Abstract
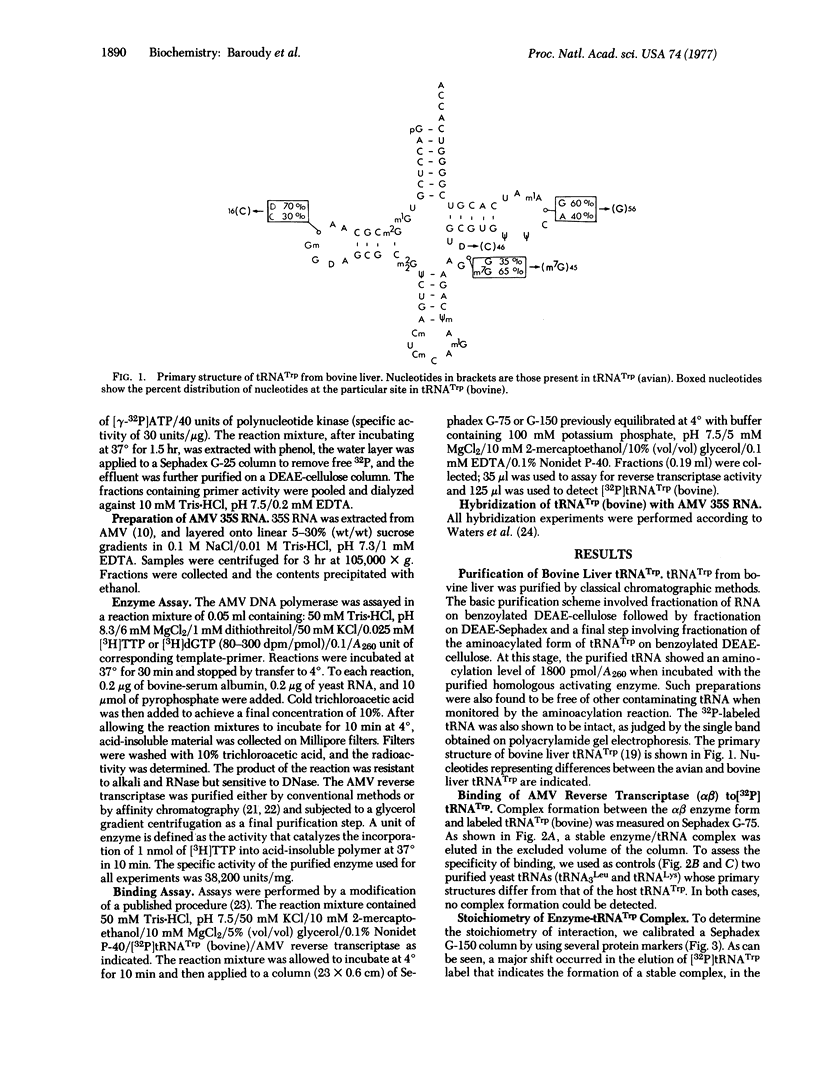
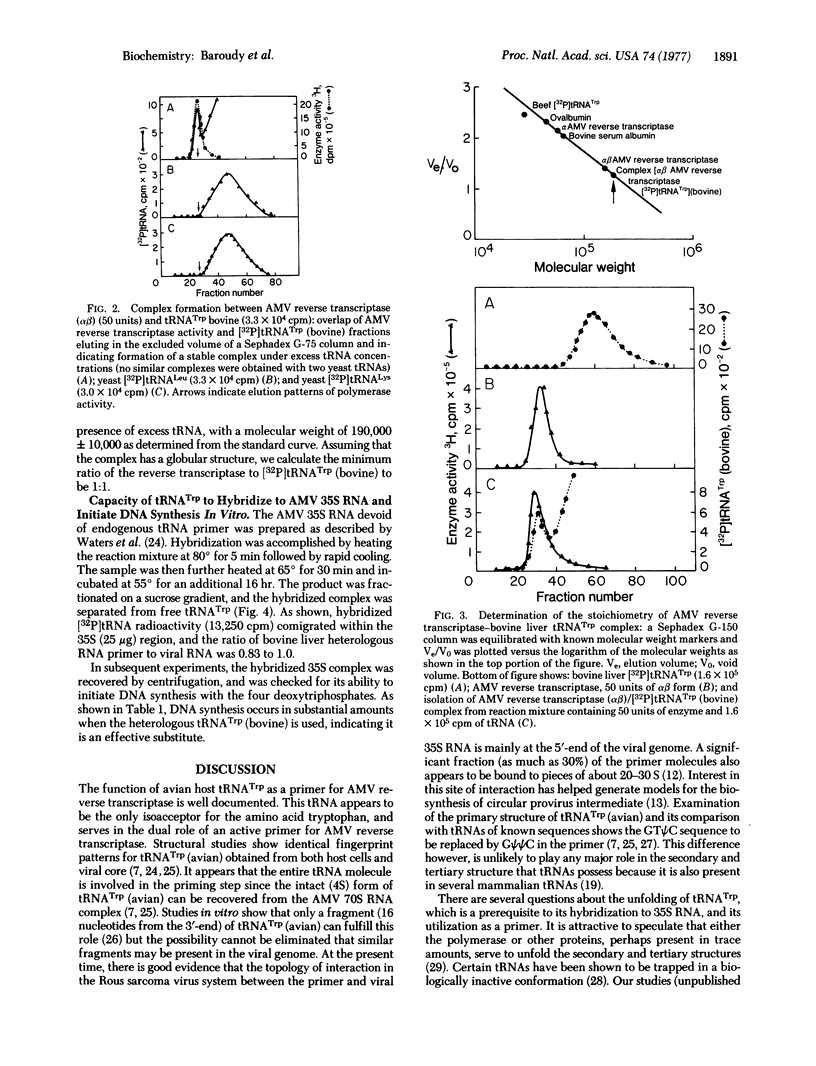
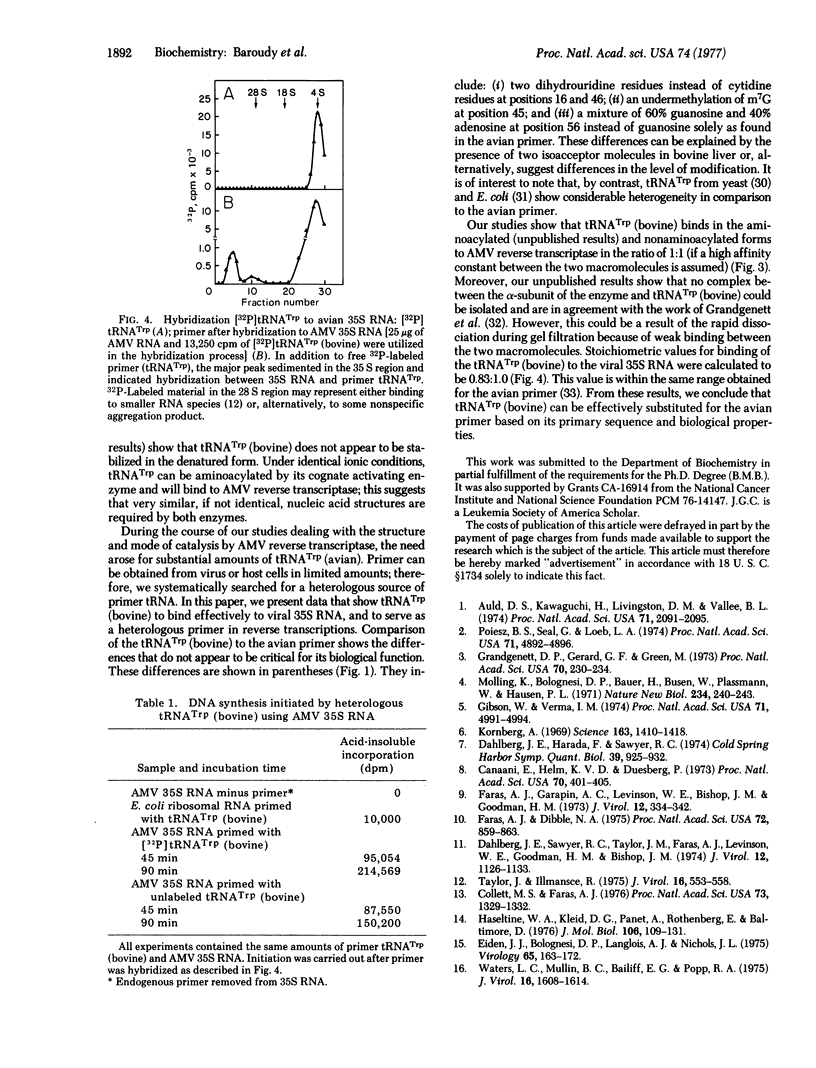
The primary structures for tTNATrp (bovine) and primer tRNATrp (avian) show only minor differences in nucleotide sequence. The heterologous tRNATrp (bovine) appears to have properties similar to the tRNATrp (avian) in its ability to bind the alphabeta from of RNA-dependent DNA nucleotidyltransferase of avian myeloblastosis virus. A stable enzyme-tRNA complex has been isolated by gel filtration. In addition, tRNATrp (bovine) can hydridize to the avian viral 35S RNA and act as a primer for transcription of the RNA. tRNATrp (bovine) can be obtained in larger amounts than the avian primer and can be used to study the interactions between the primer and the viral enzyme.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auld D. S., Kawaguchi H., Livingston D. M., Vallee B. L. RNA-dependent DNA polymerase (reverse transcriptase) from avian myeloblastosis virus: a zinc metalloenzyme. Proc Natl Acad Sci U S A. 1974 May;71(5):2091–2095. doi: 10.1073/pnas.71.5.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaani E., Helm K. V., Duesberg P. Evidence for 30-40S RNA as precursor of the 60-70S RNA of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1973 Feb;70(2):401–405. doi: 10.1073/pnas.70.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirikdjian J. G., Davis F. F. Compositional variations in the common pentanucleotide from transfer ribonucleic acids of Escherichia coli. J Biol Chem. 1970 Mar 25;245(6):1296–1301. [PubMed] [Google Scholar]
- Chirikjian J. G., Rye L., Papas T. S. Affinity chromatography of viral DNA polymerases on pyran-sepharose. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1142–1146. doi: 10.1073/pnas.72.3.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Faras A. J. Evidence for circularization of the avian oncornavirus RNA genome during proviral DNA synthesis from studies of reverse transcription in vitro. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1329–1332. doi: 10.1073/pnas.73.4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell B., Stavnezer E., Friedrich R., Bishop J. M., Goodman H. M. Nucleotide sequence that binds primer for DNA synthesis to the avian sarcoma virus genome. J Virol. 