Abstract
Background
Managing cardiovascular risk factors is important for reducing vascular complications in type 2 diabetes, even in individuals who have achieved glycemic control. Nut consumption is associated with reduced cardiovascular risk; however, there is mixed evidence about the effect of nuts on blood pressure (BP), and limited research on the underlying hemodynamics. This study assessed the effect of pistachio consumption on BP, systemic hemodynamics, and heart rate variability in adults with well‐controlled type 2 diabetes.
Methods and Results
We enrolled 30 adults (40 to 74 years) with type 2 diabetes in a randomized, crossover, controlled feeding study. After a 2‐week run‐in period, participants consumed a low‐fat control diet (27% fat) containing low‐fat/high‐carbohydrate snacks and a moderate‐fat diet (33% fat) containing pistachios (20% of total energy) for 4 weeks each, separated by a 2‐week washout. Following each diet period, we assessed BP, systemic hemodynamics, and heart rate variability at rest and during acute mental stress, and, in a subset of participants (n=21), 24‐hour ambulatory BP. BP at rest and during stress did not differ between treatments. The pistachio diet significantly reduced total peripheral resistance (−3.7±2.9%, P=0.004), increased cardiac output (3.1±2.3%, P=0.002), and improved some measures of heart rate variability (all P<0.05). Systolic ambulatory BP was significantly reduced by 3.5±2.2 mm Hg (P=0.046) following the pistachio diet, with the greatest reduction observed during sleep (−5.7±2.6 mm Hg, P=0.052).
Conclusions
A moderate‐fat diet containing pistachios modestly improves some cardiovascular risk factors in adults with well‐controlled type 2 diabetes.
Clinical Trial Registration
URL: www.clinicaltrials.gov. Unique identifier: NCT00956735.
Keywords: blood pressure, heart rate variability, hemodynamics, nutrition, type 2 diabetes mellitus
Introduction
With the global burden of diabetes continuing to grow, the resulting cardiovascular morbidity in this population is threatening to reverse the downward trend in cardiovascular mortality observed in recent years.1–2 Despite successful treatment of modifiable risk factors, individuals with type 2 diabetes retain residual risk of vascular complications. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial,3 the Action in Diabetes and Vascular Disease (ADVANCE) trial,4 and the Veterans’ Administration Diabetes Trial (VADT)5 all reported that improved glucose control does not significantly lower the risk of major cardiovascular events. Dietary modification has long been the first line of therapy recommended for risk management6 and, recently, much attention has focused on the residual risk that remains after successful pharmacological treatment of metabolic risk factors.7 In a 2013 Position Statement, the American Diabetes Association recommended 5 eating patterns for the management of diabetes: Mediterranean style, vegetarian and vegan, low‐fat, low carbohydrate, and Dietary Approaches to Stop Hypertension.8 All these eating patterns except low‐fat include nuts, but there is a notable absence of rigorous, randomized, controlled trials testing the effects of nut consumption in type 2 diabetes, particularly in regard to blood pressure.
Several epidemiological studies, including 2 prospective cohort studies (the Physicians’ Health Study9 and the Atherosclerosis Risk in Communities Study10), a cross‐sectional analysis of the PREDIMED trial,11 and data from the National Health and Nutrition Examination Survey (NHANES),12 have shown that regular nut consumption lowers blood pressure and the risk of hypertension. Approximately 2 dozen clinical trials assessed the effect of nuts on blood pressure (see Casas‐Agustench and colleagues13 for review), and the majority reported non‐significant changes in resting blood pressure over 3 to 24 weeks. Results from the PREDIMED trial show that among individuals with type 2 diabetes or ≥3 cardiovascular risk factors, a traditional Mediterranean diet containing mixed nuts (15 g/day walnuts, 7.5 g/day almonds, 7.5 g/day hazelnuts) reduces systolic blood pressure by ≈7 mm Hg14 and the 1‐year incidence of hypertension by 3.3% compared with a lower‐fat control diet.15 We have previously shown that, in adults with hypercholesterolemia, consumption of 10% of daily energy from pistachios (≈1.5 ounces/day for most adults) reduced systolic blood pressure at rest and during acute mental stress by an average of 4.8 mm Hg.16 We also observed significant shifts in systemic hemodynamics, including a dose‐dependent reduction in total peripheral resistance.
Among individuals with type 2 diabetes, management of blood pressure is key to reducing vascular complications,17 and given our previous findings in adults with hypercholesterolemia,16 regular pistachio consumption may be an effective intervention to reduce cardiovascular risk. Thus, the purpose of the present study was to compare the effects of a moderate‐fat diet containing 20% of daily energy from pistachios to a low‐fat control diet on blood pressure and systemic hemodynamics in adults with well‐controlled type 2 diabetes. We selected this population as proof‐of‐concept that dietary changes can provide additional health benefits, even in adults who are early in the progression of metabolic disease. We hypothesized that daily pistachio consumption (20% of total energy) for 4 weeks would reduce total peripheral resistance at rest and during acute stress. Secondary analyses assessed the effect of pistachio consumption on 24‐hour ambulatory blood pressure and heart rate variability.
Subjects and Methods
Recruitment began in July 2009 and continued through November 2012. Adults with well‐controlled type 2 diabetes (defined below) were recruited through campus and community advertisements (Figure1). Interested individuals completed a phone interview during which trained research assistants assessed eligibility. Participants were required to be between the ages of 30 to 75 (women had to be postmenopausal) with a body mass index (BMI, kg/m2) between 18.5 and 45.0 and managing their blood glucose by (1) diet and exercise alone or (2) any diabetes medication(s) except insulin. This study was specifically designed to include individuals who were early in the progression of diabetes and had achieved moderate glycemic control (defined as glycated hemoglobin <7.4%). Initial exclusion criteria included self‐reported history of chronic disease other than type 2 diabetes (including cardiovascular disease and diabetes complications such as retinopathy and neuropathy), history of bariatric surgery; major surgery in the prior 6 months; nut or latex allergies; and use of tobacco, daily aspirin, anti‐inflammatory medications, oral steroids, hormone replacement therapy, or medication for hypertension. Individuals meeting the phone interview criteria completed a clinic screening visit that included blood pressure measurement, an electrocardiogram (ECG), and assessment of lipids, glucose, and inflammation. Individuals with blood pressure ≥160/100 mm Hg, an abnormal ECG, fasting triglycerides ≥5.65 mmol/L, or glycated hemoglobin ≥7.4% were excluded. Participants were instructed to discontinue all dietary supplements 2 weeks prior to study enrollment, except for omega‐3 supplements, which were discontinued 8 weeks previously.
