Abstract
Savanna ecosystems comprise 22% of the global terrestrial surface and 25% of Australia (almost 1.9 million km2) and provide significant ecosystem services through carbon and water cycles and the maintenance of biodiversity. The current structure, composition and distribution of Australian savannas have coevolved with fire, yet remain driven by the dynamic constraints of their bioclimatic niche. Fire in Australian savannas influences both the biophysical and biogeochemical processes at multiple scales from leaf to landscape. Here, we present the latest emission estimates from Australian savanna biomass burning and their contribution to global greenhouse gas budgets. We then review our understanding of the impacts of fire on ecosystem function and local surface water and heat balances, which in turn influence regional climate. We show how savanna fires are coupled to the global climate through the carbon cycle and fire regimes. We present new research that climate change is likely to alter the structure and function of savannas through shifts in moisture availability and increases in atmospheric carbon dioxide, in turn altering fire regimes with further feedbacks to climate. We explore opportunities to reduce net greenhouse gas emissions from savanna ecosystems through changes in savanna fire management.
Keywords: biomass burning, climate feedbacks, greenhouse gas exchange, net ecosystem carbon balance, savanna
Introduction
Tropical savanna ecosystems account for around 22% of the global land surface (Ramankutty & Foley, 1999). Annually, up to 75% of global tropical savanna landscapes are burned either by natural or anthropogenic fires (Hao et al., 1990) and accordingly, 50% of the total annual amount of biomass burned globally takes place in the savanna region (Hao & Liu, 1994). The wet-dry tropics of northern Australia include extensive areas of savanna vegetation, which occupy approximately 1.9 million km2. This area accounts for 12% of the world's tropical savanna ecosystems, making this savanna biome of global significance. In this region, fire is arguably the greatest natural and anthropogenic environmental disturbance, with vast tracts burnt each year through lightning strikes and by pastoralists, aboriginal landholders and conservation managers (Russell-Smith et al., 2003; Andersen et al., 2005).
While these frequent savanna fires are extensive in area, they are of relatively low intensity when compared to the infrequent but intense fires of southern Australia (Williams et al., 1998). Fire intensity is seasonal, with early dry season fires being of low intensity (<1000 kW m−1) and causing minimal canopy damage. As the dry season progresses, the fuel load accumulates and cures, generating greater fire intensities. Consequently, by the late dry season and premonsoonal period (August–October), fire intensity can be an order of magnitude greater than those in the early dry season (Williams et al., 1998). Such late dry season fires usually burn over very large fronts and cause more damage, resulting in crown scorching of over 90%. Such intense fires reduce foliage cover and blacken the soil (see typical example from Howard Springs – Fig.1).
Figure 1.

Unburnt and burnt savanna at Howard Springs (12°29′39.12″S 131°09′09″E) a typical Eucalypt open forest. Dominant overstorey species Eucalyptus miniata and Eucalyptus tetrodonta with a sorghum tall grass understorey.
The land surface is the interface for the exchange of radiation, heat, moisture, CO2, aerosols, and other trace gases with the atmosphere (Fig.2). Fire (Beringer et al., 2003; Chambers et al., 2005) and other disturbances (Hutley & Beringer, 2011; Hutley et al., 2013) change the ecosystem characteristics such as structure, species composition, and physiological function (Beringer et al., 2011a). These changes result in altered biophysics, including energy partitioning (e.g. an enhanced sensible heat flux) and shifts in albedo (Beringer et al., 2003; Jin & Roy, 2005). In addition, the aerodynamic properties of the ecosystem may change, affecting surface-atmosphere coupling. For example, a fire that causes a loss of canopy leaf area, will lead to a subsequent reduction in canopy photosynthesis and evapotranspiration, which will greatly influence postfire fluxes of water and carbon. Therefore, the influence of fire on ecosystem structure, and function, and biophysical processes, have implications at a range of scales (Fig.2) (Bonan, 2008; Beringer et al., 2011a).
Figure 2.

The important linkages between the land surface and the earth system. Modification of ecosystems through fire will influence on ecosystem properties (structure, composition and function) and then through biophysical and biogeochemical feedbacks at multiple scales from local boundary layer to global climate. The important exchanges are listed using black boldface text. Reprinted from Beringer et al. (2011a, p. 1467).
Variability in ecosystem characteristics modifies surface–atmosphere exchanges, which in turn influence the overlying atmospheric boundary layer. At the local scale, enhanced sensible heat fluxes over patches of burnt landscape can induce and affect mesoscale circulation systems (Knowles, 1993). Variations in atmospheric heating rates above burnt and unburnt savanna generate horizontal pressure gradients which drive atmospheric motion at a range of scales. At the regional scale, savanna fires can have significant impacts on water, energy, and CO2 exchanges (e.g. Lynch & Wu, 2000) and as a result, are likely to have important feedbacks to regional climate. For example, spatial variability in ecosystem characteristics can generate contrasts that influence regional-scale climate systems such as the Australian monsoon (Lynch et al., 2007). Previous studies have focussed on the influence of land use and land-cover change on these coupled dynamics (Evans et al., 2011; Pielke et al., 2011; Mahmood et al., 2014). However, the extent and frequency of fires in Australia make this a crucial yet under represented research issue.
Ecosystems also interact with the earth through biogeochemical cycling (C, N, P, etc.) (Fig.2). Ecosystems can be sinks or sources of CO2, trace gases and aerosols and can therefore enhance or diminish the overall greenhouse gas concentration in the atmosphere. Burning of savannas makes a positive contribution to global CO2 concentrations through the emission of greenhouse gases (GHG), while the ensuing regrowth makes a negative contribution as CO2 is assimilated from the atmosphere. The impact of fire on ecosystem productivity and non-CO2 trace gases is poorly understood (Beringer et al., 2007), in particular the influence of below-ground process such as termite gas exchange and greenhouse gas emissions from soil (Jamali et al., 2011). Furthermore, alterations in global greenhouse gas concentrations influence climate and the global circulation. Therefore, understanding the biogeochemical and biophysical processes of savanna ecosystems and the influence of fire (and other disturbances) is important for assessing interactions between climate, greenhouse gas budgets, and water budgets from regional to global scales (Arneth et al., 2010).
The objective of this article is to review our understanding of the impact fire has upon biophysical and biogeochemical properties in Australian savannas at multiple scales, from leaf level physiology to regional climate. We use an earth system framework to elucidate the impact of fires in savannas on (i) emissions from biomass burning, (ii) leaf to ecosystem carbon budgets, (iii) long-term regional carbon budgets, (iv) soil non-CO2 greenhouse gas exchange, (v) energy and water cycles, (vi) local climate and the atmospheric boundary layer and (vii) regional climate feedbacks. The focus of the article is on biophysics and biogeochemistry rather than ecological drivers, because these have already been documented in previous studies, including ecological theory (Sankaran et al., 2004), evolutionary ecology (Bowman et al., 2010), phenology (Williams et al., 1997), environmental drivers (Williams et al., 1996), nutrient cycling (Holt & Coventry, 1990), plant demographics (Prior et al., 2009; Midgley et al., 2010) and fire (Williams et al., 1999; Yates et al., 2008; Murphy et al., 2010). The spatial variability in savanna ecosystem characteristics have been previously documented by Hutley et al. (2011) and a description of the spatial patterns and processes across the landscape is given in Beringer et al. (2011a, b). While acknowledging the scale of the Australian savanna ecosystems, we draw examples from the tropical savanna region of Australia where we have sufficient information to assess many of the connections in an earth system framework.
Biomass burning and emissions
Spatial and temporal patterns of fire emissions in north Australian savannas result from both strongly seasonal but annually reliable rainfall periods followed by drought which are conducive to frequent fires, be they mostly anthropogenic ignitions or lightning strikes. The frequency of fire occurrence across Australia is shown in Fig.3, derived from 15 years (1997–2011) of Advanced Very High Resolution Radiometer (AVHRR) data (updated from Maier & Russell-Smith, 2012). Australian tropical savannas (outlined in red in Fig.3) experience a high occurrence of fires, with some regions exceeding 0.5 fires per annum (i.e. they experience a fire every second year). At the continental scale, fire frequency shows high correlation with total annual rainfall and seasonality, while at the regional scale savanna fires are more strongly influenced by anthropogenic ignition patterns (Russell-Smith et al., 2007). When averaged annually, 18% of Australia's 1.9 million km2 of tropical savannas (Fig.3) were fire affected over the period 1997–2011. Over two-thirds of fires currently occur in the late dry season months (August–November) under relatively severe fire weather conditions (Russell-Smith et al., 2013).
Figure 3.
Fire frequency of large fire events (generally >4 km2) in Australia for the period 1997–2013 derived from AVHRR burnt area mapping. Frequencies range from less than 0.1 pa (dark blue) to 1 pa (dark red). Areas in white have not been marked as burnt during the mapping period. Tropical savannas are outlined in red (updated from Maier & Russell-Smith, 2012, p. 84). A histogram illustrates the spatial heterogeneity of fire frequency.
Globally, it is estimated that landscape and biomass fires contribute CO2 emissions of between 2 and 4 Pg C yr−1 (Bowman et al., 2009). This is equivalent to around 20–40% of the 9.5 ± 0.5 Pg C yr−1 emissions from fossil-fuel combustion in 2011 (Le Quéré et al., 2012). Recent estimates suggest that around half of global fire carbon emissions come from Africa, with South America contributing between 15 and 27%, and Australia <10% (Schultz et al., 2008; Van Der Werf et al., 2010). Applying the calculation procedures that underpin Australia's National Greenhouse Gas Inventory (NGGI) (CoA (Commonwealth of Australia), 2013; Russell-Smith et al., 2009, 2014), we calculate that, over the period 1997–2013, Australian tropical savanna fires contributed 0.088 Pg C yr−1 to this total (through all gases), being <5% of global landscape and biomass fire emissions. When spread out over the Australian savanna biome (Fig.3) the emissions are 0.46 tC ha−1 yr−1 (Fig.7).
Figure 7.

The net biome productivity (NBP) for Australian savanna, estimated as the sum of the individual components of GPP, Ra and Rh derived from the Australian continental terrestrial carbon budget (Haverd et al., 2013b) and includes the disturbance and lateral transport terms. Carbon losses and uncertainties for cyclones and termites are estimated from observations as per Hutley et al. (2013). Fire estimates are calculated in this article. All unit are tC ha−1 yr−1 except ET and P which are mm yr−1. Non-CO2 greenhouse gases are not presented here and the impact of disturbance presented here is via CO2 exchange only. The size of the arrows are proportional to the size of the flux. Uncertainty associated with each component are given (*low, **medium and ***high) based on our expert opinion.
Net annual CO2 emissions from savanna fires are conventionally regarded as CO2 neutral on the assumption that wet (growing) season growth balances out dry season emissions from associated burns (Ciais et al., 2011). However, such an assumption is not met when savanna carbon stocks are degraded under high frequency and/or high intensity fire regimes over longer time scales (Beringer et al., 2007; Cook & Meyer, 2009). Moreover, savanna fires generate substantial emissions of the relatively long-lived GHG methane (CH4) and nitrous oxide (N2O) (Schulze et al., 2009), which can ultimately react to produce tropospheric ozone (O3), itself a significant contributor to global warming contributor (Finlayson-Pitts, 1997). Applying procedures referenced above for calculating Australia's NGGI, we estimate that, over the period 1997–2013, emissions from Australian tropical savanna fires contributed 0.28 Tg CH4 yr−1 of methane and 0.28 Pg CO2 yr−1 of carbon dioxide (ANGA (Australian National Greenhouse Accounts), 2013) of which the CO2 emissions are not accountable under the NGGI.
