Abstract
Background
Changes in quality of life (QoL) after catheter ablation for long‐standing persistent atrial fibrillation (LSPAF) are not well described. We sought to compare QoL improvement after catheter ablation of paroxysmal atrial fibrillation (PAF) versus that after LSPAF.
Methods and Results
A total of 261 PAF and 126 LSPAF ablation recipients were prospectively followed for arrhythmia recurrence, QoL, hospital stay, and sick leave. In PAF versus LSPAF groups, 1.3±0.6 versus 1.6±0.7 procedures were performed per patient (P<0.00001) during a 3‐year follow‐up. Good arrhythmia control was achieved in 86% versus 87% of patients (P=0.69) and in 69% versus 69% of patients not receiving antiarrhythmic drugs (P=0.99). The baseline QoL was better in the PAF than in the LSPAF group (European Quality of Life Group instrument self‐report questionnaire visual analog scale: 66.4±14.2 versus 61.0±14.2, P=0.0005; European Quality of Life Group 3‐level, 5‐dimensional descriptive system: 71.4±9.2 versus 67.7±13.8, P=0.002). Postablation 3‐year increase in QoL was significant in both groups (all P<0.00001) and significantly lower in PAF versus LSPAF patients (visual analog scale: +5.0±14.5 versus +10.2±12.8, P=0.001; descriptive system: +5.9±14.3 versus +9.3±13.9, P=0.03). In multivariate analysis, LSPAF, less advanced age, shorter history of AF and good arrhythmia control were consistently associated with postablation 3‐year improvement in QoL. Days of hospital stay for cardiovascular reasons and days on sick leave per patient/year were significantly reduced in both groups.
Conclusions
Patients with LSPAF had worse baseline QoL. The magnitude of QoL improvement after ablation of LSPAF was significantly greater compared with after ablation of PAF, particularly when good arrhythmia control was achieved without the use of antiarrhythmic drugs.
Keywords: atrial fibrillation, long‐standing persistent, paroxysmal, quality of life
Introduction
Symptom reduction and improvement in quality of life (QoL) constitute the major incentives for catheter ablation of atrial fibrillation (AF).1–2 Postablation QoL improvement has been demonstrated in prior trials including in patients with paroxysmal AF (PAF)3–8 or persistent AF.9–11 Although some studies of persistent AF included certain proportions of patients with long‐standing persistent AF (LSPAF), data on QoL after catheter ablation for purely LSPAF are limited. This lack of data is reflected in current guidelines by different indications for catheter ablation for persistent AF and LSPAF.1–2 Generally lower ablation efficacy and doubts about the reversibility of hemodynamic and functional impairment associated with LSPAF continue to raise concerns about clinical benefits from successful LSPAF ablation beyond the restoration of sinus rhythm (SR).
We have recently shown a significant hemodynamic, functional, and QoL postablation improvement in a large population with LSPAF.12 However, QoL improvement between patients with LSPAF and PAF has not been compared. In this long‐term prospective study, we hypothesized that the postablation improvement in QoL and morbidity is not inferior in LSPAF patients with primary extensive ablation compared with PAF patients, who mostly undergo pulmonary vein isolation alone. We also hypothesized that QoL improvement is dependent not only on good arrhythmia control but also on patients’ baseline characteristics, which may help preprocedural patient selection for invasive procedures.
Methods
Population
We consecutively scrutinized 285 patients with PAF and 127 patients with LSPAF who underwent their first AF ablation at 2 centers between January 2007 and July 2009. The majority of LSPAF patients included in this study were previously investigated for global hemodynamic, functional, and QoL benefit.12 Study investigations were approved by the local ethics committee, and all patients gave written informed consent.
Electroanatomic Mapping and Catheter Ablation
During the procedure, a 10‐pole circular catheter (Lasso; Biosense Webster) and a mapping/ablation catheter (NaviStar ThermoCool; Biosense Webster) were inserted via 2 nonsteerable transseptal sheaths into the left atrium (LA). Heparin was given to maintain activated clotting time of 300 to 400 seconds.
Electroanatomic imaging was performed by the CARTO system (Biosense Webster). Radiofrequency energy was applied with a Stockert (Biosense Webster) generator with irrigation of 17 to 30 mL/min and temperature and power limits of 42°C and 35 W, respectively. Irrigation and power were limited to 20 mL/min and 20 to 25 W, respectively, inside the coronary sinus (CS).
Patients with PAF underwent wide‐area pulmonary vein (PV) isolation validated with use of the circular catheter. They received supplementary ablation for extra PV arrhythmic sources when manifested during the procedure. Likewise, cavotricuspid isthmus ablation was performed when typical atrial flutter was previously documented or occurred during the procedure. Ablation strategy in patients with LSPAF has been described in detail elsewhere.12 Briefly, mandatory wide‐area PV isolation with mitral isthmus, LA roof, and cavotricuspid isthmus ablation were successively performed in all patients. When AF continued, a stepwise approach consisting of CS ablation/isolation and additional linear and electrogram‐guided ablation was performed with the desired end point of SR restoration through ablation.
The end point of repeat procedures, in addition to SR restoration by ablation, PV reisolation, and restoration of conduction block across linear lesions, included noninducibility of AF or atrial tachycardia (AT) by incremental pacing up to 300 bpm and intravenous challenge with isoproterenol and/or adenosine.
Follow‐up
Patients were regularly seen at the outpatient department every 3 months during the first year and every 6 months subsequently. Class I or III antiarrhythmic drugs (AADs) were discontinued at the 3‐month visit in patients with uneventful follow‐up. In patients with stable SR and absence of other conditions favoring permanent anticoagulation, warfarin was stopped 3 months after PAF ablation and 6 months after LSPAF ablation in the case of preserved LAA function (outflow velocity ≥40 cm/s). ECG documentation consisted of standard 12‐lead ECG plus 24‐hour ECG recordings before each visit. In addition, transtelephonic ECG monitoring (3‐week episodic recorder or 7‐day loop recorder) was performed twice a year. Arrhythmia recurrence was defined as documented AF/AT lasting >30 seconds. Good arrhythmia control at the end of the follow‐up was defined as the absence of any arrhythmia during the minimum of the past 6 months and ≤1 electrical or pharmacological cardioversion in the past 2 years. To evaluate the impact of SR on QoL, rhythm status assessment was performed annually. The patients were classified as AF/AT free either in the absence of AF/AT recurrence in the previous 12 months or when successful repeat ablation was done ≥3 months before QoL assessment, which was deemed sufficient to experience the benefits of a recent ablation procedure.
Symptoms, QoL, Hospital Stay, and Sick Leave
QoL was assessed by using the European Quality of Life Group instrument self‐report questionnaire consisting of 2 parts: (1) EQ‐VAS (visual analog scale of 0 to 100 for recording an individual's rating of his or her current health‐related QoL state) and (2) the 3‐level, 5‐dimensional descriptive system (EQ‐5D), which evaluates mobility, self‐care, usual activities, pain/discomfort, and anxiety/depression on a 0‐to‐100 scale.13 Through the questionnaire supplement, the patients provided AF‐related symptoms, count of days spent in hospital for cardiovascular reasons (excluding hospital admissions for AF ablation), and sick leave burden within the past 12 months retrieved from the discharge medical and sick leave records. Carefully instructed patients completed the questionnaire without assistance from medical staff at baseline and annually afterward. The questionnaires were gathered and evaluated by an independent investigator, while the physicians performing ablation were blinded to the QoL. The proportion of patients with ≥1 hospitalizations, the number of days spent in hospital, and the sick leave burden per patient/year were assessed annually.
Statistical Analysis
Continuous variables were expressed as mean±SD or median with interquartile range. Simple comparisons were performed by using the 2‐tailed t test for dependent or independent samples or by Mann–Whitney U test, as appropriate. Categorical variables were expressed as percentages and compared by using χ2 test. Kaplan–Meier analysis with log‐rank statistics was used for the subgroup comparison of cumulative arrhythmia‐free survival after the first and final ablations.