Figure 1.
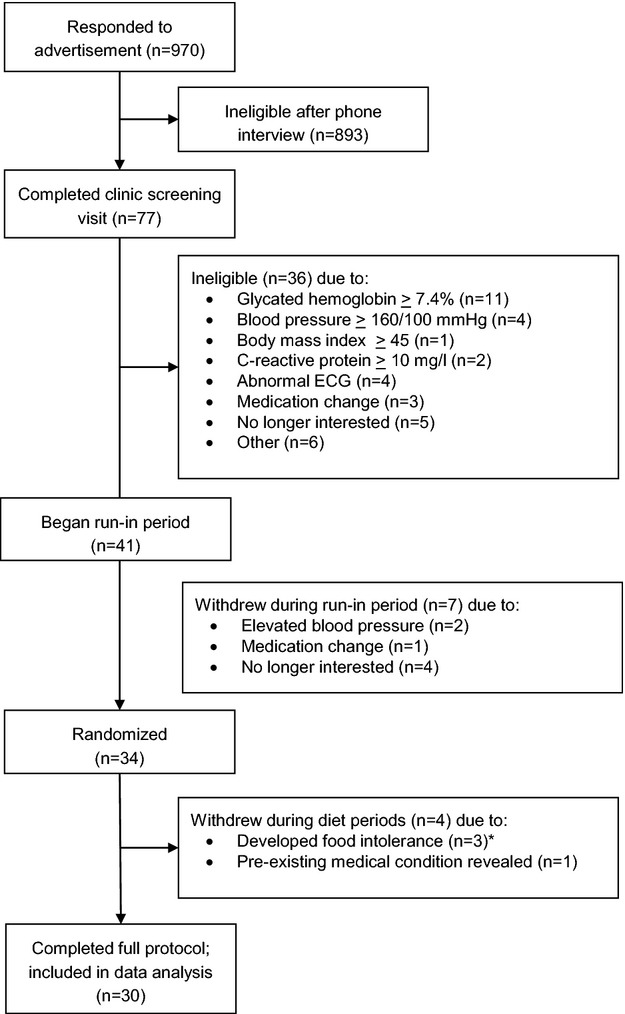
CONSORT diagram of recruitment and study completion. *Of these, 2 developed intolerances to tomatoes and 1 experienced an allergic reaction to pistachios. It was confirmed that this participant reported no history of nut allergies prior to study enrollment, but during questioning that followed the allergic reaction, this participant stated he had not previously eaten pistachios.
In January 2010, due to low enrollment, the criteria were revised to allow individuals taking a single drug for hypertension to participate when (1) their primary care physician gave written permission for them to discontinue drug monotherapy during the study, and (2) seated, resting blood pressure remained below 160/100 mm Hg for the duration of the study. In November 2010, after discovery of significant inflammation in a participant (who, in the interest of safety, was withdrawn from the study and referred for follow‐up care; Figure1), the criteria were revised to exclude individuals with C‐reactive protein (CRP) ≥10.0 mg/L.
Thirty participants (50% female) completed the full study, and their baseline characteristics are presented in Table 1. Written informed consent was obtained from all participants, and approval for the study was granted by the Institutional Review Board of the Pennsylvania State University. All data were collected at the Clinical Research Center at the Pennsylvania State University between July 2009 and March 2013. This study was registered at ClinicalTrials.gov (NCT00956735).
Table 1.
Baseline Characteristics of the Participants
| Mean±SD | |
|---|---|
| % Female | 50.0 |
| Age, y | 56.1±7.8 |
| Body mass index, kg/m2 | 31.2±6.1 |
| Resting systolic blood pressure*, mm Hg | 116.2±13.6 |
| Resting diastolic blood pressure, mm Hg | 71.0±5.4 |
| Fasting glucose, mmol/L | 5.9±21.6 |
| Glycated hemoglobin, % | 6.2±0.5 |
| Total cholesterol, mmol/L | 4.2±1.0 |
| LDL cholesterol, mmol/L | 2.5±0.9 |
| HDL cholesterol, mmol/L | 1.1±0.3 |
| Triglycerides, mmol/L | 1.6±0.8 |
| % Taking diabetes medication(s) | |
| None | 16.7 |
| 1* | 66.7 |
| 2 or more | 16.7 |
| % Taking statin therapy | 43.3 |
No participants were taking anti‐hypertensive medications during the study. HDL indicates high‐density lipoprotein; LDL, low‐density lipoprotein.
Of these, 90% were on metformin only.
Intervention
The study employed a 2‐period, randomized, crossover, controlled‐feeding design. A 2‐week run‐in period preceded the first test diet to establish baseline values for a typical Western diet. Test diets were presented in a counterbalanced order for 4 weeks each, with order determined by simple randomization (www.randomization.com) and equal allocation to each treatment order assignment. The study coordinator (KAS) generated the random allocation sequence and assigned participants to the interventions. Due to the nature of the dietary intervention, metabolic kitchen staff and participants were aware of treatment assignments; however, technicians who measured outcome variables were blinded to the diet assignments. At the end of each diet period (including the run‐in period), participants underwent testing to assess blood pressure, cardiovascular reactivity to acute mental stress, heart rate variability, and 24‐hour ambulatory blood pressure. Short compliance breaks (average of 2 weeks) separated the diet periods.