In addition, savanna fires release black carbon aerosols; for example, Beringer et al. (1995) calculated that, during 1992, savanna fires in the Northern Territory produced a large quantity (5.23 ± 0.37 × 109 g) of total particulate matter <2.5 μm in diameter. These black carbon aerosols potentially have strong positive radiative forcing (Ramanathan & Carmichael, 2008) and may change the surface albedo of savanna areas thereby increasing solar energy absorption (Govaerts, 2002).
Leaf to landscape carbon budgets
Leaf carbon
Canopy performance is significantly altered following fire events. This is caused both by the reduction in functional leaf area due to senescence of scorched leaves, and by altered gas exchange characteristics of newly expanding leaves that flush to replace those killed by the fire. The new foliage that emerges in the weeks to months following a fire is not immediately photosynthetically competent with leaf respiration rates exceeding leaf photosynthesis rates in the weeks and months following fire (Cernusak et al., 2006). Thus, the overstorey trees must not only expend carbon resources in constructing new foliage, but additionally suffer an opportunity cost associated with net negative assimilation during the canopy reconstruction phase.
What might the carbon cost of reconstructing the canopy after a fire be? Using the following assumptions, a rough estimate can be produced: canopy scorch is 0.5 m2 of foliage per m2 ground area, corresponding to about 80% of dry season canopy cover in the mesic savannas of northern Australia; specific leaf area is 5 m2 kg−1, typical for savanna eucalypts in northern Australia (Cernusak et al., 2006, 2011); the carbon mass fraction of new foliage is 0.5; however, 1.25 g of carbon is required to produce 1 g of foliage carbon due to growth respiration costs. Under these assumptions, the carbon cost of replacing scorched foliage would be roughly 60 g C m−2 ground area, or about 5% of the annual gross primary productivity (GPP) of the overstorey. This calculation is simplified, insofar as it does not take into account the loss of foliage that would occur during the dry season in the absence of fire due to natural senescence, but nevertheless it is indicative.
The opportunity cost associated with reduced carbon assimilation during the canopy reconstruction phase, when emerging foliage is not fully photosynthetically competent, is more difficult to quantify. It depends on the time courses of leaf expansion, the rates at which gains in photosynthetic capacity proceed as new leaves expand, and the water becoming available for transpiration. Negative to very low rates of net photosynthesis can persist in emerging eucalypt leaves until they have nearly fully expanded, although this will likely vary somewhat among species (Choinski et al., 2003). Beringer et al. (2007) suggested that the reduction in canopy photosynthesis could be similar in magnitude to the carbon cost of replacing burned foliage. Thus, the total cost to the carbon balance of overstorey savanna trees associated with dry season fires in northern Australian savannas could be on the order of 120 g C m−2 ground area, or about 10% of the annual GPP of the overstorey.
Leaf-scale water-use efficiency is also likely to be reduced for the period of time during which the canopy is being re-established. Expanding eucalypt leaves tend to have lower water-use efficiency than fully expanded leaves. This is caused by relatively high intercellular CO2 concentrations in expanding leaves associated with low photosynthetic capacity and high respiration rates (Cernusak et al., 2009). At the ecosystem scale, canopy transpiration has been observed to recover to prefire rates faster than canopy C assimilation (Beringer et al., 2007), likely associated with the trajectory in intercellular CO2 concentrations as expanding leaves develop.
Canopy carbon
Savannas represent a large fraction of the total tropical vegetation biomass and are highly responsive to their local environments. The rate of canopy carbon uptake at seasonal and shorter timescales is strongly controlled by local environmental drivers such as soil moisture or rainfall (Eamus et al., 1999; Cook & Heerdegen, 2001; Kanniah et al., 2013a), nutrient availability (Sankaran et al., 2005), solar radiation (Kanniah et al., 2010a, 2012, 2013b), vapour pressure deficit (Eamus et al., 2001) and fire (Beringer et al., 2007). Fire affects the radiative balance of the ecosystem immediately due to combustion of the grass-dominated understorey vegetation and blackening of the soil surface.
Previous studies have shown that low intensity fires (<1000 kW m−1) at a mesic savanna site (Howard Springs) caused minimal upper canopy damage and had a small impact on the surface energy balance and only a slight increase in Bowen ratio (ratio of sensible to latent heat fluxes). However, moderate fires (1000–3000 kW m−1) resulted in complete upper canopy scorch and almost total defoliation in the weeks following (Beringer et al., 2003) (Fig.1b). Consequently, at the Howard Springs site, canopy transpiration was reduced and energy partitioning altered. High intensity fires resulted in decreased evapotranspiration (Fig.10) and carbon uptake (Fig.4) at Howard Springs (Beringer et al., 2007). After fire, the Bowen ratio was found to increase greatly due to large increases in sensible heat fluxes. These changes in surface energy exchange following fire, when applied at the landscape scale, may have important impacts on climate through local changes in circulation patterns and changes in regional heating, precipitation and monsoon circulation (Beringer et al., 2003).
Figure 10.

Comparison of (a) daily total evapotranspiration [ET] and (b) daily total sensible heat flux for the moderate intensity fire site and for an unburnt control. The fire occurred on day 218 and the data are shown for days 5 through 10 following the fire. Reprinted from Beringer et al. (2003, p. 336).
Figure 4.

Changes to net ecosystem productivity (NEP) following annual fire using a case study during 2003. The savanna changes from a sink to source after fire and remains a source for approximately 70 days despite the canopy being rebuilt and evapotranspiration returning to prefire levels after 40 days. The difference between the observed tower fluxes and the neural network (NN) model estimates of the fire free condition gives an estimate of the indirect impact of fire (the loss of canopy productivity and the cost of rebuilding the canopy) on the canopy. The integrated effect of fire is to reduce NEP on average (2001–2006 events) by 0.7 tC ha−1. Reprinted from Beringer et al. (2007, p. 1000).
Aerosols generated from savanna burning have been found to significantly affect the direct and diffuse components of solar radiation as well as its spectral composition (Eck, 2003; Kanniah et al., 2010a), which could feedback to affect the canopy GPP of savannas (Kanniah et al., 2012). For example, Kanniah et al. (2010b) found that smoke aerosols and humidity haze produced varying aerosol optical depths (0.1–0.4) which enhanced the fraction of diffuse radiation from 11 to 22% and resulted in an increase change in net ecosystem exchange. The effect of aerosols on regional circulation is discussed later.
Long-term regional savanna NPP and NEP
In this section, we assess components of the long-term net primary productivity (NPP) and net ecosystem production (NEP) in the regional savanna carbon budget, using a land surface model. We define NEP as GPP minus ecosystem respiration (Re), in the absence of disturbance (Chapin et al., 2006). Re is the sum of autotrophic (Ra) and heterotrophic (Rh) respiration. As described in Haverd et al. (2013a, b), components of NPP and NEP were derived using the BIOS2 modelling environment, constrained by multiple observation types, and forced using remotely sensed estimates of leaf area index (LAI) and meteorology from the Bureau of Meteorology's Australian Water Availability Project data set (BoM AWAP) (Jones et al., 2009). BIOS2 is a fine-spatial-resolution (0.05°) offline modelling environment, including a modification of the Community Atmosphere Biosphere Land Exchange land surface scheme (Wang et al., 2011) incorporating the Soil–Litter–Iso model (Haverd & Cuntz, 2010) plus the Carnegie–Ames–Stanford Approach with Carbon–Nitrogen–Phosphorus (CASA–CNP) biogeochemical model (Wang et al., 2010). This scheme is used in the Australian Community Climate and Earth System Simulator. BIOS2 parameters are constrained and predictions are evaluated using multiple observation sets from across the Australian continent, including streamflow from 416 gauged catchments, eddy flux data (CO2 and H2O exchange) from 12 Australian OzFlux sites (including three from the savanna region), litterfall data, and soil, surface litter and biomass carbon pool data.
The spatial variability in carbon and water exchanges as simulated by the model are given in Fig.5 for the Australian savanna region using the boundaries of Fox et al. (2001). The figure illustrates the strong gradients in the carbon (Fig.5b, d) and water components (Fig.5c) that are driven by the steep decline in annual rainfall totals (Fig.5a) from the coast to the interior (around 1 mm km−1). The resulting patterns of biomass follow the rainfall gradient but are also very spatially heterogeneous (see the histogram insets in Fig.5). This illustrates the potential difficulty in spatial scaling of savanna processes, function and structure. From the modelled output, we then calculated the mean of the components (GPP, Rh, Ra, and ET) for the Australian savanna, where ET is evapotranspiration.
Figure 5.
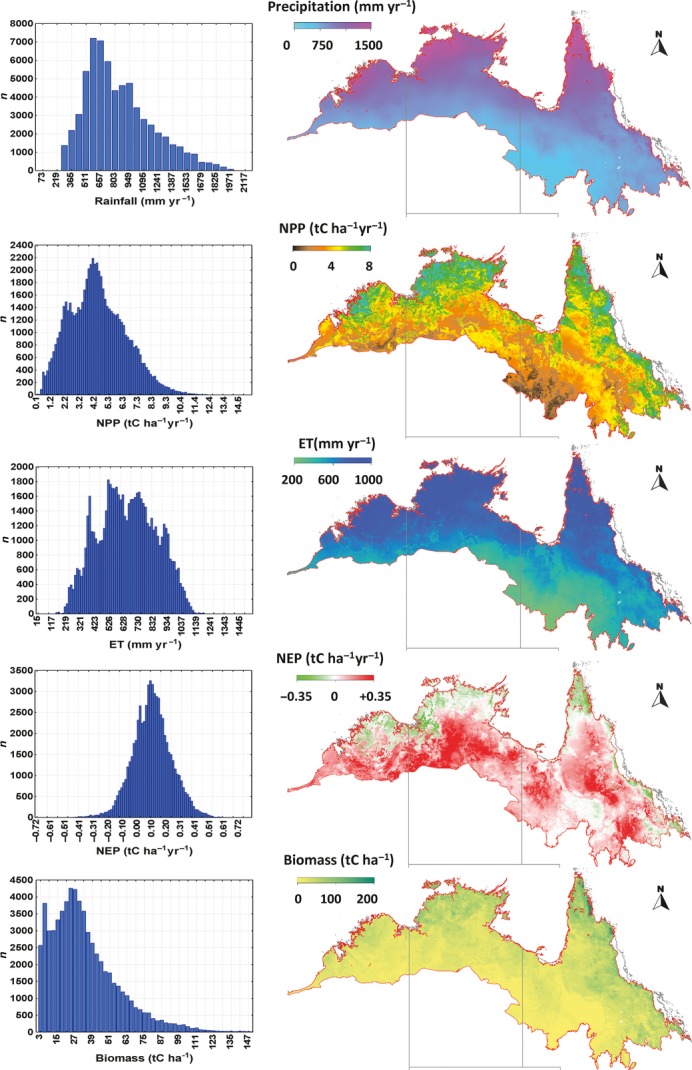
Maps of annually averaged quantities from the BIOS2 model (Haverd et al., 2013a, b) for the period 1990–2011. The model estimates are constrained and predictions are evaluated using multiple observation sets from across the Australian continent, including streamflow from 416 gauged catchments, eddy flux data (CO2 and H2O) from 12 OzFlux sites, litterfall data and data on soil, litter and biomass carbon pools. (i) Precipitation; (ii) net primary production; (iii) evapotranspiration; (iv) net ecosystem productivity (v) and biomass. Histograms illustrate the spatial heterogeneity of the spatial quantities.
