]
- van den Buuse M, Ruimschotel E, Martin S, Risbrough VB, Halberstadt AL. Enhanced effects of amphetamine but reduced effects of the hallucinogen, 5-MeO-DMT, on locomotor activity in 5-HT(1A) receptor knockout mice: implications for schizophrenia. Neuropharmacology. 2011;61:209–216. doi: 10.1016/j.neuropharm.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- Vinkers CH, Olivier B, Bouwkecht JA, Groenink L, Olivier JDA. Stree-induced hyperthermia, the serotonin system and anxiety. The Open Pharmacol J. 2010;4:15–29. [Google Scholar]
- Wieronska JM, Stachowicz K, Branski P, Palucha-Poniewiera A, Pilc A. On the mechanism of anti-hyperthermic effects of LY379268 and LY487379, group II mGlu receptors activators, in the stress-induced hyperthermia in singly housed mice. Neuropharmacology. 2012;62:322–331. doi: 10.1016/j.neuropharm.2011.07.042. [DOI] [PubMed] [Google Scholar]
- Winstock AR, Kaar S, Borschmann R. Dimethyltryptamine (DMT): prevalence, user characteristics and abuse liability in a large global sample. J Psychopharmacol. 2014;28:49–54. doi: 10.1177/0269881113513852. [DOI] [PubMed] [Google Scholar]
- Winter JC, Amorosi DJ, Rice KC, Cheng K, Yu AM. Stimulus control by 5-methoxy-N,N-dimethyltryptamine in wild-type and CYP2D6-humanized mice. Pharmacology, biochemistry, and behavior. 2011;99:311–315. doi: 10.1016/j.pbb.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter JC, Filipink RA, Timineri D, Helsley SE, Rabin RA. The paradox of 5-methoxy-N,N-dimethyltryptamine: an indoleamine hallucinogen that induces stimulus control via 5-HT1A receptors. Pharmacol Biochem Behav. 2000;65:75–82. doi: 10.1016/s0091-3057(99)00178-1. [DOI] [PubMed] [Google Scholar]
- Wu C, Jiang XL, Shen HW, Yu AM. Effects of CYP2D6 status on harmaline metabolism, pharmacokinetics and pharmacodynamics, and a pharmacogenetics-based pharmacokinetic model. Biochemical pharmacology. 2009;78:617–624. doi: 10.1016/j.bcp.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu AM, Idle JR, Herraiz T, Kupfer A, Gonzalez FJ. Screening for endogenous substrates reveals that CYP2D6 is a 5-methoxyindolethylamine O-demethylase. Pharmacogenetics. 2003a;13:307–319. doi: 10.1097/01.fpc.0000054094.48725.b7. [DOI] [PubMed] [Google Scholar]
- Yu AM, Idle JR, Krausz KW, Kupfer A, Gonzalez FJ. Contribution of individual cytochrome P450 isozymes to the O-demethylation of the psychotropic beta-carboline alkaloids harmaline and harmine. The Journal of pharmacology and experimental therapeutics. 2003b;305:315–322. doi: 10.1124/jpet.102.047050. [DOI] [PubMed] [Google Scholar]