The nutrient composition of the study diets is presented in Table 2. Participants were assigned to calorie levels (range: 1800 to 3900 kcal/day) according to the Harris‐Benedict equation,18 and adjustments were made as needed for weight stability. The run‐in diet was a nutritionally adequate diet with total fat based on a typical Western diet (36% total fat, 11.5% saturated fat, and 278 mg/day of cholesterol). The control diet was an American Heart Association's Therapeutic Lifestyle Changes diet,19 with reduced total fat (26.9% of energy), saturated fat (6.7% of energy), and cholesterol (186 mg/day). The pistachio diet was designed by replacing low‐fat or fat‐free carbohydrate snacks (ie, pretzels, string cheese, etc.) in the control diet with pistachios equivalent to 20% of daily energy (range: 59 to 128 g, depending on calorie assignment). The pistachios were provided by the American Pistachio Growers and grown in California. Nutrient content of the pistachios was analyzed by an independent laboratory (Covance) and the nutrient content of the pistachio diet reported in Table 2 was adjusted based on these results. One‐half of the daily dose of pistachios was provided as roasted, salted snacks and the other half (roasted, unsalted) was incorporated into study foods (eg, ground pistachios added to taco meat, chopped pistachios added to muffins). The nutrient content of the pistachio diet compared with the control diet, therefore, reflects the nutrient content of the pistachios (ie, rich in total fat, monounsaturated fat, polyunsaturated fat, fiber, and potassium). Daily amounts of saturated fat and dietary cholesterol were similar to the control diet. All meals and snacks were prepared in the Metabolic Kitchen at the Pennsylvania State University Clinical Research Center. For 3 to 5 days each week (depending on travel distance), participants ate 1 meal per day in the Metabolic Kitchen and had the other meals prepared and packed for off‐site consumption. Participants completed daily compliance questionnaires regarding consumption of study and non‐study foods, which were reviewed by the dietary staff, and returned food containers were checked for uneaten study foods. The compliance questionnaires and lack of study foods returned indicated that adherence to the diets was very good.
Table 2.
Nutrient Composition of the Diets*
| Run‐In | Control | Pistachio | |
|---|---|---|---|
| Energy, kcal | 2100 | 2100 | 2100 |
| Total fat, g (%) | 85.6 (35.8) | 64.0 (26.9) | 78.5 (33.2) |
| Saturated fat, g (%) | 27.5 (11.5) | 16.0 (6.7) | 15.9 (6.8) |
| Monounsaturated fat, g (%) | 31.3 (13.1) | 26.0 (10.9) | 31.2 (13.1) |
| Polyunsaturated fat, g (%) | 20.2 (8.4) | 16.8 (7.1) | 24.6 (10.4) |
| Protein, g (%) | 85.0 (16.0) | 95.6 (18.1) | 88.5 (16.6) |
| Carbohydrate, g (%) | 261.5 (48.2) | 302.8 (55.1) | 270.6 (50.7) |
| Fiber, g | 23.4 | 31.2 | 35.6 |
| Sodium, mg | 3791 | 3039 | 2553 |
| Potassium, mg | 2338 | 3767 | 4045 |
| Cholesterol, mg | 278 | 186 | 172 |
Values calculated using Nutrient Data System for Research (NDSR, 2009) and adjusted according to the nutrient analyses conducted on the study pistachios by an independent laboratory (Covance).
Systemic Hemodynamic Measures
At the end of each diet period, systemic hemodynamics were assessed in a seated position during a 20‐minute rest period, 2 stress tasks, and two 10‐minute recovery periods. Participants listened to the same classical music during the rest and recovery periods for each visit. The first stress task was the Paced Auditory Serial Addition Task (PASAT), during which participants were instructed to add together the last 2 numbers played from an audio recording and state the sum aloud.20 This task lasted for 5 minutes, and the speed at which the numbers were played increased at each testing session to account for habituation effects. The second stress task was the hand cold pressor, during which participants placed their right hand into 4°C water for 2.5 minutes. Each task was followed by a 10‐minute recovery period. Throughout these 5 tasks (baseline rest, math, recovery, cold pressor, recovery), systolic and diastolic blood pressures (SBP, DBP) were obtained at 1‐ to 2‐min intervals (depending on task) with an automatic oscillometric blood pressure monitor attached to the left arm (Dinamap, Critikon Pro 100, GE Medical Systems). Impedance cardiography utilizing a tetrapolar band configuration with spot ECG electrodes was used to estimate heart rate and stroke volume (Hutcheson Impedance Cardiograph and the Cardiac Output Program, Bio‐Impedance Technology, Inc) at the same time as the blood pressure assessments. Cardiac output and total peripheral resistance were calculated according to standard formulae.21 To control for the acute effect of eating on systemic hemodynamics,22 participants consumed a standard snack (10% of daily calories) that matched the macronutrient content of the corresponding treatment diet 2 hours prior to the cardiovascular reactivity testing. During the pistachio treatment period, this snack included 6.4 to 9.6 g of pistachios (depending on calorie assignment).
Heart Rate Variability Measures
Heart rate variability was assessed in the resting state and during 2 acute stress tasks (mental arithmetic and hand cold pressor, described above) at the end of each diet period. Three electrocardiogram electrodes were placed according to guidelines for impedance cardiography.21 The electrocardiogram was used to obtain raw interbeat intervals (R‐R) recorded at a frequency of 1000 Hz. The R‐R interval sequences were visually inspected, and the data considered artifactual was manually replaced by interpolated or extrapolated data. The square root of the mean squared differences of successive R‐R intervals (RMSSD, ms) was calculated using commercial software (Nevrokard, Medistar Inc). Frequency‐domain measures of HRV (using autoregressive spectra) were calculated using standard methods.23 Oscillations in the high frequency band (0.15 to 0.4 Hz) are thought to reflect vagal modulation of heart rate, whereas oscillations occurring in the low frequency band (0.05 to 0.15 Hz) may reflect a complex interplay between sympathetic and parasympathetic modulation of heart rate as well as baroreceptor activity.24 The HRV variables examined in the frequency domain include high frequency (ms2), low frequency (ms2), the ratio of low frequency to high frequency HRV (LF:HF), and total power (ms2).
