Abstract
Background
While self‐reported trans–fatty acid (TFA) consumption is linked to coronary heart disease (CHD), relationships between objective biomarkers of TFA subtypes (t‐16:1n9, total t‐18:1, and cis/trans‐(c/t‐), t/c‐ and t/t‐18:2) and cardiovascular disease (CVD) or total mortality are not well established.
Methods and Results
We evaluated 2742 adults in the Cardiovascular Health Study, aged 74±5 years and free of prevalent CVD, with plasma phospholipid TFA measures in 1992. Incident fatal and nonfatal CHD events, CVD and non‐CVD mortality, and total mortality were centrally adjudicated through 2010. Risks were assessed using Cox proportional hazards. During 31 494 person‐years, 1735 total deaths and 639 total CHD events occurred. In the multivariate model including mutual adjustment for the 5 TFA subtypes, circulating t/t‐18:2 was associated with higher total mortality (extreme quintile hazard ratio (HR)=1.23, 95% CI=1.04 to 1.44, P‐trend=0.01), CVD mortality (HR=1.40, 95% CI=1.05 to 1.86, P‐trend=0.02), and total CHD (HR=1.39, 95% CI=1.06 to 1.83, P‐trend=0.01). t/c‐18:2 was positively related to total mortality (HR=1.19, P‐trend=0.05), total CHD (HR=1.67, P‐trend=0.002), and nonfatal CHD (HR=2.06, P‐trend=0.002) after mutual adjustment; these associations were insignificant without mutual adjustment. Neither t‐16:1n9 nor t‐18:1 was significantly associated with total mortality or CVD, nor was c/t‐18:2 if we excluded early cases.
Conclusions
Among circulating TFAs, t/t‐18:2 was most adversely associated with total mortality, mainly due to the increased risk of CVD. t/c‐18:2 was also positively associated with total mortality and CHD, but only after adjustment for other TFAs. These results highlight the need for further investigation of dietary sources, nondietary determinants, and health effects of specific TFA subtypes, especially t‐18:2 isomers.
Keywords: cardiovascular diseases, coronary disease, nutrition, total mortality, trans–fatty acids
Introduction
The US Food and Drug Administration recently announced a preliminary determination that partially hydrogenated vegetable oil (PHVO), the primary source of industrial trans–fatty acids (TFA) in processed foods, will no longer be “generally recognized as safe” for use in food.1 This was based on evidence that consumption of TFA (unsaturated fatty acids with 1 or more carbon double‐bonds in the trans configuration) is associated with risk of coronary heart disease (CHD), adverse lipid effects,2–5 endothelial dysfunction,6–7 and pro‐inflammatory responses.6,8–10 The US Food and Drug Administration's action and most research to date have focused on total TFA, predominantly representing t‐18:1 (an 18‐carbon fatty acid with a single trans double‐bond).11–16 However, different TFA subtypes (of varying chain length and unsaturation) and different TFA isomers (of similar chain length and unsaturation, but different location of double bonds) may have both divergent dietary sources17 and health effects.18–22 For example, prior case–control studies suggested that trans‐isomers of linoleic acid (t‐18:2), but not of oleic acid (t‐18:1) or palmitoleic acid (t‐16:1), were associated with CHD death and sudden cardiac arrest.18–19 How exposure to these different TFA subtypes relates to other key outcomes, such as total mortality or non–cardiovascular disease (CVD) mortality, is not established. In addition, although circulating t‐18:2 appear especially relevant for CVD risk,18–19,22 prior studies evaluated only the sum of these fatty acids, and it is unknown how different individual t‐18:2 isomers, including c/t‐, t/c‐, and t/t‐18:2n6,9 (t/t‐18:2), relate to CHD, CVD death, and total mortality.
