Abstract
Background
Brain‐type natriuretic peptide (BNP) and the amino‐terminal fragment of its prohormone (NT‐proBNP) are known predictors of cardiovascular outcomes in patients with coronary heart disease; however, the relative prognostic value of these 2 biomarkers for secondary events remains unclear.
Methods and Results
In 983 participants with stable coronary heart disease, we evaluated the association of BNP and NT‐proBNP with time to hospitalization for heart failure, nonfatal myocardial infarction, stroke or transient ischemic attack, cardiovascular death, and combined major adverse cardiovascular events (MACE). During an average follow‐up of 6.5±3.3 years, both BNP and NT‐proBNP were associated with increased risk of MACE in a multivariable‐adjusted model (hazard ratio per standard deviation of log BNP: 1.58; 95% CI: 1.32 to 1.89; hazard ratio per standard deviation of log NT‐proBNP: 1.84; 95% CI: 1.52 to 2.24). When added to traditional risk factors, NT‐proBNP predicted MACE better than BNP (C statistic: 0.76 versus 0.72, P<0.001). Similarly, the addition of NT‐proBNP resulted in a greater net reclassification improvement for predicting MACE than the addition of BNP (65% for NT‐proBNP, 56% for BNP).
Conclusions
Both BNP and NT‐proBNP were significant predictors of MACE in stable coronary heart disease; however, NT‐proBNP was superior to BNP for net risk reclassification for MACE.
Keywords: adverse cardiovascular outcomes, BNP, NT‐proBNP, risk assessment, stable coronary heart disease
Introduction
Brain natriuretic peptide (BNP) and the amino‐terminal fragment of its prohormone (NT‐proBNP) are released in equimolar amounts from the myocardium.1 Both peptides predict adverse cardiovascular (CV) events in heart failure (HF),2 acute coronary syndromes,3–4 and stable coronary heart disease (CHD)5–10; however, the relative prognostic utility of one versus the other for secondary events in patients with stable CHD is unclear.
Prior studies comparing the prognostic value of BNP and NT‐proBNP in stable CHD have yielded conflicting results. One study of patients with stable CHD found that both biomarkers performed equally well for the prediction of HF and all‐cause mortality during 1 year of follow‐up.9 In another study, both BNP and NT‐proBNP were independently related to incident HF, but only NT‐proBNP was associated with an increased risk of stroke and CV mortality.8 These findings suggest that although BNP and NT‐proBNP are related, they may not be interchangeable as predictors of adverse CV events. In addition to differences in biologic half‐lives, assay stability, and clearance mechanisms, there may be differences in secretion patterns in response to myocardial hypoxia or in the setting of structural heart disease.11–13 Because of the conflicting data on differences in the prognostic value of BNP and NT‐proBNP, we sought to evaluate the independent prognostic value of BNP and NT‐proBNP in well‐characterized, stable outpatients with CHD enrolled in a prospective cohort study with long‐term follow‐up.
Methods
Study Participants
The Heart and Soul Study is a prospective cohort study evaluating the role of psychosocial factors on CV outcomes in 1024 patients with CHD. Methods for this study have been previously described in detail.14 Participants with CHD were included and defined as (1) history of myocardial infarction, (2) angiographic evidence of at least 50% stenosis in 1 coronary vessel or more, (3) evidence of inducible ischemia by treadmill electrocardiography or nuclear perfusion stress imaging, or (4) a history of coronary revascularization. Patients were excluded if they were unable to walk more than 1 block, had a history of acute coronary syndrome within the prior 6 months, or intended to move from the locality in the subsequent 3 years. We further limited analysis to the 983 participants with both BNP and NT‐proBNP levels measured at baseline. The institutional review board at each of the sites approved this protocol. All participants provided written informed consent.
Measurement of NT‐proBNP and BNP Levels
Blood samples were drawn after an overnight fast, with patients instructed to continue their regularly prescribed medications. Samples were originally collected during 2000 to 2002 and stored at −70°C until January 2005. The Roche Diagnostics Elecsys proBNP assay was used to measure NT‐proBNP. The assay ranges from 5 to 35 000 pg/mL. The intra‐assay and interassay coefficients of variation ranged, respectively, from 2.7% and 3.2% at NT‐proBNP concentrations of 175 pg/mL to 1.8% and 2.3% at NT‐proBNP concentrations of 4962 pg/mL.
We used the Alere Triage BNP fluorescence immunoassay (Alere Inc) to measure BNP in frozen plasma samples thawed to room temperature in November 2013. Of note, the plasma samples used to measure BNP had undergone 1 freeze–thaw cycle previously. The assay range was 5 to 5000 pg/mL. The interassay coefficient of variation was 10.1% for 28.8 pg/mL, 12.4% for 586 pg/mL, and 16.2% for 1180 pg/mL. The laboratory technicians who measured BNP and NT‐proBNP at 2 different times were located at 2 different sites and were blinded to the characteristics of the patients and the results of the echocardiographic and the other natriuretic peptide measurements.
Other Measurements
Self‐reported age, sex, ethnicity, past medical history, and smoking status were assessed by questionnaire. Glomerular filtration rate was calculated using the Chronic Kidney Disease Epidemiolology Collaboration equation.15 Levels of high‐density lipoprotein cholesterol and low‐density lipoprotein cholesterol were determined from plasma samples drawn after an overnight fast.
