Summary
Objective
The objective of this study was to compare the short term outcomes of robotic with conventional on pump coronary artery bypass grafting (CABG).
Methods
The study population included 2091 consecutive patients who underwent either conventional or robotic CABG from January 2007 to March 2012. Pre-operative, intra-operative and 30-day post-operative variables were collected for each group. In order to compare the incidence of rapid recovery between conventional and robotic CABG, the surrogate variables of early discharge and discharge to home (versus rehabilitation or acute care facility) were evaluated. A multivariate logistic regression analysis was utilized.
Results
One hundred and fifty robotic and 1,619 conventional CABG cases were analyzed. Multivariate logistic regression analysis demonstrated that robotic surgery was a strong predictor of lower 30-day complications (OR = 0.24, p=0.005), short length of stay (OR 3.31, p < 0.001), and decreased need for an acute care facility (OR 0.55, p = 0.032). In the presence of complications (NY State Complication Composite), the robotic technique was not associated with a change in discharge status.
Conclusions
In this retrospective review robotic CABG was associated with a lower 30-day complication rate, a shorter length of stay and a lower incidence of acute care facility discharge than conventional on pump CABG. It may suggest a more rapid recovery to pre-operative status after robotic surgery: however, only a randomized prospective study could confirm the advantages of a robotic approach
Keywords: robotic CABG, hybrid revascularization, rapid recovery, NY State 30 day Complication Composite, discharge status
Introduction
Conventional coronary artery bypass grafting is a time-tested procedure which results in superior long-term symptom relief and improved survival in selected patients when compared to both medical therapy and percutaneous coronary intervention (PCI) (1 - 3). The strongest predictor of improved survival conferred by CABG is a patent left internal thoracic artery (LITA) to left anterior descending coronary artery (LAD) anastomosis (4 - 6). Nonetheless, conventional CABG performed on cardiopulmonary bypass through a sternotomy is still associated with a higher morbidity and longer recovery times compared to conventional PCI.
Minimally invasive coronary revascularization procedures aim to confer the long-term benefits of LITA-LAD grafting while preserving the minimally invasive advantages of PCI. Robotic CABG is one of the most popular minimally invasive revascularization procedures in which LITA takedown, pericardiotomy and vessel identification are performed robotically. The subsequent LITA-LAD revascularization is then performed on the beating heart through a small left anterior thoracotomy with minimal rib spreading. The major indications for isolated LITA-LAD revascularization via robotic CABG are 1) isolated LAD/ diagonal disease, 2) isolated ostial left main disease, 3) partial revascularization in high risk patients and 4) hybrid coronary revascularization (HCR) in patients with multi-vessel disease. The major indication for conventional CABG is multivessel coronary artery disease where according to the specific coronary anatomy the other methods of revascularization are difficult or not feasible, and urgency of the procedure does not allow waiting time for a robot and/or surgeon to be available.
Although the short-term benefits of such a minimally invasive approach might seem intuitive, no prospective randomized studies have been performed comparing robotic and conventional CABG. The following study aims to evaluate the 30-day complications, early recovery rate and discharge status of patients undergoing either robotic or conventional on pump CABG.
Methods
This study was approved by the Institutional Review Board. All patients undergoing CABG at a single institution were prospectively studied from January 2007 to March 2012. Patients undergoing emergency surgery, re-operative CABG, CABG and concomitant procedures or primary off-pump CABG through a sternotomy were excluded from the initial analysis. The data was extracted from a de-identified prospective, cardiac surgery database, which was populated with data elements from both the New York State and Society of Thoracic Surgeons (STS) databases (7). The surgical technique of robotic CABG has been published previously (8, 9).
The primary end-point of this study was to analyze discharge disposition. The secondary end-points were 30-day mortality, NY State post-operative Complications Composite (operative mortality, permanent stroke, deep sternal wound infection, renal failure requiring dialysis, prolonged ventilation more than 24 hours, unplanned reoperation or PCI and reoperation for bleeding) and length of stay 6 days or less as the surrogate variable of early discharge. Discharge status was defined as either discharge to home or discharge to an acute care facility (rehabilitation or nursing home). The 30-day mortality was defined as death during the index admission or during the first 30 days after discharge from the index admission.