To address these key gaps in knowledge, we investigated prospective associations of plasma phospholipid TFA levels, objective biomarkers of exposure, with total mortality, CVD and non‐CVD mortality, and CHD incidence in the Cardiovascular Health Study (CHS), a community‐based prospective cohort of older US adults. Based on evidence from mechanistic studies and effects on risk factors,18–19,22 we hypothesized that t‐18:2, especially t/t‐18:2, would be associated with greater mortality and CVD risk, whereas t‐18:1 and t‐16:1n9 would not be. In addition, we hypothesized that circulating TFA subtypes would not be significantly associated with non‐CVD mortality. Given potentially diverse dietary sources of different TFA subtypes and isomers,16,23 establishing their health effects is crucial for both scientific understanding of how fatty acids influence health as well as for informing policy priorities.
Methods
Design and Population
CHS is a National Heart, Lung, and Blood Institute–sponsored, community‐based, multicenter prospective cohort of older US adults.24 Briefly, 5201 ambulatory, noninstitutionalized adults aged 65 and over were randomly selected and enrolled in 1989–1990 from Medicare eligibility lists in 4 US communities (Forsyth County, NC; Sacramento County, CA; Washington County, MD; Allegheny County, PA); an additional 687 black participants were similarly recruited and enrolled in 1992–1993. Among all eligible adults contacted, 57% agreed to enroll. Each center's institutional review committee approved the study, and all participants provided informed written consent.
Among cohort participants alive at the 1992–1993 study visit, we measured fatty acids in 3941 (71% of 5553) participants from blood samples drawn in 1992–1993, considered the baseline year for this analysis. For the present analysis, we excluded 1199 participants with prevalent CVD (coronary heart disease, stroke, transient ischemic attack, congestive heart failure, or atrial fibrillation) at this visit, resulting in 2742 participants evaluated in this analysis.
Fatty Acid Measurements
Plasma phospholipid fatty acid compositions were measured at the Fred Hutchinson Cancer Research Center using previously described methods (Data S2: Fatty acid measurements).25 Total lipids were extracted from plasma using the method of Folch,26 and the phospholipid fraction was isolated by 1‐dimensional thin layer chromatography. Fatty acid methyl esters were prepared by direct transesterification and separated using gas chromatography to quantify 45 distinct fatty acid peaks. Measured TFAs included 16‐carbon monounsaturated (t‐16:1n7, t‐16:1n9), 18‐carbon monounsaturated (t‐18:1n6, 18:1n7, 18:1n8, 18:1n9, 18:1n10‐12), and 18‐carbon polyunsaturated (t/t‐, c/t‐, t/c‐18:2) isomers. Given our focus on the industrial TFAs, we decided a priori to evaluate t‐16:1n7 in a separate investigation, due to its non‐PHVO dietary sources17 and inverse associations with metabolic risk.27–28 For this analysis, we evaluated t‐16:1n9, the 3 t‐18:2 isomers, and the sum of the t‐18:1 isomers (t‐18:1n6, 18:1n7, 18:1n8, 18:1n9, 18:1n10‐12), given their very high intercorrelations (r>0.83). These selected 5 TFA subtypes reflect a comprehensive list of the known TFAs coming from PHVO. Laboratory coefficients of variation were 5% for t‐16:1n9, 2% for total t‐18:1, and 8% for total t‐18:2.
While all TFA measurements were performed using stored blood from 1992 to 1993, the laboratory assays were tested at 2 time points: in 1999–2002 for a prior case–control study of CHD, and in 2007–2012 for all available samples in the cohort. We developed a calibration equation to evaluate and account for potential laboratory drift using duplicate measurements across these 2 time periods in a subset of participants. Laboratory drift was identified for t‐16:1n9 and t/t‐18:2, and calibration equations were used to correct for potential drift (Data S1: Method of assessment and correction for laboratory drifts). We also performed sensitivity analyses excluding the 285 participants with earlier measures.
We assessed long‐term reproducibility by comparing within‐person TFA levels in 100 subjects across blood samples drawn in 1992–1993, 1998–1999, and 2005–2006. The 6‐year and 13‐year correlations with baseline levels were 0.53 and 0.46 for t‐16:1n9, 0.65 and 0.60 for t‐18:1, 0.54 and 0.48 for c/t‐18:2, 0.35 and 0.22 for t/c‐18:2, and 0.36 and 0.43 for t/t‐18:2. The within‐person correlations of 0.40 to 0.60 compare favorably to similar correlations over time for other common CVD risk factors such as blood pressure and cholesterol levels.29–30 Conversely, the lower within‐person reliability for t/c‐18:2 would cause greater exposure misclassification over time and attenuation of true associations toward the null.