Echocardiography
A complete resting 2‐dimensional echocardiogram and Doppler ultrasound examination was performed using an Acuson Sequoia Ultrasound System (Siemens Medical Solutions USA, Inc). Standard 2‐dimensional parasternal short axis, apical 2‐chamber, apical 4‐chamber, and subcostal views were obtained. All echocardiograms were interpreted by a single expert reader.
Left ventricular (LV) end‐diastolic and end‐systolic volumes were approximated using the modified biplane methods of discs. LV ejection fraction was calculated as follows:
The truncated ellipsoid method was used to estimate LV mass, which was then indexed to body surface area.16 Mitral inflow velocities (E and A) were calculated using pulse‐wave Doppler in the apical 4‐chamber view, with the sample volume positioned between the mitral leaflet tips. Mitral inflow E‐ and A‐wave velocities, E‐wave deceleration time, and the ratio of pulmonary venous systolic‐to‐diastolic flow velocity‐time integral were used to group LV diastolic function into categories of “normal,” “mild dysfunction,” “moderate dysfunction,” or “severe dysfunction.”17 Of the 983 participants, 149 participants could not be assigned unambiguously to an LV diastolic function category and thus had a missing value for this covariate.
Clinical Outcomes
The outcome in this study was time to HF hospitalization, nonfatal myocardial infarction (MI), stroke (cerebral vascular accident [CVA]) or transient ischemic attack (TIA), and CV death. HF was defined as hospitalization for signs and symptoms of HF. MI was defined using standard diagnostic criteria.18 CVA/TIA was defined as a new neurological deficit not known to be secondary to brain trauma, tumor, infection, or other cause. Death and cause of death were verified by death certificates and review of medical records. The outcome assessment and adjudication process has been described previously in greater detail.5
Statistical Analyses
We modeled BNP and NT‐proBNP as quartiles and as log‐transformed linear variables. Samples with values for either BNP or NT‐proBNP below the lower limit of detection (5 pg/mL) were entered into the analyses as 5 pg/mL. Differences in participant characteristics were compared using chi‐square tests for dichotomous variables and analysis of variance for continuous variables.
We used demographically adjusted and multivariable‐adjusted Cox proportional hazards models to evaluate the association of BNP and NT‐proBNP with the outcome variables. The multivariable models were adjusted for Framingham secondary events clinical risk factors: for men, these include age, natural log of the ratio of total cholesterol to high‐density lipoprotein cholesterol, and diabetes; for women, these include age, natural log of the ratio of total cholesterol to high‐density lipoprotein cholesterol, natural log of systolic blood pressure, diabetes, and smoking.19 The multivariable models were also adjusted for traditional CV risk factors including age, sex, ethnicity, current smoking status, history of diabetes mellitus, history of HF, history of MI, body mass index, systolic blood pressure, low‐density lipoprotein, estimated glomerular filtration rate, LV mass index, LV ejection fraction, and LV diastolic function category. For the quartile analysis, the entire sample (quartiles I through IV) was included, and the P value for the trend of hazard ratios across quartiles, with quartile I as the referent, was calculated.
We evaluated the C statistic, which is equivalent to the area under the receiver operating characteristic curve, for BNP and NT‐proBNP as linear log‐transformed variables in Framingham secondary events clinical risk factor–adjusted logistic regression models for the prediction of adverse CV outcomes. We further determined the incremental prognostic value of BNP and NT‐proBNP using the category‐free net reclassification improvement (NRI) analysis. A baseline multivariable logistic regression model with Framingham secondary events clinical risk factors was used to generate the probability of each participant having an adverse CV outcome (P0). The probabilities were then recalculated for the outcome with the addition of BNP or NT‐proBNP into the clinical model (P1). If P1>P0, then the person was considered to have an upward reclassification, and if P1<P0 then the person was considered to have a downward reclassification. If P1=P0, then no reclassification occurred. The NRI was then calculated using the following formula: NRI=[P (up|outcome)−P (down|outcome)]+[P (down|nonoutcome)−P (up|nonoutcome)].20–22 Finally, we quantified the separation of events from nonevents by the addition of BNP or NT‐proBNP into the clinical model by calculating the integrated discrimination improvement (IDI). Mean probabilities were calculated for an event in those participants who develop events and for an event in those who do not develop events using the baseline multivariable logistic regression model with Framingham secondary events clinical risk factors (old model) and the clinical model plus BNP or NT‐proBNP (new model). The IDI was then calculated for BNP and for NT‐proBNP using the formula IDI=(Pnew, events−Pnew, nonevents)−(Pold, events−Pold, nonevents), in which Pnew, events is the mean of the new model‐based predicted probabilities of an event for those who develop events, Pold, events is the corresponding quantity based on the old model without BNP or NT‐proBNP, Pnew, nonevents is the mean of the new model‐based predicted probabilities of an event for those who do not develop events, and Pold, nonevents is the corresponding quantity based on the old model without BNP or NT‐proBNP. Calibration metrics for the risk models were estimated using the Hosmer‐Lemeshow goodness‐of‐fit test and deciles of fitted risk values. All analyses were performed using IBM SPSS version 20.0 and STATA version 12.0 (StataCorp).
Results
The study participants were predominantly older, white, and male with a high prevalence of hypertension and previous MI (Table 1). Most participants did not report a history of HF. Levels of BNP and NT‐proBNP were strongly correlated with each other (r=0.90; P<0.001).
Table 1.