Continuous variables were assessed for distribution, compared by the Student t-test if normally distributed and by the Mann-Whitney test if not normally distributed. Categorical variables were compared by Pearson chi-square test or Fisher exact test. P-values less than 0.05 were considered significant. A multi-variate logistic regression analysis was performed for three outcome variables: NY State Complication Composite, discharge status and short length of stay (6 days or less), for a total of three models analyzed. Robotic CABG was evaluated as the independent predictor of outcome adjusted for STS reportable perioperative variables entered in the three full models (age, gender, race (White or not-White), ethnicity (Hispanic vs. not-Hispanic), body mass index (BMI), history of cerebro-vascular disease, congestive heart failure (CHF), diabetes, renal failure requiring dialysis, chronic obstructive lung disease (COPD), previous myocardial infarction (MI) within 30 days, left ventricular ejection fraction (LVEF), urgency status (elective vs. urgent), number of diseased coronary arteries, left main coronary artery disease, an intra-aortic balloon pump (IABP) and socioeconomical status (SES) . Since the presence of perioperative complications is very likely to affect the discharge status and the LOS, it was added as a predictor in the last two models. The variables insignificant by the Wald test were sequentially removed from the models if they were not confounders. The criterion for confounder effect was the change in β coefficient of the primary variable of interest of more than 15 % after removal of the potential confounder variable. The assumption of linearity in the logit for continuous variables was assessed by fractional polynomials. The continuous variable was considered to meet the assumption of linearity if the p-value for the linear model was higher than 0.05. Hosmer and Lemeshow goodness of fit (GOF) test was utilized to assess that the model fit the assumption, with p > 0.05 significant for fit. Interactions were considered significant if the Wald test had p-value < 0.2 for interaction term. To evaluate the combined effect of the previously mentioned predictors, the models were run again with robotic CABG and conventional on-pump CABG adjusted for perioperative morbidity and mortality (PROMM) scores, calculated by the STS.
Similarly separate analysis was performed where robotic CABG was compared to off-pump CABG done through the median sternotomy. The same variables were entered in the models as in the original analysis and the other steps of modeling were repeated. The STATA statistical software 12.1 was used (STATA Corp., College Station, TX).
Results
Two thousand ninety one CABG's were performed from January 2007 to March 1, 2012. We excluded 322 cases: 232 off-pump sternotomy procedures (these cases were analyzed separately) and 90 on-pump sternotomy procedures: 51 emergency procedures and 3 emergent salvage procedures as well as 31 redo surgeries and 5 combined CABG carotid procedures. Thus, we had 1769 cases remaining: 150 robotic CABG and 1619 conventional CABG (Figure 1). All robotic CABG operations were performed by one surgeon and conventional CABG procedures were performed by the same surgeon and 4 additional surgeons. In the robotic CABG group, 7 patients (4.67 %) required institution of femoral-femoral bypass due to intramyocardial LAD. One patient (0.67 %) required conversion to median sternotomy and a three vessel CABG was done off pump. This patient was left in the robotic CABG group as intention to treat. From 150 robotic CABG patients, 51 (34.0 %) had isolated LAD disease, and 8 (5.33 %) had isolated left main artery lesions; thus, one vessel CABG was considered as definitive treatment; 39 patients (26 %) had other lesions but these lesions were not suitable for revascularization by surgery or PCI; 56 patients (37.33 %) had hybrid coronary revascularization (HCR). Among the HCR patients, 33 (22 %) had staged revascularization with robotic CABG first and PCI to follow from 2 to 255 days after surgery. Fourteen patients (9.33 %) had a staged HCR with PCI performed first and robotic CABG performed anywhere from 18 to 251 days following the PCI. Three patients had simultaneous HCR. Twenty-nine (56.9 %) HCR's were completed on the same admission. The majority of conventional CABG patients were planned as a complete surgical revascularization; however, 30 patients (1.85 %) in the conventional CABG group had planned PCI during the same admission.
Figure 1.
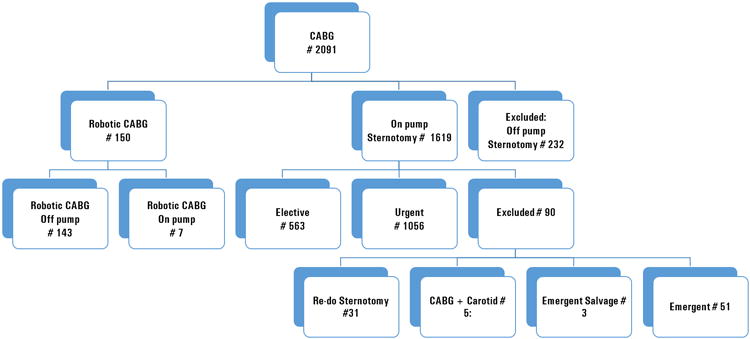
Distribution of cases in robotic and conventional coronary artery bypass graft groups, excluded cases marked.
