Abstract
Background
β2‐Microglobulin and cystatin C may have advantages over creatinine in assessing risk associated with kidney function. We therefore investigated whether emerging filtration markers, β2‐microglobulin and cystatin C, are prospectively associated with risk of the development of peripheral artery disease (PAD).
Methods and Results
We conducted nested case‐control studies among women within the Nurses’ Health Study (1990–2010) and among men within the Health Professionals Follow‐up Study (1994–2008) with the use of archived blood samples collected before PAD diagnosis. During follow‐up, symptomatic PAD was confirmed in 144 women and 143 men. Controls were matched 3:1 based on age, race, smoking status, fasting status, and date of blood sampling. Conditional logistic regression models were used to estimate relative risks (RRs) and were adjusted for plasma creatinine and cardiovascular risk factors. In women, the RRs (95% CI) per 1‐SD) increment were 1.16 (0.85 to 1.58) for β2‐microglobulin and 0.94 (0.69 to 1.28) for cystatin C. Corresponding RRs in men were 1.50 (1.08 to 2.09) for β2‐microglobulin and 1.54 (1.07 to 2.22) for cystatin C. There was no association between creatinine and PAD risk in women, whereas the association in men (RR 1.41, 95% CI 1.10 to 1.81) disappeared after adjustment for either β2‐microglobulin or cystatin C. In pooled analyses of men and women, only β2‐microglobulin was associated with PAD risk (RR 1.31, 95% CI 1.04 to 1.64).
Conclusions
In pooled analyses, β2‐microglobulin was associated with an increased risk of symptomatic PAD; a similar association with cystatin C was observed only in men. The findings suggest that β2‐microglobulin may capture the atherosclerosis‐promoting or atherosclerosis‐related elements of kidney dysfunction better than creatinine.
Keywords: creatinine, cystatin C, kidney, peripheral artery disease, β2‐microglobulin
Introduction
An estimated 10 million US adults have peripheral artery disease (PAD),1 a manifestation of systemic atherosclerosis that has been linked to reduced functional capacity2 and increased risk of cardiovascular morbidity and mortality.3–4 Preventable or treatable risk factors in the initiation and development of PAD include cigarette smoking, type 2 diabetes, hypertension, and hypercholesterolemia, which together may account for ≈75% of the excess PAD risk.5 Besides these “traditional” risk factors, kidney function has also been associated with incident PAD risk in a number of studies6–7 and is recognized as a PAD risk factor.8
The clinical standard for the assessment of kidney function is the creatinine‐based estimated glomerular filtration rate (eGFR).9 However, creatinine (molecular weight of 113 Da) is a byproduct of muscle breakdown and tends to overestimate the eGFR in individuals with lower creatinine generation due to loss of muscle mass (such as those with PAD).10–11 In addition, renal tubules actively secrete creatinine,12 further reducing the value of creatinine to estimate GFR.
Both β2‐microglobulin, a subunit of the major histocompatibility class I complex, and cystatin C, a cysteine protease inhibitor, have been proposed as alternative markers for the detection of renal impairment.13–14 Owing to their relatively low molecular weight compared with, for example, albumin (66 kDa), β2‐microglobulin (12 kDa) and cystatin C (13 kDa) pass freely through the glomerular filtration barrier.15–16 Furthermore, these filtration markers are produced at a relatively constant rate and are neither affected by muscle mass nor subject to tubular secretion.17–18
In previous observational studies, both cystatin C and β2‐microglobulin have been shown to predict cardiovascular events and mortality more strongly than creatinine.19–22 However, evidence for an association between cystatin C and future risk of PAD is sparse and conflicting.23–24 Also, although circulating β2‐microglobulin has previously been identified as a factor strongly linked to the presence and severity of PAD in cross‐sectional studies,25–26 there are no studies that have prospectively examined this association.
We, therefore, investigated the associations between 3 markers of kidney function (ie, β2‐microglobulin, cystatin C, and creatinine) with future risk of the development of PAD in 2 large and well‐characterized cohorts of men and women.
Methods
Study Population
The Nurses’ Health Study (NHS) is a prospective cohort study of 121 701 female nurses aged 30 to 55 years at recruitment in 1976. The Health Professionals Follow‐up Study (HPFS) is a prospective cohort study of 51 529 male dentists, optometrists, pharmacists, podiatrists, osteopathic physicians, and veterinarians aged 40 to 75 years at recruitment in 1986. Of these individuals, 32 826 women provided a blood sample in 1990 and 18 224 men provided blood specimens in 1994. We excluded individuals who had a history of cardiovascular disease, including myocardial infarction; surgical/percutaneous revascularization of the coronary, carotid, or peripheral beds; confirmed PAD; stroke; and transient ischemic attack.
The case‐control analytic datasets include all incident symptomatic PAD cases, for a total of 144 cases and 432 controls in the NHS and 143 cases and 429 controls in the HPFS. Controls were matched 3:1 to cases on age, race, smoking history, month of blood draw (within 3 months), and fasting status, following the nested case‐control design described by Prentice and Breslow.27 We selected controls at random, conditional on the matching factors, from participants free of cardiovascular diseases at the time the incident case occurred (risk set sampling). We restricted all cases and controls to those free of cardiovascular diseases at baseline to ensure identification of only incident cases. This included angina, myocardial infarction, surgical/percutaneous revascularization of the coronary, carotid, or peripheral beds, claudication, stroke, and transient ischemic attack, which are all assessed biennially. Because older age categories had fewer participants, we relaxed the age match range year‐by‐year if necessary to a maximum of within 3 years. The institutional review boards of the Brigham and Women's Hospital, Harvard School of Public Health, and Beth Israel Deaconess Medical Center approved the study protocol. Voluntary responses to mailed questionnaires served as the participants’ informed consent.
Handling of Blood Samples and Measurement of Biochemical Variables
Blood samples were shipped overnight with a cold pack to the central laboratory, centrifuged on arrival, aliquotted, and stored in liquid nitrogen at −130°C to −196°C. NHS specimens were anticoagulated with heparin, and HPFS were anticoagulated with EDTA. Of all samples, 97% of those from the NHS and 95% of those from the HPFS were received within 24 hours; cystatin C and creatinine levels are known to remain stable in whole blood even when stored at room temperature up to 48 hours before separation.28 All analyses were performed in a laboratory certified by the Centers for Disease Control and Prevention/National Heart, Lung, and Blood Institute Lipid Standardization Program with commercially available analytic systems. Measurements of β2‐microglobulin, cystatin C, and creatinine were performed on the Roche P Modular system (Roche Diagnostics) via an immunoturbidimetric technique (β2‐microglobulin and cystatin C) or enzymatic method (creatinine), using reagents and calibrators from Roche (β2‐microglobulin and creatinine) or from Genzyme (cystatin C). Interassay coefficients of variation for β2‐microglobulin, cystatin C, and creatinine were 5.9%, 3.0%, and 2.2% for NHS and 5.8%, 8.1%, and 6.5% for HPFS, respectively. The determination of high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, high‐sensitivity C‐reactive protein (CRP), triglycerides, and hemoglobin A1c has been described in greater detail elsewhere.29
Ascertainment of PAD
Participants reported the occurrence of diagnosed medical conditions, including claudication and revascularization for arterial disease of the leg, during the previous 2 years on biennial mailed questionnaires. We collected medical records from treating physicians and hospitals for those who reported either condition. Blinded professionals reviewed and confirmed PAD diagnoses using medical records.
We defined PAD as arterial disease below the aortic bifurcation (ie, excluding abdominal aortic aneurysm and renal artery stenosis). Confirmed PAD required ≥1 of the following (in order of severity/certainty) in the medical record: (1) report of amputation, bypass, or other revascularization procedure for occlusive artery disease, (2) angiogram showing ≥50% stenosis of ≥1 artery with congruent symptoms in the ipsilateral limb, (3) ankle‐brachial index <0.90 at rest, or (4) physician's diagnosis.