Figure6(i–iii) shows the annual time series of precipitation, NPP and NEP (1911–2011) for Australian savannas. The savanna region defined in the study by Haverd et al. (2013a) is characterized by high inter-annual variability (IAV; Smith, 2004) in precipitation (638 ± 137 mm yr−1, 1σ), which contributes to the high IAV in NPP (4.35 ± 0.62 tC ha−1 yr−1, 1σ) and NEP (0.092 ± 0.36 tC ha−1 yr−1, 1σ) and is consistent with previously understood drivers of savannas carbon fluxes exchanges (Kanniah et al., 2010a). For comparison, the gross C-CO2 emissions (tC) from biomass burning are equivalent to around 9% of the savanna NPP. Figure6(iv) shows the same time series, presented as 10-year running means, converted to percentile rank. This reveals a strong correlation between precipitation and NPP at the decadal time-scale. The percentile rank time series of decadally averaged NEP can deviate significantly from that of NPP (e.g. around 1984 and 2000), corresponding to periods of high heterotrophic respiration following long periods of biospheric carbon accumulation.
Figure 6.

Annual time series of regionally averaged savanna (i) precipitation; (ii) net primary productivity (NPP) and (iii) net ecosystem production (NEP) (NPP – heterotrophic respiration in the absence of disturbance). Shading represents 1-sigma uncertainties on the mean, and includes contributions from parameter uncertainties and forcing uncertainties, as evaluated in Haverd et al. (2013a, b). (iv) Percentile rank time series of 10-year averaged precipitation, NPP and NEP. Each point is the percentile rank of the variable (precipitation, NPP or NEP), averaged over a 10-year window, centred at the time on the x-axis. A point having a percentile rank of 100% means that all other points in the time series have lower values.
The average NEP trend (1990–2011) for the Australian savanna biome was significantly positive (0.135 ± 0.055 tC ha−1 yr−1, 1σ) and slightly higher than the Australian continental average value for the same period (0.117 ± 0.036 tC ha−1 yr−1, 1σ). This is largely attributable to the CO2 fertilization effect (i.e. the positive response of NPP to rising CO2) (Haverd et al., 2013b). The NEP values, by definition, exclude the influence of disturbances such as fire, which are discussed in the following section.
Net ecosystem carbon balance and net biome production
Net ecosystem production is defined above as the difference between GPP and Re (Chapin et al., 2006). However, many additional other processes are also involved in the terrestrial carbon balance, including fire, methane fluxes, dissolved organic carbon (DOC), dissolved inorganic carbon (DIC) and particulate organic carbon (POC) losses to rivers, volatile organic carbon emissions (VOC), erosion, disturbances (e.g. insect outbreaks, storms, cyclones) and land-use change. In most terrestrial ecosystems, carbon is accumulated fairly steadily over time by net plant growth, but is lost in relatively infrequent, large emission events associated with episodic disturbance. The resulting net accumulation is described by the net ecosystem carbon balance (NECB), defined by Chapin et al. (2006) as NEP less these episodic carbon losses from additional natural and anthropogenic disturbances. Thus, the NECB is representative of long-term ecosystem productivity, and better represents a system that experiences frequent disturbance. We follow the convention that NEP and NECB are negative for carbon losses from the ecosystem to the atmosphere. The net biome productivity (NBP) is then NECB extrapolated to larger spatial scales (Chapin et al., 2006).
Net ecosystem carbon balance and NBP are important variables to quantify for Australian savannas as these ecosystems are subjected to disturbance processes that range in temporal scales from hours to months (herbivory via termites, insects, grazers both native, and introduced), annual to decadal (fire) and for coastal and subcoastal savannas, decadal to century time scales, via impacts from extreme storm events and cyclones (Williams & Douglas, 1995; Cook & Goyens, 2008; Hutley et al., 2013). Disturbance processes play a fundamental role in savanna dynamics and are important for maintaining tree and grass coexistence (House et al., 2003). Fire frequency, herbivory and climatic variability are drivers of tree recruitment and growth, with high levels of disturbance resulting in demographical bottlenecks that constrain the growth and recruitment of woody components, resulting in grass persistence or even dominance, processes that are evident in the Australian Eucalypt dominant savanna (Prior et al., 2010).
In Australian savannas, both vertebrate and invertebrate herbivory are important. While these systems lack the large herbivores of African savannas, cattle grazing is a major land use. Grazing pressure is generally low across the Australian savanna, with the exception of central Queensland where stocking rates can be as high as 100 DSE km−2 (standardized as dry-sheep equivalents; Bastin, 2008). In the Northern Territory and Kimberly region of Western Australia, rates are typically 2–10 DSE km−2 with large tracks of the high rainfall areas (>1200 mm) not grazed. Since they consume grass, cattle reduce fuel for fire and create ecological space for shrub and tree invasion (House et al., 2003). In the absence of disturbance, particularly fire, savannas tend to become more woody, although canopy closure may be limited by rainfall (not in mesic northern savannas) or herbivory (Murphy & Bowman, 2012).
A key component of NECB is the rate of tree and shrub mortality and recruitment, which is strongly influenced by fire regime (Liedloff & Cook, 2011). Extensive experimental assessments of the impacts of fire frequency and fire intensity on tree and shrub dynamics have been undertaken since the early 1990s. Williams et al. (1999) demonstrated that total live-stem basal area can increase marginally in both control (unburnt) and early dry season burnt plots in open Eucalyptus woodland in northern Australia. In contrast, substantial live-stem basal area declines (−27%) were observed over the 4 year experimental study in plots receiving hot, late season burns. In addition, there was an increase in tree mortality with increasing fire intensity (Williams et al., 1999). A similar trend (of increasing loss of stem basal area and survival with late compared to early fires) was also reported by Prior et al. (2010) and Murphy et al. (2010).
The intensity and frequency of fires also impacts savanna structure. Frequently burned sites tend to have annual grasses dominating, but the absence of burning is associated with a decrease in annual grass cover and either an increase or decrease in perennial grass covers (Russell-Smith et al., 2003). For frequent, low- to moderate-intensity (<2500 kW m−1) fire regimes, Eucalyptus and Corymbia dominated savanna were structurally stable (Russell-Smith et al., 2003). Long-term exclusion of fire (23 years) can result in grass cover declines and tree stem density increases, including invasion of rainforest taxa (Russell-Smith et al., 2003; Scott et al., 2012a).
This transition from open eucalypt woodlands to a taller, more closed forest less dominated by Eucalypt species is associated with reduced fire frequency arising from the reduced grassy fuel production in the shaded understory. Long-term changes in savanna structure can also be inferred from the analyses of Prior et al. (2010), who showed that cooler, early dry season fires result in the largest loss of living stems in small trees, while late fires have the largest impact on intermediate (3–5 m tall) and very tall (>20 m) trees. Furthermore, recruitment rates were reduced by fire and a recruitment bottleneck occurred in response to fire because of the differential effects of fire on small, medium and tall trees (Prior et al., 2010). Increased stem density and canopy cover are associated with increased GPP and NPP globally and therefore increased savanna tree standing biomass and canopy cover will be reflected in NECB.
To estimate savanna NECB, direct observations of NEP as well as carbon losses via fire and other loss pathways are required at the stand scales. These can then be scaled to regional and biome scales estimates (NBP) via remote sensing and modelling. There are few estimates of savanna NECB and NBP available (Table1). Several stand scale studies have been conducted in high rainfall (>1400 mm annual rainfall) coastal sites in the Northern Territory of Australia. NECB has been estimated at a long-term eddy covariance flux tower at the Howard Springs site in the Northern Territory, using 5 years of data from 2001 to 2006 (Beringer et al., 2007). The impact of fire on the latent energy exchange of the canopy was evident for 40 days while foliage regrew; however, the site remained a source for 70 days during this recovery phase. Fire had direct impacts through GHG emissions as well as indirect effects through the loss of productivity due to reduced functional leaf area index and the carbon costs of rebuilding the canopy following fire (Fig.4). Accounting for these indirect (canopy rebuilding) and direct (consumption of biomass) fire impacts, allowed them to calculate a NECB of +2.0 tC ha−1 yr−1 for that high rainfall site.
Table 1.
NBP and NECB estimates for Australian savannas. A positive value denotes a net carbon sink
| Source | Method | Spatial scale | Temporal scale | NECB/NBP* (tC ha−1 yr−1) |
|---|---|---|---|---|
| Barrett (2011) | Model (VAST1.2) | Biome (NBP) | 3 years | +0.016 to +0.20 |
| Beringer et al. (2007) | Flux data | Stand (NECB) | 5 years | +2.0 |
| Hutley et al. (2005) | Flux data | Stand (NECB) | 2 years | +1.54 |
| Williams et al. (2004) | Spatial extrapolation | Region (NBP) | Annual mean | +2.12 to −0.67 |
| Chen et al. (2003) | Inventory | Stand (NECB) | Annual mean | +1.1 |
*NBP, net biome productivity; NECB, net ecosystem carbon balance.
The Howard Springs site is only representative of mesic, coastal savannas (>1400 mm annual rainfall) and there is much variation in savanna structure and composition (Hutley et al., 2011) down to the 500–600 mm rainfall isohyet (Fox et al., 2001). Williams et al. (2004) used flux tower derived estimates of NEP and applied this to a savanna region of western Arnhemland in the Northern Territory where the spatial and temporal dynamics of the fire were managed and associated emissions accounted (Russell-Smith et al., 2009). This study provided a robust estimate of NBP at a regional scale (32 484 km2) and the range of NEP and burnt areas were varied to estimate NBP under a variety of scenarios. NBP for this Arnhemland region ranged from a source (−0.67 tC ha−1 yr−1) to a weak carbon sink.
At a savanna biome scale (1.91 × 108 ha), a similar result was obtained by Barrett (2011) using a modelling approach, whereby a wide range of NEP and NBP values were simulated. Data-assimilation methods were applied with the Vegetation and Soil carbon Transfer (VAST) model (Barrett, 2002) and GPP, NPP, NEP were estimated over a 20 year period. Satellite derived estimates of burnt area and carbon emissions were then used to estimate an NBP of 0–0.2 tC ha−1 yr−1. These estimates of NBP are close to the global savanna NEP estimate of 0.14 tC ha−1 yr−1 of Grace et al. (2006).
In this article, we calculate a new NBP estimate for the Australian savannas using the spatial estimates of NEP from the BIOS2 model and explicitly accounting for the losses of carbon from fire, termites, cyclones and transport (Fig.7). Carbon losses from cyclones and termites are estimated from observations as per Hutley et al. (2013) and fire loss is calculated as described in the emissions section above. Non-CO2 greenhouse gases are not presented here and the impact of disturbance presented here is via CO2 exchange only. This integration suggests the Australian savannas are a net carbon source of −0.63 tC ha−1 yr−1, with fire losses the largest disturbance pathway. Cyclonic impacts are small in comparison, given the return interval of ca. 500 years for large catastrophic events (Hutley et al., 2013) and limited area of impact (50 km from the coastline). Loss of carbon via lateral or fluvial transport of DOC, DIC and POC is likely to be low, although there is considerable uncertainty given the paucity of data describing those fluxes (Bass et al., 2013; Haverd et al., 2013b). In addition, the production and fate of soil black carbon (charcoal) is largely unknown but may be significant given the frequent fire. The production of black carbon for savanna and grassland fire is generally <3% of biomass, but there is considerable uncertainty (Grace et al., 2006). One of only a few estimates comes from southern Africa where, Kuhlbusch et al. (1996) found that 0.6–1.5% of the exposed biomass carbon was converted to black carbon, however, the mass of wind and/or fluvially exported ash is unknown. A proportion (%) of black carbon is buried within the soil and becomes a component of the total soil organic carbon pool and can reduce the temperature sensitivity and decrease the turnover time of soil carbon (Lehmann et al., 2008).