Ambulatory Blood Pressure Monitoring
At study enrollment, participants were invited to participate in an optional sub‐study of ambulatory blood pressure. Twenty‐one participants agreed, and there was no significant difference in resting blood pressure between individuals who accepted and declined participation in this sub‐study. Near the end of each diet period, but while still on the relevant diet, participants were fitted with an ambulatory blood pressure monitor (model 90207; Spacelabs Healthcare) and brachial cuff that was worn for an average of 21.3±0.16 hours. Automated readings were taken every 20 minutes during the day (6 am to 10 pm) and every 30 minutes overnight (10 pm to 6 am). To test differences between daytime and nighttime blood pressure, wake and sleep times were conservatively estimated to be 10 am to 9 pm (wake) and 1 am to 5:30 am (sleep).25 The average number of successful readings obtained in a 24‐hour period was 50±2, with 26±1 wake readings and 8±1 sleep readings.
Statistical Analyses
This study was designed with 80% power and alpha set at 0.05 to detect significant between‐treatment differences in total peripheral resistance of 62.2 dyne‐s/cm5 (SD of the difference=116), based on our previous study.16 Treatment effects were tested per protocol (only participants completing the entire study were included in the analysis) using the mixed models procedure in SAS (v9.3). Differences in baseline characteristics according to treatment order assignment were examined for all variables and uniformly non‐significant (data not shown). For the outcome analyses, diet, diet period (first or second), (for relevant analyses) task (rest, math, recovery 1, cold pressor, recovery 2), and their interactions were entered as fixed effects; subject was a random effect. Diet by task and diet by period interactions were uniformly non‐significant (no evidence of carryover). All analyses were adjusted for age, sex, BMI, and the baseline (run‐in) value. α<0.05 was considered statistically significant, and Tukey tests were used to adjust for multiple comparisons. Tables and figures reflect means±standard errors.
Results
Blood pressure and systemic hemodynamics at rest and during acute mental stress are presented in Table 3. Regardless of treatment, systolic and diastolic blood pressure, heart rate, cardiac output, and total peripheral resistance increased during the stress tasks (all P<0.0001). There were no differences between the treatments in resting measurements for any variables. However, when data from the rest period, stress tasks, and recovery periods were included in a repeated‐measures analysis, stroke volume and cardiac output were significantly greater following the pistachio diet (+3.1±2.5%, P=0.011 and +3.1±2.3%, P=0.002) than the control diet. In contrast, total peripheral resistance was significantly lower following the pistachio diet than the control diet (−3.7±2.9%, P=0.004).
Table 3.
Systemic Hemodynamics at Rest and During Acute Mental Stress
| Variable | Condition | Baseline | Control | Pistachio | Mean Difference Pistachio—Control | Condition P Value* | Treatment P Value* |
|---|---|---|---|---|---|---|---|
| Systolic BP, mm Hg | Rest | 116.2±2.5 | 112.1±2.2 | 112.3±1.9 | 0.3±2.0 | ||
| Math | 135.0±4.1 | 127.8±3.9 | 128.0±3.4 | 0.2±3.6 | |||
| Recovery | 118.5±2.6 | 114.6±2.4 | 114.7±2.3 | 0.0±2.4 | |||
| Cold | 146.7±4.2 | 139.6±4.1 | 139.9±4.1 | 0.3±4.1 | |||
| Recovery | 119.8±2.8 | 117.6±2.5 | 115.7±2.4 | −1.9±2.5 | |||
| Average | 127.0±1.8 | 122.1±1.6 | 121.9±1.5 | −0.2±1.6 | 0.0001 | 0.76 | |
| Diastolic BP, mm Hg | Rest | 71.0±0.9 | 69.2±0.9 | 69.2±1.1 | 0.0±1.0 | ||
| Math | 78.9±1.5 | 76.2±1.4 | 75.9±1.2 | −0.4±1.3 | |||
| Recovery | 70.3±1.0 | 70.1±0.9 | 69.2±0.9 | −1.0±0.9 | |||
| Cold | 83.6±1.6 | 82.0±1.5 | 81.4±1.4 | −0.7±1.5 | |||
| Recovery | 71.7±1.3 | 70.0±1.0 | 69.8±0.9 | −0.2±1.0 | |||
| Average | 75.0±0.7 | 73.4±0.7 | 73.0±0.6 | −0.4±0.6 | 0.0001 | 0.28 | |
| Heart rate, bpm | Rest | 63.8±1.1 | 64.7±1.4 | 64.9±1.4 | 0.1±1.4 | ||
| Math | 72.0±1.4 | 71.9±1.5 | 71.5±1.4 | −0.4±1.4 | |||
| Recovery | 64.4±1.2 | 65.5±1.4 | 65.7±1.5 | 0.1±1.4 | |||
| Cold | 71.1±1.3 | 70.1±1.6 | 71.0±1.6 | 0.9±1.6 | |||
| Recovery | 64.7±1.2 | 64.3±1.3 | 64.9±1.4 | 0.5±1.4 | |||
| Average | 67.2±0.6 | 67.3±0.7 | 67.6±0.7 | 0.2±0.7 | 0.0001 | 0.43 | |
| Stroke volume, mL/beat | Rest | 68.3±3.1 | 66.1±3.5 | 67.9±3.3 | 1.7±3.4 | ||
