Abstract
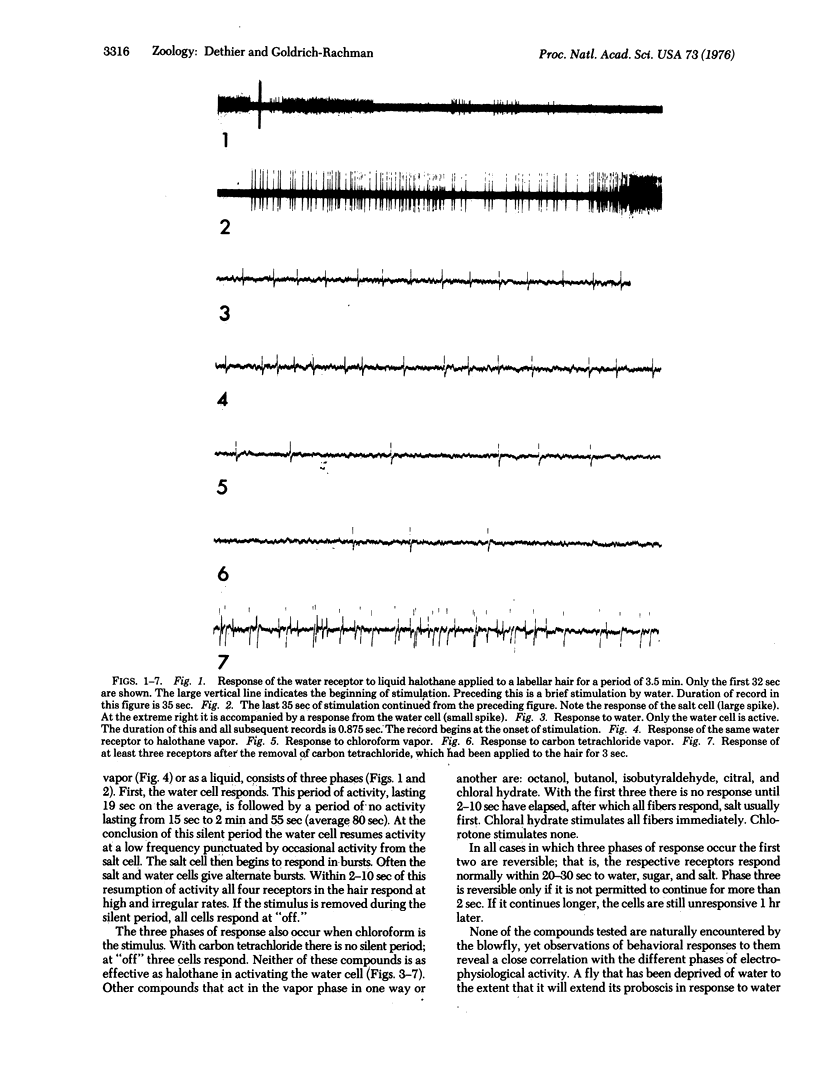
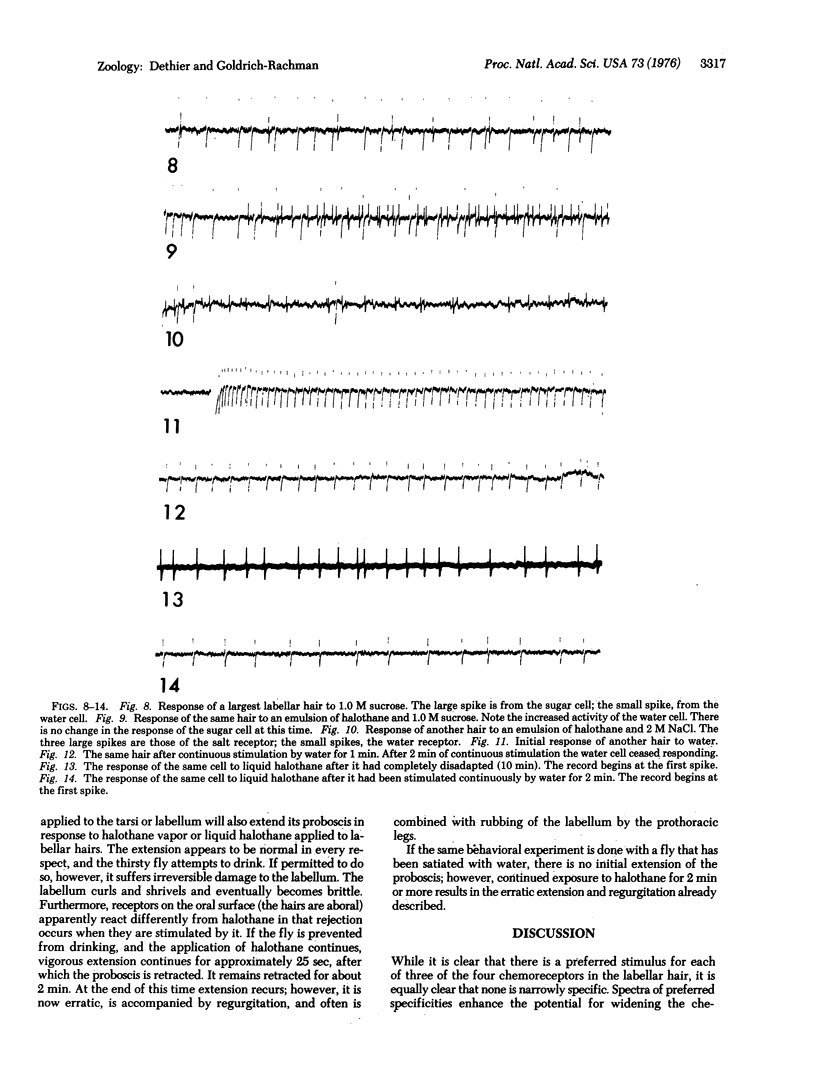
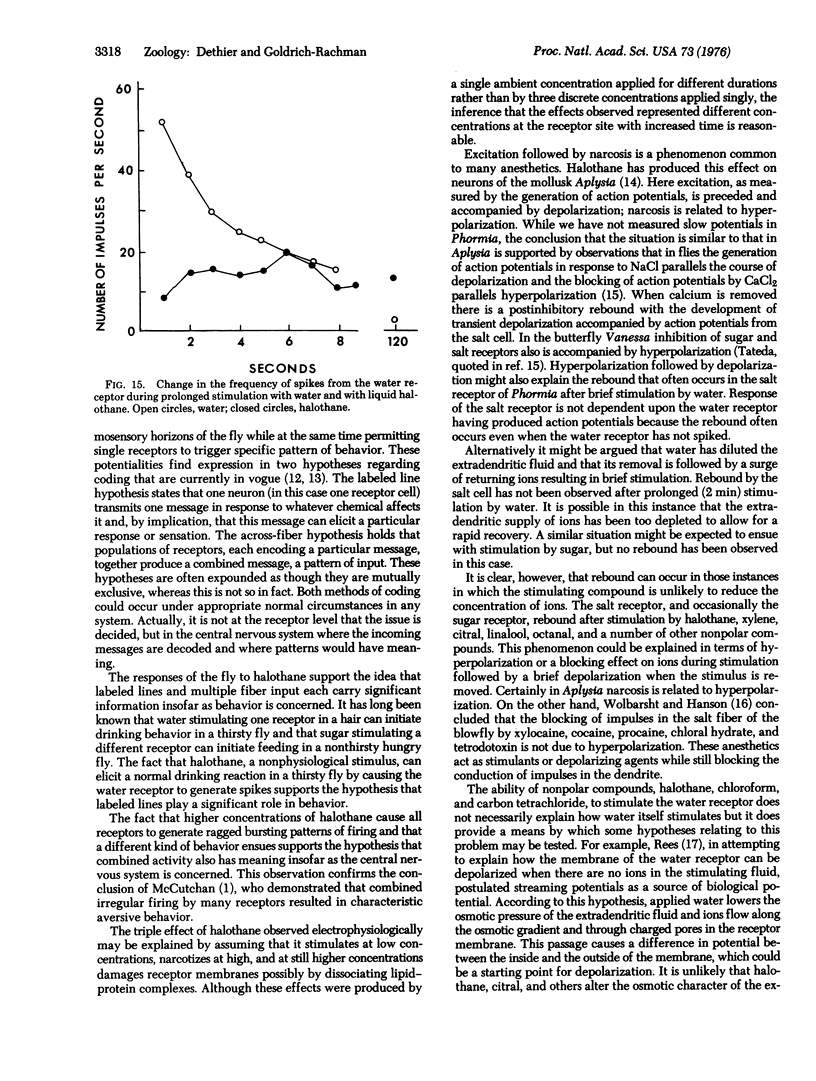
Halothane, chloroform, and carbon tetrachloride, in the vapor and liquid phases, stimulate the water receptor of the blowfly Phormia regina. There are three successive phases of response to long-lasting stimulation by halothane: stimulation of the water receptor for the first 19 sec, narcosis for the next 80 sec, and stimulation of all receptors after 80 sec. The behavior of the fly is correlated with these phases. A thirsty fly extends its proboscis and attempts to drink during the first phase, withdraws its proboscis during the second, and extends in a manner characteristic of aversion in the third. A water-satiated fly responds only in the third phase. These results indicate that both the labeled line and the across-fiber hypothesis of sensory coding apply to the blowfly. At the level of sensory transduction the data do not rule out the possibility that streaming potentials are normally involved in stimulation of the water receptor. They are also consistent with a hypothesis that neutral narcotics stimulate the water receptor by facilitating the passage of sodium ions through the dendritic membrane.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chalazonitis N. Selective actions of volatile anesthetics on synaptic transmission and autorhythmicity in single identifiable neurons. Anesthesiology. 1967 Jan-Feb;28(1):111–123. [PubMed] [Google Scholar]