Assessment of Covariables
Questionnaires provided information about anthropometric data and lifestyle, including weight, detailed information about smoking, parental history of myocardial infarction, alcohol consumption, physical activity per week, medical history concerning medication use, and self‐reported history of diabetes, hypertension, and hypercholesterolemia. Participants reported past smoking, time since quitting, and the average number of cigarettes smoked per day. Pack‐years were calculated as years of smoking multiplied by the average number of packs smoked per day and updated biennially. We calculated body mass index (BMI) by dividing weight (kg) by squared height (m2). Physical activity was expressed as metabolic equivalent task‐hours based on self‐reported types and durations of activities over the previous year. Previous studies demonstrated that the validity and reproducibility of all these self‐reported measures are high.30–34 We calculated the eGFR based both on the creatinine‐based Chronic Kidney Disease Epidemiology Collaboration (eGFRCKD‐EPI) equation35 and on a more recent, combined creatinine–cystatin C (eGFRCREAT‐CYS) equation.36
Statistical Analyses
Continuous data are summarized as either mean±SD for normally distributed variables or median and interquartile range for non‐normally distributed variables. Categorical data are expressed as percentages. We used generalized linear mixed models and Cochran–Mantel–Haenszel tests to compare continuous variables and categorical variables by case‐control status, respectively, accounting for clustering by matching status. Associations between β2‐microglobulin, cystatin C, and creatinine and PAD risk factors were examined using age‐adjusted Spearman partial correlation coefficients.
To examine the prospective association of β2‐microglobulin, cystatin C, and creatinine with subsequent PAD risk, we treated these markers as both a categorical (in quartiles) and a continuous (per 1‐SD increase in log‐transformed marker) variable. Log‐transformation of the markers led to a superior model fit. To test for a linear trend in the categorical analysis, we treated the natural log‐transformed quartile median as a linear variable. We also examined the possibility of nonlinear associations between the 3 biomarkers and PAD nonparametrically with restricted cubic splines.37 In tests for nonlinearity, we used the likelihood ratio test, comparing the model with only the linear term to the model with the linear and the cubic spline terms. We used conditional logistic regression to estimate the odds ratio (OR) and 95% CI for PAD associated with increasing concentrations of these markers. Because the controls were selected using risk set sampling, the ORs derived from conditional logistic regression provide unbiased estimates of the incidence rate ratio as a measure of relative risk (RR). We included covariables in our models as linear variables if appropriate or as categorical if they were discrete or their association with PAD was nonlinear. Adjustment for smoking intensity did not provide further information after accounting for pack‐years. Analyses that defined hypertension based on either a physician's diagnosis or a patient's antihypertensive medication use (thiazides, β‐blockers, calcium channel blockers, and angiotensin‐converting enzyme or angiotensin II receptor antagonists) yielded similar results.
To pool the RR estimates for women and men, we used meta‐analyses with fixed effects or random effects when the test for heterogeneity was significant. We tested interactions with cross‐product terms between (continuous) log‐transformed concentrations of the kidney function markers and selected variables in multivariable‐adjusted conditional or unconditional logistic regression models with additional adjustment for the matching factors. All P‐values presented are 2‐tailed, and P‐values <0.05 were considered statistically significant. All analyses were performed using SAS statistical software (version 9.3; SAS Institute).
Results
Baseline characteristics of PAD cases and controls are shown in Table 1. As expected, both female and male cases had higher levels of CRP, triglycerides, and hemoglobin A1c and were more likely to have a history of diabetes, hypertension, or hypercholesterolemia than were matched controls. In men, β2‐microglobulin and cystatin C levels were significantly higher among cases than among controls, but in women, β2‐microglobulin and cystatin C levels did not differ between cases and controls. Creatinine was higher in male cases but lower in female cases compared to their matched controls. Based on eGFR categories of the Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline,9 66% of the women and 50% of the men had a normal or high kidney function (eGFRCKD‐EPI ≥90 mL min−1 per 1.73 m−2) and 33% and 46%, respectively, had a mildly reduced kidney function (eGFRCKD‐EPI between 60 and 89 mL min−1 per 1.73 m−2).
Table 1.
Baseline Characteristics of Women and Men Who Developed Symptomatic Peripheral Artery Disease During Follow‐up (Cases) and Matched Controls
| Characteristics | Women | Men | ||||
|---|---|---|---|---|---|---|
| Cases | Controls | P Value | Cases | Controls | P Value | |
| N | 144 | 431 | 143 | 429 | ||
| Age, y | 60.0±5.1 | 60.0±5.2 | Matched | 65.4±8.1 | 65.3±8.1 | Matched |
| Ethnicity, nonblack | 144 (100%) | 431 (100%) | Matched | 142 (99%) | 429 (100%) | Matched |
| Smoking status, n | ||||||
| Never | 30 (21%) | 90 (21%) | Matched | 23 (16%) | 82 (19%) | Matched |
| Former | 56 (39%) | 169 (39%) | 78 (55%) | 242 (56%) | ||
| Current | 58 (40%) | 172 (40%) | 32 (22%) | 90 (21%) | ||
| β2‐Microglobulin, mg/L | 1.91 (1.72 to 2.21) | 1.89 (1.65 to 2.17) | 0.28 | 1.99 (1.74 to 2.35) | 1.80 (1.59 to 2.06) | <0.001 |
| Cystatin C, mg/L | 0.95 (0.85 to 1.06) | 0.92 (0.84 to 1.04) | 0.50 | 1.04 (0.91 to 1.22) | 0.96 (0.86 to 1.07) | <0.001 |
| Creatinine, mg/dL | 0.67±0.12 | 0.70±0.12 | 0.04 | 0.92±0.20 | 0.88±0.19 | 0.04 |
| eGFR, mL min−1 per 1.73 m−2* | 93±12 | 91±12 | 0.05 | 85±15 | 88±13 | 0.01 |
| C‐reactive protein, mg/L | 2.56 (1.25 to 4.70) | 1.62 (0.73 to 3.33) | 0.07 | 2.24 (1.18 to 3.52) | 1.18 (0.51 to 2.26) | 0.005 |
| HDL cholesterol, mg/dL | 60.5±19.9 | 62.1±17.1 | 0.42 | 41.7±11.5 | 48.5±14.2 | <0.001 |
| LDL cholesterol, mg/dL | 148±44 | 142±38 | 0.16 | 139±35 | 131±33 | 0.02 |
| Triglycerides, mg/dL | 110 (85 to 161) | 106 (73 to 145) | 0.34 | 143 (105 to 195) | 115 (80 to 165) | 0.001 |
| Hemoglobin A1c, % | 5.48 (5.28 to 5.72) | 5.33 (5.17 to 5.55) | <0.001 | 5.56 (5.34 to 5.96) | 5.41 (5.24 to 5.59) | <0.001 |
| Pack‐years of smoking, y | 34 (5 to 50) | 18 (2 to 37) | <0.001 | 26 (5 to 45) | 18 (1 to 35) | <0.001 |
| History of type 2 diabetes, n | 19 (13%) | 12 (3%) | <0.001 | 28 (20%) | 16 (4%) | <0.001 |
| History of hypertension, n | 68 (47%) | 137 (32%) | <0.001 | 70 (49%) | 130 (30%) | <0.001 |
| History of hypercholesterolemia, n | 84 (58%) | 200 (46%) | 0.01 | 82 (57%) | 187 (44%) | 0.005 |
| Parental history of myocardial infarction before age 60 y, n | 31 (22%) | 61 (14%) | 0.03 | 22 (15%) | 44 (10%) | 0.09 |
| Body mass index, kg/m2 | 25.3±4.5 | 24.8±4.0 | 0.20 | 25.8±3.3 | 25.6±4.4 | 0.41 |
| Physical activity, MET‐h per week | 12.8 (4.7 to 24.9) | 13.5 (4.9 to 27.9) | 0.78 | 22.7 (8.0 to 43.8) | 27.4 (10.3 to 52.8) | 0.003 |
| Alcohol consumption, g/d | 1.9 (0 to 11.0) | 2.1 (0 to 9.9) | 0.92 | 7.6 (0.9 to 17.8) | 9.8 (1.8 to 20.3) | 0.48 |
Matching factors, besides age, race, and smoking status, included date of blood draw and fasting status. Data are expressed as mean (SD), median (interquartile range), or number (percentage). P values are derived from mixed linear models for continuous variables and Cochran–Mantel–Haenszel tests for categorical variables controlling for matched sets. eGFR indicates estimated glomerular filtration rate; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; MET‐h, metabolic equivalent hours.
eGFR was calculated according to the Chronic Kidney Disease Epidemiology Collaboration equation.