Demographics and co-morbidities are presented in Table 1. Robotic CABG and conventional CABG cohorts did not have statistically significant differences in patients' age, weight, height, BMI, gender distribution, frequency of diabetes, COPD, cerebrovascular disease, CHF, end-stage renal disease (ESRD) requiring dialysis and liver disease. However, the cohorts had statistically significant differences in the number of diseased coronary vessels, left main coronary artery disease, LVEF, prevalence of Hispanic and White population, previous MI, urgency status, class of angina and SES. The outcome end points of postoperative 30-day mortality rate, stroke, deep sternal wound infection, other major infection rate, new renal failure requiring dialysis, reoperation for bleeding, postoperative atrial fibrillation rate, rate of reoperation and/or PCI for graft occlusion and reoperation for bleeding were not statistically different between robotic and conventional CABG groups (Table 2). NY State post-operative Complication Composite rate (2.67 % in robotic CABG group and 11.54 % in conventional group, p = 0.001), discharge to acute care facility rate (16.0 % in robotic CABG group vs. 25.83 % in conventional group, p = 0.008) and prolonged ventilation rates (0.67 % in robotic CABG group vs. 4.79 % in conventional group, p < 0.021) were significantly lower in the robotic CABG group compared to the conventional group (Table 2). The length of stay (LOS) from admission to discharge, from the day of surgery to discharge, anesthesia time and surgery time were significantly lower in robotic than conventional CABG (Table 2). Three hundred twenty nine patients (20.3 %) in the conventional group received intraoperative blood transfusions compared to only 9 patients (6.0 %) in the robotic CABG group (p < 0.001). Three hundred forty five patients (21.28 %) in the conventional group received postoperative blood transfusion while only 16 patients (10.67 %) received postoperative blood transfusion in the robotic group (p = 0.002).
Table 1.
Baseline characteristics of study population.
| Variables | Robotic CABG (150) | Conventional CABG (1619) | P value |
|---|---|---|---|
| PROMM % (25 - 75 %) | 10.3 (7.3 - 15.5) | 12.7 (8.9 - 20.0) | < 0.001 1 |
| Age (mean ± SD) | 64.76 ± 12.47 | 63.33 ± 10.41 | 0.20 2 |
| Weight (mean ± SD) | 81.22 ± 19.71 | 81.20 ± 18.75 | 0.97 2 |
| Height (mean ± SD) | 169.23 ± 11.21 | 167.37 ± 10.26 | 0.05 2 |
| BMI (mean ± SD) | 28.1 ± 4.87 | 29.07 ± 10.47 | 0.27 2 |
| LVEF (mean ± SD) | 54.2 ± 10.58 | 49.36 ± 12.94 | < 0.001 2 |
| Number of Diseased Vessels | 2.73 ± 1.08 | 3.61 ± 0.84 | < 0.001 2 |
| Left Main Disease (%) | 22 (14.67 %) | 489 (30.17 %) | < 0.001 3 |
| Gender Male (%) | 103 (68.67 %) | 1,088 (66.0 %) | 0.67 3 |
| Diabetes (%) | 76 (50.67 %) | 861 (53.13 %) | 0.57 3 |
| COPD (%) | 21 (14.0 %) | 208 (12.9 %) | 0.71 3 |
| Cerebrovascular Disease (%) | 26 (17.33 %) | 228 (14.07 %) | 0.28 3 |
| CHF (%) | 30 (20.0 %) | 256 (15.79 %) | 0.18 3 |
| Dialysis (%) | 7 (4.67 %) | 67 (4.13 %) | 0.76 3 |
| PCI this admission (%) | 29 (19.33 %) | 30 (1.85 %) | < 0.0013 |
| PCI before admission (%) | 46 (30.67 %) | 301 (18.57 %) | < 0.0013 |
| Ethnicity - Hispanic (%) | 41 (27.52 %) | 684 (42.33) | < 0.0013 |
| Race: None-White (%) | 82 (54.67 %) | 1113 (68.68 %) | < 0.001 3 |
| SES (25 - 75 %) | - 1.62 (from - 4.65 to - 0.04) | - 2.30 (from - 5.79 to - 0.56) | 0.03 1 |
| Urgency status (%) | 58 (38.67 %) | 1058 (65.27 %) | <0.0013 |
| Previous MI (%) | 59 (39.33 %) | 860 (52.5 %) | 0.002 3 |
| Liver Disease (%) | 2 (1.3 %) | 3 (0.19 %) | 0.06 4 |
| Angina Class (0/I/II/III/IV %) | 16.33/23.81/31.29/21.77/6.8 | 7.79/15.46/32.11/32.67/11.97 | <0.0013 |
Mann-Whitney test;
Two-tail unpaired t-test;
Pearson chi square test;
Fisher exact test. Robotic endo-ACAB - robotic endoscopic atraumatic coronary artery bypass; BMI – body mass index, LVEF – left ventricular ejection fraction, COPD – chronic obstructive lung disease, CHF – congestive heart failure, MI – myocardial infarction, SES socioeconomical status.
Table 2.