Baseline Characteristics of the 983 Study Participants With Stable Coronary Heart Disease*
| Age, y | 67 (11) |
| Male (n, %) | 800 (81) |
| Ethnicity (n, %) | |
| White | 592 (60) |
| Black | 160 (16) |
| Hispanic | 113 (12) |
| Other | 117 (12) |
| Diabetes mellitus (n, %) | 257 (26) |
| Hypertension (n, %) | 691 (70) |
| Current smoking (n, %) | 195 (20) |
| History of heart failure (n, %) | 173 (18) |
| History of myocardial infarction (n, %) | 524 (53) |
| BMI, kg/m2 | 28 (5) |
| SBP, mm Hg | 133 (21) |
| DBP, mm Hg | 75 (11) |
| LDL, mg/dL | 104 (34) |
| HDL, mg/dL | 46 (14) |
| Creatinine, mg/dL | 1.14 (0.64) |
| eGFR, mL/min | 71 (22) |
| NT‐proBNP*, pg/mL | 174 (74, 460) |
| BNP*, pg/mL | 42 (18, 102) |
| LV mass index, g/m2 | 100 (34) |
| LVEF, % | 62 (10) |
| LV diastolic function* (n, %) | |
| Normal | 508 (61) |
| Impaired relaxation | 215 (22) |
| Pseudo‐normal | 59 (6) |
| Restrictive | 52 (5) |
BNP indicates brain‐type natriuretic peptide; BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; LV, left ventricular; LVEF, left ventricular ejection fraction; NT‐proBNP, amino‐terminal fragment of the BNP prohormone; SBP, systolic blood pressure.
Mean (SD) reported for continuous variables.
Reported as median (interquartile range)
Of the 983 participants, 149 did not have adequate echocardiographic information for categorization of diastolic function.
During a mean follow‐up of 6.5±3.3 years, there were 133 MIs, 178 HF hospitalizations, 67 CVA/TIAs, 144 CV deaths, and 346 MACE.
When modeled as continuous variables, both BNP and NT‐proBNP were strongly associated with HF hospitalization, nonfatal MI, CVA/TIA, CV death, and MACE (Table 2). These associations were attenuated but remained significant with adjustment for clinical risk factors. In the fully adjusted models that included estimated glomerular filtration rate and LV structural and functional parameters, both BNP and NT‐proBNP were significantly associated with HF hospitalization, nonfatal MI, CV death, and MACE, but neither was associated with CVA/TIA.
Table 2.
Association of BNP and NT‐proBNP (Entered as Log‐Transformed Continuous Variables, Per 1SD Increase) With Cardiovascular Outcomes
| Log BNP | Log NT‐proBNP | |||
|---|---|---|---|---|
| HR* (95% CI) | P Value | HR* (95% CI) | P Value | |
| HF | ||||
| Model 1* | 3.18 (2.65 to 3.80) | <0.001 | 3.14 (2.70 to 3.65) | <0.001 |
| Model 2* | 3.08 (2.56 to 3.70) | <0.001 | 3.14 (2.69 to 3.68) | <0.001 |
| Model 3* | 2.81 (2.12 to 3.74) | <0.001 | 2.81 (2.09 to 3.78) | <0.001 |
| Nonfatal MI | ||||
| Model 1* | 1.65 (1.36 to 2.00) | <0.001 | 1.80 (1.51 to 2.16) | <0.001 |
| Model 2* | 1.60 (1.32 to 1.95) | <0.001 | 1.76 (1.46 to 2.12) | <0.001 |
| Model 3* | 1.43 (1.09 to 1.88) | 0.01 | 1.77 (1.30 to 2.41) | <0.001 |
| CVA/TIA | ||||
| Model 1* | 1.64 (1.25 to 2.15) | 0.001 | 1.66 (1.28 to 2.14) | <0.001 |
| Model 2* | 1.64 (1.24 to 2.16) | <0.001 | 1.68 (1.29 to 2.18) | <0.001 |
| Model 3* | 1.37 (0.93 to 2.01) | 0.11 | 1.40 (0.92 to 2.13) | 0.12 |
| CV death | ||||
| Model 1* | 1.90 (1.57 to 2.30) | <0.001 | 2.36 (1.99 to 2.81) | <0.001 |
| Model 2* | 1.90 (1.56 to 2.32) | <0.001 | 2.40 (2.00 to 2.89) | <0.001 |
| Model 3* | 1.61 (1.22 to 2.13) | 0.001 | 2.19 (1.63 to 2.94) | <0.001 |
| Any event | ||||
| Model 1* | 1.96 (1.73 to 2.21) | <0.001 | 2.20 (1.97 to 2.46) | <0.001 |
| Model 2* | 1.94 (1.71 to 2.20) | <0.001 | 2.19 (1.95 to 2.45) | <0.001 |
| Model 3* | 1.58 (1.32 to 1.89) | <0.001 | 1.84 (1.52 to 2.24) | <0.001 |
BNP indicates brain‐type natriuretic peptide; CI, confidence interval; CV, cardiovascular; CVA, cerebral vascular accident; HF, heart failure; HR, hazard ratio; MI, myocardial infarction; NT‐proBNP, amino‐terminal fragment of the BNP prohormone; TIA, transient ischemic attack.
Adjusted for age, sex and ethnicity.
Adjusted for age, natural log of the ratio of total cholesterol to high‐density lipoprotein cholesterol, and diabetes for men and age, natural log of the ratio of total cholesterol to high‐density lipoprotein cholesterol, natural log of systolic blood pressure, diabetes, and smoking for women.
Adjusted for age, sex, ethnicity, diabetes mellitus, current smoking, history of heart failure, history of myocardial infarction, body mass index, systolic blood pressure, low‐density lipoprotein, estimated glomerular filtration rate, left ventricular mass index, left ventricular ejection fraction, and left ventricular diastolic function.