Cystatin C and β2‐microglobulin were strongly correlated with each other (ρ=0.74, P<0.001 for women; ρ=0.77, P<0.001 for men) (Table 2) and highly correlated with creatinine, although not as strongly as with each other. Both β2‐microglobulin and cystatin C were correlated with age and modestly with BMI, CRP, and triglycerides but not with pack‐years of smoking in both men and women. Besides an inverse correlation with pack‐years of smoking, no consistent correlations were observed between creatinine and cardiovascular risk factors in men and women. Both unadjusted Spearman's rank correlation coefficients and Pearson product‐moment correlation coefficients are shown in Table 3.
Table 2.
Spearman Partial Correlation Coefficients Between β2‐Microglobulin, Cystatin C, and Creatinine and Selected Cardiovascular Risk Factors Among 431 Control Women and 429 Control Men
| Women | Men | |||||
|---|---|---|---|---|---|---|
| β2‐Microglobulin | Cystatin C | Creatinine | β2‐Microglobulin | Cystatin C | Creatinine | |
| β2‐Microglobulin | 1.00 | 1.00 | ||||
| Cystatin C | 0.74‡ | 1.00 | 0.77‡ | 1.00 | ||
| Creatinine | 0.37‡ | 0.46‡ | 1.00 | 0.44‡ | 0.50‡ | 1.00 |
| C‐reactive protein | 0.19‡ | 0.18‡ | −0.01 | 0.24‡ | 0.21‡ | −0.02 |
| HDL cholesterol | −0.17‡ | −0.24‡ | 0.01 | −0.10* | −0.07 | 0.03 |
| LDL cholesterol | 0.10* | 0.16‡ | 0.09 | −0.04 | 0.00 | 0.05 |
| Triglycerides | 0.16‡ | 0.20‡ | 0.12* | 0.13† | 0.17‡ | 0.08 |
| Hemoglobin A1c | 0.11* | −0.00 | −0.06 | −0.06 | −0.01 | −0.05 |
| Body mass index | 0.14† | 0.24‡ | 0.05 | 0.16† | 0.18‡ | 0.06 |
| Physical activity | −0.10* | −0.13† | 0.02 | −0.07 | −0.06 | 0.01 |
| Pack‐years of smoking | 0.00 | 0.10* | −0.15† | 0.04 | 0.07 | −0.08 |
| Age | 0.28‡ | 0.23‡ | 0.09 | 0.45‡ | 0.39‡ | 0.14† |
Correlations are age‐adjusted except age. HDL indicates high‐density lipoprotein; LDL, low‐density lipoprotein.
*P<0.05, †P<0.01, ‡P<0.001.
Table 3.
Spearman's Rank Correlation Coefficients and Pearson Product‐Moment Correlation Coefficients Between β2‐Microglobulin, Cystatin C, and Creatinine and Selected Cardiovascular Risk Factors Among 431 Control Women and 429 Control Men
| Spearman | Pearson | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Women | Men | Women | Men | |||||||||
| β2‐Microglobulin | Cystatin C | Creatinine | β2‐Microglobulin | Cystatin C | Creatinine | β2‐Microglobulin | Cystatin C | Creatinine | β2‐Microglobulin | Cystatin C | Creatinine | |
| β2‐Microglobulin | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| Cystatin C | 0.76‡ | 1.00 | 0.81‡ | 1.00 | 0.76‡ | 1.00 | 0.87‡ | 1.00 | ||||
| Creatinine | 0.38‡ | 0.47‡ | 1.00 | 0.45‡ | 0.51‡ | 1.00 | 0.41‡ | 0.50‡ | 1.00 | 0.71‡ | 0.73‡ | 1.00 |
| C‐reactive protein | 0.20‡ | 0.19‡ | −0.00 | 0.28‡ | 0.25‡ | −0.00 | 0.09 | 0.06 | −0.03 | 0.28‡ | 0.20‡ | 0.11* |
| HDL cholesterol | −0.15† | −0.22‡ | 0.01 | −0.07 | −0.05 | 0.03 | −0.11* | −0.17‡ | 0.03 | −0.06 | −0.04 | 0.01 |
| LDL cholesterol | 0.12† | 0.18‡ | 0.10* | −0.04 | −0.00 | 0.04 | 0.10* | 0.16‡ | 0.09 | 0.04 | 0.01 | 0.06 |
| Triglycerides | 0.16‡ | 0.20‡ | 0.12* | 0.10* | 0.14† | 0.05 | 0.09 | 0.14† | 0.09 | 0.08 | 0.10* | 0.04 |
| Hemoglobin A1c | 0.16† | 0.05 | −0.04 | −0.01 | 0.03 | −0.04 | 0.13† | 0.06 | −0.04 | −0.04 | −0.04 | −0.11* |
| Body mass index | 0.14† | 0.24‡ | 0.05 | 0.11* | 0.14‡ | 0.06 | 0.17‡ | 0.24‡ | 0.06 | 0.02 | 0.01 | −0.02 |
| Physical activity | −0.08 | −0.11* | 0.03 | −0.09 | −0.07 | 0.01 | −0.12* | −0.15† | −0.02 | −0.04 | −0.04 | −0.00 |
| Pack‐years of smoking | −0.03 | 0.106 | −0.16‡ | 0.04 | 0.06 | −0.08 | −0.03 | 0.07 | −0.16‡ | −0.02 | 0.04 | −0.08 |
| Age | 0.28‡ | 0.23‡ | 0.09 | 0.45‡ | 0.39‡ | 0.14† | 0.30‡ | 0.25‡ | 0.09 | 0.33‡ | 0.35‡ | 0.17 |
HDL indicates high‐density lipoprotein; LDL, low‐density lipoprotein.
*P<0.05, †P<0.01, ‡P<0.001.
During a median follow‐up of 15 years (interquartile range 11 to 17 years), PAD was detected in 144 women, and during a median follow‐up of 7 years (interquartile range 4 to 10 years) PAD was detected in 143 men. Of the 144 PAD cases among women, 74 (51%) cases were confirmed by medical records indicating surgery or revascularization procedures, 24 (17%) cases by angiogram, 39 (27%) cases by ankle‐brachial index, and 7 (5%) cases by physician diagnosis. Of the 143 PAD cases among men, medical reports of amputation, bypass, or other revascularization procedure confirmed 87 (61%) PAD cases; angiogram or Doppler ultrasound, 15 (10%) cases; ankle‐brachial index <0.90, (16%) 23 cases; and physician diagnosis, 18 (13%) cases.
Because there was no evidence for nonlinear associations between markers of kidney function and future risk of PAD, we conducted analyses per 1‐SD increase in log‐transformed markers. Higher circulating levels of β2‐microglobulin, cystatin C, and creatinine were associated with increased risk of symptomatic PAD in men but not in women (Table 4). RRs among women tended to be higher for β2‐microglobulin than the other 2 measures, and the difference between men and women was significant for cystatin C but not for β2‐microglobulin (Figure). Although expected given the limited variation in age, sex, and ethnicity (the main determinants of eGFRCKD‐EPI besides creatinine) between cases and matched controls, the associations with risk of PAD were similar if kidney function was estimated using the eGFRCKD‐EPI formula rather than using circulating creatinine (Table 5). Associations were also similar when we modeled the inverse of β2‐microglobulin or cystatin C (Table 5), as surrogate of eGFR, compared with the log‐transformed values of β2‐microglobulin or cystatin C, respectively.