Quantifying uncertainties around these estimates is difficult. A thorough assessment of model uncertainties around NEP is given in Haverd et al. (2013a) but is not available for the disturbance and transport components. Therefore, in this case we provide a qualitative indication of uncertainties of the components in Fig.7 and we suggest that the disturbance terms have high uncertainty. In Australia, we have an excellent capability in fire remote sensing and ecology, however improvements can always be made. The general uncertainties in fire emissions lie in the inability to determine (i) spatial extent of all fire events, (ii) fire intensity, (iii) biomass consumed and (iv) appropriate emissions factors. Fluvial transport and black carbon production, transport and consumption should be quantified to establish a more robust cycling for savannas.
Climate, biomass and fire interactions
To understand the current and future links between climate, biomass and fire interactions of trees and grasses, we employed the adaptive dynamic vegetation model (aDVGM) of Scheiter & Higgins (2009). aDVGM simulates plant physiological processes and adds novel processes that allow plants to dynamically adjust carbon allocation and leaf phenology to the environmental conditions. The model is an individual-based model that simulates state variables such as biomass, height and photosynthetic rates of single plants which are then integrated to the stand scale. Fire (intensity, timing and frequency), demographics, phenology, carbon and water pools and fluxes are simulated. The aDVGM requires precipitation, temperature, atmospheric CO2 concentration, humidity, wind speed, soil carbon, soil nitrogen and it typically simulates vegetation at 1 ha stands. It has been validated in African savannas (Scheiter & Higgins, 2009). A number of experiments were undertaken by Scheiter et al. (2014) and a summary is provided below and in Fig.8.
Figure 8.

Summary of the effects of climate change, precipitation seasonality, fire regime (as described by fire return interval and fire timing) and subsequent fire intensity on tree/grass biomass in Australian savannas. A dynamic vegetation model (aDVGM – Scheiter & Higgins, 2009) was applied to a number of experiments which varied precipitation seasonality, fire return interval (FRI) and fire timing (Scheiter et al., 2014). Climate drivers were taken from output from the SRES A1B from the MPI Hamburg ECHAM5 model (Roeckner, 2013). In this scenario by 2100, carbon dioxide concentration [CO2] increased to ca. 700 ppm, air temperature (T) increased on average by 4°, mean annual precipitation (P) increased by 13%. Simulations were conducted using the following experiments: #1 FRI ranges from 1 to 10 years (10 year FRI minus 1 year FRI); #2 Fire timing from early to late dry season ignitions (early minus late); #3 Increased precipitation seasonality from current to more than current (see Scheiter et al. 2014); #4 Full factorial simulations varying climate change drivers T,P, and [CO2] where we report the anova effect sizes as partial eta squares for the impact of [CO2], T and P on tree biomass (#5) and grass biomass (#6). Here, modifications of fire intensity (#7) resulting from changes in FRI and fire timing are shown. Changes in fire intensity feedback to affect tree/grass biomass which is incorporated as seen in the biomass arrows. Values show the difference in biomass or fire intensity for the different experiments and the arrow sizes are proportional to those values. Dashed arrows indicate a negative value. All biomass units are in tC ha−1 over the simulation and fire intensity is in units of W m−1.
Simulations were conducted to examine the effects of climate change, precipitation seasonality, fire regime (as described by fire return interval and fire timing) and subsequent fire intensity on tree/grass biomass in Australian savannas. Experiments were undertaken which varied precipitation seasonality, fire return interval and fire timing (Scheiter et al., 2014). Climate drivers were taken from the SRES A1B scenario from the MPI Hamburg ECHAM5 model (Roeckner, 2013). In this scenario by 2100, carbon dioxide concentration [CO2] increased to ca. 700 ppm, air temperature (T) increased on average by 4° C and mean annual precipitation (P) increased by 13%. A description of the experiments is provided in the caption for Fig.8. The model showed that under the single climate scenario that both tree and grass biomass increased by 18.6 and 0.7 tC ha−1 yr−1, respectively (Fig.8). A full factorial set of simulations that varied climate change drivers T, P, and [CO2] showed that most of the increase in tree biomass was due to the CO2 concentration increase (anova effect size as partial eta squares of 0.97), whereas grass biomass increased due mainly to the temperature increase (anova effect size as partial eta squares of 0.94). Increasing the seasonality of precipitation reduced the growing season and resulted in a decrease in biomass of both trees and grasses with a feedback to lower fire intensities due to lower fuel loads. We then examined the effect of fire regime on tree and grass biomass and found that changing fires to occur in the early dry season or reducing fire frequency both increased the tree biomass by 20.3 and 4.9 tC ha−1 yr−1, respectively. Increases in tree biomass were due to woody thickening (an increase in standing biomass of woody species) which is a global phenomenon most commonly observed in arid and semiarid regions including savannas and shrublands (Witt et al., 2009; Scott et al., 2012b). As a result of competition the grass biomass decreased slightly. Interestingly the fire intensity increased with longer return times (due to increased fuel loads) but decreased in early dry season fires (due to higher fuel moisture content).
As demonstrated by recent north Australian experience, there is substantial scope for mitigating savanna burning emissions through the implementation of strategic early-mid dry season fire management, especially if there is a market incentive. For example, the 28 000 km2 Western Arnhem Land fire Abatement (WALFA) project reduced emissions of Kyoto-accountable GHG (CH4, N2O) by 38% over the first 7 years of implementation, relative to the preproject 10-year emissions baseline (Russell-Smith et al., 2013). This was achieved through the imposition of a more conservative fire regime emphasizing prescribed early season management to reduce late season wildfires. Although WALFA operates under a voluntary market offset arrangement with a multi-national corporate, Australian greenhouse policy, currently in flux, is likely to continue to support market incentives given that non-CO2 emissions from savanna burning are included in Australia's NGGI.
Soil greenhouse gas exchange
Soils in savanna ecosystems contribute to the production and consumption of the GHG CO2, CH4 and N2O via soil microbial processes and subterranean termite activity. In addition to direct GHG emissions during biomass combustion (Biomass burning and emissions), fire may also affect soil-atmosphere exchange of GHGs in the long-term (Castaldi et al., 2006, 2010) by altering soil carbon inputs, nutrient inputs, surface microbial activity, surface moisture and temperature. However, the effect of fire upon savanna soil-atmosphere exchange of CO2, CH4, N2O, and other trace gases is unclear and often contradictory (Anderson & Poth, 1998; Pinto, 2002).
A detailed study on the effect of fire on soil based GHG emissions was conducted by Livesley et al. (2011) at the Howard Springs site over a 16 month period, from October 2007 to January 2009. Soil GHG fluxes were measured at high temporal resolution before and after an experimental fire. Fluxes were estimated using automated chambers connected to a field-based gas chromatograph connected to automated chambers. These were supplemented by monthly manual chamber measurements on unburnt and experimentally burnt plots to provide greater replication and spatial coverage. There was no apparent impact of fire upon soil CO2 emissions following either of the two experimental burns, one conducted in September 2007 (data not shown) and one in August 2008 (Fig.9). The savanna soil generally acted as a CH4 sink, and this did not change after fire. However, relatively large CH4 emissions were observed in a short 24 h period directly following the fire as the ash bed smouldered. The fire treatments had no impact on the negligible soil N2O exchange rates.
Figure 9.

Soil CO2 (panel a) CH4 (panel b) and N2O (panel c) exchange recorded with an automated measuring system from August 2008 to December 2009 at Howard Springs, NT. Panel (d) shows soil water content, soil temperature and rainfall in the same time period. Measurements were made in an area of unburnt savanna woodland and an area that received a controlled burn on 27 August 2008 (Livesley et al., 2011). Fire did not show a significant effect on soil CO2, CH4 and N2O exchange other than an immediate spike in CH4 emissions in the 24 h period after the controlled burn.
The moderate intensity of these savanna fires at Howard Springs did not alter soil properties enough to change the biogeochemical processes involved in the production and consumption of soil GHGs. Similar results were observed in South American (Pinto, 2002) and South African (Zepp et al., 1996) savannas systems, where no significant effect of fire on soil respiration flux was detected. Significant changes in soil respiration can only be expected after prolonged fire treatments (annual burn or long-term fire prevention) (Pinto, 2002). However, a separate study in Australia's Northern Territory demonstrated that frequent fires (annually) can potentially lower soil respiration in the wet season following a fire (Richards et al., 2012), as reduced overstorey carbon led to reduced belowground carbon inputs and consequently reduced soil respiration.
Soil CH4 oxidation (uptake) activity is often greatest between 10 and 20 cm down the soil profile (Potter et al., 1996), which is beyond the thermal impact of a low or medium intensity fire. This may explain why fire had no apparent effect on soil CH4 fluxes at Howard Springs (Livesley et al., 2011), even though there was a significant decrease in soil surface moisture levels (0–5 cm) and a significant increase in surface temperature levels in the fire treatments. In South American and South African savannas, soil CH4 oxidation was similarly unresponsive to fire events (Zepp et al., 1996; Anderson & Poth, 1998) although the mechanisms involved were unknown (Castaldi et al., 2006). The temporary absence of termite activity after fire may lead to a net increase in soil CH4 uptake, as microbial oxidation is no longer offset by termite CH4 emissions (Poth et al., 1995). Alternatively, CH4 uptake may increase after fire as soil diffusivity increases after surface organic material has combusted. Soil diffusivity is one of the main controllers of soil CH4 uptake (Smith et al., 2003; Von Fischer et al., 2009; Stiehl-Braun et al., 2011), limiting the amount of CH4 and oxygen that can reach the methanotrophic bacteria.
Forest or woodland fires often lead to an increase in soil NO3− and NH4+ content (Attiwill & Adams, 1993) which may provide a substrate for nitrification or denitrification processes and thus increased N2O emissions. Livesley et al. (2011) observed an increase in NH4+, but no change in NO3− after Howard Springs savanna fires but soil N2O fluxes remained negligible (±1.0 μg N m−2 h−1) suggesting tight N cycling in these savannas (Bustamante et al., 2006). Many savanna fire studies have measured an increase in soil inorganic N but no discernible increase in N2O flux (Levine et al., 1996; Anderson & Poth, 1998; Pinto, 2002; Andersson, 2003).
The research suggests that fire in savanna systems can potentially impact soil respiration (decrease) as well as soil CH4 uptake (increase). The specific circumstances under which these impacts can be observed are not apparent and the mechanisms involved are unclear and should be subject to further research. There is reasonable evidence to suggest that fire has no, or very little, impact upon savanna soil N2O emissions.
Energy and water balances
The previous sections have provided an assessment of the effects of fire on the carbon balance and GHG exchanges across leaf to biome scales. However, there are also significant impacts on the radiation and energy balance of savannas following fire. When flying above Australia's tropical savanna late in the dry season, the broad spatial extent of blackened landscape is clearly visible, occasionally interspersed with unburned landscape, supporting a hypothesis of fire-scar driven atmospheric circulations at a range of scales. Although there have been many previous studies of Australian savanna energy and water balances (Hutley et al., 2000; Beringer & Tapper, 2002; Leuning et al., 2005) there have been few documenting fire impacts on these important surface processes. We have previously measured radiation, energy and carbon exchange over unburned and burned (both before and after low and moderate intensity fires) open woodland savanna at Howard Springs, Australia (Beringer et al., 2003). Fire affected the radiation balance immediately following fire through consumption of the grass-dominated understory and blackening of the surface. Albedo almost halved following fire (from 0.12 to 0.07 and from 0.11 to 0.06 for the moderate and low intensity sites respectively), but the recovery of albedo was dependent on the initial fire intensity. The low intensity fire caused little canopy damage with little impact on the surface energy balance and only a slight increase in Bowen ratio. However, the moderate fire resulted in a comprehensive canopy scorch and almost complete leaf drop in the days and weeks following fire. The shutdown of most leaves within the canopy reduced transpiration and altered energy partitioning markedly, with much less energy partitioned into the evaporative heat fluxes and much more into sensible heating of the atmosphere (Fig.10). Leaf death and shedding also resulted in a cessation of ecosystem carbon uptake and the savanna turned from a sink to a source of carbon to the atmosphere because of the continued ecosystem respiration (previous section). Postfire, the Bowen ratio increased greatly due to large increases in sensible heat fluxes. A subsequent study by Wendt et al. (2007) at the same site confirmed these results and also showed substantial increases in ground heat flux following the fire, because of removal of shading and insulating vegetation and increased surface heating because of the reduced albedo.