Participants with levels of BNP or NT‐proBNP in the highest quartile had the greatest incidence of these events during the follow‐up period (Figure). In a demographically adjusted model, the highest quartiles of BNP and NT‐proBNP were both strongly associated with an increased risk of HF hospitalization, MI, CVA/TIA, and CV death and the combined outcome when compared with their respective lowest quartiles (Table 3). With further adjustment for additional clinical risk factors, the associations of the highest quartiles of BNP and NT‐proBNP with HF hospitalization (15‐fold and 26‐fold increased risk, respectively), nonfatal MI (approximately 4‐fold increased risk for both), CVA/TIA (approximately 2.5‐fold and 4‐fold increased risk, respectively), CV death (4‐fold and 7‐fold increased risk, respectively), and MACE (3‐fold and 4‐fold increased risk, respectively) were somewhat attenuated but remained significant. In the fully adjusted models that included estimated glomerular filtration rate and LV structural and functional parameters, the highest quartile of both BNP and NT‐proBNP remained predictive of HF hospitalization, nonfatal MI, CV death, and MACE, whereas only the highest quartile of NT‐proBNP remained significantly associated with CVA/TIA (Table 3).
Figure 1.
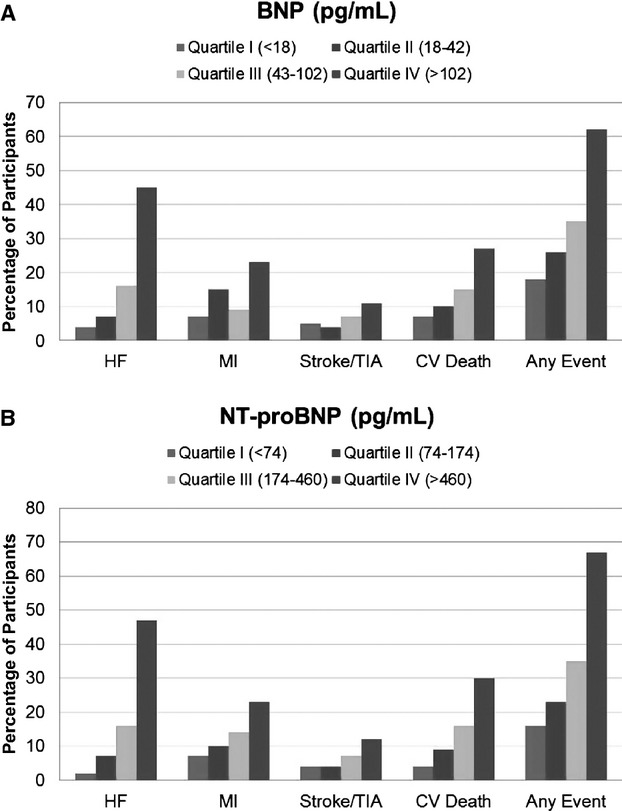
Percentage of participants with cardiovascular outcomes by quartiles of BNP and NT‐proBNP. Bar graph illustrating the percentage of participants in each quartile of BNP level (A) and NT‐proBNP level (B) with hospitalization for HF, nonfatal MI, stroke or TIA, CV death, or any of these events. BNP indicates brain‐type natriuretic peptide; CV, cardiovascular; HF, heart failure; MI, myocardial infarction; NT‐proBNP, amino‐terminal fragment of the BNP prohormone; TIA, transient ischemic attack.
Table 3.
Association of BNP and NT‐proBNP With Cardiovascular Outcomes
| BNP (Quartile IV vs I) | NT‐proBNP (Quartile IV vs I) | |||
|---|---|---|---|---|
| HR* (95% CI) | P Value* | HR* (95% CI) | P Value* | |
| HF | ||||
| Model 1* | 14.79 (7.38 to 29.66) | <0.001 | 26.83 (11.62 to 61.93) | <0.001 |
| Model 2* | 15.21 (7.54 to 30.68) | <0.001 | 25.69 (11.10 to 59.42) | <0.001 |
| Model 3* | 6.33 (1.10 to 4.24) | <0.001 | 9.82 (3.69 to 26.17) | <0.001 |
| Nonfatal MI | ||||
| Model 1* | 3.57 (2.02 to 6.31) | <0.001 | 4.20 (2.02 to 6.31) | <0.001 |
| Model 2* | 3.55 (1.98 to 6.38) | <0.001 | 3.97 (2.17 to 7.26) | <0.001 |
| Model 3* | 2.16 (1.10 to 4.24) | 0.10 | 2.39 (1.15 to 4.96) | 0.04 |
| CVA/TIA | ||||
| Model 1* | 2.42 (1.20 to 4.90) | 0.005 | 3.69 (1.71 to 7.96) | <0.001 |
| Model 2* | 2.46 (1.19 to 5.10) | 0.006 | 3.73 (1.67 to 8.31) | <0.001 |
| Model 3* | 1.84 (0.77 to 4.39) | 0.08 | 3.01 (1.12 to 8.08) | 0.02 |
| CV death | ||||
| Model 1* | 3.66 (2.10 to 6.40) | <0.001 | 7.19 (3.71 to 13.90) | <0.001 |
| Model 2* | 3.90 (2.19 to 6.93) | <0.001 | 6.82 (3.51 to 13.23) | <0.001 |
| Model 3* | 2.17 (1.10 to 4.28) | 0.01 | 3.24 (1.50 to 6.99) | 0.001 |
| Any event | ||||
| Model 1* | 3.09 (2.31 to 4.14) | <0.001 | 4.29 (3.18 to 5.79) | <0.001 |
| Model 2* | 3.36 (2.49 to 4.53) | <0.001 | 4.36 (3.21 to 5.93) | <0.001 |
| Model 3* | 2.02 (1.41 to 2.87) | <0.001 | 2.43 (1.66 to 2.56) | <0.001 |
BNP indicates brain‐type natriuretic peptide; CV, cardiovascular; CVA, cerebral vascular accident; HF, heart failure; HR, hazard ratio; MI, myocardial infarction; NT‐proBNP, amino‐terminal fragment of the BNP prohormone; TIA, transient ischemic attack.