Beside the main study groups according to AF type (PAF and LSPAF), other 3 subgroups were defined by the final rhythm status categorized as good arrhythmia control: (1) presently off AADs, (2) presently on AADs, or (3) absent. In addition, 2 smaller subgroups were composed of patients who were classified as being either consistently AF/AT free or consistently non–AF/AT free across all annual assessments.
Analysis of QoL was performed separately for EQ‐VAS and EQ‐5D indices. They were studied in the above‐mentioned study groups and subgroups. The QoL trends across all study periods in the main study groups and in the individual subgroups were investigated by using ANOVA for repeated measures. The change in QoL between baseline and 3‐year follow‐up visit was investigated by using 2‐factorial ANOVA with AF type and rhythm status (as described) as independent variables. Scheffè's test for post‐hoc comparisons was used in ANOVA statistics.
Pearson's univariate correlation was used to assess the association between baseline–to–3‐year change in QoL and baseline characteristics of patients or their relevant outcome measures. This analysis was performed in total population and for PAF and LSPAF subgroups. All factors that correlated (P<0.10) with QoL change according to any of these 3 univariate analyses entered the multivariate regression models and were analyzed with the stepwise forward method. Individual factors were included as either continuous or categorical variables. All categorical factors were binary except the baseline–to–3‐year change in AADs (Class I or III) treatment status, which had 3 categories: AADs discontinued/not changed/resumed. A P‐value <0.05 was considered significant. All analyses were performed using the STATISTICA version 12 software (Statsoft, Inc).
Results
Baseline Characteristics and Symptoms
Of consecutive 285 PAF and 127 LSPAF patients, 261 PAF and 126 LSPAF patients had baseline QoL assessment available and were included in the study. QoL survey response rate was 100%, 100%, and 96% in years 1, 2, and 3 after the ablation (comparable in both groups), respectively. Patients who were excluded at baseline did not differ statistically in clinical characteristics and ablation outcome from the investigated population. The same applied for the subgroups of patients with complete and incomplete QoL information. Baseline characteristics are listed in Table 1. Symptoms of palpitations, chest discomfort, dizziness, and presyncope were more frequently reported by the PAF patients. Incapacity, dyspnea, and fatigue were more common in patients with LSPAF (Table 2).
Table 1.
Baseline Characteristics
| PAF (n=261) | LSPAF (n=126) | P Value | |
|---|---|---|---|
| Females | 86 (33%) | 27 (21%) | 0.02 |
| Age, y | 57±10 (24 to 78) | 59±9 (31 to 75) | 0.04 |
| History of AF, mo | 47 IQR 28 to 80 (9 to 444) | 61 IQR 40 to 95 (13 to 504) | 0.002* |
| LSPAF duration, mo | NA | 28 IQR 18 to 47 (13 to 254) | |
| Hypertension | 148 (57%) | 87 (69%) | 0.02 |
| Diabetes mellitus | 33 (13%) | 20 (16%) | 0.39 |
| Stroke | 29 (11%) | 19 (15%) | 0.27 |
| History of heart failure | 20 (8%) | 27 (21%) | 0.0001 |
| CAD | 19 (7%) | 9 (7%) | 0.96 |
| CHADS2 score | 1.0±1.0 (0 to 4) | 1.3±1.0 (0 to 5) | 0.002 |
| CHA2DS2VASc score | 1.6±1.4 (0 to 6) | 1.9±1.3 (0 to 6) | 0.08 |
| LA anteroposterior diameter, mm | 43±6 (26 to 64) | 48±6 (33 to 68) | <0.0001 |
| LV ejection fraction, % | 58±6 (30 to 70) | 54±10 (25 to 70) | <0.0001 |
| Body mass index, kg/m2 | 28.9±5.0 (18.4 to 52.7) | 30.7±4.7 (19.4 to 44.6) | 0.0009 |
| Retired | 102 (39%) | 48 (38%) | 0.85 |
| Disabled | 18 (7%) | 15 (12%) | 0.10 |
Data shown as mean±SD or median with interquartile range (IQR) with total range or proportions (%). AF indicates atrial fibrillation; CAD, coronary artery disease; LA, left atrial; LSPAF, long‐standing persistent AF; LV, left ventricular; NA, not applicable; PAF, paroxysmal AF.
P=P‐value of t test for independent samples and *Mann–Whitney U test (PAF vs LSPAF).
Table 2.
Preablation Symptoms
| PAF (n=261) | LSPAF (n=126) | P Value | |
|---|---|---|---|
| Palpitations | 220 (84%) | 43 (34%) | <0.0001 |
| Incapacity | 137 (52%) | 107 (85%) | <0.0001 |
| Dyspnea | 140 (54%) | 104 (83%) | <0.0001 |
| Fatigue | 141 (54%) | 87 (69%) | 0.005 |
| Dizziness | 45 (17%) | 12 (10%) | 0.04 |
| Chest discomfort | 48 (18%) | 7 (6%) | 0.0007 |
| Sweating | 49 (19%) | 22 (17%) | 0.75 |
| Restless sleep | 39 (15%) | 11 (9%) | 0.09 |
| Syncope | 19 (7%) | 5 (4%) | 0.21 |
| Presyncope | 35 (13%) | 6 (5%) | 0.01 |
Data shown as counts (proportions). LSPAF indicates long‐standing persistent atrial fibrillation; PAF, paroxysmal atrial fibrillation.
P=P‐value of χ2 test (PAF vs LSPAF).
Initial and Repeat Ablation and Long‐term Results
At the initial ablation, isolation of all PVs was completed in all patients. In the LSPAF group, AF was terminated (ie, converted directly into SR or into intermediate AT) and SR was restored by ablation in 92 (73%) and 65 (52%) patients, respectively. LA roof and mitral isthmus block was completed in 110 (87%) and 107 (85%) patients, respectively.
In the PAF versus the LSPAF group, there were 1.3±0.6 versus 1.6±0.7 ablation procedures per patient (P<0.00001)―2 procedures in 23% versus 36% of patients (P=0.006) and ≥3 procedures in 4% versus 14% of patients (P=0.0004), respectively. At first repeat ablation, paroxysmal AF was targeted in 65% versus 9% (P<0.00001), persistent AF in 15% versus 39% (P=0.002), paroxysmal AT in 2% versus 3% (P=0.71), and persistent AT in 18% versus 48% (P=0.0003) of the PAF versus LSPAF patients, respectively. At second repeat ablation, these proportions were 29% versus 14% (P=0.34), 21% versus 21% (P=1.0), 0% versus 7% (P=0.40), and 50% versus 57% (P=0.72) patients, respectively.
Freedom from AF/AT recurrence was achieved in 51% versus 29% of patients (P=0.002) following the initial ablation (Figure 1A) and in 68% versus 68% of patients (P=0.99) after the last (single or repeat) ablation (Figure 1B), for PAF versus LSPAF groups, respectively. Among patients with arrhythmia recurrences, paroxysmal AF/AT prevailed in those with baseline PAF versus LSPAF (81% versus 19%, P<0.00001).
Figure 1.
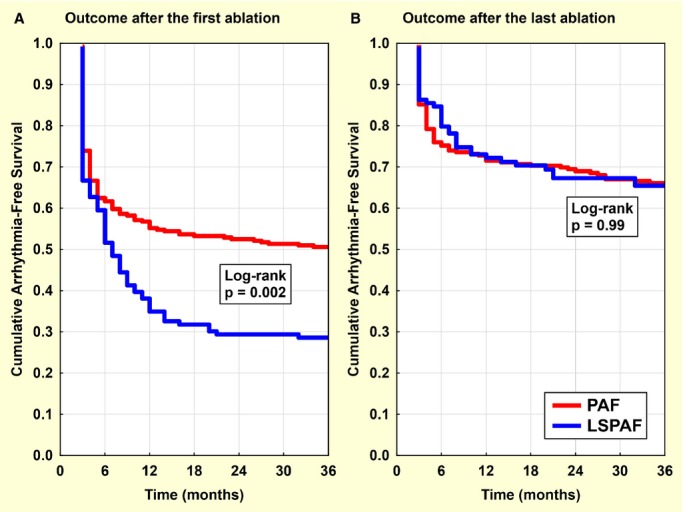
Freedom from arrhythmia after the first and last ablation. Kaplan–Meier curves for the outcome after the first (A) and the last (B) atrial fibrillation (AF) ablation. Arrhythmia‐free survival censored at 3 years in patients with paroxysmal AF (PAF) and long‐standing persistent AF (LSPAF) is compared by using log rank test.