| Math | 69.3±3.9 | 65.5±4.3 | 68.5±4.0 | 3.0±4.1 | |||
| Recovery | 67.3±3.1 | 67.4±3.8 | 68.4±3.4 | 1.0±3.6 | |||
| Cold | 68.0±3.8 | 64.6±3.6 | 67.9±3.7 | 3.3±3.7 | |||
| Recovery | 66.2±3.6 | 68.2±3.8 | 69.4±3.5 | 1.2±3.6 | |||
| Average | 67.8±1.6 | 66.4±1.7 | 68.4±1.6 | 2.1±1.6 | 0.17 | 0.011 | |
| Cardiac output, L/min | Rest | 4.36±0.20 | 4.26±0.21 | 4.36±0.20 | 0.10±0.21 | ||
| Math | 5.01±0.30 | 4.73±0.29 | 4.84±0.27 | 0.11±0.28 | |||
| Recovery | 4.32±0.20 | 4.36±0.21 | 4.45±0.20 | 0.09±0.21 | |||
| Cold | 4.82±0.27 | 4.48±0.23 | 4.77±0.25 | 0.29±0.24 | |||
| Recovery | 4.28±0.23 | 4.34±0.21 | 4.46±0.20 | 0.12±0.20 | |||
| Average | 4.55±0.11 | 4.43±0.10 | 4.57±0.10 | 0.14±0.10 | 0.0001 | 0.002 | |
| Total peripheral resistance, dyne‐s/cm5 | Rest | 1694.7±105.1 | 1695.4±107.4 | 1641.1±97.2 | −54.3±102.3 | ||
| Math | 1693.5±113.2 | 1737.8±124.9 | 1691.1±110.9 | −46.6±117.9 | |||
| Recovery | 1702.5±101.6 | 1664.8±97.5 | 1618.0±94.0 | −46.8±95.8 | |||
| Cold | 1918.5±156.8 | 1964.5±148.2 | 1852.8±158.3 | −111.7±153.3 | |||
| Recovery | 1789.1±127.1 | 1680.5±91.5 | 1622.3±91.4 | −58.2±91.4 | |||
| Average | 1757.6±54.0 | 1746.1±51.4 | 1681.8±49.6 | −64.3±50.5 | 0.0001 | 0.004 |
Data are mean±standard error, n=30. BMI indicates body mass index; BP, blood pressure.
Statistical significance assessed by PROC MIXED in SAS, after adjustment for age, sex, BMI, and baseline (run‐in) values. The condition effect tested differences in hemodynamics between resting and stress conditions. The treatment effect tested differences in hemodynamics between the Control and Pistachio diets.
Mean heart rate variability at rest and during the acute stress tasks are shown in Table 4. Both RMSSD and high‐frequency power, which reflect parasympathetic activity, were significantly higher following the pistachio diet (+13.7±4.8%, P=0.028 and +24.4±10.6%, P=0.007). Low‐frequency power, which reflects both sympathetic and parasympathetic activity, was also greater following the pistachio diet (+19.8±10.6%, P=0.041). There was no difference between the treatments in total power or the ratio of low‐ to high‐frequency power.
Table 4.
Heart Rate Variability
| Variable | Baseline | Control | Pistachio | Mean Difference Pistachio—Control | P Value* |
|---|---|---|---|---|---|
| RMSSD, mm | 24.9±1.1 | 23.9±1.1 | 27.2±1.3 | 3.3±1.2 | 0.028 |
| High frequency, ms2 | 273.5±24.4 | 253.3±25.1 | 315.1±28.8 | 61.8±27.0 | 0.007 |
| Low frequency, ms2 | 494.9±43.1 | 480.0±43.8 | 575.0±58.4 | 95.0±51.1 | 0.041 |
| Low:high frequency, ms2 | 2.7±0.2 | 2.9±0.2 | 2.5±0.2 | −0.4±0.2 | 0.59 |
| Total power, ms2 | 1256.0±87.3 | 1236.3±116.8 | 1401.0±115.4 | 164.7±116.1 | 0.10 |
Data are mean±standard error from the rest, stress tasks, and recovery periods, n=30. BMI indicates body mass index; RMSSD, square root of the mean squared differences of successive R‐R intervals.
Statistical significance for comparison between Control and Pistachio diets by PROC MIXED in SAS, after adjustment for age, sex, BMI, and baseline (run‐in) values.
Twenty‐four‐hour systolic blood pressure was significantly lower following the pistachio diet compared to the control diet (−3.0±1.8%, P=0.046; Table 5), with the largest difference occurring during sleep (−5.3±2.5%, P=0.052). Diastolic blood pressure did not differ between treatments, and there were no differences in dipping status (defined as ≥10% reduction in systolic blood pressure during sleep) between the treatments (data not shown).
Table 5.
Ambulatory Blood Pressure
| Variable | Period* | Baseline | Control | Pistachio | Mean Difference Pistachio—Control | P Value* |
|---|---|---|---|---|---|---|
| Systolic BP, mm Hg | 24‐hour | 115.8±2.0 | 117.3±2.3 | 113.8±2.0 | −3.5±2.1 | 0.046 |
| Wake | 119.1±2.3 | 122.4±2.8 | 119.4±2.3 | −3.0±2.6 | 0.23 | |
| Sleep | 107.0±2.8 | 106.7±2.9 | 101.1±2.4 | −5.7±2.6 | 0.052 | |
| Diastolic BP, mm Hg | 24‐hour | 70.0±1.2 | 71.2±1.2 | 69.2±1.2 | −2.0±1.2 | 0.11 |
| Wake | 73.0±1.5 | 74.4±1.9 | 73.5±1.7 | −0.9±1.8 | 0.66 | |
| Sleep | 62.3±1.7 | 63.0±1.6 | 58.8±1.5 | −4.1±1.5 | 0.12 |
Data are mean±standard error, n=21. BMI indicates body mass index; BP, blood pressure.
24‐hour includes all readings; wake includes readings from 10 am to 9 pm; sleep includes readings from 1 am to 5:30 am.
Statistical significance for comparison between Control and Pistachio diets by PROC MIXED in SAS, after adjustment for age, sex, BMI, and baseline (run‐in) values.