Table 4.
Relative Risks for Peripheral Artery Disease According to β2‐Microglobulin, Cystatin C, and Creatinine in Women and Men
| Kidney Function Marker | Continuous Biomarker, per 1‐SD Increment | Quartiles of Biomarker, Median (Range) | P Trend* | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| Women | ||||||
| β2‐Microglobulin, mg/L | 0.21 | 1.51 (1.05 to 1.67) | 1.78 (1.68 to 1.89) | 2.01 (1.90 to 2.16) | 2.39 (2.17 to 3.87) | |
| No. cases/controls | 144/431 | 27/114 | 40/109 | 40/101 | 37/106 | |
| Model 1 (matching factors)* | 1.15 (0.93 to 1.42) | 1.00 (Reference) | 1.59 (0.91 to 2.78) | 1.75 (0.99 to 3.10) | 1.60 (0.87 to 2.93) | 0.14 |
| Model 2 (multivariable)* | 1.06 (0.80 to 1.40) | 1.00 (Reference) | 2.12 (1.05 to 4.28) | 2.16 (1.04 to 4.49) | 1.41 (0.66 to 3.05) | 0.54 |
| Cystatin C, mg/L | 0.17 | 0.77 (0.49 to 0.83) | 0.89 (0.84 to 0.92) | 1.00 (0.93 to 1.04) | 1.12 (1.05 to 1.75) | |
| No. cases/controls | 144/431 | 35/120 | 30/98 | 42/115 | 37/98 | |
| Model 1 (matching factors)* | 1.08 (0.89 to 1.31) | 1.00 (Reference) | 1.04 (0.60 to 1.81) | 1.27 (0.76 to 2.14) | 1.32 (0.77 to 2.26) | 0.26 |
| Model 2 (multivariable)* | 0.89 (0.69 to 1.16) | 1.00 (Reference) | 0.70 (0.34 to 1.42) | 0.85 (0.45 to 1.60) | 0.72 (0.36 to 1.44) | 0.51 |
| Creatinine, mg/dL | 0.17 | 0.56 (0.35 to 0.60) | 0.64 (0.61 to 0.67) | 0.71 (0.68 to 0.74) | 0.81 (0.75 to 1.40) | |
| No. cases/controls | 144/431 | 44/97 | 30/99 | 41/121 | 28/114 | |
| Model 1 (matching factors)* | 0.80 (0.66 to 0.97) | 1.00 (Reference) | 0.64 (0.37 to 1.11) | 0.71 (0.42 to 1.18) | 0.52 (0.30 to 0.91) | 0.04 |
| Model 2 (multivariable)* | 0.89 (0.70 to 1.12) | 1.00 (Reference) | 0.80 (0.41 to 1.59) | 0.88 (0.46 to 1.68) | 0.63 (0.32 to 1.27) | 0.25 |
| Men | ||||||
| β2‐Microglobulin, mg/L | 0.22 | 1.49 (1.18 to 1.61) | 1.73 (1.62 to 1.83) | 1.97 (1.84 to 2.11) | 2.41 (2.12 to 10.24) | |
| No. cases/controls | 143/429 | 24/114 | 27/118 | 41/104 | 51/92 | |
| Model 1 (matching factors)* | 1.66 (1.35 to 2.05) | 1.00 (Reference) | 1.14 (0.62 to 2.10) | 2.25 (1.23 to 4.14) | 3.36 (1.83 to 6.19) | <0.001 |
| Model 2 (multivariable)* | 1.61 (1.23 to 2.09) | 1.00 (Reference) | 1.28 (0.58 to 2.82) | 1.92 (0.87 to 4.28) | 2.85 (1.24 to 6.56) | 0.008 |
| Cystatin C, mg/L | 0.19 | 0.81 (0.63 to 0.87) | 0.92 (0.88 to 0.97) | 1.03 (0.98 to 1.09) | 1.21 (1.10 to 3.22) | |
| No. cases/controls | 143/429 | 20/116 | 39/123 | 27/96 | 57/94 | |
| Model 1 (matching factors)* | 1.65 (1.34 to 2.05) | 1.00 (Reference) | 1.97 (1.06 to 3.65) | 1.78 (0.92 to 3.46) | 4.02 (2.18 to 7.41) | <0.001 |
| Model 2 (multivariable)* | 1.63 (1.24 to 2.15) | 1.00 (Reference) | 1.47 (0.69 to 3.14) | 1.48 (0.67 to 3.29) | 3.00 (1.40 to 6.43) | 0.004 |
| Creatinine, mg/dL | 0.18 | 0.71 (0.49 to 0.77) | 0.82 (0.78 to 0.86) | 0.91 (0.87 to 0.96) | 1.05 (0.97 to 3.04) | |
| No. cases/controls | 143/429 | 33/118 | 33/109 | 28/117 | 49/85 | |
| Model 1 (matching factors)* | 1.23 (1.03 to 1.48) | 1.00 (Reference) | 1.11 (0.63 to 1.95) | 0.87 (0.50 to 1.53) | 2.10 (1.23 to 3.58) | 0.01 |
| Model 2 (multivariable)* | 1.41 (1.10 to 1.81) | 1.00 (Reference) | 1.70 (0.81 to 3.59) | 1.15 (0.56 to 2.39) | 3.28 (1.58 to 6.82) | 0.004 |
HDL indicates high‐density lipoprotein; LDL, low‐density lipoprotein.
P for trend based on median plasma concentrations of quartiles.
Matching factors included age, race, smoking status, month of blood draw, and fasting status.
Adjusted for C‐reactive protein, HDL cholesterol, LDL cholesterol, hemoglobin A1c, triglycerides, pack‐years of smoking, type 2 diabetes, hypertension, hypercholesterolemia, parental history of myocardial infarction before age 60 years, body mass index, physical activity, and alcohol consumption.
Figure 1.
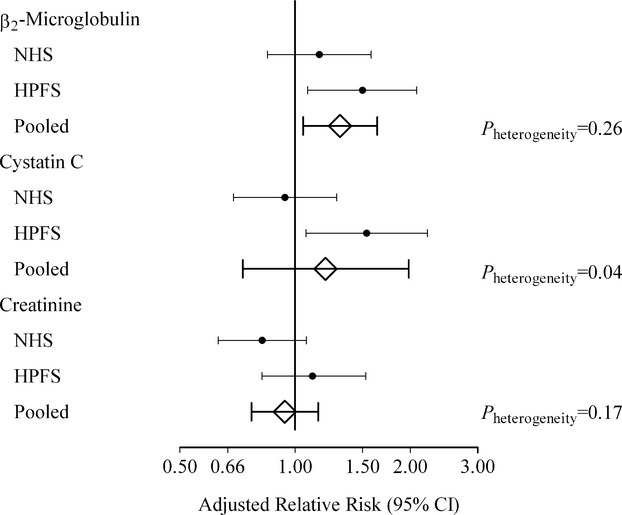
Adjusted relative risks (95% CI) for peripheral artery disease per 1‐SD increment in β2‐microglobulin, cystatin C, and creatinine. Relative risks are adjusted for C‐reactive protein; HDL and LDL cholesterol; hemoglobin A1c; triglycerides; creatinine (except creatinine, which is adjusted for β2‐microglobulin instead); pack‐years of smoking; history of type 2 diabetes, hypertension, and hypercholesterolemia; parental history of myocardial infarction before age 60 years; body mass index; physical activity; and alcohol consumption. Matching factors are age, race, smoking status, month of blood draw, and fasting status. To calculate the pooled estimates, we used meta‐analyses with fixed effects or random effects in case of significant heterogeneity (P<0.05). HDL indicates high‐density lipoprotein; HPFS, Health Professionals Follow‐up Study; LDL, low‐density lipoprotein; NHS, Nurses’ Health Study.