At annual assessments, 60% and 53% of patients at 1 year, 64% and 61% of patients at 2 years, and 66% and 67% of patients at 3 years were classified as AF/AT free for the PAF and LSPAF group, respectively. QoL assessment was performed >3 months after any repeat ablation in 75% versus 62% (P=0.008), 88% versus 87% (P=0.82), and 93% versus 95% (P=0.41) of patients at follow‐up years 1, 2, and 3 for PAF versus LSPAF patients, respectively.
At the end of follow‐up, good arrhythmia control was present in 224 (86%) and 110 (87%) patients with PAF versus LSPAF (P=0.69), 69% versus 69% off AADs (P=0.99). Use of Class I and III AADs and warfarin was significantly reduced in both groups (Table 3).
Table 3.
Antiarrhythmic Drugs and Oral Anticoagulation Therapy
| PAF | LSPAF | |||||
|---|---|---|---|---|---|---|
| Preablation | Postablation | P Value | Preablation | Postablation | P Value | |
| Amiodarone | 93 (36%) | 28 (11%) | <0.0001 | 65 (52%) | 13 (10%) | <0.0001 |
| Sotalol | 42 (16%) | 21 (9%) | 0.005 | 10 (8%) | 6 (5%) | 0.30 |
| Propafenon | 100 (38%) | 20 (9%) | <0.0001 | 0 | 1 | — |
| Flecainide | 1 | 1 | — | 0 | 0 | — |
| Dronedarone | 0 | 2 | — | 0 | 1 | — |
| Warfarin | 247 (95%) | 82 (31%) | <0.0001 | 126 (100%) | 39 (31%) | <0.0001 |
Data shown as counts (proportions). LSPAF indicates long‐standing persistent atrial fibrillation; PAF, paroxysmal atrial fibrillation.
P=P‐value of χ2 test (preablation vs postablation).
Five (1.3%) patients (3 PAF, 2 LSPAF) died >25 months after the initial ablation. None of the deaths was directly related to ablation procedure (motorcycle accident, infective endocarditis after pacemaker implantation, hemorrhagic stroke on optimum warfarin, and 2 cases of heart failure).
Quality of Life
QoL was progressively improving with significant change evident after 12 months. The rate of improvement was steeper in LSPAF patients who had significantly worse baseline QoL, so that intergroup differences in QoL disappeared at months 24 and 36 (Table 4, Figure 2). The baseline–to–3‐year differences of QoL indices were significantly greater in LSPAF versus PAF patients (9.3±13.9 versus 5.9±14.3 for EQ‐5D, P=0.03, and 10.2±12.8 versus 5.0±14.5 for EQ‐VAS; P=0.001) and remained significant after the adjustment for final rhythm status (ANOVA P=0.03 and P=0.0007, respectively).
Table 4.
Quality of Life
| EQ‐VAS | EQ‐5D | |||||
|---|---|---|---|---|---|---|
| PAF | LSPAF | P Value | PAF | LSPAF | P Value | |
| Baseline | 66.4±14.2 | 61.0±14.2 | 0.0005 | 71.4±9.2 | 67.7±13.8 | 0.002 |
| 1 year | 69.0±13.9 | 65.8±14.6 | 0.04 | 74.2±11.3 | 73.1±15.0 | 0.40 |
| 2 years | 73.1±15.0 | 70.5±14.5 | 0.11 | 77.7±14.8 | 75.9±15.2 | 0.26 |
| 3 years | 71.4±16.4 | 71.1±14.2 | 0.86 | 77.2±15.4 | 77.1±14.0 | 0.95 |
Data shown as mean±SD. 5D indicates 5‐dimensional descriptive system; LSPAF, long‐standing persistent atrial fibrillation; PAF, paroxysmal atrial fibrillation; VAS, visual analog scale.
P=P‐value of t test for independent samples (PAF vs LSPAF).
Figure 2.
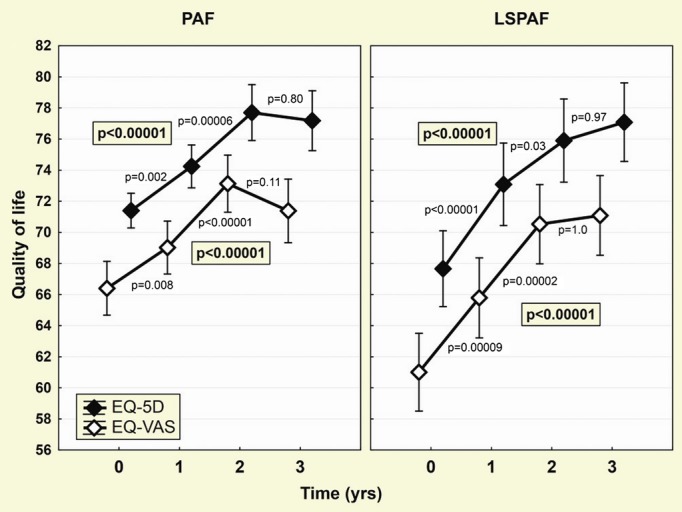
Improvement in quality of life (QoL) during the follow‐up. Evolution of both QoL measures (EQ‐VAS and EQ‐5D) during 3‐year follow‐up is shown separately for patients with paroxysmal AF (PAF) and long‐standing persistent AF (LSPAF). Points and whiskers represent mean and 95% CI. Global effects (P‐values shown inside the box) were assessed by ANOVA for repeated measures. Remaining P‐values are for post‐hoc comparison of fractional differences (Scheffè's test). 5D indicates 5‐dimensional descriptive system; AF, atrial fibrillation; EQ, European Quality of Life Group; VAS, visual analog scale.
Subgroup analysis in smaller subgroups of patients, who were consistently AF/AT free (n=140 in PAF group, n=72 in LSPAF group) or consistently non–AF/AT free (n=48 in PAF group, n=28 in LSPAF group) across all annual assessments (Figure 3), showed that baseline–to–3‐year improvement in QoL was highly significant in AF/AT‐free PAF and LSPAF patients (EQ‐5D: 9.6±14.4 and 10.4±12.8; EQ‐VAS: 7.0±14.5 and 11.5±13.5, respectively; all P<0.00001). In non–AF/AT‐free patients, there was no improvement in QoL compared with baseline in the PAF group (EQ‐5D: −0.5±12.2, P=0.74; EQ‐VAS: 1.3±13.8, P=0.46), while LSPAF patients exhibited modest improvement (EQ‐5D: 7.1±17.2, P=0.046; EQ‐VAS: 3.8±12.6, P=0.14). On ANOVA, there was no significant difference between PAF and LSPAF patients.
Figure 3.