Discussion
In this randomized, crossover, controlled‐feeding study, we have shown that consuming 20% of total energy from pistachios for 4 weeks modifies systemic hemodynamics, increases heart rate variability, and reduces 24‐hour systolic ambulatory blood pressure in adults with well‐controlled type 2 diabetes, but does not lower resting blood pressure or diastolic ambulatory blood pressure relative to a low‐fat control diet. Our goal was to demonstrate that diet can improve some cardiovascular risk factors, even in adults early in disease progression with well‐controlled type 2 diabetes. This is also the primary limitation of this study: we acknowledge that our results may not be relevant to individuals with more advanced disease. However, we offer evidence of an effective, preventative, low‐risk strategy in a group that remains at risk of vascular events in spite of their glycemic control.
Previous studies of nuts and blood pressure have relied on the traditional clinic method of measuring blood pressure under resting conditions. However, blood pressure during acute mental stress is an independent predictor of cardiovascular risk,26 and the shifts we observed in underlying hemodynamics (reduced total peripheral resistance and increased cardiac output) could have important clinical implications even in the absence of blood pressure reductions. In the majority of patients with essential hypertension, total peripheral resistance is elevated while cardiac output is normal.27 Peripheral resistance is an important contributor to left ventricular hypertrophy, which is an independent predictor of cardiovascular outcomes. Furthermore, a reduction in left ventricular hypertrophy lowers cardiovascular risk independent of changes in blood pressure.28 Based on a meta‐analysis of 50 studies, the medications that most effectively reverse left ventricular hypertrophy are those that reduce peripheral resistance (angiotension‐converting enzyme inhibitors and calcium channel blockers).29 Thus, it is plausible that the reduction in total peripheral resistance that we observed following pistachio consumption could contribute to a reversal of left ventricular hypertrophy. Unfortunately, we did not measure left ventricular mass in the present study, and it is unlikely that a clinically significant change in left ventricular mass could occur during a 4‐week dietary intervention. Future research about the mechanisms underlying the hemodynamic shifts that we observed should include longer diet periods and assessment of left ventricular mass and remodeling.
To our knowledge, this is the first study on nuts to include ambulatory blood pressure monitoring, which is a better predictor of target organ damage than traditional clinic blood pressure measurements.30 We observed reductions of both 24‐hour and sleep systolic blood pressure following the pistachio diet. Blood pressure normally decreases (or “dips”) during sleep, and individuals who do not display this physiological change can be 66% more likely to experience a cardiovascular event.31 Therefore, a significant reduction in 24‐hour blood pressure that is due primarily to a reduction in sleep blood pressure could be particularly beneficial to cardiovascular health. Unfortunately, ambulatory assessment of cardiac output and peripheral resistance is difficult to perform, so it is unknown how the underlying hemodynamics shifted during the ambulatory monitoring to reduce blood pressure. The observed reduction in ambulatory systolic blood pressure was small, but such a change on a population level is estimated to reduce coronary heart disease and stroke mortality by 5% to 8%.32 Importantly, this finding indicates that among adults with type 2 diabetes and normal blood pressure at baseline (Table 1), pistachio consumption can further reduce blood pressure and potentially lower residual vascular risk. These findings must be replicated in a larger sample, including individuals with more advanced diabetes.
Heart rate variability is also a recognized cardiovascular risk factor,33–35 and we observed improvements in 3 indices of heart rate variability following pistachio consumption: RMSSD, high frequency, and low frequency. Both RMSSD and high frequency reflect vagal tone, and low frequency is believed to reflect both sympathetic and parasympathetic function. In the Atherosclerosis Risk In Communities studies, individuals with type 2 diabetes who were in the lowest quartile of either high or low frequency heart rate variability were 50% to 80% more likely to develop coronary heart disease during an 8‐year follow‐up.36 The effect of pistachios on heart rate variability that we observed in the present study may be a yet unexplored mechanism through which nuts benefit cardiovascular health.
Given that this study utilized a controlled‐feeding protocol with pistachios, we can only speculate which component(s) of pistachios may be responsible for the observed changes. Like all nuts, pistachios have a heart‐healthy fatty acid profile (low in saturated fat, high in unsaturated fat) and are rich in plant protein, fiber, vitamins, and minerals. Pistachios are a source of potassium, magnesium, and phytosterols. Notably, in the present study the ratio of sodium to potassium was substantially lower for the pistachio diet (0.63) than for the control diet (0.81). This difference was due to the high potassium content of pistachios (≈600 mg for 69 g/day for the 2100 calorie level) and the sodium content of the control diet snacks (ie, pretzels, string cheese, etc.) that were replaced by the pistachios (≈500 mg per day for the 2100 calorie diet). It is possible that a single bioactive component of pistachios is responsible for the observed changes in cardiovascular risk factors, but it is more likely that the macro‐ and micronutrient profiles combined contribute to their health benefits.
As is common in clinical nutrition trials, this study examined the effect of a dietary intervention on multiple cardiovascular risk factors, which increase the risk of Type I error. The a priori primary outcome of the study was cardiovascular responses to stress, which included the blood pressure, systemic hemodynamics, and heart rate variability data. We reported unadjusted P values for these statistical tests, but we did explore the effect of multiple comparisons (n=17 dependent variables) on our results using the Benjamini‐Hochberg False Discovery Rate method.37 With this approach, statistical significance for the difference between the pistachio and control diets was maintained for stroke volume (P=0.046), cardiac output (P=0.034), total peripheral resistance (P=0.037), and high‐frequency heart rate variability (P=0.041), but not RMSSD (P=0.09), low‐frequency heart rate variability (P=0.11), or ambulatory systolic blood pressure (24‐hour and sleep, P=0.11 for both). Therefore, our findings for heart rate variability and, particularly, ambulatory blood pressure (given that it included only a sub‐sample of the participants) should be interpreted with caution and must be replicated in future studies.