1976 Aug;19(2):548–558. doi: 10.1128/jvi.19.2.548-558.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg J. E., Harada F., Sawyer R. C. Structure and properties of an RNA primer for initiation of Rous sarcoma virus DNA synthesis in vitro. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):925–932. doi: 10.1101/sqb.1974.039.01.108. [DOI] [PubMed] [Google Scholar]
- Dahlberg J. E., Sawyer R. C., Taylor J. M., Faras A. J., Levinson W. E., Goodman H. M., Bishop J. M. Transcription of DNA from the 70S RNA of Rous sarcoma virus. I. Identification of a specific 4S RNA which serves as primer. J Virol. 1974 May;13(5):1126–1133. doi: 10.1128/jvi.13.5.1126-1133.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden J. J., Bolognesi D. P., Langlois A. J., Nichols J. L. The initial nucleotide sequence of DNA transcribed from avian myeloblastosis virus 70 S RNA by RNA-dependent DNA polymerase. Virology. 1975 May;65(1):163–172. doi: 10.1016/0042-6822(75)90016-1. [DOI] [PubMed] [Google Scholar]
- Faras A. J., Dibble N. A. RNA-directed DNA synthesis by the DNA polymerase of Rous sarcoma virus: structural and functional identification of 4S primer RNA in uninfected cells. Proc Natl Acad Sci U S A. 1975 Mar;72(3):859–863. doi: 10.1073/pnas.72.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faras A. J., Garapin A. C., Levinson W. E., Bishop J. M., Goodman H. M. Characterization of the low-molecular-weight RNAs associated with the 70S RNA of Rous sarcoma virus. J Virol. 1973 Aug;12(2):334–342. doi: 10.1128/jvi.12.2.334-342.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier M., Dorizzi M., Sarger C., Labouresse J. Purification of tRNATrp, tRNAVal, and partial purification of tRNAIle and tRNAMfet from beef liver. Biochimie. 1976;58(10):1159–1165. doi: 10.1016/s0300-9084(76)80114-9. [DOI] [PubMed] [Google Scholar]
- Fresco J. R., Adams A., Ascione R., Henley D., Lindahl T. Tertiary structure in transfer ribonucleic acids. Cold Spring Harb Symp Quant Biol. 1966;31:527–537. doi: 10.1101/sqb.1966.031.01.068. [DOI] [PubMed] [Google Scholar]