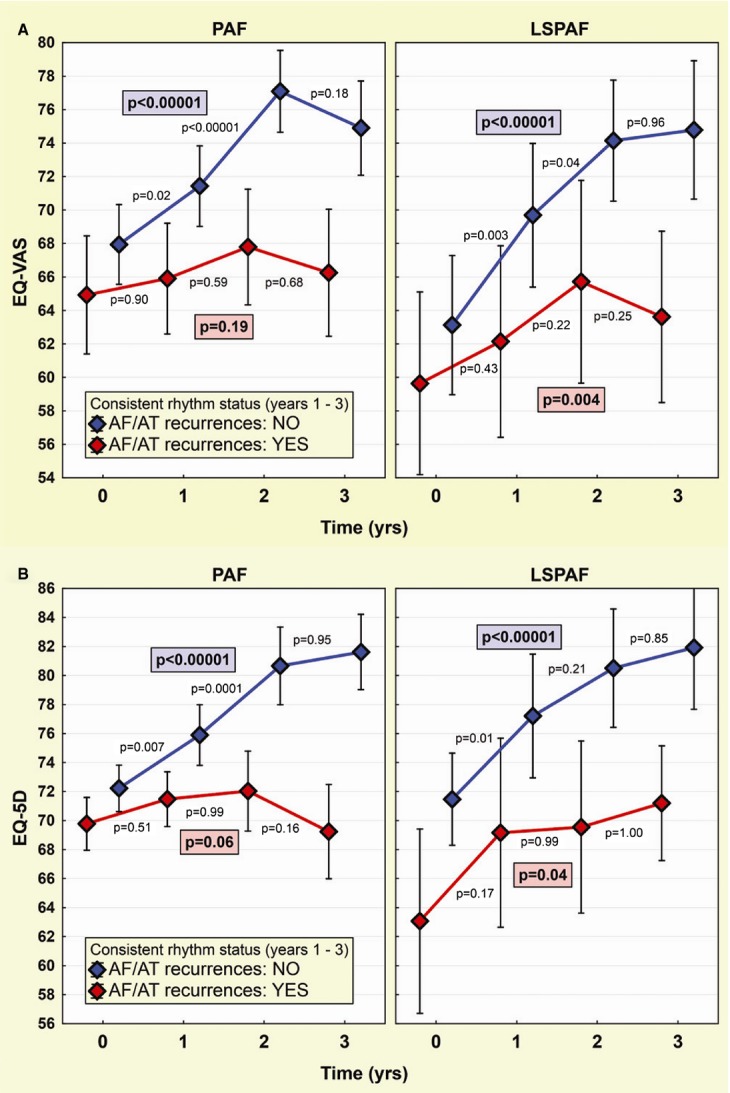
Change in quality of life in subgroups by prevailing rhythm status. Evolution of EQ‐VAS (A) and EQ‐5D (B) is shown separately for patients with paroxysmal AF (PAF) and long‐standing persistent AF (LSPAF). QoL trends (means and 95% CIs) are plotted for AF/AT‐free patients (blue) vs those with AF/AT recurrences (red) consistently at all annual QoL assessments. Layout of P‐values as in Figure 2. 5D indicates 5‐dimensional descriptive system; AF, atrial fibrillation; AT, atrial tachycardia; QoL, quality of life; EQ, European Quality of Life Group; VAS, visual analog scale.
Another subgroup analysis of QoL change according to the final good arrhythmia control is summarized in Figures 4 and 5 and Table 5. For both PAF and LSPAF patients, the baseline–to–3‐year improvement in QoL was highly significant in case of good arrhythmia control off AADs and completely missing in the absence of good arrhythmia control. When good arrhythmia control on AADs was achieved, the borderline QoL benefit was observed in LSPAF patients only. By ANOVA, there was no significant difference between PAF and LSPAF patients except the improvement in EQ‐VAS in the setting of good arrhythmia control off AADs, which was significantly greater in LSPAF versus PAF patients (P=0.01).
Figure 4.
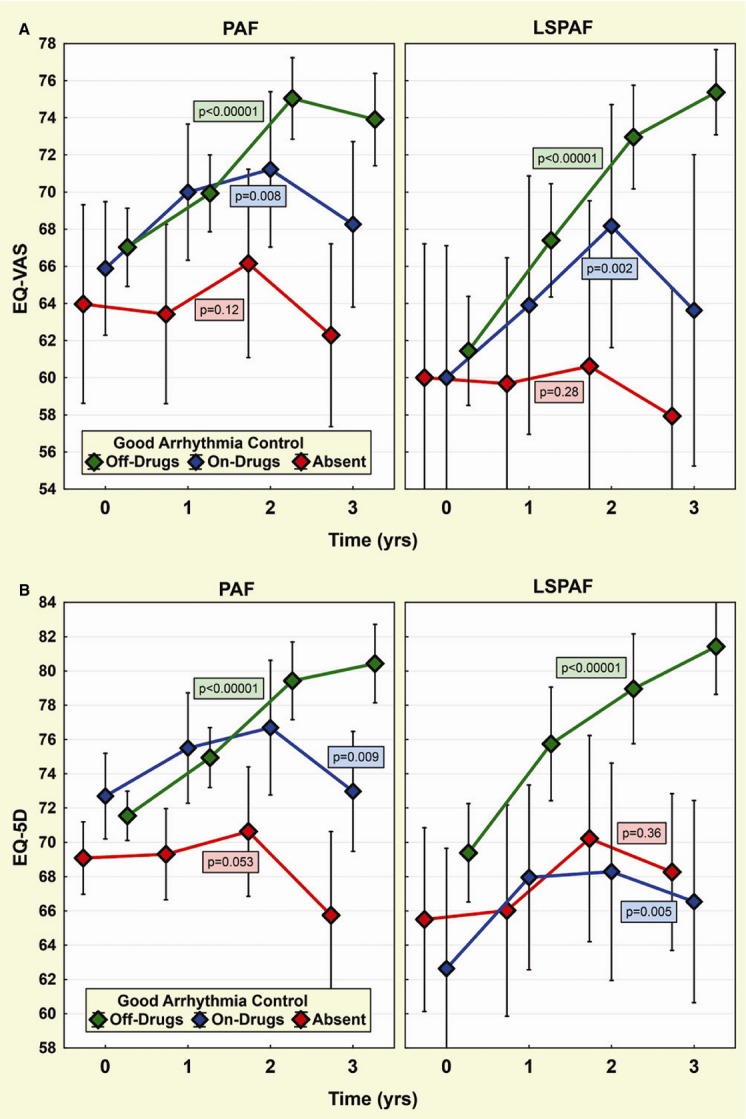
Change in quality of life in subgroups by final good arrhythmia control. Trends in EQ‐VAS (A) and EQ‐5D (B) for patients with paroxysmal AF (PAF) and long‐standing persistent AF (LSPAF) are plotted analogically to Figure 3. Three subgroups are composed according to good arrhythmia control (off drugs/on drugs/absent) at the end of follow‐up. Layout of P‐values as in Figure 2. 5D indicates 5‐dimensional descriptive system; AF, atrial fibrillation; EQ, European Quality of Life Group; VAS, visual analog scale.
Figure 5.
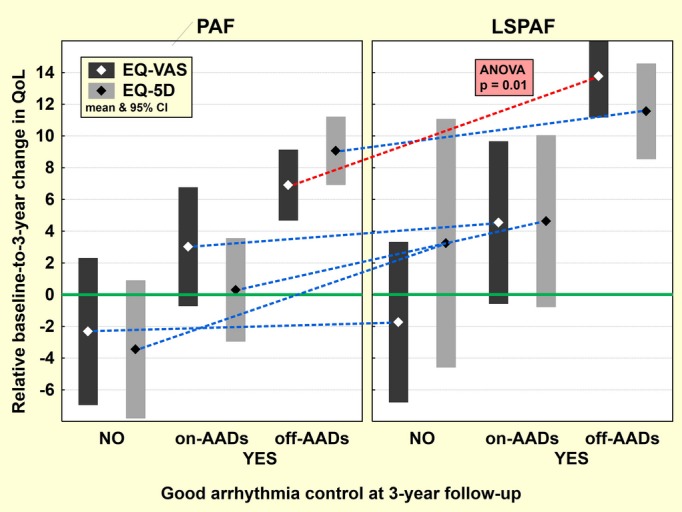
Relative change in quality of life in subgroups by final good arrhythmia control. Relative baseline–to–3‐year change in both QoL measures (EQ‐VAS and EQ‐5D) for patients with paroxysmal AF (PAF) and long‐standing persistent AF (LSPAF) is shown for 3 subgroups composed according to good arrhythmia control (off drugs/on drugs/absent) at the end of follow‐up. Points and bars represent mean and 95% CI. 5D indicates 5‐dimensional descriptive system; AADs, antiarrhythmic drugs; AF, atrial fibrillation; QoL, quality of life; EQ, European Quality of Life Group; VAS, visual analog scale.