The present study has a few limitations. First, the sample included only adults with well‐controlled (average HbA1c <6.5% at baseline) and relatively “early” diabetes (17% taking no diabetes medication, 66% on a single diabetes medication, and no insulin users). We relied on self‐reported and staff‐monitored compliance to the study diets and procedures and did not assess adherence with biological measures. We are unable to draw conclusions about pistachio consumption in amounts other than the 20% of energy daily that was evaluated here. We compared the pistachio diet to a low‐fat control diet that was designed in accordance with current recommendations for type 2 diabetes,8 but did not test it against other dietary patterns such as the Mediterranean diet. Potassium and sodium levels of the control and pistachio diets differed, which may have influenced the observed results on hemodynamics. We did not measure urinary potassium and sodium, and therefore cannot comment on how these levels may have been affected by the treatment periods. Future studies should match sodium and potassium levels between test diets and include measurement of urinary excretions. The controlled‐feeding protocol enabled us to assess the efficacy of pistachio consumption in 4 weeks, but does not provide information on the effectiveness of a pistachio intervention over a longer period of time. Finally, the total sample was relatively small, and only a portion of the subjects completed the ambulatory blood pressure monitoring. Future studies should enroll larger samples, include ambulatory blood pressure as a primary outcome, and test the effectiveness of pistachio consumption on cardiovascular risk factors in a free‐living setting.
In conclusion, this carefully controlled randomized clinical trial indicates that replacing low‐fat snacks with pistachios equal to 20% of energy daily modifies systemic hemodynamics, increases heart rate variability, and reduces ambulatory blood pressure in adults with well‐controlled type 2 diabetes. This study provides further evidence that daily nut consumption benefits multiple cardiovascular risk factors and may be an effective strategy for reducing residual vascular risk.
Sources of Funding
The study was supported primarily by the American Pistachio Growers (Fresno, CA), and, in part, by grants UL1RR033184/UL1TR000127 (PSU) and F31AG043224 (Sauder) from the NIH.
Disclosures
All authors have received grant and other research support for the present study from American Pistachio Growers (Fresno, CA). Pistachios for the present study were donated by American Pistachio Growers (Fresno, CA).
Acknowledgments
The project described was supported by the National Center for Research Resources, Grant UL1RR033184, and is now at the National Center for Advancing Translational Sciences, Grant UL1TR000127. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The expertise provided by Constance Geiger, PhD, is appreciated. The authors’ responsibilities were as follows: Drs West, Kris‐Etherton, and Ulbrecht designed the research; Sauder and McCrea conducted the research; Sauder analyzed the data; Sauder and West wrote the paper; and West had primary responsibility for final content. All authors read and approved the final manuscript.
References
- 1.Ford ES, Capewell S. Coronary heart disease mortality among young adults in the U.S. from 1980 through 2002: concealed leveling of mortality rates. J Am Coll Cardiol. 2007; 50:2128-2132. [DOI] [PubMed] [Google Scholar]
- 2.O'Flaherty M, Ford E, Allender S, Scarborough P, Capewell S. Coronary heart disease trends in England and Wales from 1984 to 2004: concealed levelling of mortality rates among young adults. Heart. 2008; 94:178-181. [DOI] [PubMed] [Google Scholar]
- 3.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail‐Beigi F, Grimm RH, Jr, Probstfield JL, Simons‐Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008; 358:2545-2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008; 358:2560-2572. [DOI] [PubMed] [Google Scholar]
- 5.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009; 360:129-139. [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association. Standards of medical care in diabetes–2014. Diabetes Care. 2014; 37suppl 1:S14-S80. [DOI] [PubMed] [Google Scholar]
- 7.Fruchart JC, Sacks F, Hermans MP, Assmann G, Brown WV, Ceska R, Chapman MJ, Dodson PM, Fioretto P, Ginsberg HN, Kadowaki T, Lablanche JM, Marx N, Plutzky J, Reiner Z, Rosenson RS, Staels B, Stock JK, Sy R, Wanner C, Zambon A, Zimmet P. The Residual Risk Reduction Initiative: a call to action to reduce residual vascular risk in patients with dyslipidemia. Am J Cardiol. 2008; 102:1K-34K. [DOI] [PubMed] [Google Scholar]
- 8.Evert AB, Boucher JL, Cypress M, Dunbar SA, Franz MJ, Mayer‐Davis EJ, Neumiller JJ, Nwankwo R, Verdi CL, Urbanski P, Yancy WS., Jr Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013; 36:3821-3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Djousse L, Rudich T, Gaziano JM. Nut consumption and risk of hypertension in US male physicians. Clin Nutr. 2009; 28:10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weng LC, Steffen LM, Szklo M, Nettleton J, Chambless L, Folsom AR. A diet pattern with more dairy and nuts, but less meat is related to lower risk of developing hypertension in middle‐aged adults: the Atherosclerosis Risk in Communities (ARIC) study. Nutrients. 2013; 5:1719-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibarrola‐Jurado N, Bullo M, Guasch‐Ferre M, Ros E, Martinez‐Gonzalez MA, Corella D, Fiol M, Warnberg J, Estruch R, Roman P, Aros F, Vinyoles E, Serra‐Majem L, Pinto X, Covas MI, Basora J, Salas‐Salvado JPREDEIMED Study Investigators. Cross‐sectional assessment of nut consumption and obesity, metabolic syndrome and other cardiometabolic risk factors: the PREDIMED study. PLoS One. 2013; 8:e57367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Neil CE, Keast DR, Nicklas TA, Fulgoni VL., III Nut consumption is associated with decreased health risk factors for cardiovascular disease and metabolic syndrome in U.S. adults: NHANES 1999–2004. J Am Coll Nutr. 2011; 30:502-510. [DOI] [PubMed] [Google Scholar]