Mortality, discharge status and NY State post-operative Complication Composite, hospital length of stay (LOS), LOS after surgery, anesthesia time and surgical time in robotic and conventional coronary artery bypass (CABG).
| Outcome | Robotic CABG (n = 150) | Conventional CABG (n = 1619) | P value |
|---|---|---|---|
| Hospital Mortality (%) | 0 (0) | 29 (1.79) | 0.167 1 |
| 30 Days Mortality Rate (%) | 0 (0) | 32 (2.041) | 0.105 1 |
| Discharged in acute care facility (%) | 24 (16.0) | 411 (25.83) | 0.008 2 |
| NY State post-operative complication composite (%) | 4 (2.67) | 187 (11.54) | 0.001 2 |
| Stroke under 24 hours (%) | 1 (0.67) | 19 (1.17) | 1.00 1 |
| Stroke over 24 hours (%) | 1 (0.67) | 16 (0.99) | 1.00 1 |
| Deep Sternal Wound Infection (%) | 0 (0) | 29 (1.79) | 0.17 1 |
| Postoperative Infection/Sepsis/Endocarditis (%) | 0 (0) | 14 (0.86) | 0.62 1 |
| Postoperative GI complications (%) | 0 (0) | 11 (0.68) | 0.62 1 |
| Renal failure requiring dialysis (%) | 0 (0) | 21 (1.24) | 0.25 1 |
| Post-operative respiratory failure (%) | 1 (0.67) | 76 (4.79) | 0.021 2 |
| Unplanned reoperation or PCI (%) | 0 (0) | 24 (1.48) | 0.24 1 |
| Reoperation for bleeding (%) | 2 (1.33) | 44 (2.72) | 0.43 1 |
| Postoperative atrial fibrillation (%) | 19 (12.67) | 289 (17.84) | 0.11 2 |
| LOS after Surgery 6 days or less (%) | 129 (86.0) | 1033 (63.73) | < 0.001 2 |
| LOS, median (25 – 75 %) | 6 (4 - 9) | 9 (6 - 13) | < 0.001 3 |
| LOS after Surgery, median (25 – 75 %) | 3 (4 - 6) | 6 (5 - 7) | < 0.001 3 |
| Anesthesia time in hours, mean ± SD | 5.8 ± 1.1 | 6.7 ± 1.3 | < 0.001 4 |
| Surgery time in hours, mean ± SD | 3.7 ± 1.1 | 4.8 ± 1.2 | < 0.001 4 |
Fisher's exact test;
Pearson chi square test;
Mann-Whitney test;
Two-tailed unpaired t-test; Robotic endo-ACAB - robotic endoscopic atraumatic coronary artery bypass; PCI – percutaneous coronary intervention, GI – gastrointestinal.
The logistic regression model for NY State Complication Composite rate demonstrated robotic technique, diabetes, CHF, urgent status and IABP as statistically significant independent predictors of complications (see OR and p-values in Table 3). Robotic CABG (OR = 0.24, p = 0.005) was strongly associated with decreased NY State Complication Composite rate, while diabetes (OR = 1.47, p = 0.019), CHF (OR = 2.26, p < 0.001), urgent status (OR = 1.45, p = 0.039) and IABP (3.07, p < 0.001) were associated with increased NY State Complication Composite rate. Removed variables were not confounders, and there was no statistically significant interactions in this model.
Table 3.
Logistic regression model for NY State post-operative Complication Composite.
| Variables | OR | P value | CI |
|---|---|---|---|
| Robotic CABG | 0.24 | 0.005 | 0.09 - 0.65 |
| Diabetes | 1.47 | 0.019 | 1.07 - 2.02 |
| CHF | 2.26 | < 0.001 | 1.59 - 3.21 |
| Urgent vs. Elective Status | 1.45 | 0.039 | 1.02 - 2.05 |
| IABP | 3.07 | < 0.001 | 1.71 - 5.50 |
Robotic CABG - Robotic coronary artery bypass grafting versus conventional CABG, OR – odd ratio, CI – 95 % confidence interval, CHF – congestive heart failure, IABP – intra-aortic balloon pump. The variables included in the full models were age, gender, race (White or not-White), ethnicity (Hispanic vs. not-Hispanic), BMI, history of cerebro-vascular disease, CHF, diabetes, renal failure requiring dialysis, chronic obstructive lung disease (COPD), previous MI within 30 days, left ventricular ejection fraction (LVEF), urgency status (elective vs. urgent), number of diseased coronary arteries, left main coronary artery disease, and IABP. The variables insignificant by the Wald test (p > 0.05) were sequentially removed from the models if they were not confounders.
Logistic regression model for discharge status demonstrated the number of diseased coronary arteries as a confounder and they remained in the model in spite of a lack of statistical significance. Significant interaction between robotic technique and NY State Complication Composite was suspected (p = 0.179 for interaction term), and the model was stratified by the presence of NY State Complications. In the stratified model, with no complications robotic CABG (OR = 0.55, p = 0.032), male gender (OR = 0.40, p < 0.001) and LVEF (OR= 0.98, p < 0.004) were strongly associated with a decreased rate of discharge to an acute care facility (Table 4). Age was replaced in the model in 4 quartiles (29 – 57, 58 – 64, 65 – 72, and 73 – 94) and BMI was dichotomized to 30.0 as criteria for obesity, since these variables did not meet assumption of linearity in this model. The age in the 3rd and 4th quartiles, BMI more than 30.0, dialysis, COPD and cerebrovascular disease, were associated with an increased rate of discharge to an acute care facility in the absence of complications. In the model with complications, robotic CABG was no longer a significant predictor of discharge status and other comorbidity variables also lost their significance, except age 3rd (OR = 2.95, p = 0.029) and 4th quartiles (OR = 4.55, p = 0.008) were associated with increased discharge in an acute care facility.