Table 5.
Relative Risks for Peripheral Artery Disease According to eGFRCKD‐EPI, eGFRCREAT‐CYS, Inverse of β2‐Microglobulin, and Inverse of Cystatin C in Women and Men
| Continuous, per 1‐SD Decrement | Quartiles, Median (Range) | P Trend* | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| Women | ||||||
| eGFRCKD‐EPI, mL·min−1·1.73 m−2 | 12 | 104 (100 to 121) | 97 (94 to 100) | 92 (86 to 94) | 77 (41 to 85) | |
| No. cases/controls | 144/431 | 39/105 | 37/106 | 44/101 | 24/119 | |
| Model 1 (matching factors)* | 0.81 (0.65 to 1.01) | 1.00 (Reference) | 0.88 (0.48 to 1.60) | 1.12 (0.61 to 2.04) | 0.51 (0.27 to 0.98) | 0.03 |
| Model 2 (multivariable)* | 0.87 (0.67 to 1.12) | 1.00 (Reference) | 1.02 (0.47 to 2.21) | 1.49 (0.70 to 3.16) | 0.62 (0.28 to 1.41) | 0.16 |
| eGFRCREAT‐CYS, mL·min−1·1.73 m−2 | 11 | 103 (97 to 125) | 94 (91 to 97) | 87 (83 to 91) | 78 (53 to 83) | |
| No. cases/controls | 144/431 | 39/105 | 37/106 | 44/101 | 24/119 | |
| Model 1 (matching factors)* | 0.92 (0.75 to 1.13) | 1.00 (Reference) | 0.79 (0.45 to 1.39) | 0.91 (0.52 to 1.57) | 0.85 (0.48 to 1.50) | 0.63 |
| Model 2 (multivariable)* | 0.86 (0.66 to 1.12) | 1.00 (Reference) | 0.63 0.30 to 1.33) | 0.78 (0.38 to 1.61) | 0.60 (0.29 to 1.27) | 0.27 |
| 1/β2‐microglobulin, L/mg | 0.11 | 0.66 (0.60 to 0.95) | 0.56 (0.53 to 0.59) | 0.50 (0.46 to 0.53) | 0.42 (0.26 to 0.46) | |
| No. cases/controls | 144/431 | 29/118 | 37/99 | 40/103 | 38/111 | |
| Model 1 (matching factors)* | 1.18 (0.94 to 1.47) | 1.00 (Reference) | 1.55 (0.88 to 2.72) | 1.63 (0.93 to 2.86) | 1.48 (0.82 to 2.68) | 0.21 |
| Model 2 (multivariable)* | 1.09 (0.82 to 1.45) | 1.00 (Reference) | 2.16 (1.04 to 4.46) | 2.17 (1.05 to 4.48) | 1.26 (0.59 to 2.69) | 0.59 |
| 1/cystatin C, L/mg | 0.19 | 1.31 (1.20 to 2.04) | 1.12 (1.08 to 1.19) | 1.00 (0.96 to 1.06) | 0.89 (0.57 to 0.95) | |
| No. cases/controls | 144/431 | 34/106 | 36/122 | 32/96 | 42/107 | |
| Model 1 (matching factors)* | 1.09 (0.89 to 1.33) | 1.00 (Reference) | 0.92 (0.54 to 1.58) | 1.06 (0.60 to 1.86) | 1.24 (0.73 to 2.12) | 0.41 |
| Model 2 (multivariable)* | 0.90 (0.69 to 1.17) | 1.00 (Reference) | 0.62 (0.31 to 1.25) | 0.75 (0.38 to 1.50) | 0.61 (0.30 to 1.25) | 0.26 |
| Men | ||||||
| eGFRCKD‐EPI, mL·min−1·1.73 m−2 | 13 | 101 (96 to 117) | 92 (90 to 96) | 86 (81 to 90) | 72 (19 to 80) | |
| No. cases/controls | 143/429 | 36/107 | 28/114 | 29/115 | 50/93 | |
| Model 1 (matching factors)* | 1.32 (1.08 to 1.61) | 1.00 (Reference) | 0.78 (0.43 to 1.42) | 0.81 (0.43 to 1.53) | 1.78 (0.97 to 3.26) | 0.01 |
| Model 2 (multivariable)* | 1.54 (1.17 to 2.02) | 1.00 (Reference) | 0.77 (0.36 to 1.66) | 0.95 (0.44 to 2.06) | 2.52 (1.16 to 5.48) | 0.005 |
| eGFRCREAT‐CYS, mL·min−1·1.73 m−2 | 15 | 101 (94 to 117) | 89 (84 to 94) | 80 (74 to 84) | 66 (16 to 74) | |
| No. cases/controls | 143/429 | 25/118 | 31/112 | 34/109 | 53/89 | |
| Model 1 (matching factors)* | 1.65 (1.32 to 2.05) | 1.00 (Reference) | 1.51 (0.81 to 2.80) | 1.85 (0.99 to 3.45) | 3.80 (2.02 to 7.16) | <0.001 |
| Model 2 (multivariable)* | 1.71 (1.28 to 2.29) | 1.00 (Reference) | 1.37 (0.62 to 3.04) | 1.39 (0.64 to 3.01) | 3.82 (1.69 to 8.66) | <0.001 |
| 1/β2‐microglobulin, L/mg | 0.11 | 0.67 (0.62 to 0.85) | 0.58 (0.55 to 0.61) | 0.51 (0.47 to 0.54) | 0.41 (0.10 to 0.47) | |
| No. cases/controls | 143/429 | 25/120 | 26/113 | 41/104 | 51/92 | |
| Model 1 (matching factors)* | 1.76 (1.41 to 2.20) | 1.00 (Reference) | 1.17 (0.63 to 2.17) | 2.28 (1.24 to 4.18) | 3.40 (1.85 to 6.26) | <0.001 |
| Model 2 (multivariable)* | 1.65 (1.24 to 2.20) | 1.00 (Reference) | 1.27 (0.56 to 2.87) | 1.91 (0.86 to 4.24) | 2.82 (1.23 to 6.47) | 0.008 |
| 1/cystatin C, L/mg | 0.18 | 1.24 (1.15 to 1.59) | 1.09 (1.03 to 1.14) | 0.97 (0.92 to 1.02) | 0.83 (0.31 to 0.91) | |
| No. cases/controls | 143/429 | 20/116 | 35/113 | 31/106 | 57/94 | |
| Model 1 (matching factors)* | 1.71 (1.37 to 2.14) | 1.00 (Reference) | 1.91 (1.02 to 3.59) | 1.86 (0.97 to 3.56) | 4.03 (2.19 to 7.43) | <0.001 |
| Model 2 (multivariable)* | 1.64 (1.23 to 2.17) | 1.00 (Reference) | 1.41 (0.65 to 3.09) | 1.54 (0.71 to 3.32) | 3.00 (1.40 to 6.43) | 0.004 |
CKD‐EPI indicates Chronic Kidney Disease Epidemiology Collaboration; CREAT‐CYS, creatinine–cystatin C; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
P for trend based on median of quartiles.
Matching factors included age, race, smoking status, month of blood draw, and fasting status.
Adjusted for HDL cholesterol, LDL cholesterol, C‐reactive protein, hemoglobin A1c, triglycerides, pack‐years of smoking, type 2 diabetes, hypertension, hypercholesterolemia, parental history of myocardial infarction before age 60 years, body mass index, physical activity, and alcohol consumption.