Other Risk Factors
At baseline in 1992–1993, sociodemographic characteristics, medical history, and lifestyle risk factors were collected during a 90‐minute home interview;24 and body–mass index, waist circumference, and blood pressure were measured by standardized methods during a clinical examination. Fasting total cholesterol, high‐density lipoprotein (HDL) cholesterol, triglycerides, and C‐reactive protein (CRP)31 were measured centrally; and low‐density lipoprotein (LDL) cholesterol was calculated according to the Friedewald equation.32 Dietary habits were assessed 3 years earlier, in 1989–1990, using a picture‐sort food frequency questionnaire validated against 6 detailed 24‐hour diet recall interviews spaced ≈1 month apart.33–34
Ascertainment of Events
Participants were followed by means of annual study‐clinic examinations with interim phone contacts for 10 years, and telephone contacts every 6 months thereafter. Follow‐up for vital status was 100% complete; <1% of all person‐time was missing and censored early. All potential CVD events and deaths were reviewed and classified by centralized adjudication committees of physicians using information from participant or proxy interviews, medical records, physician questionnaires, death certificates, medical examiner forms, Centers for Medicare & Medicaid Services hospitalizations, and available diagnostic tests and consultations. Algorithms and methods for mortality follow‐up and confirmation and classification of CHD in CHS have been described.35–36 CVD mortality was defined as death due to atherosclerotic CHD, cerebrovascular disease, other atherosclerotic disease, and other CVD. Total incident CHD was also adjudicated, including CHD death and nonfatal myocardial infarction. Given the large number of clinical end points examined and the complexities of evaluating total and subtypes of stroke, we did not investigate stroke in this study. However, due to the substantial biological and clinical relevance of stroke as well as the heterogeneity in stroke phenotypes, we are going to separately evaluate stroke as an end point in another study.
Statistical Analysis
Fatty acids levels were evaluated in quintiles as categorical variables. Tests for trend were evaluated by assigning each participant the median value of each quintile category and evaluating this as a continuous variable. Cox proportional‐hazards were used to estimate hazard ratios of events, with time‐at‐risk until the first event of interest, censoring at other causes of death in analyses of cause‐specific mortality or at the latest date of adjudicated follow‐up. The proportional hazard assumption was not rejected on the basis of Schoenfeld residuals for each TFA biomarker. To minimize potential confounding, we adjusted for age, sex, race, education, enrollment site, diabetes, hypertension, smoking, alcohol consumption, physical activity, body–mass index, waist circumference, phospholipid omega‐6 and omega‐3 fatty acids, and estimated dietary saturated and monounsaturated fat consumption. Because alcohol consumption could be nonlinearly related to CHD,37–38 we also modeled alcohol consumption with quadratic terms or as categorical variable (<1, 1 to 14, 14+ servings/week) in the multivariate model. In addition, we further adjusted for levels of LDL cholesterol, HDL cholesterol, and CRP as potential mediators in the multivariate model, because circulating TFAs could influence the levels of LDL cholesterol, HDL cholesterol, and CRP, which could further affect the risk of total mortality and CVD. The extent of attenuation in the effect estimates after the adjustment can help reflect the effects of TFAs attributable to the mediators in the model.39 Missing covariate information (most factors <2%; dietary factors = 4% to 10%) was imputed by best‐subset‐regression using multiple demographic/risk variables. Potential nonlinear associations were evaluated using restricted cubic splines.40
Because different TFA subtypes shared some common dietary sources and were partly intercorrelated,17 associations for 1 subtype could be confounded by the influence of other subtypes.19 We therefore performed analyses with and without mutual adjustment for the various TFA subtypes. We also performed sensitivity analyses evaluating total t‐18:2 (summing c/t‐, t/c‐, and t/t‐18:2); censoring the cohort at the midpoint of follow‐up (8 years) to minimize misclassification due to exposure variation over time; and excluding events within the first 2 years of follow‐up to minimize reverse causation from pre‐existing subclinical disease. We also performed analyses corrected for regression dilution due to within‐individual variation in TFA levels over time to obtain estimates of association due to usual levels of TFA exposure.41 We explored potential effect modification by age, sex, or race using multiplicative interaction terms and likelihood ratio testing, with Bonferroni correction for these exploratory multiple comparisons. Analyses were performed using Stata 10.1, with 2‐tailed α=0.05 except for exploratory tests for effect modification (2‐tailed α=0.00056).