Despite decreases in canopy ET following high intensity fire, at a catchment scale, there appears to be little impact of fire on water yield, although this finding is based on only two studies (Townsend & Douglas, 2000, 2004). Water yield from catchments subjected to fire exclusion and early- and late-dry season fire regimes showed no difference between the three treatments (Townsend & Douglas, 2000). Woody cover was reduced in the catchment with late dry season burning, with the more open canopy favouring enhanced grass growth cover. These high water using C4 grasses (Hutley et al., 2000) may have compensated for any reduced tree water use, with limited impact on water yield resulting. The lengthy period between burning and wet season runoff may also have mitigated the impact of fire (Townsend & Douglas, 2000).
It is interesting to speculate upon the impact of fire on surface roughness. Intuitively it might be expected that aerodynamic roughness would decrease following savanna fire, particularly an intense savanna fire, as a result of removal of surface vegetation as well as leaf material in the tree canopy. Such changes have been observed in other parts of the world, including boreal forest (Chambers et al., 2005). To our knowledge, there have been no published direct meteorological measurements of surface roughness within Australia's tropical savanna following fire. However, N. Tapper, M. Katurji, J. Beringer, L.B. Hutley, (unpublished data) found no discernable change in turbulence spectra that could be linked to changes in aerodynamic roughness before, and immediately after, a low intensity fire that left the tree canopy intact at a site near Adelaide River, Northern Territory. It might be expected that a more intense fire would produce changes in aerodynamic roughness that would impact turbulence.
The changes in surface energy exchange following fire when applied at the landscape scale might be expected to have impacts on climate through local changes in circulation patterns and changes in regional heating, precipitation and monsoon circulation.
Fire, local climate and boundary layer processes
As shown above, fire scars can radically alter the surface energy budget of tropical savanna by reducing surface albedo, increasing available energy for partitioning into sensible and latent heat fluxes, as well as by increasing ground heat flux. Changes such as these can alter atmospheric heating rates and boundary-layer conditions, which can ultimately feedback to affect the local and regional climate. We have previously measured radiative and energy fluxes and boundary layer profiles over burnt and unburnt tropical savanna near Howard Springs (Wendt et al., 2007). At the burnt site, a moderate intensity fire, estimated between 1000 and 3500 kW m−1, initially affected the land surface by removing all understory vegetation, charring and blackening the ground surface, scorching the overstorey canopy and reducing the albedo (Wendt et al., 2007). Tethered balloon measurements showed that, despite the presence of premonsoonal rain events occurring during the measurement period, the lower boundary layer over the burnt site was up to 2 °C warmer than that over the unburnt site during the middle of the day and this warming extended to at least 500 m above the surface (Fig.11). This increase in boundary-layer heating, when applied to fire scars at the landscape scale, may can have the ability to form or alter local mesoscale circulations and ultimately create a feedback to regional heating and precipitation patterns that may affect larger-scale processes such as the Australian monsoon (Pielke et al., 2011). For example, a similar effect has been observed across the Western Australian ‘Rabbit Proof Fence’ where native vegetation on one side contrasts with adjacent agricultural fields, which generated altered boundary-layers and modified precipitation patterns (Lyons et al., 1993; Evans et al., 2011).
Figure 11.

Mean atmospheric temperature profiles from 13 days of simultaneous soundings from the burnt and unburnt sites (between days 247 and 276, 2005). Profiles show mean (±SE) temperature data for each 25 m layer. The burnt site profile is shown as a red line and the unburnt site profile as a blue line. The dashed lines show the average for the nocturnal sounding (0100) and the solid lines show the daytime sounding at (1200). Data from Wendt et al. (2007).
Regional climate feedbacks
Related to fire events and the accompanying strong alteration of surface properties is an impact on regional climate and associated processes and feedbacks. Betts (2009) gives a general review on land–surface–atmosphere coupling in observations and models while Seneviratne et al. (2010) place an emphasis on the soil-moisture temperature and precipitation feedbacks under a changing climate. Mcalpine et al. (2009) provide a synthesis on how natural climate variability, climate change as well as land use and land-cover change have an impact on Australian climate as well as boundary layer processes. Albeit specifically in relation to fire events, the potential for feedbacks between changes in land surface properties following fire (as described above) and the regional climate have been investigated by Görgen et al. (2006). Here, a fire-regrowth scheme was implemented in the soil-canopy component of the Conformal-Cubic Atmosphere Model (C-CAM; McGregor & Dix, 2001) with a grid resolution of 65 km over Australia. Surface properties were modified in the model by the fire intensity and its spatial and temporal extent (timing of the event, and the length of the regrowth period) were mapped using the AVHRR satellite. Albedo, roughness length, LAI, and fractional vegetation coverage were modified to simulate the impacts of these biophysical factors on surface energy balances and flux partitioning.
In an initial sensitivity experiment, they simulated a large-scale and high intensity fire in the late dry-season with a long regrowth period extending well into the wet season (Görgen et al., 2006). On average, the fire caused a change of about 150 W m−2 in net radiation, which led to increased soil temperature, larger turbulent fluxes, higher mixing ratios in the boundary layer, increased wind speeds and an increased boundary-layer height. Moisture availability was the limiting factor in convective precipitation responses. Precipitation increases of around 15% were statistically significant during the premonsoon season (Fig.12a). These changes were associated with an intensification of the Pilbara heat low (Fig.12b) and the potential for increased lateral inflow of moist oceanic air. In a different study with less strong and more uniform surface property changes simulating fires, Notaro et al. (2011) found similar changes in boundary layer processes but the response on precipitation during the premonsoon season was opposite.
Figure 12.

Average (1979–1999) impacts of changed surface conditions on regional meteorological conditions in a C-CAM run with 80% burned area and fire intensity vs. control run without fires. (a) Altered November premonsoon circulation patterns at 850 hPa. (b) Statistically significant (dotted) precipitation increase [mm day−1], October–November–December (OND) and December–January–February (DJF). Reprinted from Görgen et al. (2006, p. 10 and 11).
The implications of these findings for Australian monsoon precipitation and circulation were further investigated using a factorial experimental design to fully characterize the response of the system across a realistic range of fire and regrowth characteristics in 90 independent 21-year experiments (Abramson et al., 2006; Lynch et al., 2007). It was found that the total area receiving monsoon precipitation could increase over northern Australia by up to 30% in the presence of large, high intensity fires late in the dry season. Indeed, the timing of the fire accounted for 58% of the variance in monsoon precipitation, followed by the area (18%) and the intensity (15%). Fires above 40% of the maximum possible size that occur late in the dry season had a strong positive impact on monsoonal circulation as quantified by the Australian Monsoon Index (Fig.13). Furthermore, due to an increase in moisture convergence the uplift intensity, rather than the moisture availability, controlled the precipitation variability. Late dry season fires of high intensity did significantly affect the simulated Australian Monsoon Index from an average of 0.03 to 0.43 m s−1 (Fig.13). Finally, and perhaps most significantly, these findings clearly indicate that the impacts depend upon key thresholds in intensity and area of the savanna fire regime.
Figure 13.
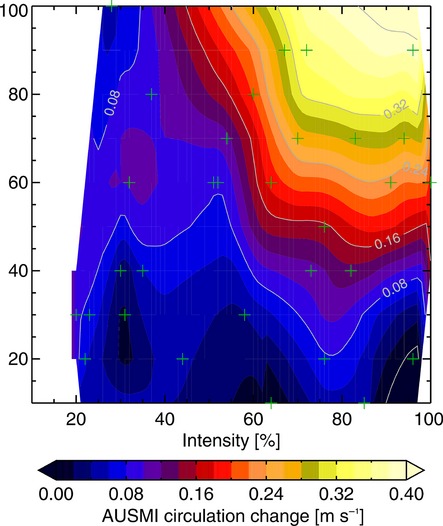
Simulated differences between the fire scenario and the reference simulation of the response of the Australian Monsoon Index (AUSMI) [m s−1] to variation in fire intensity and burned area. AUSMI is obtained by averaging the daily mean 850 hPa zonal wind speed from the equator to 10°S and from 120°E to 150°E, the zone of monsoonal reversal of the flow in and out of Australia. The crosses indicate the population of the simulation space consisting only experiments with a timing of the fire-event later than the Julian day 250 (7 September). The contoured response metric is then Gaussian low pass filtered linear interpolations based on Delauney triangulations. Reprinted from Lynch et al. (2007, p. 4).
In addition to surface properties, changes in direct and indirect aerosol emissions associated with landscape fires also exert multiple effects on different spatial and temporal scales. Bowman et al. (2009) give a general overview of those effects. Ward et al. (2012) provide global estimates of the radiative forcing associated with aerosols from fires using sensitivity experiments with the Community Atmosphere Model (CAM). From 1850 to 2000 radiative forcing amounted to 0.5 W m−2. Again using CAM, Tosca et al. (2013) found that simulations using prescribed fire emissions and aerosol optical depths that were 10% greater than present resulted in increased tropospheric heating and a decreased surface temperature. This altered the Hadley circulation and then modified precipitation patterns and amounts. Simulations including fires vs. those without resulted in a cooling of between 0.05 and 0.3 K across parts of northern Australia as well as positive precipitation anomalies of up to 0.3 mm day−1 occur over Western Australia. Precipitation decreases were statistically not significant and amounted to −0.2 mm day−1 in the Northern Territory and the Gulf of Carpentaria region. In regional model experiments Tummon et al. (2010) showed for South Africa that direct and semidirect effects of biomass burning on radiation and aerosol optical depth may reduce surface radiative forcing and thereby decrease latent and sensible heat fluxes and PBL height. The radiative absorption in these experiments led to a tropospheric warming and thereby stabilization with a decrease in precipitation. In a similar experiment for equatorial Asia, touching on northern Australia, Tosca et al. (2010) found similar feedback mechanisms. In Mitchell et al. (2013), recent observational data are presented that give evidence for a fairly homogeneous distribution of elevated fire-induced aerosol levels during the late dry season.
Conclusions
This article has demonstrated that the modification of the savanna land surface via fire influences the rest of the earth system via biophysical and biogeochemical cycles with feedbacks to regional and global climate. The future status of savannas worldwide remains uncertain as they are particularly threatened by land-use change and disturbance (fire, cyclones, grazing, invasive species). We show that the savanna NEP is a small sink but that the magnitude of the disturbance components are sufficient to change the system to a modest source. Changes in the frequency and magnitude of disturbance from climate change could easily tip the balance one way or the other. There is a strong need to reduce uncertainties in estimates of fire, termites and cyclones and understand how these may interact with environmental change (climate change and invasive species). Moreover, there remain possibly important terms (black carbon and VOC's) that we do not yet know enough about. We suggest that a priority for future research is to understand how these agents affect biophysical and biogeochemical cycling and how these may interact with climate change in the future. We suggest that climate change will confound projections to quantify how savannas may respond to future environmental perturbations. For example, reductions in rainfall would have consequences for grass and tree biomass (and hence fuel load) with a feedback to fire intensity (reduction), which may reduce the impact of fire and therefore alter biophysical and biogeochemical processes in the short term. Long- term shifts in fire frequency and intensity will have a flow on effect upon savanna demography, thereby altering savanna structure and function that in turn modifies land surface properties such as leaf area index, surface roughness and flux partitioning. This then has the potential to feedback from local to global climate both mechanically (local) and through secondary effects driven by regional circulation changes.