- 13.Casas‐Agustench P, Lopez‐Uriarte P, Ros E, Bullo M, Salas‐Salvado J. Nuts, hypertension and endothelial function. Nutr Metab Cardiovasc Dis. 2011; 21suppl 1:S21-S33. [DOI] [PubMed] [Google Scholar]
- 14.Estruch R, Martinez‐Gonzalez MA, Corella D, Salas‐Salvado J, Ruiz‐Gutierrez V, Covas MI, Fiol M, Gomez‐Gracia E, Lopez‐Sabater MC, Vinyoles E, Aros F, Conde M, Lahoz C, Lapetra J, Saez G, Ros E. Effects of a Mediterranean‐style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med. 2006; 145:1-11. [DOI] [PubMed] [Google Scholar]
- 15.Salas‐Salvado J, Fernandez‐Ballart J, Ros E, Martinez‐Gonzalez MA, Fito M, Estruch R, Corella D, Fiol M, Gomez‐Gracia E, Aros F, Flores G, Lapetra J, Lamuela‐Raventos R, Ruiz‐Gutierrez V, Bullo M, Basora J, Covas MI. Effect of a Mediterranean diet supplemented with nuts on metabolic syndrome status: one‐year results of the PREDIMED randomized trial. Arch Intern Med. 2008; 168:2449-2458. [DOI] [PubMed] [Google Scholar]
- 16.West SG, Gebauer SK, Kay CD, Bagshaw DM, Savastano DM, Diefenbach C, Kris‐Etherton PM. Diets containing pistachios reduce systolic blood pressure and peripheral vascular responses to stress in adults with dyslipidemia. Hypertension. 2012; 60:58-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parati G, Bilo G, Ochoa JE. Benefits of tight blood pressure control in diabetic patients with hypertension: importance of early and sustained implementation of effective treatment strategies. Diabetes Care. 2011; 34suppl 2:S297-S303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris JA, Benedict FG. A biometric study of human basal metabolism. Proc Natl Acad Sci USA. 1918; 4:370-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001; 285:2486-2497. [DOI] [PubMed] [Google Scholar]
- 20.Gronwall DM. Paced auditory serial‐addition task: a measure of recovery from concussion. Percept Mot Skills. 1977; 44:367-373. [DOI] [PubMed] [Google Scholar]
- 21.Sherwood A, Allen MT, Fahrenberg J, Kelsey RM, Lovallo WR, van Doornen LJ. Methodological guidelines for impedance cardiography. Psychophysiology. 1990; 27:1-23. [DOI] [PubMed] [Google Scholar]
- 22.Sauder KA, Johnston ER, Skulas‐Ray AC, Campbell TS, West SG. Effect of meal content on heart rate variability and cardiovascular reactivity to mental stress. Psychophysiology. 2012; 49:470-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boardman A, Schlindwein FS, Rocha AP, Leite A. A study on the optimum order of autoregressive models for heart rate variability. Physiol Meas. 2002; 23:325-336. [DOI] [PubMed] [Google Scholar]
- 24.Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, van der Molen MW. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997; 34:623-648. [DOI] [PubMed] [Google Scholar]
- 25.Spruill TM, Gerin W, Ogedegbe G, Burg M, Schwartz JE, Pickering TG. Socioeconomic and psychosocial factors mediate race differences in nocturnal blood pressure dipping. Am J Hypertens. 2009; 22:637-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta‐analysis of prospective evidence. Hypertension. 2010; 55:1026-1032. [DOI] [PubMed] [Google Scholar]
- 27.Beevers G, Lip GY, O'Brien E. ABC of hypertension: the pathophysiology of hypertension. BMJ. 2001; 322:912-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Devereux RB, Wachtell K, Gerdts E, Boman K, Nieminen MS, Papademetriou V, Rokkedal J, Harris K, Aurup P, Dahlof B. Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA. 2004; 292:2350-2356. [DOI] [PubMed] [Google Scholar]
- 29.Schmieder RE, Schlaich MP, Klingbeil AU, Martus P. Update on reversal of left ventricular hypertrophy in essential hypertension (a meta‐analysis of all randomized double‐blind studies until December 1996). Nephrol Dial Transplant. 1998; 13:564-569. [DOI] [PubMed] [Google Scholar]
- 30.Franklin SS, Gustin W, IV, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age‐related changes in blood pressure. The Framingham Heart Study. Circulation. 1997; 96:308-315. [DOI] [PubMed] [Google Scholar]
- 31.Verdecchia P, Angeli F, Mazzotta G, Garofoli M, Ramundo E, Gentile G, Ambrosio G, Reboldi G. Day‐night dip and early‐morning surge in blood pressure in hypertension: prognostic implications. Hypertension. 2012; 60:34-42. [DOI] [PubMed] [Google Scholar]
- 32.Stamler R. Implications of the INTERSALT study. Hypertension. 1991; 17:I16-I20. [DOI] [PubMed] [Google Scholar]
- 33.Liao D, Cai J, Barnes RW, Tyroler HA, Rautaharju P, Holme I, Heiss G. Association of cardiac autonomic function and the development of hypertension: the ARIC study. Am J Hypertens. 1996; 9:1147-1156. [DOI] [PubMed] [Google Scholar]
- 34.Liao D, Cai J, Rosamond WD, Barnes RW, Hutchinson RG, Whitsel EA, Rautaharju P, Heiss G. Cardiac autonomic function and incident coronary heart disease: a population‐based case‐cohort study. The ARIC Study. Am J Epidemiol. 1997; 145:696-706. [DOI] [PubMed] [Google Scholar]
- 35.Tsuji H, Larson MG, Venditti FJ, Jr, Manders ES, Evans JC, Feldman CL, Levy D. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996; 94:2850-2855. [DOI] [PubMed] [Google Scholar]
- 36.Liao D, Carnethon M, Evans GW, Cascio WE, Heiss G. Lower heart rate variability is associated with the development of coronary heart disease in individuals with diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Diabetes. 2002; 51:3524-3531. [DOI] [PubMed] [Google Scholar]
- 37.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. 1995; 57:259-300. [Google Scholar]