Table 4.
Logistic regression model for discharge status in acute care facility in patients without NY State post-operative Complication Composite.
| Variables | OR | P value | CI |
|---|---|---|---|
| Robotic CABG | 0.55 | 0.032 | 0.31 - 0.95 |
|
| |||
| Age | |||
| 2-d quartile | 1.26 | 0.279 | 0.83 - 1.94 |
| 3-d quartile | 2.72 | < 0.001 | 1.85 - 4.01 |
| 4-th quartile | 6.82 | < 0.001 | 4.61 - 10.08 |
|
| |||
| Male gender | 0.4 | < 0.001 | 0.30 - 0.52 |
|
| |||
| BMI more than 30.0 | 1.67 | < 0.001 | 1.27 - 2.20 |
|
| |||
| Dialysis | 4.06 | < 0.001 | 2.23 - 7.41 |
|
| |||
| COPD | 1.69 | 0.004 | 1.18 - 2.42 |
|
| |||
| Cerebrovascular disease | 1.73 | 0.001 | 1.24 - 2.43 |
|
| |||
| LVEF | 0.98 | 0.004 | 0.97 - 0.99 |
|
| |||
| Number of diseased coronary arteries | |||
| 2 | 0.89 | 0.824 | 0.32 - 2.49 |
| 3 | 1.29 | 0.595 | 0.50 - 3.33 |
| 4 | 1.43 | 0.454 | 0.56 - 3.68 |
| 5 | 1.71 | 0.293 | 0.63 - 4.63 |
Robotic CABG - Robotic coronary artery bypass grafting versus conventional CABG, OR – odd ratio, CI – 95 % confidence interval, BMI – body mass index, LVEF – left ventricular ejection fraction, COPD – chronic obstructive lung disease. The LVEF (p = 0.342) and BMI (p = 0.061 for linearity) met the assumption of linearity in logit by the fractional polynomials test, but we dichotomized BMI to 30 as criteria to obesity, since p value for very close to be significant for none linearity. The fractional polynomials test did not demonstrate that the assumption of linearity in logit met for age (p = 0.037). Thus, age was entered in model in 4 quartiles (29 – 57, 58 – 64, 65 – 72, 73 – 94). The variables included in the full models were age, gender, race (White or not-White), ethnicity (Hispanic vs. not-Hispanic), BMI, history of cerebro-vascular disease, CHF, diabetes, renal failure requiring dialysis, COPD, previous MI within 30 days, LVEF, urgency status (elective vs. urgent), number of diseased coronary arteries, left main coronary artery disease, and intra-aortic balloon pump (IABP). The variables insignificant by the Wald test (p > 0.05) were sequentially removed from the models. The number of diseased coronary arteries was not significant predictor of discharge status but was a confounder and for that reason left in the model.
The logistic regression model demonstrated robotic CABG (OR = 3.31, p < 0.001), Hispanic ethnicity (OR 1.48, p = 0.001) and LVEF (OR = 1.01, p = 0.031) were strongly associated with rapid discharge (LOS < 6 days). The NY state Complication Composite, non-White race, age, BMI more than 30, CHF, cerebrovascular disease, dialysis and IABP (see OR and p values in Table 5) were associated with a decreased rate of rapid discharge. There was no evidence of interaction in this model.
Table 5. Logistic regression model for short LOS (less than 6 days).
| Variables | OR | P value | CI |
|---|---|---|---|
| Robotic CABG | 3.31 | < 0.001 | 2.00 - 5.50 |
| NY State Complication Composite | 0.13 | < 0.001 | 0.09 - 0.19 |
| Age | 0.96 | < 0.001 | 0.95 - 0.97 |
| BMI more than 30.0 | 0.72 | 0.006 | 0.57 - 0.91 |
| Race Not-White vs. White | 0.62 | < 0.001 | 0.48 - 0.81 |
| Ethnicity Hispanic vs. Not-Hispanic | 1.48 | 0.001 | 1.17 - 1.89 |
| Dialysis | 0.34 | < 0.001 | 0.20 - 0.91 |
| CHF | 0.49 | < 0.001 | 0.36 - 0.67 |
| Cerebrovascular disease | 0.72 | 0.04 | 0.93 - 0.98 |
| LVEF | 1.01 | 0.031 | 1.00 - 1.02 |
| IABP | 0.36 | 0.001 | 0.20 - 0.68 |
Robotic CABG - Robotic coronary artery bypass grafting versus conventional CABG, OR – odd ratio, CI – 95 % confidence interval, BMI – body mass index, CHF – congestive heart failure, LVEF – left ventricular ejection fraction, IABP – intra-aortic balloon pump. The variables included in the full models were age, gender, race (White or not-White), ethnicity (Hispanic vs. not-Hispanic), BMI, history of cerebro-vascular disease, CHF, diabetes, renal failure requiring dialysis, chronic obstructive lung disease (COPD), previous MI within 30 days, LVEF, urgency status (elective vs. urgent), number of diseased coronary arteries, left main coronary artery disease, and IABP. The variables insignificant by the Wald test (p > 0.05) were sequentially removed from the models if they were not confounders. Age (p = 0.753) and LVEF (p = 0.549) met assumption of linearity in fractional polynomials test and entered as continuous variables. BMI barely met assumption of linearity (p = 0.055) and was to 30 as criteria for obesity and entered to the model.