Table 5.
Baseline–to–3‐Year Change in Quality of Life in Subgroups by AF Type and Final Good Arrhythmia Control
| AF Type | Good Arrhythmia Control | N | EQ‐5D | EQ‐VAS | ||||
|---|---|---|---|---|---|---|---|---|
| Change | P 1 | P 2 | Change | P 1 | P 2 | |||
| PAF | Off AADs | 174 | +9.1±14.3 | <0.00001 | 0.19 | +6.9±14.9 | <0.00001 | 0.0003 |
| On AADs | 42 | +0.3±10.4 | 0.85 | 0.14 | +3.0±12.0 | 0.11 | 0.63 | |
| Absent | 34 | −3.4±12.4 | 0.12 | 0.10 | −2.3±13.3 | 0.31 | 0.88 | |
| LSPAF | Off AADs | 84 | +11.6±13.8 | <0.00001 | +13.8±12.0 | <0.00001 | ||
| On AADs | 22 | +4.6±12.2 | 0.09 | +4.5±11.5 | 0.08 | |||
| Absent | 15 | +3.2±14.1 | 0.39 | −1.7±9.1 | 0.47 | |||
Data shown as mean±SD. 5D indicates 5‐dimensional descriptive system; AAD, antiarrhythmic drug; AF, atrial fibrillation; LSPAF, long‐standing persistent AF; PAF, paroxysmal AF; VAS, visual analog scale.
P1=P‐value of t test for dependent samples (baseline vs 3‐year follow‐up). P2=P‐value of t test for independent samples (PAF vs LSPAF).
Predictors of QoL Improvement
Univariate correlates of relative QoL change at the end of the study are shown in Table 6. When baseline factors only were investigated multivariately, greater QoL improvement was independently associated with less advanced age, presence of LSPAF, and shorter history of AF (Table 7). Outcome measures (good arrhythmia control and warfarin discontinuation) were also independent predictors of QoL improvement (Table 8). Their inclusion into the multivariate analysis did not considerably influence the association of baseline factors with QoL outcome. Other factors, like arterial hypertension, LA diameter, and body mass index, appeared to be inconsistent multivariate correlates of QoL improvement with borderline statistical significance.
Table 6.
Univariate Correlates of Quality of Life Change Between Baseline and 3‐Year Follow‐up
| Change in EQ‐VAS (Year 3–Baseline) | Change in EQ‐5D (Year 3–Baseline) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Population | PAF | LSPAF | Total Population | PAF | LSPAF | |||||||
| R | P Value | R | P Value | R | P Value | R | P Value | R | P Value | R | P Value | |
| Age, y | −0.19 | <0.00001 | −0.22 | <0.00001 | −0.19 | 0.04 | −0.24 | <0.00001 | −0.28 | <0.00001 | −0.19 | 0.03 |
| Female | −0.03 | 0.53 | 0.06 | 0.37 | −0.21 | 0.02 | −0.07 | 0.15 | −0.06 | 0.34 | −0.07 | 0.45 |
| LSPAF | 0.17 | 0.001 | NA | NA | NA | NA | 0.11 | 0.03 | NA | NA | NA | NA |
| AF history, mo | −0.13 | 0.01 | −0.18 | 0.006 | −0.10 | 0.25 | −0.16 | 0.003 | −0.14 | 0.03 | −0.23 | 0.01 |
| LSPAF, mo | NA | NA | NA | NA | −0.26 | 0.004 | NA | NA | NA | NA | −0.30 | 0.001 |
| BMI, kg/m2 | −0.05 | 0.36 | −0.04 | 0.51 | −0.18 | 0.053 | −0.10 | 0.055 | −0.10 | 0.13 | −0.18 | 0.048 |
| LA, mm | −0.07 | 0.20 | −0.18 | 0.005 | −0.08 | 0.40 | −0.07 | 0.18 | −0.12 | 0.054 | −0.13 | 0.16 |
| LVEF, % | 0.00 | 0.99 | 0.11 | 0.07 | −0.03 | 0.75 | 0.00 | 0.93 | 0.07 | 0.27 | −0.02 | 0.81 |
| LVEF ≤40% | 0.06 | 0.28 | −0.07 | 0.30 | 0.10 | 0.29 | 0.06 | 0.25 | 0.05 | 0.43 | 0.03 | 0.73 |
| Hypertension | −0.08 | 0.11 | −0.16 | 0.01 | 0.04 | 0.69 | −0.19 | 0.0002 | −0.21 | 0.001 | −0.19 | 0.03 |
| Diabetes mellitus | −0.05 | 0.35 | −0.05 | 0.43 | −0.07 | 0.43 | −0.11 | 0.03 | −0.05 | 0.41 | −0.25 | 0.007 |
| Stroke/TIA | −0.03 | 0.51 | −0.06 | 0.37 | −0.02 | 0.86 | −0.09 | 0.09 | −0.06 | 0.35 | −0.16 | 0.08 |
| CAD | −0.08 | 0.15 | −0.10 | 0.11 | −0.03 | 0.74 | 0.01 | 0.91 | 0.01 | 0.87 | −0.01 | 0.94 |
| Disability | 0.04 | 0.41 | 0.03 | 0.68 | 0.03 | 0.72 | 0.02 | 0.75 | −0.03 | 0.59 | 0.07 | 0.42 |
| Retired | −0.10 | 0.06 | −0.10 | 0.11 | −0.10 | 0.30 | −0.19 | 0.0002 | −0.20 | 0.002 | −0.18 | 0.047 |
| Sick leave | 0.12 | 0.02 | 0.12 | 0.06 | 0.10 | 0.29 | 0.12 | 0.02 | 0.11 | 0.08 | 0.11 | 0.24 |
| Hospitalization | 0.06 | 0.28 | 0.06 | 0.37 | 0.03 | 0.77 | 0.00 | 0.94 | 0.03 | 0.67 | −0.06 | 0.50 |
| Good AF/AT control | 0.24 | <0.00001 | 0.20 | 0.001 | 0.35 | <0.00001 | 0.23 | <0.00001 | 0.26 | 0.00003 | 0.16 | 0.07 |
| AADs change (−1/0/1) | −0.02 | 0.74 | −0.08 | 0.21 | −0.01 | 0.88 | −0.16 | 0.003 | −0.20 | 0.001 | −0.15 | 0.09 |
| Warfarin cessation | 0.21 | 0.00005 | 0.11 | 0.07 | 0.42 | <0.00001 | 0.26 | <0.00001 | 0.26 | 0.00004 | 0.25 | 0.006 |
5D indicates 5‐dimensional descriptive system; AAD, Class I/III antiarrhythmic drug (categories: −1=discontinued, 0=not changed, 1=resumed); AF, atrial fibrillation; AT, atrial tachycardia; BMI, body mass index; CAD, coronary artery disease; LA, left atrial; LSPAF (months), duration of continuous AF without intervening sinus rhythm; LSPAF, long‐standing persistent AF; LVEF, left ventricular ejection fraction; NA, not applicable; P, P‐value of correlation; PAF, paroxysmal AF; R, Pearson's correlation coefficient; TIA, transient ischemic attack; VAS, visual analog scale.
Table 7.