Although the RRs increased slightly after further accounting for circulating creatinine, β2‐microglobulin and cystatin C remained unassociated with PAD risk among women (Figure). For comparison, the corresponding multivariable‐adjusted RR for each 1‐SD increment in CRP was 1.48 (95% CI 1.14 to 1.94) in women and 1.39 (95% CI 1.08 to 1.79) in men. In exploratory analyses when models were mutually adjusted for both β2‐microglobulin and cystatin C, the RRs per each 1‐SD increment were 1.44 (95% CI 0.94 to 2.22) for β2‐microglobulin and 0.73 (0.48 to 1.12) for cystatin C in women and 1.31 (95% CI, 0.84 to 2.03) for β2‐microglobulin and 1.26 (0.77 to 2.06) for cystatin C in men. In further analyses, we found no interactions of β2‐microglobulin or cystatin C with creatinine, conventional cardiovascular risk factors, or duration of follow‐up in women and men (Table 6).
Table 6.
P for Interaction Values Between β2‐Microglobulin and Cystatin C and Selected Risk Factors in the Association With Symptomatic PAD
| Risk Factor | Women | Men | ||
|---|---|---|---|---|
| β2‐Microglobulin | Cystatin C | β2‐Microglobulin | Cystatin C | |
| Age | 0.46 | 0.36 | 0.53 | 0.55 |
| Current smoker (yes/no) | 0.52 | 0.61 | 0.65 | 0.64 |
| Creatinine | 1.00 | 0.99 | 0.30 | 0.25 |
| C‐reactive protein | 0.89 | 0.85 | 0.15 | 0.22 |
| HDL cholesterol | 0.26 | 0.48 | 0.14 | 0.10 |
| LDL cholesterol | 0.89 | 0.61 | 0.15 | 0.08 |
| Body mass index | 0.44 | 0.86 | 0.37 | 0.11 |
| Physical activity | 0.47 | 0.73 | 0.13 | 0.20 |
HDL indicates high‐density lipoprotein; LDL, low‐density lipoprotein.
In pooled analyses of men and women, the RRs per each 1‐SD increment were 1.31 (95% CI 1.04 to 1.64) for β2‐microglobulin and 1.19 (95% CI 0.74 to 1.93) for cystatin C in multivariable‐adjusted models including adjustment for creatinine (Figure). When we adjusted for eGFRCKD‐EPI instead of circulating creatinine, the pooled RRs for β2‐microglobulin (RR 1.28, 95% CI 1.02 to 1.60) and cystatin C (RR 1.13, 95% CI 0.89 to 1.43) remained essentially the same. Similar results were obtained for β2‐microglobulin (RR 1.33, 95% CI 1.05 to 1.70) and for cystatin C (RR 1.20, 95% CI 0.73 to 1.96) when we excluded subjects with an eGFRCKD‐EPI <60 mL min−1 per 1.73 m−2. The pooled multivariable‐adjusted estimate for creatinine (RR 1.09, 95% CI 0.73 to 1.64) decreased further after additionally accounting for either β2‐microglobulin (RR 0.94, 95% CI 0.77 to 1.15) or cystatin C (RR 0.98, 95% CI 0.79 to 1.21).
Discussion
In 2 large nested case‐control studies, circulating β2‐microglobulin and cystatin C and, to a lesser extent, creatinine were associated with incident PAD in men, although creatinine was no longer associated with risk after adjustment for either β2‐microglobulin or cystatin C. Circulating cystatin C and creatinine were completely unassociated with risk of PAD among women. In pooled analyses among men and women, β2‐microglobulin was associated with a higher risk of the development of symptomatic PAD. The association of β2‐microglobulin with PAD risk remained after accounting for circulating creatinine and for other established cardiovascular risk factors. Moreover, β2‐microglobulin was also associated with PAD risk in individuals with eGFRs above the clinical cutoff for chronic kidney disease (eGFRCKD‐EPI ≥60 mL min−1 per 1.73 m−2), extending the possible range over which kidney function is related to risk.
Using high‐throughput proteomic profiling, a cross‐sectional study that included both men and women demonstrated that β2‐microglobulin correlated strongly with PAD severity, independent of other risk factors.25 Our present study complements and extends this finding by showing a prospective association between β2‐microglobulin and future risk of symptomatic PAD among subjects free of diagnosed cardiovascular or kidney disease. This increased risk of systemic atherosclerosis concords with positive associations between β2‐microglobulin and risk of coronary heart disease in postmenopausal women38 and in the general population.22 Among Japanese community‐dwelling nondisabled elderly, a strong dose–response relationship was found between serum β2‐microglobulin and mortality risk.21 Our data indicate that the association of β2‐microglobulin with incident symptomatic PAD is largely independent of circulating creatinine or creatinine‐based eGFR. This may suggest that β2‐microglobulin represents kidney function that is not well captured by circulating creatinine. Indeed, β2‐microglobulin is correlated more strongly with GFR measured by inulin than is serum creatinine.18,39 Another possibility that cannot be excluded by the present data is that β2‐microglobulin reflects other, non‐GFR determinants of PAD risk. Given the modest correlations with CRP, β2‐microglobulin may be a marker of local or systemic inflammation, but the observed association with PAD remained after adjustment for CRP. In addition, it is possible that circulating β2‐microglobulin reflects amyloid deposition and aggregation in the vessel wall as part of the atherosclerotic process.40–41 Perhaps most intriguingly, the repetitive ischemia–reperfusion injury of the limbs that occurs as PAD develops may promote β2‐microglobulin shedding from endothelial cell surfaces due to its noncovalent binding with the major histocompatibility complex class I α‐chain without direct attachment to the cell membrane.25
Few prospective studies have investigated the association of cystatin C with risk of incident PAD among apparently healthy subjects. In >4000 community‐dwelling elderly subjects of the Cardiovascular Health Study, cystatin C was associated with an increased risk of lower extremity PAD procedures (bypass surgery, angioplasty, or amputation) only among subjects in the highest quintile (threshold for cystatin C >1.27 mg/L).24 In a nested case‐control study with 133 symptomatic PAD cases in the Physicians Health Study, no evidence was found for an increased risk with elevated cystatin C levels. However, the 95th cutoff percentile for cystatin C based on the distribution of the controls was <1.08 mg/L.23 Although we did not find evidence for a nonlinear association, the higher PAD risk in our study among men appeared to be statistically significant when comparing the first (<0.83 mg/L) cystatin C quartile with the fourth (>1.10 mg/L), but no such association was observed in women. These results suggest that only particularly high levels of cystatin C may be associated with increased risk of PAD, although differences between studies hamper direct comparisons.
The findings on β2‐microglobulin and PAD risk may have applications in the clinical setting. First, determination of β2‐microglobulin is already widely available and routinely measured in clinical settings for hematological and lymphoproliferative diseases (eg, multiple myeloma and lymphoma42–44). Measurement of β2‐microglobulin for the early detection of PAD may thus be easily incorporated into routine care cardiovascular risk prediction. Of note, measurement of cystatin C is not yet common in most clinical laboratories. Second, these findings suggest that (preservation of) kidney function may be an additional target45–46 to reduce the risk of PAD. It cannot be excluded that lower kidney function may be a consequence rather than a cause of atherosclerosis, particularly if renal arteries occlude in parallel with arteries of the lower extremities. The observation, however, that kidney function is associated with PAD risk even after extensive adjustment for cardiovascular risk factors suggests a role for kidney function itself as PAD risk promoter.
Relative risks of all 3 markers of kidney function tended to be higher in the HPFS (men) than in the NHS (women). Besides sex, there are other differences between the 2 studies that merit attention. First, plasma was stored in heparin tubes for NHS samples versus in EDTA tubes for HPFS samples, which may have created differential measurement of these markers. This is unlikely, though, because baseline correlations between these markers with each other and with other confounding variables were almost exactly the same for the women in NHS as for the men in HPFS. Also, the coefficients of variation of the measurements for all 3 markers were relatively low for both HPFS and NHS. It is also unlikely that differences in end point definitions may explain the stronger associations because definitions were similar between the 2 studies and positive associations of comparable magnitude between well‐known PAD risk factors, such as for CRP and lipoprotein(a),47 were observed in both studies. Third, eGFR was systematically higher in women, and in these analyses, only β2‐microglobulin tended to be positively associated with risk. This suggests that it may provide more useful discrimination at high levels of eGFR than other markers of kidney function. Nevertheless, the stronger associations among men could reflect differences in PAD pathophysiology between sexes, although a recent meta‐analysis indicated that there is no reason to assume that kidney function is a less important risk factor for other forms of cardiovascular disease in women than in men.48 Future studies should address whether markers of kidney function universally perform better in men or whether β2‐microglobulin is a superior marker to assess risk of the development of PAD.