Adjusted for age, sex and ethnicity.
Adjusted for age, natural log of the ratio of total cholesterol to high‐density lipoprotein cholesterol, and diabetes for men and age, natural log of the ratio of total cholesterol to high‐density lipoprotein cholesterol, natural log of systolic blood pressure, diabetes, and smoking for women.
Adjusted for age, sex, ethnicity, diabetes mellitus, current smoking, history of HF, history of MI, body mass index, systolic blood pressure, low‐density lipoprotein, estimated glomerular filtration rate, left ventricular mass index, left ventricular ejection fraction, and left ventricular diastolic function.
P value is for trend across quartiles.
Calibration metrics for risk models used in the receiver operating characteristic curve analyses were as follows: BNP alone, P=0.01; NT‐proBNP alone, P=0.08; clinical risk factors, P=0.007; clinical risk factors plus BNP, P=0.20; clinical risk factors plus NT‐proBNP, P=0.16. In receiver operating characteristic curve analyses, NT‐proBNP had a stronger association with MACE than did BNP (Table 4). We next evaluated and compared the marginal contribution of adding BNP and NT‐proBNP to a clinical risk factor model that included age, natural log of the ratio of total cholesterol to high‐density lipoprotein cholesterol, and diabetes for men and age, natural log of the ratio of total cholesterol to high‐density lipoprotein cholesterol, natural log of systolic blood pressure, diabetes, and smoking for women. The addition of BNP and the addition of NT‐proBNP to the clinical predictors improved the C statistic significantly for the prediction of MACE, with a significantly higher C statistic for the model including clinical risk factors and NT‐proBNP than the model including clinical risk factors and BNP. Improvement in the category‐free NRI for the prediction of MACE was stronger for NT‐proBNP (Table 5). For NT‐proBNP, the improvement in NRI was driven more by downward risk classification of patients without MACE (35%) than by upward risk classification of patients with MACE (29%). As with the NRI, the IDI was higher with the addition of NT‐proBNP to the clinical model (0.16; 95% CI: 0.11 to 0.20) than with the addition of BNP (0.11; 95% CI: 0.07 to 0.14).
Table 4.
Prediction of Adverse Cardiovascular Events Assessed by Receiver Operating Characteristic Curve Analysis
| Model | C Statistic (95% CI) | Comments |
|---|---|---|
| 1. BNP alone | 0.70 (0.66 to 0.74) | |
| 2. NT‐proBNP alone | 0.74 (0.70 to 0.77) | P<0.001 vs BNP alone |
| 3. Clinical risk factors* | 0.63 (0.60 to 0.67) | |
| 4. Clinical risk factors*+BNP | 0.72 (0.69 to 0.76) | P<0.001 vs clinical risk factors |
| 5. Clinical risk factors*+NT‐proBNP | 0.76 (0.73 to 0.79) | P<0.001 vs clinical risk factors |
| P<0.001 for model 4 vs 5 |
BNP indicates brain‐type natriuretic peptide; CI, confidence interval; NT‐proBNP, amino‐terminal fragment of the BNP prohormone.
Clinical risk factor model includes age, natural log of the ratio of total cholesterol to high‐density lipoprotein cholesterol, and diabetes for men and age, natural log of the ratio of total cholesterol to high‐density lipoprotein cholesterol, natural log of systolic blood pressure, diabetes, and smoking for women.
Table 5.
Net Reclassification Improvement in Predicting Risk of Cardiovascular Events With the Addition of BNP or NT‐proBNP to a Clinical Risk Prediction Model*
| Proportion of Patients With Events Who Were Reclassified (95% CI) | Proportion of Patients Without Events Who Were Reclassified (95% CI) | Net Reclassification Improvement | |
|---|---|---|---|
| +BNP | 29% (22%, 38%) | 27% (20%, 35%) | 56% (45%, 70%) |
| +NT‐proBNP | 29% (21%, 39%) | 35% (29%, 43%) | 65% (52%, 80%) |
BNP indicates brain‐type natriuretic peptide; CI, confidence interval; NT‐proBNP, amino‐terminal fragment of the BNP prohormone.
Clinical risk prediction model includes age, natural log of the ratio of total cholesterol to high‐density lipoprotein cholesterol, and diabetes for men and age, natural log of the ratio of total cholesterol to high‐density lipoprotein cholesterol, natural log of systolic blood pressure, diabetes, and smoking for women.
Discussion
We evaluated the association of BNP and NT‐proBNP with adverse CV events in a cohort of patients with stable CHD and found that both BNP and NT‐proBNP were associated with adverse CV events, including HF hospitalizations, MI, CVA/TIA, and CV deaths; however, NT‐proBNP was superior to BNP for reclassifying risk of MACE. Our study expands the literature by performing a thorough head‐to‐head comparison between BNP and NT‐proBNP for the prediction of adverse CV events over a long follow‐up period in a large, well‐characterized, outpatient population with stable CHD.