The separate logistic regression models with robotic CABG adjusted for PROMM in four quartiles for outcomes the NY State Complication Composite, discharge status and rapid discharge demonstrated results very similar to our initial analysis in terms of OR and significance (Table 6A, B and C).
Table 6 A.
Logistic regression model for NY State postoperative Complication Composite robotic CABG versus conventional CABG adjusted for Predicted Perioperative Morbidity Mortality scores.
| Variables | OR | P value | CI |
|---|---|---|---|
| Robotic CABG | 0.25 | 0.007 | 0.09 - 0.68 |
| PROMM score | |||
| 2-d quartile | 1.58 | 0.072 | 0.96 - 2.60 |
| 3-d quartile | 1.75 | 0.025 | 1.07 - 2.90 |
| 4-th quartile | 4.3 | < 0.001 | 2.76 - 6.72 |
CABG – coronary artery bypass grafting, PROMM - perioperative morbidity and mortality, OR – odd ratio, CI – 95 % confidence interval.
Table 6B.
Logistic regression model for discharge status in acute care facility in patients without NY State postoperative Complication Composite adjusted for Predicted Perioperative Morbidity Mortality scores.
| Variables | OR | P value | CI |
|---|---|---|---|
| Robotic CABG | 0.59 | 0.046 | 0.35 - 0.99 |
| PROMM score | |||
| 2-d quartile | 1.92 | 0.001 | 1.30 - 2.84 |
| 3-d quartile | 3.21 | < 0.001 | 2.21 - 4.64 |
| 4-th quartile | 7.04 | < 0.001 | 4.90 - 10.14 |
CABG – coronary artery bypass grafting, PROMM - perioperative morbidity and mortality, OR – odd ratio, CI – 95 % confidence interval.
Table 6 C.
Logistic regression model for rapid discharge (LOS less than 6 days) in patients without NY State post-operative Complication Composite.
| Variables | OR | P value | CI |
|---|---|---|---|
| Robotic CABG | 2.51 | < 0.001 | 1.53 - 4.13 |
| PROMM score | |||
| 2-d quartile | 0.69 | 0.026 | 0.50 - 0.96 |
| 3-d quartile | 0.39 | < 0.001 | 0.29 - 0.54 |
| 4-th quartile | 0.2 | < 0.001 | 0.15 - 0.28 |
CABG – coronary artery bypass grafting, PROMM - perioperative morbidity and mortality, OR – odd ratio, CI – 95 % confidence interval.
When only robotic and off-pump CABG were included in the analysis, the results of logistic regression models for the same outcomes were again very similar: robotic CABG was an independent predictor of fewer complications, less nursing home discharge and more often rapid recovery (Table 7A, B and C).
Table 7 A.
Logistic regression model for NY State post-operative Complication Composite in robotic CABG versus off-pump sternotomy CABG
| Variables | OR | P value | CI |
|---|---|---|---|
| Robotic CABG | 0.28 | 0.023 | 0.09 - 0.83 |
| Age | 1.06 | 0.007 | 1.02 - 1.11 |
| Dialysis | 5.59 | 0.004 | 1.74 - 17.96 |
CABG – coronary artery bypass grafting, OR – odd ratio, CI – 95 % confidence interval.
Table 7 B.
Logistic regression model for discharge status in robotic CABG versus off-pump sternotomy CABG (in patients without NY State post-operative Complication Composite)
| Variables | OR | P value | CI |
|---|---|---|---|
| Robotic CABG | 0.45 | 0.01 | 0.24 - 0.83 |
| Age | 1.09 | < 0.001 | 1.06 - 1.13 |
| Male gender | 0.4 | 0.002 | 0.23 - 0.72 |
| BMI | 1.07 | 0.007 | 1.02 - 1.12 |
| Dialysis | 9.4 | < 0.001 | 2.82 - 31.33 |
CABG – coronary artery bypass grafting, BMI – body mass index, OR – odd ratio, CI – 95 % confidence interval.
Table 7 C.