Strengths of the current study are the large number of biochemical and traditional cardiovascular risk factors for which we could adjust in our analyses, the prospective design, the long‐term follow‐up, the use of risk set sampling to select controls, the homogeneity of the 2 study populations, and the use of adjudicated events with a pronounced atherosclerotic origin. Besides these strengths, several potential limitations warrant to be mentioned. First, we used symptomatic PAD as end point. Subclinical or asymptomatic PAD, which may have otherwise been detected through an abnormal ankle‐brachial index or pulse examination, is likely to have been missed. However, outcomes included in this analysis were confirmed by medical records, reducing the likelihood of false‐positive cases at the expense of a risk of a falsely low incidence rate. Second, participants in both cohorts were predominantly white; extrapolation of our results to other race/ethnicity subgroups should therefore be done with caution. Blacks, for instance, have higher circulating creatinine but not β2‐microglobulin or cystatin C levels14 and are at higher risk of the development of PAD.8 Third, sex was completely confounded by the 2 cohorts. Therefore, it is impossible to know if the differences in significance between men and women were due to differences in study design or true sex differences. Fourth, although β2‐microglobulin and cystatin C clearly outperformed creatinine in assessing PAD risk, particularly among the men, we cannot directly determine whether that reflects better estimation of GFR or simply nonrenal effects. Fifth, although CRP was strongly associated with risk in both cohorts, we adjusted for only CRP, which may be an imperfect measure of inflammation. Finally, we did not have data on albuminuria, which could potentially have captured other aspects of chronic kidney disease.
Conclusions
The present study identified an association between circulating β2‐microglobulin and future risk of symptomatic PAD in pooled analyses of men and women. When the 2 sexes were studied separately, β2‐microglobulin and cystatin C were associated with risk of symptomatic PAD in men but not in women. The associations of these emerging markers of kidney function with PAD risk appeared to be independent of circulating creatinine or creatinine‐based eGFR, which is the current clinical standard for the assessment of kidney function and well‐known cardiovascular risk factors. Cystatin C and particularly β2‐microglobulin may have greater potential than creatinine for the early detection of systemic atherosclerotic diseases.
Supplementary Material
Appendix Beta2-microglobulin, Cystatin C, and Creatinine and Risk of Symptomatic Peripheral Artery Disease.
Sources of Funding
This study was supported by grants P01 CA87969, R01 CA49449, R01 HL034594, R01 HL091874, R01 HL35464, and UM1 CA167552 from the National Institutes of Health and by research grant CH 001 from the Top Institute (TI) Food and Nutrition, the Netherlands (Drs Joosten and Bakker).
Disclosures
Dr Cooke is the inventor of patents owned by Stanford University related to the use of β2‐microglobulin and cystatin C in diagnostic assays.
References
- 1.Pande RL, Perlstein TS, Beckman JA, Creager MA. Secondary prevention and mortality in peripheral artery disease: National Health and Nutrition Examination Study, 1999 to 2004. Circulation. 2011; 124:17-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDermott MM, Liu K, Greenland P, Guralnik JM, Criqui MH, Chan C, Pearce WH, Schneider JR, Ferrucci L, Celic L, Taylor LM, Vonesh E, Martin GJ, Clark E. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA. 2004; 292:453-461. [DOI] [PubMed] [Google Scholar]
- 3.Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, Folsom AR, Hirsch AT, Dramaix M, deBacker G, Wautrecht JC, Kornitzer M, Newman AB, Cushman M, Sutton‐Tyrrell K, Lee AJ, Price JF, d'Agostino RB, Murabito JM, Norman PE, Jamrozik K, Curb JD, Masaki KH, Rodriguez BL, Dekker JM, Bouter LM, Heine RJ, Nijpels G, Stehouwer CD, Ferrucci L, McDermott MM, Stoffers HE, Hooi JD, Knottnerus JA, Ogren M, Hedblad B, Witteman JC, Breteler MM, Hunink MG, Hofman A, Criqui MH, Langer RD, Fronek A, Hiatt WR, Hamman R, Resnick HE, Guralnik J. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta‐analysis. JAMA. 2008; 300:197-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: morbidity and mortality implications. Circulation. 2006; 114:688-699. [DOI] [PubMed] [Google Scholar]
- 5.Joosten MM, Pai JK, Bertoia ML, Rimm EB, Spiegelman D, Mittleman MA, Mukamal KJ. Associations between conventional cardiovascular risk factors and risk of peripheral artery disease in men. JAMA. 2012; 308:1660-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fried LF, Shlipak MG, Crump C, Bleyer AJ, Gottdiener JS, Kronmal RA, Kuller LH, Newman AB. Renal insufficiency as a predictor of cardiovascular outcomes and mortality in elderly individuals. J Am Coll Cardiol. 2003; 41:1364-1372. [DOI] [PubMed] [Google Scholar]
- 7.O'Hare AM, Vittinghoff E, Hsia J, Shlipak MG. Renal insufficiency and the risk of lower extremity peripheral arterial disease: results from the Heart and Estrogen/Progestin Replacement Study (HERS). J Am Soc Nephrol. 2004; 15:1046-1051. [DOI] [PubMed] [Google Scholar]
- 8.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR. Inter‐society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007; 45:S5-S67. [DOI] [PubMed] [Google Scholar]
- 9. Kidney disease: improving global outcomes (KDIGO) CKD work group. KDIGO clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013. suppl 3:1-150. [Google Scholar]
- 10.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011; 80:17-28. [DOI] [PubMed] [Google Scholar]
- 11.Astor BC, Levey AS, Stevens LA, Van Lente F, Selvin E, Coresh J. Method of glomerular filtration rate estimation affects prediction of mortality risk. J Am Soc Nephrol. 2009; 20:2214-2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stevens LA, Schmid CH, Greene T, Li L, Beck GJ, Joffe MM, Froissart M, Kusek JW, Zhang YL, Coresh J, Levey AS. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009; 75:652-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jovanovic D, Krstivojevic P, Obradovic I, Durdevic V, Dukanovic L. Serum cystatin C and beta2‐microglobulin as markers of glomerular filtration rate. Ren Fail. 2003; 25:123-133. [DOI] [PubMed] [Google Scholar]
- 14.Juraschek SP, Coresh J, Inker LA, Levey AS, Kottgen A, Foster MC, Astor BC, Eckfeldt JH, Selvin E. Comparison of serum concentrations of beta‐trace protein, beta2‐microglobulin, cystatin C, and creatinine in the US population. Clin J Am Soc Nephrol. 2013; 8:584-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filler G, Bokenkamp A, Hofmann W, Le Bricon T, Martinez‐Bru C, Grubb A. Cystatin C as a marker of GFR—history, indications, and future research. Clin Biochem. 2005; 38:1-8. [DOI] [PubMed] [Google Scholar]
- 16.Vincent C, Chanard J, Caudwell V, Lavaud S, Wong T, Revillard JP. Kinetics of 125I‐beta 2‐microglobulin turnover in dialyzed patients. Kidney Int. 1992; 42:1434-1443. [DOI] [PubMed] [Google Scholar]
- 17.Randers E, Kristensen JH, Erlandsen EJ, Danielsen H. Serum cystatin C as a marker of the renal function. Scand J Clin Lab Invest. 1998; 58:585-592. [DOI] [PubMed] [Google Scholar]