Although both BNP and NT‐proBNP levels have been found to be inversely associated with long‐term survival in patients with stable CHD,5–6,10,23 it has not been established whether one outperforms the other for the prediction of adverse CV events in stable CHD. Richards et al found that BNP and NT‐proBNP performed similarly in their ability to predict HF and all‐cause mortality in patients with stable CHD (n=1049).9 This study, however, was limited to those 2 outcomes and to a 12‐month follow‐up period. In another relevant study, Omland et al examined a population with stable CHD and preserved systolic function enrolled in the Prevention of Events With Angiotensin‐Converting Enzyme Inhibition (PEACE) trial (n=3761) and found that although both BNP and NT‐proBNP predicted HF events over a median follow‐up period of 4.8 years, NT‐proBNP was also associated with greater risk of CV death and stroke.8 In this study, neither marker appeared to significantly increase the risk of MI. In our study, we noted an independent association between both BNP and NT‐proBNP and the combined outcome of HF hospitalization, MI, CVA/TIA, or CV death. Moreover both BNP and NT‐proBNP were associated with the individual outcomes of HF, MI, and CV death. Although neither BNP nor NT‐proBNP was associated with CVA/TIA when modeled as a continuous variable, the highest levels of NT‐proBNP were predictive of CVA/TIA independent of clinical predictors and LV structure and function. The differences between the results of our study and those reported by Richards et al9 and Omland et al8 may be due differences in the populations studied, in the follow‐up periods, in the covariates used, or in the assays used to measure BNP and NT‐proBNP levels.
In our receiver operating characteristic curve analyses, the addition of both NT‐proBNP and BNP to a clinical model composed of risk factors for secondary events in the Framingham study resulted in significant improvement of the C statistic. For these models containing clinical risk factors and BNP or NT‐proBNP, there was no evidence of poor model calibration (goodness of fit P>0.10).The significantly improved C statistic likely reflects the known robustness of natriuretic peptide levels for the prediction of MACE; however, the addition of NT‐proBNP resulted in a significantly greater improvement in the C statistic than the addition of BNP. Moreover, the results of the category‐free NRI and IDI analyses suggest that NT‐proBNP may provide greater incremental prognostic value than BNP when added to clinical predictors for the prediction of adverse CV events. Our findings expand on those of a previous study that showed NT‐proBNP provides incremental prognostic information for the prediction of adverse CV events in lower risk patients with CV risk factors.24 The magnitude of the NRI achieved with the addition of NT‐proBNP to traditional clinical risk factors and echocardiographic predictors is similar to that obtained with the addition of flow‐mediated dilation or coronary artery calcium scores to the Framingham risk score.25–26
Differences in half‐life and clearance mechanisms between the 2 biomarkers may explain why BNP and NT‐proBNP may not be equivalent markers of adverse CV outcomes.27 Although both BNP and NT‐proBNP levels are thought to be inversely related to renal function, BNP is further cleared by neutral endopeptidases and the natriuretic peptide C receptor.28 However, several studies have also shown that BNP and NT‐proBNP levels are similarly affected in patients with mild and moderate renal dysfunction.29–30 In addition, the half‐life of NT‐proBNP is significantly longer than that of BNP.27 These differences may contribute to the higher circulating NT‐proBNP levels observed in many physiologic and pathologic states. Despite these differences, we found that both BNP and NT‐proBNP were similarly associated with adverse CV outcomes after adjustment for renal function.
Although both BNP and NT‐proBNP show significant biological variation, the intraindividual variability is greater for BNP than NT‐proBNP in stable outpatients with HF.31 This, together with its longer in vivo half‐life, may make NT‐proBNP a better reflector of longer term physiologic changes than BNP. In addition, although NT‐proBNP is very stable under refrigerated storage, there are concerns that BNP may degrade faster than NT‐proBNP at room temperature as well as in frozen samples.28,32 This potential difference in rates of degradation may have influenced our findings because NT‐proBNP levels were measured in 2005 and BNP levels were measured in 2013; however, we have previously established the long‐term stability of proteins in the frozen plasma samples in the Heart and Soul Study. To test both the reliability of our samples and the stability of frozen proteins, we measured NT‐proBNP levels (using the Roche Diagnostics Elecsys proBNP assay) in frozen plasma samples in 2005 and in 2012 and found that, among 979 participants, the correlation was 0.95 between the 2 NT‐proBNP measurements. We repeated a similar analysis with cystatin C by measuring levels in 2006 and in 2010 and found that, among 989 participants, the correlation was 0.96 between the 2 cystatin C measurements. Moreover, the strong correlation between NT‐proBNP and BNP levels in the current study, similar to or better than that reported in previous studies in which the BNP and NT‐proBNP levels were measured at closer time points,8–9 argues against significant degradation of BNP. Although there were significant differences in the analytic characteristics of the assays that we used to measure BNP and NT‐proBNP levels, this strong correlation between BNP and NT‐proBNP levels across a wide range of values also argues against these real and potential differences in analytic characteristics of the 2 assays fully explaining the differences that we observed in the performance of the 2 biomarkers as prognostic indicators.