- Gibson W., Verma I. M. Studies on the reverse transcriptase of RNA tumor viruses. Structural relatedness of two subunits of avian RNA tumor viruses. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4991–4994. doi: 10.1073/pnas.71.12.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandgenett D. P., Gerard G. F., Green M. A single subunit from avian myeloblastosis virus with both RNA-directed DNA polymerase and ribonuclease H activity. Proc Natl Acad Sci U S A. 1973 Jan;70(1):230–234. doi: 10.1073/pnas.70.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandgenett D. P., Vora A. C., Faras A. J. Different states of avian myeloblastosis virus DNA polymerase and their binding capacity to primer rRNATrp. Virology. 1976 Nov;75(1):26–32. doi: 10.1016/0042-6822(76)90004-0. [DOI] [PubMed] [Google Scholar]
- Harada F., Sawyer R. C., Dahlberg J. E. A primer ribonucleic acid for initiation of in vitro Rous sarcarcoma virus deoxyribonucleic acid synthesis. J Biol Chem. 1975 May 10;250(9):3487–3497. [PubMed] [Google Scholar]
- Haseltine W. A., Kleid D. G., Panet A., Rothenberg E., Baltimore D. Ordered transcription of RNA tumor virus genomes. J Mol Biol. 1976 Sep 5;106(1):109–131. doi: 10.1016/0022-2836(76)90303-x. [DOI] [PubMed] [Google Scholar]
- Hirsh D. Tryptophan transfer RNA as the UGA suppressor. J Mol Biol. 1971 Jun 14;58(2):439–458. doi: 10.1016/0022-2836(71)90362-7. [DOI] [PubMed] [Google Scholar]
- Hung P. P., Lee S. G. Isolation of nucleic acid-binding protein: stimulation of reverse transcriptase-catalysed DNA synthesis. Nature. 1976 Feb 12;259(5543):499–502. doi: 10.1038/259499a0. [DOI] [PubMed] [Google Scholar]
- Kacian D. L., Watson K. F., Burny A., Spiegelman S. Purification of the DNA polymerase of avian myeloblastosis virus. Biochim Biophys Acta. 1971 Sep 24;246(3):365–383. doi: 10.1016/0005-2787(71)90773-8. [DOI] [PubMed] [Google Scholar]
- Keith G., Roy A., Ebel J. P., Dirheimer G. The nucleotide sequences of two tryptophane-tRNAs from brewer's yeast. FEBS Lett. 1971 Oct 1;17(2):306–308. doi: 10.1016/0014-5793(71)80171-0. [DOI] [PubMed] [Google Scholar]
- Kornberg A. Active center of DNA polymerase. Science. 1969 Mar 28;163(3874):1410–1418. doi: 10.1126/science.163.3874.1410. [DOI] [PubMed] [Google Scholar]