Additional research priorities include the need to quantify additional processes to obtain a comprehensive NBP, such as non-CO2 fluxes, DOC and DIC losses to rivers, VOC, black carbon, and sediment transport (Randerson et al., 2002). Although this article focussed on the ecosystem scale, it is expected that grass and trees will respond differently to future environmental change and disturbance. Consequently that we may see shifts in the tree:grass ratio due to changes in rainfall and increasing atmospheric CO2. Increasing temperature and changing growing season length will also modify fire regimes that will subsequently feedback to further alter tree and grass dynamics. Therefore, an understanding of the complex suite of feedbacks is required using a process based approach to better understand potential responses. Despite their importance for the earth system and human well-being, savannas represent a gap in earth observations because of the major difficulty in the remote assessment of vegetation structure and dynamics of two such distinct functional groups (tree and grass) (Hanan & Hill, 2012). The challenges for remote sensing observations in savannas were highlighted in a recent workshop on the ‘Challenges and Opportunities in remote sensing of Global Savannas’ in Fort Collins in 2010 and a subsequently NASA white paper (Hanan & Hill, 2012) on tree/grass systems. In addition, savannas are a challenge for modelling communities to simulate in global climate and vegetation models (Beringer et al., 2011a).
The challenge will be to manage our savanna ecosystems to provide ecosystem services in the face of changing fire regimes and the potential interaction between climate change, invasive species and land management. Currently ‘our capacity to manage fire remains imperfect and may become more difficult in the future as climate change alters fire regimes. This risk is difficult to assess, however, because fires are still poorly represented in global models’ (Bowman et al., 2009, p. 481). The solution requires an earth system science framework to provide a holistic understanding of linkages between physical and human systems to provide tools for assessment, management and policy.
Acknowledgments
This study was funded by the Australian Research Council (FF0348550, DP0344744, DP0772981, DP130101566, LP0774812, LP100100073 and SR0566976). Beringer is funded under an ARC FT (FT1110602). Support for collection and archiving was provided through the Australia Terrestrial Ecosystem Research Network (TERN) (http://www.tern.org.au). The support of the Cooperative Research Centre for Enterprise Distributed Systems, the Department of Communications, Information Technology and the Arts under a GranetNet grant, and the Australian Partnership for Advanced Computing, are also gratefully acknowledged. The Australian Climate Change Science Programme supported VH, JGC, and MR. We thank the two anonymous reviewers for their constructive feedback.
References
- Abramson D, Lynch A, Takemiya H. Deploying scientific applications to the PRAGMA grid testbed: strategies and lessons. In: Turner SJ, Lee BS, Wentong C, et al., editors. Sixth IEEE International Symposium on Cluster Computing and the Grid (CCGRID'06) The Institute of Electrical and Electronics Engineers; 2006. pp. 241–248. 16–19 May. [Google Scholar]
- Andersen AN, Cook GD, Corbett LK, et al. Fire frequency and biodiversity conservation in Australian tropical savannas: implications from the Kapalga fire experiment. Austral Ecology. 2005;30:155–167. [Google Scholar]
- Anderson IC, Poth MA. Controls on fluxes of trace gases from Brazilian cerrado soils. Journal of Environment Quality. 1998;27:1117. [Google Scholar]
- Andersson M. Soil emissions of nitrous oxide in fire-prone African savannas. Journal of Geophysical Research. 2003;108:4630. [Google Scholar]
- ANGA (Australian National Greenhouse Accounts) 2013. National Inventory Report 2011, Vol. 1.
- Arneth A, Sitch S, Bondeau A, et al. From biota to chemistry and climate: towards a comprehensive description of trace gas exchange between the biosphere and atmosphere. Biogeosciences. 2010;7:121–149. [Google Scholar]
- Attiwill PM, Adams MA. Nutrient cycling in forests. New Phytologist. 1993;124:561–582. doi: 10.1111/j.1469-8137.1993.tb03847.x. [DOI] [PubMed] [Google Scholar]
- Barrett DJ. Steady state turnover time of carbon in the Australian terrestrial biosphere. Global Biogeochemical Cycles. 2002;16:55–1–55–21. [Google Scholar]
- Barrett DJ. Timescales and dynamics of carbon in Australia's savannas. In: Hill MJ, Hanan NP, editors. Ecosystem Function in Savannas: Measurement and Modeling at Landscape to Global Scales. Boca Raton, FL: CRC Press; 2011. pp. 347–366. [Google Scholar]
- Bass AM, O’ Grady D, Berkin C, Leblanc M, Tweed S, Nelson PN, Bird MI. High diurnal variation in dissolved inorganic C, δ13C values and surface efflux of CO2 in a seasonal tropical floodplain. Environmental Chemistry Letters. 2013;11:399–405. [Google Scholar]
- Bastin G. ACRIS Livestock Density Update 2003–2008. Canberra: Department of Sustainability, Environment, Water, Population and Communities; 2008. [Google Scholar]
- Beringer J, Tapper N. Surface energy exchanges and interactions with thunderstorms during the Maritime Continent Thunderstorm Experiment (MCTEX) Journal of Geophysical Research-Atmospheres. 2002;107:4552. [Google Scholar]
- Beringer J, Packham D, Tapper NJ. Biomass burning and resulting emissions in the Northern Territory, Australia. International Journal of Wildland Fire. 1995;5:229–235. [Google Scholar]
- Beringer J, Hutley LB, Tapper NJ, Coutts A, Kerley A, O'Grady AP. Fire impacts on surface heat, moisture and carbon fluxes from a tropical savanna in northern Australia. International Journal of Wildland Fire. 2003;12:333–340. [Google Scholar]
- Beringer J, Hutley LB, Tapper NJ, Cernusak LA. Savanna fires and their impact on net ecosystem productivity in North Australia. Global Change Biology. 2007;13:990–1004. [Google Scholar]
- Beringer J, Hacker J, Hutley LB, et al. SPECIAL – savanna patterns of energy and carbon integrated across the landscape. Bulletin of the American Meteorological Society. 2011a;92:1467–1485. [Google Scholar]
- Beringer J, Hutley LB, Hacker JM, et al. Patterns and processes of carbon, water and energy cycles across northern Australian landscapes: from point to region. Agricultural and Forest Meteorology. 2011b;151:1409–1416. [Google Scholar]
- Betts AK. Land-surface-atmosphere coupling in observations and models. Journal of Advances in Modeling Earth Systems. 2009;1:1–18. [Google Scholar]
- Bonan GB. Forests and climate change: forcings, feedbacks, and the climate benefits of forests. Science. 2008;320:1444–1449. doi: 10.1126/science.1155121. [DOI] [PubMed] [Google Scholar]
- Bowman DMJS, Balch JK, Artaxo P, et al. Fire in the earth system. Science (New York, N.Y.) 2009;324:481–484. doi: 10.1126/science.1163886. [DOI] [PubMed] [Google Scholar]
- Bowman DMJS, Brown GK, Braby MF, et al. Biogeography of the Australian monsoon tropics. Journal of Biogeography. 2010;37:201–216. [Google Scholar]
- Bustamante MMC, Medina E, Asner GP, Nardoto GB, Garcia-Montiel DC. Nitrogen cycling in tropical and temperate savannas. In: Martinelli LA, Howarth RW, editors. Nitrogen Cycling in the Americas: Natural and Anthropogenic Influences and Controls. Dordrecht, the Netherlands: Springer; 2006. pp. 209–237. Available at: http://link.springer.com/content/pdf/bfm%3A978-1-4020-5517-1%2F1.pdf (accessed 15 September 2013) [Google Scholar]
- Castaldi S, Ermice A, Strumia S. Fluxes of N2O and CH4 from soils of savannas and seasonally-dry ecosystems. Journal of Biogeography. 2006;33:401–415. [Google Scholar]
- Castaldi S, de Grandcourt A, Rasile A, Skiba U, Valentini R. Fluxes of CO2, CH4 and N2O from soil of burned grassland savannah of central Africa. Biogeosciences Discussions. 2010;7:4089–4126. [Google Scholar]
- Cernusak LA, Hutley LB, Beringer J, Tapper NJ. Stem and leaf gas exchange and their responses to fire in a north Australian tropical savanna. Plant Cell and Environment. 2006;29:632–646. doi: 10.1111/j.1365-3040.2005.01442.x. [DOI] [PubMed] [Google Scholar]
- Cernusak LA, Tcherkez G, Keitel C, et al. Why are non-photosynthetic tissues generally 13 C enriched compared with leaves in C 3 plants? Review and synthesis of current hypotheses. Functional Plant Biology. 2009;36:199. doi: 10.1071/FP08216. [DOI] [PubMed] [Google Scholar]
- Cernusak LA, Hutley LB, Beringer J, Holtum JAM, Turner BL. Photosynthetic physiology of eucalypts along a sub-continental rainfall gradient in northern Australia. Agricultural and Forest Meteorology. 2011;151:1462–1470. [Google Scholar]
- Chambers SD, Beringer J, Randerson JT, Chapin FS. Fire effects on net radiation and energy partitioning: contrasting responses of tundra and boreal forest ecosystems. Journal of Geophysical Research-Atmospheres. 2005;110:9106. [Google Scholar]
- Chapin FS, Woodwell GM, Randerson JT, et al. Reconciling carbon-cycle concepts, terminology, and methods. Ecosystems. 2006;9:1041–1050. [Google Scholar]
- Chen X, Hutley LB, Eamus D. Carbon balance of a tropical savanna of northern Australia. Oecologia. 2003;137:405–416. doi: 10.1007/s00442-003-1358-5. [DOI] [PubMed] [Google Scholar]
- Choinski JS, Jr, Ralph P, Eamus D. Changes in photosynthesis during leaf expansion in Corymbia gummifera. Australian Journal of Botany. 2003;51:111. [Google Scholar]
- Ciais P, Bombelli A, Williams M, et al. The carbon balance of Africa: synthesis of recent research studies. Philosophical Transactions. Series A, Mathematical, Physical, and Engineering Sciences. 2011;369:2038–2057. doi: 10.1098/rsta.2010.0328. [DOI] [PubMed] [Google Scholar]
- CoA (Commonwealth of Australia) Carbon Credits (Carbon Farming Initiative) Reduction of Greenhouse Gas Emissions through Early Dry Season Savanna Burning—1.1) Methodology Determination 2013. Canberra, ACT, Australia: Commonwealth of Australia; 2013. [Google Scholar]
- Cook GD, Goyens CMAC. The impact of wind on trees in Australian tropical savannas: lessons from Cyclone Monica. Austral Ecology. 2008;33:462–470. [Google Scholar]
- Cook GD, Heerdegen RG. Spatial variation in the duration of the rainy season in monsoonal Australia. Journal of Climatology. 2001;21:1723–1732. [Google Scholar]
- Cook G, Meyer C. Fire, fuels and greenhouse gases. In: Russell-Smith J, Whitehead PJ, Cooke P, editors. Culture, Ecology and Economy of Savanna Fire Management in Northern Australia: Rekindling the Wurrk Tradition. Melbourne, Vic., Australia: CSIRO Publications; 2009. pp. 313–327. [Google Scholar]
- Eamus D, Myers B, Duff G, Williams D. Seasonal changes in photosynthesis of eight savanna tree species. Tree Physiology. 1999;19:665–671. doi: 10.1093/treephys/19.10.665. [DOI] [PubMed] [Google Scholar]
- Eamus D, Hutley LB, O'Grady AP. Daily and seasonal patterns of carbon and water fluxes above a north Australian savanna. Tree Physiology. 2001;21:977–988. doi: 10.1093/treephys/21.12-13.977. [DOI] [PubMed] [Google Scholar]
- Eck TF. Variability of biomass burning aerosol optical characteristics in southern Africa during the SAFARI 2000 dry season campaign and a comparison of single scattering albedo estimates from radiometric measurements. Journal of Geophysical Research. 2003;108:8477. [Google Scholar]
- Evans JP, Pitman AJ, Cruz FT. Coupled atmospheric and land surface dynamics over southeast Australia: a review, analysis and identification of future research priorities. International Journal of Climatology. 2011;31:1758–1772. [Google Scholar]
- Finlayson-Pitts BJ. Tropospheric air pollution: ozone, airborne toxics, polycyclic aromatic hydrocarbons, and particles. Science. 1997;276:1045–1051. doi: 10.1126/science.276.5315.1045. [DOI] [PubMed] [Google Scholar]