- Dethier V. G. Sensitivity of the contact chemoreceptors of the blowfly to vapors. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2189–2192. doi: 10.1073/pnas.69.8.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethier V. G. The specificity of the labellar chemoreceptors of the blowfly and the response to natural foods. J Insect Physiol. 1974 Sep;20(9):1859–1869. doi: 10.1016/0022-1910(74)90215-7. [DOI] [PubMed] [Google Scholar]
- EVANS D. R., MELLON D., Jr Electrophysiological studies of a water receptor associated with the taste sensilla of the blow-fly. J Gen Physiol. 1962 Jan;45:487–500. doi: 10.1085/jgp.45.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson R. P. Stimulus coding in topographic and nontopographic afferent modalities: on the significance of the activity of individual sensory neurons. Psychol Rev. 1968 Nov;75(6):447–465. doi: 10.1037/h0026752. [DOI] [PubMed] [Google Scholar]
- Goldrich N. R. Behavioral responses of Phormia regina (Meigen) to labellar stimulation with amino acids. J Gen Physiol. 1973 Jan;61(1):74–88. doi: 10.1085/jgp.61.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORITA H. Initiation of spike potentials in contact chemosensory hairs of insects. III. D.C. stimulation and generator potential of labellar chemoreceptor of calliphora. J Cell Comp Physiol. 1959 Oct;54:189–204. doi: 10.1002/jcp.1030540209. [DOI] [PubMed] [Google Scholar]
- MORITA H., YAMASHITA S. Generator potential of insect chemoreceptor. Science. 1959 Oct 9;130(3380):922–922. doi: 10.1126/science.130.3380.922. [DOI] [PubMed] [Google Scholar]
- Normann T. C., Duve H. Experimentally induced release of a neurohormone influencing hemolymph trehalose level in Calliphora erythrocephala (Diptera). Gen Comp Endocrinol. 1969 Jun;12(3):449–459. doi: 10.1016/0016-6480(69)90161-0. [DOI] [PubMed] [Google Scholar]
- PFAFFMANN C. Gustatory nerve impulses in rat, cat and rabbit. J Neurophysiol. 1955 Sep;18(5):429–440. doi: 10.1152/jn.1955.18.5.429. [DOI] [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- Shiraishi A., Kuwabara M. The effects of amino acids on the labellar hair chemosensory cells of the fly. J Gen Physiol. 1970 Dec;56(6):768–782. doi: 10.1085/jgp.56.6.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLBARSHT M. L., HANSON F. E. ELECTRICAL ACTIVITY IN THE CHEMORECEPTORS OF THE BLOWFLY. 3. DENDRITIC ACTION POTENTIALS. J Gen Physiol. 1965 Mar;48:673–683. doi: 10.1085/jgp.48.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]