Logistic regression model for rapid discharge (LOS less than 6 days) in robotic CABG versus off-pump sternotomy CABG.
| Variables | OR | P value | CI |
|---|---|---|---|
| Robotic CABG | 4.17 | < 0.001 | 2.34 - 7.43 |
| Age | 0.96 | 0.001 | 0.94 - 0.98 |
| Dialysis | 0.14 | 0.001 | 0.05 - 0.44 |
| CHF | 0.5 | 0.035 | 0.26 - 0.95 |
| LVEF | 1.03 | 0.005 | 1.01 - 1.05 |
CABG – coronary artery bypass grafting, OR – odd ratio, CI – 95 % confidence interval, CHF – congestive heart failure, LVEF – left ventricular ejection fraction.
Discussion
Robotic CABG aims to decrease the surgical trauma of LITA-LAD revascularization by both eliminating the cardiopulmonary bypass machine and avoiding a sternotomy incision. The goal of the present study was to evaluate the 30day outcomes of robotic versus conventional CABG. Although some have argued that the most appropriate comparison group for a robotic CABG population of patients is an off pump CABG through a sternotomy (10 - 13), the fact remains that conventional CABG on cardiopulmonary bypass remains the revascularization procedure of choice for 80% of patients undergoing CABG in the United States. Equivalent results between on pump and off pump CABG in multiple randomized studies (14 - 16) remains the ongoing driver for surgeons to resist the additional technical demands imposed by a beating heart procedure.
The short operative times, together with the avoidance of a sternotomy and CPB, appear to make robotic CABG a different surgical insult than either conventional multi-vessel off pump CABG or conventional on pump CABG. No prospective randomized study has ever compared conventional and robotic CABG for either short or long term outcomes. Some may argue that these are completely different patient groups and that most patients undergoing robotic CABG are patients with single vessel disease that would never be considered for conventional CABG. Approximately one third of the patients in our robotic CABG cohort had only single vessel LAD disease with the majority of those patients having in stent restenosis. However, an additional two-thirds had multi-vessel disease and robotic CABG was part of a hybrid coronary revascularization. Most patients with multi-vessel disease who underwent only robotic CABG had either disease in a single small vessel or concomitant chronic total occlusions in one or more territories and would likely have been considered for conventional CABG.
The results of this study should be considered in the context of its limitations, specifically lack of randomization and the differences between the groups. However, with all its drawbacks the study demonstrated that the robotic CABG is a strong predictor of lower 30-day complications, short LOS and discharge to home, which further support the easier recovery of this procedure. Discharge status is becoming an important outcome measure considering its economic impact in the modern healthcare system. It may be influenced not only by medical but economical status, but socioeconomical status (SES) was not significant predictor in any of our models, besides patients were obtained by the same referral base and from the same urban population. These results are even more important when it is considered that this patient population comes primarily from an extremely underserved area where social issues can severely impact successful discharge to pre-operative residence.
It should be noted that the present study did not aim to compare robotic and conventional CABG directly as the small number of robotic patients compared to the conventional CABG patients made a thorough propensity analysis difficult. Rather, predictors of early recovery and complications were evaluated in multivariate models. It is clear that other factors also affect both complication rate and recovery. The fact that a robotic approach is a powerful driver in these models implies that in higher risk patients, robotic CABG can potentially impact the greatest on recovery.
In distinction to previous studies of minimally invasive CABG, the total operative time of robotic CABG in our study was lower compared to conventional CABG. Very short operative times with robotic CABG (2 - 3 hours total) can be achieved with experience, and previous studies have shown the steep learning curve of this operation (10, 13). The present cohort of patients represents the primary surgeon's experience at this single institution and does not represent 5 previous years of experience with the procedure and over 150 prior robotic CABG's performed. As such, this cohort represents an experience which has already been matured through the learning curve of a new procedure.
There are several limitations to our study. The first one is that it is retrospective and nonrandomized thereby introducing the inherent biases and confounding that this entails. The robotic group is relatively small with less number of coronary arteries diseased and significantly better LV function than the conventional CABG patient group. The second one is that one group is much larger than the other and all robotic cases are performed by one surgeon, while the other cases performed by him and five other surgeons. Thus, we had to rely on logistic regression analysis to adjust for differences in several baseline characteristics between two groups. The decision to perform the robotic CABG or HCR procedure was based mainly on coronary anatomy and the cardiologists' familiarity with robotic CABG or the HCR option. We can speculate that the first factor played a major role in the decision and all eligible patients were offered the minimally invasive option. But we cannot know for sure that the improved outcomes in the robotic CABG group are due to operative technique and not coronary anatomy, presence of comorbidities, body habitus or other immeasurable factors. Likewise, the follow up was not available beyond the 30-day period and data on repeated revascularizations or long term outcomes for both procedures is not available. We do understand that this information is very important and going to address it in our future work. Ongoing long-term studies of robotic CABG and HCR promise to clarify these important long-term outcomes.
In conclusion we would like to emphasize that a prospective randomized study would be needed to precisely define the advantages of a robotic approach.