- Fox ID, Neldner VJ, Wilson GW, et al. The Vegetation of the Australian Tropical Savannas. Brisbane, Qld: Environmental Protection Agency; 2001. [Google Scholar]
- Görgen K, Lynch AH, Marshall AG, Beringer J. Impact of abrupt land cover changes by savanna fire on northern Australian climate. Journal of Geophysical Research. 2006;111:19106. [Google Scholar]
- Govaerts YM. Impact of fires on surface albedo dynamics over the African continent. Journal of Geophysical Research. 2002;107:4629. [Google Scholar]
- Grace J, San Jose J, Meir P, Miranda HS, Montes RA, Jose JS. Productivity and carbon fluxes of tropical savannas. Journal of Biogeography. 2006;33:387–400. [Google Scholar]
- Hanan N, Hill MJ. Savannas in a Changing Earth System: The NASA Terrestrial Ecology Tree-Grass Project. Washington, USA: White Paper for the NASA Terrestrial Ecology Program, Earth Science Division; 2012. [Google Scholar]
- Hao WM, Liu M-H. Spatial and temporal distribution of tropical biomass burning. Global Biogeochemical Cycles. 1994;8:495–503. [Google Scholar]
- Hao WM, Liu M-H, Crutzen PJ. Estimates of annual and regional releases of CO2, and other trace gases to the atmosphere from fires in the tropics, based on FAO statistics for the period 1975–1980. In: Goldammer JG, editor. Fire in the Tropical Biota, Ecological Studies 84. New York: Springer-Verlag; 1990. pp. 440–462. [Google Scholar]
- Haverd V, Cuntz M. Soil–Litter–Iso: a one-dimensional model for coupled transport of heat, water and stable isotopes in soil with a litter layer and root extraction. Journal of Hydrology. 2010;388:438–455. [Google Scholar]
- Haverd V, Raupach MR, Briggs PR, et al. Multiple observation types reduce uncertainty in Australia's terrestrial carbon and water cycles. Biogeosciences. 2013a;10:2011–2040. [Google Scholar]
- Haverd V, Raupach MR, Briggs PR, et al. The Australian terrestrial carbon budget. Biogeosciences. 2013b;10:851–869. [Google Scholar]
- Holt JA, Coventry RJ. Nutrient cycling in Australian savannas. Journal of Biogeography. 1990;17:427–432. [Google Scholar]
- House JI, Archer S, Breshears DD, Scholes RJ. Conundrums in mixed woody-herbaceous plant systems. Journal of Biogeography. 2003;30:1763–1777. [Google Scholar]
- Hutley LB, Beringer J. Disturbance and climatic drivers of carbon dynamics of a north Australian tropical savanna. In: Hill MJ, Hanan NP, editors. Ecosystem Function in Savannas: Measurement and Modeling at Landscape to Global Scales. Boca Raton, FL: CRC/Taylor and Francis Press; 2011. pp. 57–75. [Google Scholar]
- Hutley LB, O'Grady AP, Eamus D. Evapotranspiration from Eucalypt open-forest savanna of Northern Australia. Functional Ecology. 2000;14:183–194. [Google Scholar]
- Hutley LB, Leuning R, Beringer J, Cleugh HA. The utility of the eddy covariance techniques as a tool in carbon accounting: tropical savanna as a case study. Australian Journal of Botany. 2005;53:663. [Google Scholar]
- Hutley LB, Beringer J, Isaac PR, Hacker JM, Cernusak LA. A sub-continental scale living laboratory: spatial patterns of savanna vegetation over a rainfall gradient in northern Australia. Agricultural and Forest Meteorology. 2011;151:1417–1428. [Google Scholar]
- Hutley LB, Evans BJ, Beringer J, Cook GD, Maier SM, Razon E. Impacts of an extreme cyclone event on landscape-scale savanna fire, productivity and greenhouse gas emissions. Environmental Research Letters. 2013;8:045023. [Google Scholar]
- Jamali H, Livesley SJ, Grover SP, Dawes TZ, Hutley LB, Cook GD, Arndt SK. The importance of termites to the CH4 balance of a tropical savanna woodland of northern Australia. Ecosystems. 2011;14:698–709. [Google Scholar]
- Jin Y, Roy DP. Fire-induced albedo change and its radiative forcing at the surface in northern Australia – art. no. L13401. Geophysical Research Letters. 2005;32:13401. [Google Scholar]
- Jones DA, Wang W, Fawcett R. High-quality spatial climate data-sets for Australia. Australian Meteorological and Oceanographic Journal. 2009;58:233–248. [Google Scholar]
- Kanniah KD, Beringer J, Hutley LB. The comparative role of key environmental factors in determining savanna productivity and carbon fluxes: a review, with special reference to northern Australia. Progress in Physical Geography. 2010a;34:459–490. [Google Scholar]
- Kanniah KD, Beringer J, Tapper NJ, Long CN. Aerosols and their influence on radiation partitioning and savanna productivity in northern Australia. Theoretical and Applied Climatology. 2010b;100:423–438. [Google Scholar]
- Kanniah KD, Beringer J, North P, Hutley L. Control of atmospheric particles on diffuse radiation and terrestrial plant productivity: a review. Progress in Physical Geography. 2012;36:209–237. [Google Scholar]
- Kanniah KD, Beringer J, Hutley LB. Response of savanna gross primary productivity to interannual variability in rainfall: results of a remote sensing based light use efficiency model. Progress in Physical Geography. 2013a;37:642–663. [Google Scholar]
- Kanniah KD, Beringer J, Hutley L. Exploring the link between clouds, radiation, and canopy productivity of tropical savannas. Agricultural and Forest Meteorology. 2013b;182–183:304–313. [Google Scholar]
- Knowles J. The Influence of Forest-Fire Induced Albedo Differences on the Generation of Mesoscale Circulations. Fort Collins, CO, USA: Colorado State University; 1993. [Google Scholar]
- Kuhlbusch TAJ, Andreae MO, Cachier H, Goldammer JG, Lacaux J-P, Shea R, Crutzen PJ. Black carbon formation by savanna fires: measurements and implications for the global carbon cycle. Journal of Geophysical Research. 1996;101:23651. [Google Scholar]
- Le Quéré C, Andres RJ, Boden T, et al. The global carbon budget 1959–2011. Earth System Science Data Discussions. 2012;5:1107–1157. [Google Scholar]
- Lehmann J, Skjemstad J, Sohi S, et al. Australian climate-carbon cycle feedback reduced by soil black carbon. Nature Geoscience. 2008;1:832–835. [Google Scholar]
- Leuning R, Cleugh HA, Zegelin SJ, Hughes D. Carbon and water fluxes over a temperate Eucalyptus forest and a tropical wet/dry savanna in Australia: measurements and comparison with MODIS remote sensing estimates. Agricultural and Forest Meteorology. 2005;129:151–173. [Google Scholar]
- Levine JS, Winstead EL, Parsons DAB, et al. Biogenic soil emissions of nitric oxide (NO) and nitrous oxide (N2O) from savannas in South Africa: the impact of wetting and burning. Journal of Geophysical Research. 1996;101:23689. [Google Scholar]
- Liedloff A, Cook GD. Interaction of fire and rainfall variability on tree structure and carbon fluxes in savannas: application of the flames model. In: Hill MJ, Hanan NP, editors. Ecosystem Function in Savannas: Measurement and Modeling at Landscape to Global Scales. Boca Raton, USA: Taylor & Francis, Inc; 2011. pp. 293–308. [Google Scholar]
- Livesley SJ, Grover SPP, Hutley LB, et al. Seasonal variation and fire effects on CH4, N2O and CO2 exchange in savanna soils of northern Australia. Agricultural and Forest Meteorology. 2011;151:1440–1452. [Google Scholar]
- Lynch AH, Wu WL. Impacts of fire and warming on ecosystem uptake in the boreal forest. Journal of Climate. 2000;13:2334–2338. [Google Scholar]
- Lynch AH, Abramson D, Görgen K, Beringer J, Uotila P. Influence of savanna fire on Australian monsoon season precipitation and circulation as simulated using a distributed computing environment. Geophysical Research Letters. 2007;34:L20801. [Google Scholar]
- Lyons TJ, Schwerdtfeger P, Hacker JM, Foster IJ, Smith RCG, Xinmei H. Land-atmosphere interaction in a semiarid region: the Bunny fence experiment. Bulletin of the American Meteorological Society. 1993;74:1327–1334. [Google Scholar]
- Mahmood R, Pielke RA, Hubbard KG, et al. Land cover changes and their biogeophysical effects on climate. International Journal of Climatology. 2014;34:929–953. [Google Scholar]
- Maier SW, Russell-Smith J. Measuring and monitoring of contemporary fire regimes in Australia using satellite remote sensing. In: Bradstock RA, Gill AM, Williams RJ, editors. Flammable Australia: Fire Regimes, Biodiversity and Ecosystems in a Changing World. Melbourne, Vic: CSIRO Publishing; 2012. pp. 79–96. [Google Scholar]
- Mcalpine CA, Syktus J, Ryan JG, et al. A continent under stress: interactions, feedbacks and risks associated with impact of modified land cover on Australia's climate. Global Change Biology. 2009;15:2206–2223. [Google Scholar]
- McGregor JL, Dix MR. The CSIRO conformal-cubic atmospheric GCM. In: Hodnett PE, editor. IUTAM Symposium on Advances in Mathematical Modelling of Atmosphere and Ocean Dynamics Fluid Mechanics and Its Applications. Dordrecht, the Netherlands: Kluwer Academic Publishers, Springer; 2001. pp. 197–202. ) [Google Scholar]
- Midgley JJ, Lawes MJ, Chamaillé-Jammes S. TURNER REVIEW No. 19. Savanna woody plant dynamics: the role of fire and herbivory, separately and synergistically. Australian Journal of Botany. 2010;58:1. [Google Scholar]
- Mitchell RM, Forgan BW, Campbell SK, Qin Y. The climatology of Australian tropical aerosol: evidence for regional correlation. Geophysical Research Letters. 2013;40:2384–2389. [Google Scholar]
- Murphy BP, Bowman DMJS. What controls the distribution of tropical forest and savanna? Ecology letters. 2012;15:748–758. doi: 10.1111/j.1461-0248.2012.01771.x. [DOI] [PubMed] [Google Scholar]
- Murphy BP, Russell-Smith J, Prior LD. Frequent fires reduce tree growth in northern Australian savannas: implications for tree demography and carbon sequestration. Global Change Biology. 2010;16:331–343. [Google Scholar]
- Notaro M, Wyrwoll K-H, Chen G. Did aboriginal vegetation burning impact on the Australian summer monsoon? Geophysical Research Letters. 2011;38:L11704. [Google Scholar]
- Pielke RA, Pitman A, Niyogi D, et al. Land use/land cover changes and climate: modeling analysis and observational evidence. Wiley Interdisciplinary Reviews: Climate Change. 2011;2:828–850. [Google Scholar]
- Pinto AdeS. Soil emissions of N2O, NO, and CO2 in Brazilian savannas: effects of vegetation type, seasonality, and prescribed fires. Journal of Geophysical Research. 2002;107:8089. [Google Scholar]
- Poth M, Anderson IC, Miranda HS, Miranda AC, Riggan PJ. The magnitude and persistence of soil NO, N2O, CH4, and CO2 fluxes from burned tropical savanna in Brazil. Global Biogeochemical Cycles. 1995;9:503–513. [Google Scholar]
- Potter CS, Davidson EA, Verchot LV. Estimation of global biogeochemical controls and seasonality in soil methane consumption. Chemosphere. 1996;32:2219–2246. [Google Scholar]
- Prior LD, Murphy BP, Russell-Smith J. Environmental and demographic correlates of tree recruitment and mortality in north Australian savannas. Forest Ecology and Management. 2009;257:66–74. [Google Scholar]
- Prior LD, Williams RJ, Bowman DMJS. Experimental evidence that fire causes a tree recruitment bottleneck in an Australian tropical savanna. Journal of Tropical Ecology. 2010;26:595–603. [Google Scholar]
- Ramanathan V, Carmichael G. Global and regional climate changes due to black carbon. Nature Geoscience. 2008;1:221–227. [Google Scholar]
- Ramankutty N, Foley JA. Estimating historical changes in global land cover: croplands from 1700 to 1992. Global Biogeochemical Cycles. 1999;13:997–1027. [Google Scholar]
- Randerson AJT, Chapin FS, III, Harden JW, Neff JC, Harmon ME, Randerson JT. Net ecosystem production: a comprehensive measure of net carbon accumulation by ecosystems. Ecological Applications. 2002;12:937–947. [Google Scholar]
- Richards AE, Dathe J, Cook GD. Fire interacts with season to influence soil respiration in tropical savannas. Soil Biology and Biochemistry. 2012;53:90–98. [Google Scholar]
- Roeckner E. World Data Center 714 for Climate. CERA-DB. 2013. IPCC DDC AR4 ECHAM5/MPI-OM SRESA1B run1.