Baseline–to–3‐Year Change in Quality of Life: Multivariate Regression Model With Baseline Factors Only
| Change in EQ‐VAS (Year 3–Baseline) | Change in EQ‐5D (Year 3–Baseline) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Entered (Y/N) | Included (Y/N) | Coefficient | SD | P Value | Entered (Y/N) | Included (Y/N) | Coefficient | SD | P Value | |
| Regression intercept | 41.0 | 7.1 | <0.00001 | 42.3 | 6.2 | <0.00001 | ||||
| Age, y | Y | Y | −0.25 | 0.07 | 0.0009 | Y | Y | −0.34 | 0.07 | <0.00001 |
| Female | Y | N | N | |||||||
| LSPAF | Y | Y | 7.5 | 1.6 | <0.00001 | Y | Y | 5.1 | 1.5 | 0.001 |
| History of AF, mo | Y | Y | −0.027 | 0.012 | 0.02 | Y | Y | −0.032 | 0.012 | 0.009 |
| BMI, kg/m2 | Y | N | Y | Y | −0.35 | 0.14 | 0.02 | |||
| LA diameter, mm | Y | Y | −0.29 | 0.13 | 0.03 | Y | N | |||
| LVEF, % | Y | N | N | |||||||
| Hypertension | Y | N | Y | N | ||||||
| Diabetes mellitus | N | Y | N | |||||||
| Stroke/TIA | N | Y | N | |||||||
| CAD | N | N | ||||||||
Factors were entered depending on the results of univariate correlation analysis (for details, see the text) and included by stepwise forward method. 5D indicates 5‐dimensional descriptive system; AF, atrial fibrillation; BMI, body mass index; CAD, coronary artery disease; LA, left atrial; LSPAF, long‐standing persistent AF; LVEF, left ventricular ejection fraction; TIA, transient ischemic attack; VAS, visual analog scale; Y, yes; N, no.
Table 8.
Baseline–to–3‐Year Follow‐Up Change in Quality of Life: Multivariate Regression Model With Baseline and Outcome Factors
| Change in EQ‐VAS (Year 3–Baseline) | Change in EQ‐5D (Year 3–Baseline) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Entered (Y/N) | Included (Y/N) | Coefficient | SD | P Value | Entered (Y/N) | Included (Y/N) | Coefficient | SD | P Value | |
| Regression intercept | 28.9 | 7.6 | 0.0002 | 22.0 | 4.9 | <0.00001 | ||||
| Age, y | Y | Y | −0.22 | 0.07 | 0.004 | Y | Y | −0.21 | 0.08 | 0.009 |
| Female | Y | N | N | |||||||
| LSPAF | Y | Y | 6.9 | 1.6 | 0.00002 | Y | Y | 4.1 | 1.5 | 0.007 |
| History of AF, mo | Y | N | Y | Y | −0.028 | 0.012 | 0.02 | |||
| BMI, kg/m2 | Y | N | Y | N | ||||||
| LA diameter, mm | Y | Y | −0.28 | 0.13 | 0.03 | Y | N | |||
| LVEF, % | Y | N | N | |||||||
| Hypertension | Y | N | Y | Y | −3.8 | 1.5 | 0.01 | |||
| Diabetes mellitus | N | Y | N | |||||||
| Stroke/TIA | N | Y | N | |||||||
| CAD | N | N | ||||||||
| Good arrhythmia control | Y | Y | 8.4 | 2.1 | 0.00008 | Y | N | |||
| Change in AADs (−1/0/1) | N | Y | N | |||||||
| Warfarin discontinuation | Y | N | Y | Y | 6.2 | 1.5 | 0.00004 | |||
Factors were entered depending on the results of univariate correlation analysis (for details, see the text) and included by stepwise forward method. 5D indicates 5‐dimensional descriptive system; AAD, Class I/III antiarrhythmic drug (categories: −1=discontinued, 0=not changed, 1=resumed); AF, atrial fibrillation; BMI, body mass index; CAD, coronary artery disease; LA, left atrial; LSPAF, long‐standing persistent AF; LVEF, left ventricular ejection fraction; TIA, transient ischemic attack; VAS, visual analog scale; Y, yes; N, no.
Hospital Stay and Sick Leave
The proportion of patients with hospital admissions decreased from 40% before index ablation to 22%, 11%, and 11% in the subsequent 3 years and from 48% to 25%, 17%, and 8% in PAF and LSPAF patients, respectively. Accordingly, the number of days of hospital stay for cardiovascular reasons per patient/year was significantly reduced during the first postablation year, comparably in both groups (Figure 6). The number of days of sick leave per patient/year was also significantly and comparably reduced in both groups (Figure 6).
Figure 6.
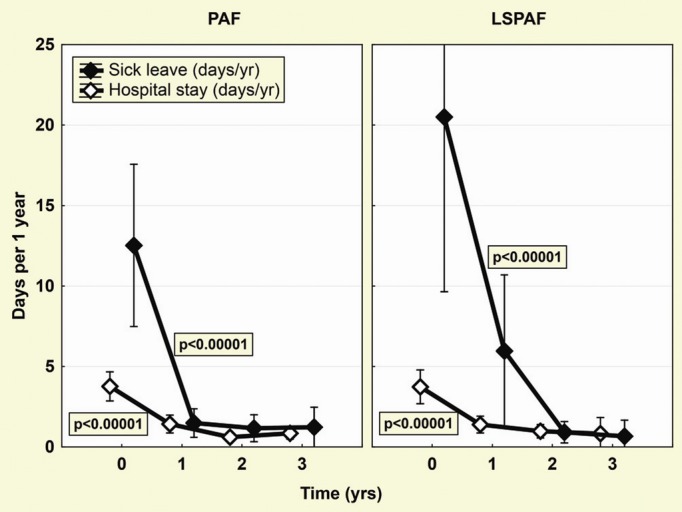
Hospitalizations and sick leave. Significant reduction of days spent in hospital or on sick leave is shown separately for patients with paroxysmal AF (PAF) and long‐standing persistent AF (LSPAF). Global effects (P‐values shown inside the box) were assessed by ANOVA for repeated measures. AF indicates atrial fibrillation.
Discussion
This study investigated how catheter ablation affects QoL in patients with PAF and LSPAF—the opposite poles in terms of arrhythmogenic substrate, clinical manifestation, and ablation success rate. Short‐term persistent AF patients were purposely excluded to avoid an overlap of investigated groups with ambiguity in clinical manifestation and degree of structural remodeling as it can occur between the patients with short‐lasting (7 days to weeks) persistent AF and PAF, on the one hand, and between AF persisting for nearly 1 year versus true LSPAF, on the other. The response rate to the QoL questionnaire during follow‐up was favorable. In the population of purely consecutive patients, only a small proportion denied to participate from the very beginning. In the others, we collected almost complete QoL data for up to 3 years of follow‐up.
The major findings of the study are as follows: (1) LSPAF patients benefited globally more from catheter ablation than did PAF patients, and this finding remained valid even after multivariate adjustment for baseline and clinical outcome factors. They complained of a different spectrum of symptoms with dominating dyspnea and incapacity associated with a significantly lower QoL at baseline and experienced steeper postablation QoL improvement to finally achieve a QoL level comparable to that of PAF patients. (2) Improvement in QoL was reduced in patients with advanced age and/or longer history of AF. (3) Improvement in QoL was paralleled by the reduction in hospitalizations for cardiovascular reasons and days of sick leave in both groups.
Ablation Outcome
Although extensive/repeat ablation(s) can effectively control heart rhythm even in patients with persistent AF,14 outcomes of LSPAF ablation have been generally worse compared with those for PAF ablation.1–2,15 In this study, ablation efficacy in the PAF group was consistent with recent less optimistic observations from studies with longer follow‐up16 and comparable to the LSPAF group. Favorable outcome in our LSPAF patients can be first explained by aiming SR restoration at the initial ablation and by finalizing the predefined set of linear lesions even in the case of AF termination at an earlier stage of the procedure. Such an extent of ablation with a strict procedural end point was not used in the majority of previous studies. Second, repeat ablation instead of medical therapy was the preferred treatment option in case of AF/AT recurrences, and arrhythmia noninducibility was always the desired end point. Third, operators with high‐volume AF‐ablation experience were predominantly involved in ablation procedures for LSPAF. Importantly, comparable final outcomes in both groups provided a favorable background for the intergroup QoL comparisons.