- Mölling K., Bolognesi D. P., Bauer H., Büsen W., Plassmann H. W., Hausen P. Association of viral reverse transcriptase with an enzyme degrading the RNA moiety of RNA-DNA hybrids. Nat New Biol. 1971 Dec 22;234(51):240–243. doi: 10.1038/newbio234240a0. [DOI] [PubMed] [Google Scholar]
- Panet A., Haseltine W. A., Baltimore D., Peters G., Harada F., Dahlberg J. E. Specific binding of tryptophan transfer RNA to avian myeloblastosis virus RNA-dependent DNA polymerase (reverse transcriptase). Proc Natl Acad Sci U S A. 1975 Jul;72(7):2535–2539. doi: 10.1073/pnas.72.7.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiesz B. J., Seal G., Loeb L. A. Reverse transcriptase: correlation of zinc content with activity. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4892–4896. doi: 10.1073/pnas.71.12.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríman J., Beaudreau G. S. Viral DNA-dependent DNA polymerase and the properties of thymidine labelled material in virions of an oncogenic RNA virus. Nature. 1970 Oct 31;228(5270):427–430. doi: 10.1038/228427a0. [DOI] [PubMed] [Google Scholar]
- Staskus K. A., Collett M. S., Faras A. J. Initiation of DNA synthesis by the avian oncornavirus RNA-directed DNA polymerase: structural and functional localization of the major species of primer RNA on the oncornavirus genome. Virology. 1976 May;71(1):162–168. doi: 10.1016/0042-6822(76)90102-1. [DOI] [PubMed] [Google Scholar]
- Székely M., Sanger F. Use of polynucleotide kinase in fingerprinting non-radioactive nucleic acids. J Mol Biol. 1969 Aug 14;43(3):607–617. doi: 10.1016/0022-2836(69)90362-3. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R. Site on the RNA of an avian sarcoma virus at which primer is bound. J Virol. 1975 Sep;16(3):553–558. doi: 10.1128/jvi.16.3.553-558.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters L. C., Mullin B. C., Bailiff E. G., Popp R. A. tRNA's associated with the 70S RNA of avian myeloblastosis virus. J Virol. 1975 Dec;16(6):1608–1614. doi: 10.1128/jvi.16.6.1608-1614.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters L. C., Mullin B. C., Ho T., Yang W. K. Ability of tryptophan tRNA to hybridize with 35S RNA of avian myeloblastosis virus and to prime reverse transcription in vitro. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2155–2159. doi: 10.1073/pnas.72.6.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]