Study Limitations
This study had several limitations. Because our study population was composed predominately of older men in an urban setting with stable CHD, these findings may not be generalizable to other populations and disease states. Moreover, HF events were defined as hospitalizations, and patients presenting with signs and symptoms of HF in the outpatient setting were not included. Some of the attenuation of risk of adverse events associated with BNP and NT‐proBNP with adjustment for parameters of LV structure and function could have resulted from the fact that 15% of the participants could not be assigned unambiguously to an LV diastolic function category and thus had a missing value for this covariate. As discussed, differences in the in vitro stability and biological variation of BNP and NT‐proBNP, in the analytic characteristics of the assays, and in the time point of testing, along with 1 additional freeze–thaw cycle for BNP, may also have played a role in producing the differences in their predictive value. However, as discussed, the long‐term stability of proteins in the frozen blood samples in the Heart and Soul Study and the strong correlation between BNP and NT‐proBNP across a range of values make it unlikely that these factors fully explain the findings observed in this study.
Conclusion
In patients with stable CHD, both BNP and NT‐proBNP are strong predictors of adverse CV events; however, when added to clinical risk factors, NT‐proBNP outperforms BNP in risk classification for adverse CV events.
Sources of Funding
The Heart and Soul Study was funded by the Department of Veterans Affairs (Washington, DC); the National Heart, Lung, and Blood Institute (grant R01 HL079235); the American Federation for Aging Research (Paul Beeson Scholars Program); the Robert Wood Johnson Foundation (Faculty Scholars Program); the Nancy Kirwan Heart Research Fund, San Francisco, California; and the Ischemia Research and Education Foundation. The NT‐proBNP assays were funded by Roche Diagnostics Corporation. The BNP assays were funded by Alere Inc.
Disclosures
M.A.W. has received research support from Roche Diagnostics, Inc and Alere, Inc.
References
- 1.Yasue H, Yoshimura M, Sumida H, Kikuta K, Kugiyama K, Jougasaki M, Ogawa H, Okumura K, Mukoyama M, Nakao K. Localization and mechanism of secretion of b‐type natriuretic peptide in comparison with those of a‐type natriuretic peptide in normal subjects and patients with heart failure. Circulation. 1994; 90:195-203. [DOI] [PubMed] [Google Scholar]
- 2.Clerico A, Fontana M, Zyw L, Passino C, Emdin M. Comparison of the diagnostic accuracy of brain natriuretic peptide (BNP) and the n‐terminal part of the propeptide of bnp immunoassays in chronic and acute heart failure: a systematic review. Clin Chem. 2007; 53:813-822. [DOI] [PubMed] [Google Scholar]
- 3.Glaser R, Peacock WF, Wu AH, Muller R, Mockel M, Apple FS. Placental growth factor and b‐type natriuretic peptide as independent predictors of risk from a multibiomarker panel in suspected acute coronary syndrome (acute risk and related outcomes assessed with cardiac biomarkers [arrow]) study. Am J Cardiol. 2011; 107:821-826. [DOI] [PubMed] [Google Scholar]
- 4.Scirica BM, Sabatine MS, Jarolim P, Murphy SA, de Lemos JL, Braunwald E, Morrow DA. Assessment of multiple cardiac biomarkers in non‐ST‐segment elevation acute coronary syndromes: observations from the merlin‐timi 36 trial. Eur Heart J. 2011; 32:697-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bibbins‐Domingo K, Gupta R, Na B, Wu AH, Schiller NB, Whooley MA. N‐terminal fragment of the prohormone brain‐type natriuretic peptide (NT‐proBNP), cardiovascular events, and mortality in patients with stable coronary heart disease. JAMA. 2007; 297:169-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kragelund C, Gronning B, Kober L, Hildebrandt P, Steffensen R. N‐terminal pro‐B‐type natriuretic peptide and long‐term mortality in stable coronary heart disease. N Engl J Med. 2005; 352:666-675. [DOI] [PubMed] [Google Scholar]
- 7.Omland T, de Lemos JA. Amino‐terminal pro‐B‐type natriuretic peptides in stable and unstable ischemic heart disease. Am J Cardiol. 2008; 101:61-66. [DOI] [PubMed] [Google Scholar]
- 8.Omland T, Sabatine MS, Jablonski KA, Rice MM, Hsia J, Wergeland R, Landaas S, Rouleau JL, Domanski MJ, Hall C, Pfeffer MA, Braunwald E. Prognostic value of B‐type natriuretic peptides in patients with stable coronary artery disease: the peace trial. J Am Coll Cardiol. 2007; 50:205-214. [DOI] [PubMed] [Google Scholar]
- 9.Richards M, Nicholls MG, Espiner EA, Lainchbury JG, Troughton RW, Elliott J, Frampton CM, Crozier IG, Yandle TG, Doughty R, MacMahon S, Sharpe N. Comparison of B‐type natriuretic peptides for assessment of cardiac function and prognosis in stable ischemic heart disease. J Am Coll Cardiol. 2006; 47:52-60. [DOI] [PubMed] [Google Scholar]
- 10.Ndrepepa G, Braun S, Niemoller K, Mehilli J, von Beckerath N, von Beckerath O, Vogt W, Schomig A, Kastrati A. Prognostic value of N‐terminal pro‐brain natriuretic peptide in patients with chronic stable angina. Circulation. 2005; 112:2102-2107. [DOI] [PubMed] [Google Scholar]