Symptoms and QoL
Several prior studies of primarily PAF patients have demonstrated significant QoL improvement after catheter ablation.3–8 This improvement was shown to persist >2 years.6–7 In contrast, data on purely LSPAF are missing. Amelioration of postablation QoL was observed in mixed populations of persistent AF and LSPAF patients or persistent AF patients only9–11; however, translation of these findings to selected populations with LSPAF in general may not be valid. One study demonstrated postablation decrease in symptom severity score by 10±5 points in LSPAF patients,9 which corresponds to the postablation improvement reported by large multicenter studies in PAF patients.5,8 Another study comprising 91% subjects with persistent/permanent AF and left ventricular dysfunction showed a significant postablation increase in physical (24±21 points) and mental (21±21 points) scores, exceeding the magnitude of postablation improvement attained in other studies of purely PAF.5–6,8,10
Our study showed significantly worse baseline QoL in patients with LSPAF, whose complaints of palpitations are typically superseded by continuous and progressive dyspnea, fatigue, and incapacity affecting QoL more adversely than episodic palpitations in patients with PAF. Importantly, this contradicts general beliefs that symptoms regress when PAF transforms into persistent AF. Steeper QoL rise in LSPAF patients resulting in postablation QoL comparable to that of PAF patients was rather unexpected. Preexisting advanced myocardial remodeling and possible deleterious ablation effects in the LSPAF patients were suspected to reduce the functional improvement despite effective rhythm control. Yet, our study does not suggest such a consequence of extensive ablation even after a long period of continuous LSPAF and even in the presence of baseline differences in left ventricular dysfunction/heart failure and LA enlargement. Overall, the study indicates that rhythm control achieved by ablation alone (ie, off drugs and associated with discontinuation of oral anticoagulation) constitutes the major determinant of QoL improvement.
There are 2 explanations for greater QoL benefit in the LSPAF patients. First, because the background health status was basically comparable between the groups, it might simply result from elimination of AF that had a greater impact on QoL in patients with LSPAF. Second, QoL in patients with LSPAF improved to some extent even with AF/AT recurrences, which was not observed in PAF patients. In the PAF patients, the recurrent AF/ATs were presumably perceived as treatment failure despite average reduction in the arrhythmic burden. On the other hand, transformation of LSPAF into paroxysmal or only episodic persistent AF/AT interrupting long periods of stable SR was recognized by patients as a significant improvement in their health status.
Predictors of QoL Improvement
LSPAF ablation as a factor independently associated with post‐ablation QoL benefit is a challenging observation. It supports the results obtained by direct comparison of QoL change in LSPAF and PAF groups. Even though subsequent subgroup analyses were less convincing in terms of greater QoL benefit in LSPAF patients, these were limited by small number of subjects. It is also appropriate to mention that QoL benefit from LSPAF versus PAF ablation was in consistent way numerically greater in all the analyzed subgroups (Figure 5). Independent association of QoL benefit with less advanced age and shorter history of AF is also a novel finding, which supports early‐intervention strategy for the management of AF.
Prior studies comparing pharmacological rhythm and rate control of persistent AF suggested a negative impact of AAD use from a different perspectives by showing no significant difference in QoL improvement between patients with final SR while receiving amiodarone versus patients with AF17 or only modest improvement of QoL and exercise performance with successful pharmacological rhythm control.18–19 That is why we anticipated that QoL improvement would be driven not only by good arrhythmia control and discontinuation of warfarin but also by discontinuation of AAD therapy. However, all of these 3 factors were, by expectation, highly intercorrelated variables resulting in apparently controversial output of multivariate analysis. Discontinuation of warfarin treatment (and not good arrhythmia control) was independently associated with improvement in EQ‐5D, which is in line with results of the study by Wokhlu et al.7 On the contrary, improvement in EQ‐VAS was independently associated with good arrhythmia control (and not with warfarin withdrawal). AADs were not associated with any QoL benefit. Such conflicting results cannot be clinically explained and likely originate from an unstable multivariate model because of the relatively strong interaction between all 3 factors.
Speculatively, regarding the use of new anticoagulants, QoL improvement accruing from their discontinuation may be lower than that from warfarin discontinuation, which might diminish the QoL benefit from ablation overall. Nevertheless, this could not influence the difference in QoL improvement between our PAF and LSPAF groups with comparable proportions of patients receiving oral anticoagulation before ablation (95% and 100%, respectively).
The study by Wokhlu et al7 also identified obesity and higher baseline Medical Outcomes Study Short Form 36 score as predictors of limited QoL improvement.7 In our study, the association of body mass index with QoL change was rather weak even in univariate analysis. We did not analyze the relationship between baseline QoL and its change during the study because such association is affected by the phenomenon of “regression to the mean,” which artificially makes such correlation positive and usually significant.
Hospital Stay and Sick Leave
Data on hospital stay and sick leave after ablation are scarce and entirely missing for LSPAF patients. Reduction of hospital admissions was previously found after AF ablation regardless of the presence/absence of arrhythmia recurrences. Nonrecurrent medically treated patients also benefited but less than nonrecurrent ablated patients. Drug‐related side effects represented the main cause (53%) of hospital admission in these patients.20 Sick leave was assessed in 1 study, which measured relative employer‐sponsored costs for AF including regression‐adjusted monthly medical, pharmacy, sick leave, and short‐term disability costs calculated 1 year before and 3 years after the index ablation. Ablation was cost‐effective with estimated total ablation‐period costs recovery 38 to 50 months after ablation, including recovery of the employee absence payment within 18 months.21 Our study confirmed significant reductions in hospital stay and sick leave after ablation for LSPAF.
Limitations
There is a lack of consensus on how to evaluate QoL in AF patients and how to administer QoL surveys. Although previous studies mainly used a different Short Form 36, the descriptive capacity of Short Form 36 and EQ‐5D can be regarded as comparable.22 Further, although the support for the use of “self‐assessment” of individual well‐being is limited with the risk of bias by adaptation behavior, patients in fact may respond more truthfully to a self‐report questionnaire than to interviewer‐administered surveys.
It may be also speculated that patients anticipating an invasive procedure may report a lower QoL. In addition, placebo effect has been suspected to overestimate the postablation QoL change. However, the “objective” EQ‐5D index is constructed to guide the responder to assess a longer period than merely preablation status. Finally, any error would be comparable in all patients and unlikely to introduce bias in favor of a particular study group.
The lack of more sensitive disease‐specific measure of AF‐related QoL is another limitation of our study. However, because patients with major structural heart diseases or other serious comorbidities were not included, it is likely that other (eg, orthopaedic) conditions impacted the generic EQ‐5D to a lesser extent.
Our study did not include a control group. However, superiority of ablation strategy over AAD treatment for QoL end point was consistently demonstrated in multiple randomized studies in symptomatic PAF patients.4–6 In our study, comparative design can, in part, substitute for the need of control group. The QoL benefit from ablation in LSPAF patients can only be suggested indirectly because of comparable or greater effect in LSPAF versus PAF patients.
Patients with LSPAF enrolled in our study represented a selected cohort of highly symptomatic subjects. The proportion of such patients in general population is unknown. Different criteria for patient selection in different centers may significantly impact the final QoL benefit. Consequently, the generalizability of our 2‐center study should be interpreted with caution.
Conclusions
This study demonstrated that overall QoL improvement in LSPAF patients after extensive ablation and subsequent pursuit for recurrent arrhythmias was at least comparable, but likely higher, than that achieved in PAF patients. The benefit was mainly associated with freedom from AF/AT in the absence of AADs suggesting that AAD‐free status should be the final goal of AF ablation procedure. The study further underscores the impact of catheter ablation on the reversal of functional compromise due to persistent arrhythmia and corroborates the hypothesis that preexisting advanced LA structural remodeling and subsequent extensive/repeat ablation may not diminish the benefits from effective rhythm control in patients with LSPAF.
Sources of Funding
This work was supported by grants from the Internal Grant Agency of Czech Ministry of Health (IGA MZ) NR9143‐3/2007 and IGA MZ NS/9684‐4/2008 and the European Regional Development Fund–Project FNUSA‐ICRC CZ.1.05/1.1.00/02.0123.
Disclosures
None.