- Russell-Smith J, Yates C, Edwards A, et al. Contemporary fire regimes of northern Australia, 1997-2001: change since Aboriginal occupancy, challenges for sustainable management. International Journal of Wildland Fire. 2003;12:283–297. [Google Scholar]
- Russell-Smith J, Yates CP, Whitehead PJ, et al. Bushfires “down under”: patterns and implications of contemporary Australian landscape burning. International Journal of Wildland Fire. 2007;16:361. [Google Scholar]
- Russell-Smith J, Murphy BP, Meyer CP, et al. Improving estimates of savanna burning emissions for greenhouse accounting in northern Australia: limitations, challenges, applications. International Journal of Wildland Fire. 2009;18:1–18. [Google Scholar]
- Russell-Smith J, Cook GD, Cooke PM, Edwards AC, Lendrum M, Meyer C(Mick), Whitehead PJ. Managing fire regimes in north Australian savannas: applying Aboriginal approaches to contemporary global problems. Frontiers in Ecology and the Environment. 2013;11:e55–e63. [Google Scholar]
- Russell-Smith J, Yates CP, Evans J, Meyer CP. Application of a “lower rainfall” savanna burning emissions abatement methodology. In: Murphy BP, Edwards AC, Meyer CP, Russell-Smith J, editors. Carbon Accounting and Management in Fire-Prone Australian Savannas. Melbourne, Vic., Australia: CSIRO Publications; 2014. (In press) [Google Scholar]
- Sankaran M, Ratnam J, Hanan NP. Tree-grass coexistence in savannas revisited – insights from an examination of assumptions and mechanisms invoked in existing models. Ecology Letters. 2004;7:480–490. [Google Scholar]
- Sankaran M, Hanan NP, Scholes RJ, et al. Determinants of woody cover in African savannas. Nature. 2005;438:846–849. doi: 10.1038/nature04070. [DOI] [PubMed] [Google Scholar]
- Scheiter S, Higgins SI. Impacts of climate change on the vegetation of Africa: an adaptive dynamic vegetation modelling approach. Global Change Biology. 2009;15:2224–2246. [Google Scholar]
- Scheiter S, Higgins SI, Beringer J, Hutley LB. Climate change and long-term fire management impacts on Australian savanna. New Phytologist. 2014 doi: 10.1111/nph.13130. . (Accepted) [DOI] [PubMed] [Google Scholar]
- Schultz MG, Heil A, Hoelzemann JJ, et al. Global wildland fire emissions from 1960 to 2000. Global Biogeochemical Cycles. 2008;22:GB2002. [Google Scholar]
- Schulze ED, Luyssaert S, Ciais P, et al. Importance of methane and nitrous oxide for Europe's terrestrial greenhouse-gas balance. Nature Geoscience. 2009;2:842–850. [Google Scholar]
- Scott RL, Serrano-Ortiz P, Domingo F, Hamerlynck EP, Kowalski AS. Commonalities of carbon dioxide exchange in semiarid regions with monsoon and Mediterranean climates. Journal of Arid Environments. 2012a;84:71–79. [Google Scholar]
- Scott K, Setterfield SA, Douglas MM, Parr CL, Schatz J, Andersen AN. Does long-term fire exclusion in an Australian tropical savanna result in a biome shift? A test using the reintroduction of fire. Austral Ecology. 2012b;37:693–711. [Google Scholar]
- Seneviratne SI, Corti T, Davin EL, et al. Investigating soil moisture-climate interactions in a changing climate: a review. Earth-Science Reviews. 2010;99:125–161. [Google Scholar]
- Smith I. An assessment of recent trends in Australian rainfall. Australian Meteorological Magazine 2. 2004;53:167–173. [Google Scholar]
- Smith KA, Ball T, Conen F, Dobbie KE, Massheder J, Rey A. Exchange of greenhouse gases between soil and atmosphere: interactions of soil physical factors and biological processes. European Journal of Soil Science. 2003;54:779–791. [Google Scholar]
- Stiehl-Braun PA, Hartmann AA, Kandeler E, Buchmann N, Niklaus PA. Interactive effects of drought and N fertilization on the spatial distribution of methane assimilation in grassland soils. Global Change Biology. 2011;17:2629–2639. [Google Scholar]
- Tosca MG, Randerson JT, Zender CS, Flanner MG, Rasch PJ. Do biomass burning aerosols intensify drought in equatorial Asia during El Niño? Atmospheric Chemistry and Physics. 2010;10:3515–3528. [Google Scholar]
- Tosca MG, Randerson JT, Zender CS. Global impact of smoke aerosols from landscape fires on climate and the Hadley circulation. Atmospheric Chemistry and Physics. 2013;13:5227–5241. [Google Scholar]
- Townsend SA, Douglas MM. The effect of three fire regimes on stream water quality, water yield and export coefficients in a tropical savanna (northern Australia) Journal of Hydrology. 2000;229:118–137. [Google Scholar]
- Townsend SA, Douglas MM. The effect of a wildfire on stream water quality and catchment water yield in a tropical savanna excluded from fire for 10 years (Kakadu National Park, North Australia) Water Research. 2004;38:3051–3058. doi: 10.1016/j.watres.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Tummon F, Solmon F, Liousse C, Tadross M. Simulation of the direct and semidirect aerosol effects on the southern Africa regional climate during the biomass burning season. Journal of Geophysical Research. 2010;115:D19206. [Google Scholar]
- Van Der Werf GR, Randerson JT, Giglio L, et al. Global fire emissions and the contribution of deforestation, savanna, forest, agricultural, and peat fires (1997–2009) Atmospheric Chemistry and Physics. 2010;10:11707–11735. [Google Scholar]
- Von Fischer JC, Butters G, Duchateau PC, Thelwell RJ, Siller R. In situ measures of methanotroph activity in upland soils: a reaction-diffusion model and field observation of water stress. Journal of Geophysical Research. 2009;114:G01015. [Google Scholar]
- Wang YP, Law RM, Pak B. A global model of carbon, nitrogen and phosphorus cycles for the terrestrial biosphere. Biogeosciences. 2010;7:2261–2282. [Google Scholar]
- Wang YP, Kowalczyk E, Leuning R, et al. Diagnosing errors in a land surface model (CABLE) in the time and frequency domains. Journal of Geophysical Research. 2011;116:G01034. [Google Scholar]
- Ward DS, Kloster S, Mahowald NM, Rogers BM, Randerson JT, Hess PG. The changing radiative forcing of fires: global model estimates for past, present and future. Atmospheric Chemistry and Physics. 2012;12:10857–10886. [Google Scholar]
- Wendt CK, Beringer J, Tapper NJ, Hutley LB. Local boundary-layer development over burnt and unburnt tropical savanna: an observational study. Boundary-Layer Meteorology. 2007;124:291–304. [Google Scholar]
- Williams RJ, Douglas M. Windthrow in a Tropical savanna in Kakadu National Park, northern Australia. Journal of Tropical Ecology. 1995;11:547–558. [Google Scholar]
- Williams RJ, Duff GA, Bowman DMJS, Cook GD. Variation in the composition and structure of tropical savannas as a function of rainfall and soil texture along a large-scale climatic gradient in the Northern Territory, Australia. Journal of Biogeography. 1996;23:747–756. [Google Scholar]
- Williams R, Myers B, Muller W, Duff G, Eamus D. Leaf phenology of woody species in a north Australian tropical savanna. Ecology. 1997;78:2542–2558. [Google Scholar]
- Williams RJ, Gill AM, Moore PHR. Seasonal changes in fire behaviour in a tropical Savanna in Northern Australia. International Journal of Wildland Fire. 1998;8:227–239. [Google Scholar]
- Williams RJ, Cook GD, Gill AM, Moore PHR. Fire regime, fire intensity and tree survival in a tropical savanna in northern Australia. Austral Ecology. 1999;24:50–59. [Google Scholar]
- Williams RJ, Hutley LB, Cook GD, Russell-Smith J, Edwards A, Chen X. Assessing the carbon sequestration potential of mesic savannas in Northern Territory, Australia: approaches, uncertainties and potential impacts of fire. Functional Plant Biology. 2004;31:415–422. doi: 10.1071/FP03215. [DOI] [PubMed] [Google Scholar]
- Witt GB, Harrington RA, Page MJ. Is “vegetation thickening” occurring in Queensland's mulga lands? A 50-year aerial photographic analysis. Australian Journal of Botany. 2009;57:572. [Google Scholar]
- Yates CP, Edwards AC, Russell-Smith J. Big fires and their ecological impacts in Australian savannas: size and frequency matters. International Journal of Wildland Fire. 2008;17:768–781. [Google Scholar]
- Zepp RG, Miller WL, Burke RA, Parsons DAB, Scholes MC. Effects of moisture and burning on soil-atmosphere exchange of trace carbon gases in a southern African savanna. Journal of Geophysical Research. 1996;101:23699. [Google Scholar]