- 18.Bianchi C, Donadio C, Tramonti G, Consani C, Lorusso P, Rossi G. Reappraisal of serum beta2‐microglobulin as marker of GFR. Ren Fail. 2001; 23:419-429. [DOI] [PubMed] [Google Scholar]
- 19.Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, Siscovick DS, Stehman‐Breen C. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005; 352:2049-2060. [DOI] [PubMed] [Google Scholar]
- 20.Shlipak MG, Matsushita K, Arnlov J, Inker LA, Katz R, Polkinghorne KR, Rothenbacher D, Sarnak MJ, Astor BC, Coresh J, Levey AS, Gansevoort RT. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013; 369:932-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shinkai S, Chaves PH, Fujiwara Y, Watanabe S, Shibata H, Yoshida H, Suzuki T. Beta2‐microglobulin for risk stratification of total mortality in the elderly population: comparison with cystatin C and C‐reactive protein. Arch Intern Med. 2008; 168:200-206. [DOI] [PubMed] [Google Scholar]
- 22.Astor BC, Shafi T, Hoogeveen RC, Matsushita K, Ballantyne CM, Inker LA, Coresh J. Novel markers of kidney function as predictors of ESRD, cardiovascular disease, and mortality in the general population. Am J Kidney Dis. 2012; 59:653-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albert MA, Rifai N, Ridker PM. Plasma levels of cystatin‐C and mannose binding protein are not associated with risk of developing systemic atherosclerosis. Vasc Med. 2001; 6:145-149. [DOI] [PubMed] [Google Scholar]
- 24.O'Hare AM, Newman AB, Katz R, Fried LF, Stehman‐Breen CO, Seliger SL, Siscovick DS, Shlipak MG. Cystatin C and incident peripheral arterial disease events in the elderly: results from the Cardiovascular Health Study. Arch Intern Med. 2005; 165:2666-2670. [DOI] [PubMed] [Google Scholar]
- 25.Wilson AM, Kimura E, Harada RK, Nair N, Narasimhan B, Meng XY, Zhang F, Beck KR, Olin JW, Fung ET, Cooke JP. Beta2‐microglobulin as a biomarker in peripheral arterial disease: proteomic profiling and clinical studies. Circulation. 2007; 116:1396-1403. [DOI] [PubMed] [Google Scholar]
- 26.Fung ET, Wilson AM, Zhang F, Harris N, Edwards KA, Olin JW, Cooke JP. A biomarker panel for peripheral arterial disease. Vasc Med. 2008; 13:217-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prentice RL, Breslow NE. Retrospective studies and failure time models. Biometrika. 1978; 65:153-158. [Google Scholar]
- 28.Spithoven EM, Bakker SJ, Kootstra‐Ros JE, de Jong PE, Gansevoort RT. Stability of creatinine and cystatin C in whole blood. Clin Biochem. 2013; 46:1611-1614. [DOI] [PubMed] [Google Scholar]
- 29.Joosten MM, Joshipura KJ, Pai JK, Bertoia ML, Rimm EB, Mittleman MA, Mukamal KJ. Total adiponectin and risk of symptomatic lower extremity peripheral artery disease in men. Arterioscler Thromb Vasc Biol. 2013; 33:1092-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ascherio A, Rimm E, Giovannucci E, Colditz G, Rosner B, Willett W, Sacks F, Stampfer M. A prospective study of nutritional factors and hypertension among US men. Circulation. 1992; 86:1475-1484. [DOI] [PubMed] [Google Scholar]
- 31.Simon KC, Chen H, Schwarzschild M, Ascherio A. Hypertension, hypercholesterolemia, diabetes, and risk of Parkinson disease. Neurology. 2007; 69:1688-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joosten MM, Chiuve SE, Mukamal KJ, Hu FB, Hendriks HF, Rimm EB. Changes in alcohol consumption and subsequent risk of type 2 diabetes in men. Diabetes. 2011; 60:74-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self‐reported waist and hip circumferences in men and women. Epidemiology. 1990; 1:466-473. [DOI] [PubMed] [Google Scholar]
- 34.Chasan‐Taber S, Rimm EB, Stampfer MJ, Spiegelman D, Colditz GA, Giovannucci E, Ascherio A, Willett WC. Reproducibility and validity of a self‐administered physical activity questionnaire for male health professionals. Epidemiology. 1996; 7:81-86. [DOI] [PubMed] [Google Scholar]
- 35.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009; 150:604-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012; 367:20-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989; 8:551-561. [DOI] [PubMed] [Google Scholar]
- 38.Prentice RL, Paczesny S, Aragaki A, Amon LM, Chen L, Pitteri SJ, McIntosh M, Wang P, Buson Busald T, Hsia J, Jackson RD, Rossouw JE, Manson JE, Johnson K, Eaton C, Hanash SM. Novel proteins associated with risk for coronary heart disease or stroke among postmenopausal women identified by in‐depth plasma proteome profiling. Genome Med. 2010; 2:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woitas RP, Stoffel‐Wagner B, Poege U, Schiedermaier P, Spengler U, Sauerbruch T. Low‐molecular weight proteins as markers for glomerular filtration rate. Clin Chem. 2001; 47:2179-2180. [PubMed] [Google Scholar]
- 40.Gorevic PD, Casey TT, Stone WJ, DiRaimondo CR, Prelli FC, Frangione B. Beta‐2 microglobulin is an amyloidogenic protein in man. J Clin Invest. 1985; 76:2425-2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takayama F, Miyazaki S, Morita T, Hirasawa Y, Niwa T. Dialysis‐related amyloidosis of the heart in long‐term hemodialysis patients. Kidney Int Suppl. 2001; 78:S172-S176. [DOI] [PubMed] [Google Scholar]
- 42.Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, Fonseca R, Rajkumar SV, Offord JR, Larson DR, Plevak ME, Therneau TM, Greipp PR. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003; 78:21-33. [DOI] [PubMed] [Google Scholar]
- 43.Delgado J, Pratt G, Phillips N, Briones J, Fegan C, Nomdedeu J, Pepper C, Aventin A, Ayats R, Brunet S, Martino R, Valcarcel D, Milligan D, Sierra J. Beta2‐microglobulin is a better predictor of treatment‐free survival in patients with chronic lymphocytic leukaemia if adjusted according to glomerular filtration rate. Br J Haematol. 2009; 145:801-805. [DOI] [PubMed] [Google Scholar]
- 44.Alexanian R, Barlogie B, Fritsche H. Beta 2 microglobulin in multiple myeloma. Am J Hematol. 1985; 20:345-351. [DOI] [PubMed] [Google Scholar]
- 45.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WRC, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, White CJ, White J, White RA. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic). Circulation. 2006; 113:e463-e654. [DOI] [PubMed] [Google Scholar]
- 46.Rooke TW, Hirsch AT, Misra S, Sidawy AN, Beckman JA, Findeiss LK, Golzarian J, Gornik HL, Halperin JL, Jaff MR, Moneta GL, Olin JW, Stanley JC, White CJ, White JV, Zierler RE. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline). Circulation. 2011; 124:2020-2045.10.1161/?CIR.0b013e31822e80c321959305 [Google Scholar]
- 47.Bertoia ML, Pai JK, Lee JH, Taleb A, Joosten MM, Mittleman MA, Yang X, Witztum JL, Rimm EB, Tsimikas S, Mukamal KJ. Oxidation‐specific biomarkers and risk of peripheral artery disease. J Am Coll Cardiol. 2013; 61:2169-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nitsch D, Grams M, Sang Y, Black C, Cirillo M, Djurdjev O, Iseki K, Jassal SK, Kimm H, Kronenberg F, Oien CM, Levey AS, Levin A, Woodward M, Hemmelgarn BR. Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: a meta‐analysis. BMJ. 2013; 346:f324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix Beta2-microglobulin, Cystatin C, and Creatinine and Risk of Symptomatic Peripheral Artery Disease.