Results
At baseline, mean±SD age was 74±5.1 years; 63.6% of participants were women, and 12.3% were African American. Intercorrelations between TFA isomers varied and were lowest for t/t‐18:2 (Table 1). Due to higher concentrations, total t‐18:1 correlated most strongly with total TFA.
Table 1.
Baseline Concentrations of Plasma Phospholipid Trans–Fatty Acid and Their Correlations in the Cardiovascular Health Study (n=2742)
| Trans‐16:1n9 | Total Trans‐18:1* | Cis/Trans‐18:2 | Trans/Cis‐18:2 | Trans/Trans‐18:2 | Total Trans Fat* | |
|---|---|---|---|---|---|---|
| Mean concentration (SD), % of fatty acids | 0.07 (0.02) | 1.97 (0.71) | 0.08 (0.02) | 0.13 (0.05) | 0.05 (0.02) | 2.48 (0.78) |
| 5th, 95th percentiles | 0.04, 0.11 | 0.96, 3.24 | 0.04, 0.12 | 0.07, 0.23 | 0.03, 0.07 | 1.19, 3.68 |
| Pearson correlation | ||||||
| Trans‐16:1n9 (cv=5%) | 1.00 | |||||
| Total trans‐18:1* (cv=2%) | 0.75 | 1.00 | ||||
| Cis/trans‐18:2 (cv=8%) | 0.47 | 0.62 | 1.00 | |||
| Trans/cis‐18:2 (cv=8%) | 0.33 | 0.50 | 0.78 | 1.00 | ||
| Trans/trans‐18:2 (cv=8%) | 0.08 | 0.09 | −0.04 | 0.02 | 1.00 | |
| Total trans fat* (cv=2%) | 0.76 | 0.99 | 0.67 | 0.56 | 0.09 | 1.00 |
Cv indicates coefficient of variation from laboratory; SD, standard deviation.
Total trans‐18:1 consisted of subtypes including trans‐18:1n6, n7, n8, n9, n10‐12 with intercorrelation between each subtype ranging from 0.83 to 0.93.
Total trans‐fatty acids were sum of trans‐16:1n7, trans‐16:1n9, total trans‐18:1, cis/trans‐18:2, trans/cis‐18:2, trans/trans‐18:2.
In unadjusted cross‐sectional analyses at baseline, higher t‐16:1n9 and t‐18:1 levels were associated with older age and lower education (Table 2). Most TFAs were associated with white race, except for t/t‐18:2, which was associated with black race. All TFAs correlated with lower alcohol consumption; associations with other lifestyle factors such as smoking status and physical activity were more modest. t‐16:1n9, t‐18:1 and t/t‐18:2 were inversely associated with body–mass index and waist circumference; while t‐16:1n9 and t‐18:1 were inversely and t/t‐18:2 was positively associated with HDL cholesterol. In addition, c/t‐ and t/c‐18:2 were positively and t/t‐18:2 was inversely associated with triglycerides. Circulating TFA levels were generally unassociated with LDL‐cholesterol levels, blood pressure, or CRP.
Table 2.