- 11.McKie PM, Rodeheffer RJ, Cataliotti A, Martin FL, Urban LH, Mahoney DW, Jacobsen SJ, Redfield MM, Burnett JC., Jr Amino‐terminal pro‐B‐type natriuretic peptide and b‐type natriuretic peptide: biomarkers for mortality in a large community‐based cohort free of heart failure. Hypertension. 2006; 47:874-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mueller T, Gegenhuber A, Poelz W, Haltmayer M. Head‐to‐head comparison of the diagnostic utility of BNP and NT‐probnp in symptomatic and asymptomatic structural heart disease. Clin Chim Acta. 2004; 341:41-48. [DOI] [PubMed] [Google Scholar]
- 13.Sakai H, Tsutamoto T, Ishikawa C, Tanaka T, Fujii M, Yamamoto T, Takashima H, Horie M. Direct comparison of brain natriuretic peptide (BNP) and N‐terminal pro‐bnp secretion and extent of coronary artery stenosis in patients with stable coronary artery disease. Circ J. 2007; 71:499-505. [DOI] [PubMed] [Google Scholar]
- 14.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health‐related quality of life: the heart and soul study. JAMA. 2003; 290:215-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, III, Zhang YL, Greene T, Levey AS. Estimating GFR using serum cystatin c alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008; 51:395-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I. Recommendations for quantitation of the left ventricle by two‐dimensional echocardiography. American society of echocardiography committee on standards, subcommittee on quantitation of two‐dimensional echocardiograms. J Am Soc Echocardiogr. 1989; 2:358-367. [DOI] [PubMed] [Google Scholar]
- 17.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009; 22:107-133. [DOI] [PubMed] [Google Scholar]
- 18.Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, Goldberg RJ, Hand MM, Jaffe AS, Julian DG, Levy D, Manolio T, Mendis S, Mensah G, Pajak A, Prineas RJ, Reddy KS, Roger VL, Rosamond WD, Shahar E, Sharrett AR, Sorlie P, Tunstall‐Pedoe H. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003; 108:2543-2549. [DOI] [PubMed] [Google Scholar]
- 19.D'Agostino RB, Russell MW, Huse DM, Ellison RC, Silbershatz H, Wilson PW, Hartz SC. Primary and subsequent coronary risk appraisal: new results from the framingham study. Am Heart J. 2000; 139:272-281. [DOI] [PubMed] [Google Scholar]
- 20.Pencina MJ, D'Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011; 30:11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pencina MJ, D'Agostino RB, Vasan RS. Statistical methods for assessment of added usefulness of new biomarkers. Clin Chem Lab Med. 2010; 48:1703-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pickering JW, Endre ZH. New metrics for assessing diagnostic potential of candidate biomarkers. Clin J Am Soc Nephrol. 2012; 7:1355-1364. [DOI] [PubMed] [Google Scholar]
- 23.Omland T, Richards AM, Wergeland R, Vik‐Mo H. B‐type natriuretic peptide and long‐term survival in patients with stable coronary artery disease. Am J Cardiol. 2005; 95:24-28. [DOI] [PubMed] [Google Scholar]
- 24.Blankenberg S, McQueen MJ, Smieja M, Pogue J, Balion C, Lonn E, Rupprecht HJ, Bickel C, Tiret L, Cambien F, Gerstein H, Munzel T, Yusuf S. Comparative impact of multiple biomarkers and n‐terminal pro‐brain natriuretic peptide in the context of conventional risk factors for the prediction of recurrent cardiovascular events in the heart outcomes prevention evaluation (hope) study. Circulation. 2006; 114:201-208. [DOI] [PubMed] [Google Scholar]
- 25.Polonsky TS, McClelland RL, Jorgensen NW, Bild DE, Burke GL, Guerci AD, Greenland P. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010; 303:1610-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow‐mediated dilation for incident cardiovascular events in a population‐based study: the multi‐ethnic study of atherosclerosis. Circulation. 2009; 120:502-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Omland T. B‐type natriuretic peptides: prognostic markers in stable coronary artery disease. Expert Rev Mol Diagn. 2008; 8:217-225. [DOI] [PubMed] [Google Scholar]
- 28.Omland T, Hagve TA. Natriuretic peptides: physiologic and analytic considerations. Heart Fail Clin. 2009; 5:471-487. [DOI] [PubMed] [Google Scholar]
- 29.Luchner A, Hengstenberg C, Lowel H, Riegger GA, Schunkert H, Holmer S. Effect of compensated renal dysfunction on approved heart failure markers: direct comparison of brain natriuretic peptide (BNP) and n‐terminal pro‐BNP. Hypertension. 2005; 46:118-123. [DOI] [PubMed] [Google Scholar]
- 30.van Kimmenade RR, Januzzi JL, Jr, Bakker JA, Houben AJ, Rennenberg R, Kroon AA, Crijns HJ, van Dieijen‐Visser MP, de Leeuw PW, Pinto YM. Renal clearance of B‐type natriuretic peptide and amino terminal pro‐B‐type natriuretic peptide a mechanistic study in hypertensive subjects. J Am Coll Cardiol. 2009; 53:884-890. [DOI] [PubMed] [Google Scholar]
- 31.Wu AH, Smith A, Wieczorek S, Mather JF, Duncan B, White CM, McGill C, Katten D, Heller G. Biological variation for N‐terminal pro‐ and B‐type natriuretic peptides and implications for therapeutic monitoring of patients with congestive heart failure. Am J Cardiol. 2003; 92:628-631. [DOI] [PubMed] [Google Scholar]
- 32.Ordonez‐Llanos J, Collinson PO, Christenson RH. Amino‐terminal pro‐B‐type natriuretic peptide: analytic considerations. Am J Cardiol. 2008; 101:9-15. [DOI] [PubMed] [Google Scholar]