References
- 1.European Heart Rhythm Association, European Association for Cardio‐Thoracic Surgery. Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al‐Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohloser SH, Kohl P, Le Heuzy JY, Ponikowski P, Rutten FH. Guidelines for the management of atrial fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010; 31:2369-2429. [DOI] [PubMed] [Google Scholar]
- 2.Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ, Jr, Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haïssaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jaïs P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow‐up, definitions, endpoints, and research trial design. Heart Rhythm. 2012; 9:632-696.22386883 [Google Scholar]
- 3.Weerasooriya R, Jaïs P, Hocini M, Scavee C, Macle L, Hsu LF, Sanders P, Garrigue S, Clementy J, Haïssaguerre M. Effect of catheter ablation on quality of life of patients with paroxysmal atrial fibrillation. Heart Rhythm. 2005; 2:619-623. [DOI] [PubMed] [Google Scholar]
- 4.Wazni OM, Marrouche NF, Martin DO, Verma A, Bhargava M, Saliba W, Bash W, Schweikert R, Brachmann J, Gunther J, Gutleben K, Pisano E, Potenza D, Fanelli R, Raviele A, Themistoclakis S, Rossillo S, Bonso A, Natale A. Radiofrequency ablation vs. antiarrhythmic drugs as first‐line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA. 2005; 293:2634-2640. [DOI] [PubMed] [Google Scholar]
- 5.Jaïs P, Cauchemez B, Macle L, Daoud E, Khairy P, Subbiah R, Hocini M, Extramiana F, Sacher F, Bordachar P, Klein G, Weerasooriya R, Clémenty J, Haïssaguerre M. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation. Circulation. 2008; 118:2498-2505. [DOI] [PubMed] [Google Scholar]
- 6.Pappone C, Vicedomini G, Augello G, Mantiso F, Savano M, Baldi M, Petretta A, Giannelli L, Calovic Z, Filuta V, Tavazzi L, Santinelli V. Radiofrequency catheter ablation and antiarrhythmic drug therapy. A prospective, randomized, 4‐year follow‐up trial: the APAF Study. Circ Arrhythm Electrophysiol. 2011; 4:808-814. [DOI] [PubMed] [Google Scholar]
- 7.Wokhlu A, Monahan KH, Hodge DO, Asirvatham SJ, Friedman PA, Munger TM, Bradley DJ, Blum CM, Haroldson JM, Packer DL. Long‐term quality of life after ablation of atrial fibrillation. The impact of recurrence, symptom relief, and placebo effect. J Am Coll Cardiol. 2010; 55:2308-2316. [DOI] [PubMed] [Google Scholar]
- 8.Reynolds MR, Walczak J, White SA, Cohen DJ, Wilber DJ. Improvements in symptoms and quality of life in patients with paroxysmal atrial fibrillation treated with radiofrequency catheter ablation versus antiarrhythmic drugs. Circ Cardiovasc Qual Outcomes. 2010; 3:615-623. [DOI] [PubMed] [Google Scholar]
- 9.Oral H, Pappone C, Chugh A, Good E, Bogun F, Pelosi F, Jr, Bates ER, Lehmann MH, Vicedomini G, Augello G, Agricola E, Sala S, Santinelli V, Morady F. Circumferential pulmonary‐vein ablation for chronic atrial fibrillation. N Engl J Med. 2006; 354:934-941. [DOI] [PubMed] [Google Scholar]
- 10.Hsu LF, Jaïs P, Sanders P, Garrigue S, Hocini M, Sacher F, Takahashi Y, Rotter M, Pasquié JL, Scavée C, Bordachar P, Clémenty J, Haïssaguerre M. Catheter ablation for atrial fibrillation in congestive heart failure. N Engl J Med. 2004; 351:2373-2383. [DOI] [PubMed] [Google Scholar]
- 11.Jones DG, Haldar SK, Hussain W, Sharma R, Francis DP, Rahman‐Haley SL, McDonagh TA, Underwood R, Markides V, Wong T. A randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in heart failure. J Am Coll Cardiol. 2013; 61:1894-1903. [DOI] [PubMed] [Google Scholar]
- 12.Fiala M, Wichterle D, Bulková V, Škňouřil L, Nevřalová R, Toman O, Dorda M, Januška J, Špinar J. A prospective evaluation of hemodynamics, functional status, and quality of life after radiofrequency catheter ablation of long‐standing persistent atrial fibrillation. Europace. 2014; 16:15-25. [DOI] [PubMed] [Google Scholar]
- 13.Greiner W, Weijnen T, Nieuwenhuizen M, Oppe S, Badia X, Busschbach J, Buxton M, Dolan P, Kind P, Krabbe P, Ohinmaa A, Parkin D, Roset M, Sintonen H, Tsuchiya A, de Charro F. A single European currency for EQ‐5D health states. Results from a six country study. Eur J Health Econ. 2003; 4:222-231. [DOI] [PubMed] [Google Scholar]
- 14.Rostock T, Salukhe TV, Steven D, Drewitz I, Hoffmann BA, Bock K, Servatius H, Müllerleile K, Sultan A, Gosau N, Meinertz T, Wegsheider K, Willems S. Long‐term single‐ and multiple‐procedure outcome and predictors of success after catheter ablation for persistent atrial fibrillation. Heart Rhythm. 2011; 8:1391-1397. [DOI] [PubMed] [Google Scholar]
- 15.Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Natale A, Packer D, Skanes A, Ambrogi F, Biganzoli E. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010; 3:32-38. [DOI] [PubMed] [Google Scholar]
- 16.Weerasooriya R, Khairy P, Litalien J, Macle L, Hocini M, Sacher F, Lellouche N, Knecht S, Wright M, Nault I, Miyazaki S, Scavee C, Clementy J, Haïssaguerre M. Catheter ablation for atrial fibrillation: are results maintained at 5 years of follow‐up? J Am Coll Cardiol. 2011; 57:160-166. [DOI] [PubMed] [Google Scholar]
- 17.Grönefeld GC, Lilienthal J, Kuck KH, Hohloser SHfor the Pharmacological Intervention in Atrial Fibrillation (PIAF) Study Investigators. Impact of rate versus rhythm control on quality of life in patients with persistent atrial fibrillation. Results from a prospective randomized study. Eur Heart J. 2003; 24:1430-1436. [DOI] [PubMed] [Google Scholar]
- 18.Hagens VE, Ranchor AV, Sonderen EV, Bosker HA, Kamp O, Tijssen JG, Kingma JH, Crijns HJ, Van Gelder ICfor the RACE Study Group. Effect of rate or rhythm control on quality of life in persistent AF. J Am Coll Cardiol. 2004; 43:241-247. [DOI] [PubMed] [Google Scholar]
- 19.Singh SN, Tang XC, Singh BN, Dorian P, Reda DJ, Harris CL, Fletcher RD, Sharma SC, Atwood JE, Jacobson AK, Lewis HD, Lopez B, Raisch DW, Ezekowitz MDfor SAFE‐T Investigators. Quality of life and exercise performance in patients in sinus rhythm versus persistent atrial fibrillation: a Veterans Affairs Cooperative Studies Program Substudy. J Am Coll Cardiol. 2006; 48:721-730. [DOI] [PubMed] [Google Scholar]
- 20.Pappone C, Rosanio S, Augello G, Gallus G, Vicedomini G, Mazzone P, Gulletta S, Gugliotta F, Pappone A, Santinelli V, Tortoriello V, Sala S, Zangrillo A, Crescenzi G, Benussi S, Alfieri O. Mortality, morbidity, and quality of life after circumferential pulmonary vein ablation for atrial fibrillation: outcomes from a controlled nonrandomized long‐term study. J Am Coll Cardiol. 2003; 42:185-197. [DOI] [PubMed] [Google Scholar]
- 21.Kleinman NL, Rohrbacker NJ, White SA, March JL, Reynolds MR. Economic impact to employers of treatment options for cardiac arrhythmias in the US health system. J Occup Environ Med. 2011; 53:405-414. [DOI] [PubMed] [Google Scholar]
- 22.Quercioli C, Mesina G, Barbini E, Carriero G, Fani M, Nante N. Importance of sociodemographic and morbidity aspects in measuring health‐related quality of life: performance of three tools. Eur J Health Econ. 2009; 10:389-397. [DOI] [PMC free article] [PubMed] [Google Scholar]