Acknowledgments
The work was supported by the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), through CTSA grant numbers UL1TR000086, TL1RR000087, and KL2TR000088. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
The work was supported by the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), through CTSA grant numbers UL1TR000086, TL1RR000087, and KL2TR000088.
Footnotes
Disclosure: V. S. Srinivas, MD is on the Board of Abbott Laboratories, Green Oaks, IL USA. Galina Leyvi, MD, Stephen Forest, MD, Mark Greenberg, MD, Nan Wang, BS, Alec Mais, Max Snyder, MD, and Joseph J. DeRose Jr. MD, declare no conflicts of interest.
Conflict of interest: none declared.
References
- 1.CASS Principal Investigators and their associates: Coronary Artery Surgery Study (CASS): A randomized trial of coronary bypass surgery. Survival data. Circulation. 1983;68:939–950. doi: 10.1161/01.cir.68.5.939. [DOI] [PubMed] [Google Scholar]
- 2.The Bypass Angioplasty Revascularization Investigation (BARI) Investigators: Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. N Engl J Med. 1996;335:217–225. doi: 10.1056/NEJM199607253350401. [DOI] [PubMed] [Google Scholar]
- 3.Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–972. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 4.Mohan R, Amsel BJ, Walter PJ. Coronary artery bypass grafting in the elderly: A review of studies on patients older than 64, 69 or 74 years. Cardiology. 1992;80:215–225. doi: 10.1159/000175005. [DOI] [PubMed] [Google Scholar]
- 5.Etienne PY, Glineur D, Papadatos S, et al. Comparison of minimally invasive direct coronary artery bypass surgery with implantation of drug-eluting stents in patients with left anterior descending coronary artery disease. Innovations. 2009;4(6):340–344. doi: 10.1097/IMI.0b013e3181c49e8b. [DOI] [PubMed] [Google Scholar]
- 6.Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 1986;314:1–6. doi: 10.1056/NEJM198601023140101. [DOI] [PubMed] [Google Scholar]
- 7.Edwards FH, Grover FL, Shroyer AL, et al. The Society of Thoracic Surgeons National Cardiac Surgery Database: Current Risk Assessment. Ann Thorac Surg. 1997;63:903–908. doi: 10.1016/s0003-4975(97)00017-9. [DOI] [PubMed] [Google Scholar]
- 8.DeRose JJ. Current State of Integrated “Hybrid” Coronary Revascularization. Semin Thorac Cardiovasc Surg. 2009;21:229–236. doi: 10.1053/j.semtcvs.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Bonatti J, Lehr EJ, Vesely MJ, et al. Hybrid coronary revascularization – techniques and outcome. Eur Surg. 2011;43:198–204. [Google Scholar]
- 10.Hu S, Li Q, Gao P, et al. Simultaneous Hybrid Revascularization Versus Off-Pump Coronary Artery Bypass for Multivessel Coronary Artery Disease. Ann Thorac Surg. 2011;91:432–439. doi: 10.1016/j.athoracsur.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 11.Halkos ME, Vassiliades TA, Douglas JS, et al. Hybrid coronary revascularization versus off-pump coronary artery bypass grafting for the treatment of multivessel coronary artery disease. Ann Thorac Surg. 2011;92:1695–1702. doi: 10.1016/j.athoracsur.2011.05.090. [DOI] [PubMed] [Google Scholar]
- 12.Kon ZN, Brown EN, Tran R, et al. Simultaneous hybrid coronary revascularization reduces postoperative morbidity compared with results from conventional off-pump coronary artery bypass. J Thorac Cardiovasc Surg. 2008;135:367–375. doi: 10.1016/j.jtcvs.2007.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bachinsky WB, Abdelsalam M, Boga G, et al. Comparative study of same sitting hybrid coronary artery revascularization versus off-Pump coronary artery bypass in multivessel coronary artery disease. J Interv Cardiol. 2012;25:460–468. doi: 10.1111/j.1540-8183.2012.00752.x. [DOI] [PubMed] [Google Scholar]
- 14.Houlind K, Kjeldsen BJ, Madsen SN, et al. On-pump versus off-pump coronary artery bypass surgery in elderly patients: results from the Danish on-pump versus off-pump randomization study. Circulation. 2012;125:2431–2439. doi: 10.1161/CIRCULATIONAHA.111.052571. [DOI] [PubMed] [Google Scholar]
- 15.Diegeler A, Börgermann J, Kappert U, et al. Off-pump versus on-pump coronary-artery bypass grafting in elderly patients. N Engl J Med. 2013;368:1189–1198. doi: 10.1056/NEJMoa1211666. [DOI] [PubMed] [Google Scholar]
- 16.Lamy A, Devereaux PJ, Prabhakaran D, et al. Effects of off-pump and on-pump coronary-artery bypass grafting at 1 year. N Engl J Med. 2013 Mar 28;368(13):1179–1188. doi: 10.1056/NEJMoa1301228. [DOI] [PubMed] [Google Scholar]