Baseline Characteristics of the 2742 Participants, by Quintile of Each Plasma Phospholipid Trans–Fatty Acid Concentration
| Trans‐16:1n9 | Total Trans‐18:1* | Cis/Trans‐18:2 | Trans/Cis‐18:2 | Trans/Trans‐18:2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| I | V | I | V | I | V | I | V | I | V | |
| Range, % total fatty acids | 0.02–0.05 | 0.09–0.20 | 0.23–1.37 | 2.55–5.70 | 0.01–0.06 | 0.10–0.26 | 0.02–0.09 | 0.17 to 0.62 | 0.011 to 0.036 | 0.053 to 0.085 |
| N adults in quintile | 550 | 548 | 549 | 548 | 550 | 528 | 562 | 548 | 594 | 536 |
| Age, y | 73.0±4.6 | 74.7±5.4* | 73.5±4.9 | 74.7±5.4* | 74.2±5.3 | 74.2±5.4 | 73.8±5.1 | 73.9±4.9 | 73.0±4.6 | 74.3±5.4* |
| Gender, %male | 38 | 33 | 36 | 37 | 47 | 34* | 41 | 36 | 31 | 39* |
| Race, %white | 87 | 92* | 81 | 93* | 73 | 97* | 78 | 93* | 94 | 69* |
| Education, % >high school | 51 | 41* | 53 | 36* | 46 | 42 | 48 | 43 | 46 | 47 |
| Diabetes mellitus*, % | 11 | 10 | 16 | 10* | 20 | 8* | 19 | 11* | 12 | 16 |
| Hypertension*, % | 41 | 36 | 43 | 38 | 41 | 35 | 43 | 35 | 38 | 45 |
| Current smoker, % | 10 | 11 | 10 | 12 | 9 | 10 | 10 | 12 | 10 | 10 |
| Former smoker, % | 45 | 38 | 48 | 37* | 47 | 42 | 43 | 44 | 43 | 42 |
| Alcohol, drinks/week | 4.4±7.2 | 0.9±2.6* | 5.1±12.1 | 0.9±3.7* | 2.8±5.9 | 1.5±4.4* | 2.94±10.8 | 1.64±4.4* | 3.3±11.0 | 1.6±4.2* |
| Physical activity, kcal/week | 1318±1803 | 901±1150* | 1126±1619 | 947±1194 | 1167±1537 | 1127±1491 | 1141±1602 | 1020±1291 | 999±1243 | 1032±1420 |
| Body–mass index, kg/m2 | 27.1±4.4 | 25.9±4.1* | 27.1±4.7 | 25.6±4.2* | 26.8±4.6 | 26.6±4.6 | 26.4±4.5 | 26.8±4.7 | 27.6±4.6 | 26.0±4.7* |
| Waist circumference, cm | 97.8±13.4 | 95.2±11.8* | 97.9±13.8 | 94.7±11.9* | 97.2±13.4 | 96.8±12.9 | 96.2±13.2 | 97.4±13.0 | 98.8±13.1 | 93.9±13.5* |
| Systolic blood pressure, mm Hg | 135.2±20.1 | 137.9±22.1 | 135.7±19.1 | 138.1±22.4 | 135.2±20.1 | 137.7±21.2 | 134.9±20.2 | 137.7±21.5 | 135.1±20.2 | 137.8±21.8 |
| Diastolic blood pressure, mm Hg | 72.2±11.0 | 71.2±11.3 | 72.0±11.6 | 70.8±11.0 | 71.9±11.7 | 71.1±11.4 | 71.4±11.2 | 71.7±11.3 | 71.2±12.1 | 72.4±11.5 |
| LDL cholesterol, mg/dL | 125.8±34.0 | 130.0±32.9 | 124.4±36.3 | 128.6±32.3* | 127.6±34.7 | 125.9±32.8 | 125.5±33.1 | 129.2±33.8 | 127.9±33.5 | 124.1±34.7 |
| HDL cholesterol, md/dL | 56.8±15.5 | 53.2±13.2* | 58.0±16.1 | 53.1±14.0* | 55.0±14.4 | 54.0±14.3 | 55.1±14.6 | 53.2±13.9 | 53.4±14.2 | 57.7±14.9* |
| Triglyceride, mg/dL | 144.6±95.8 | 135.3±72.8 | 143.5±93.8 | 138.8±80.4 | 124.8±69.7 | 156.7±88.2* | 127.0±69.6 | 156.8±91.1* | 180.1±99.5 | 109.2±71.4* |
| C‐reactive protein, mg/L | 6.0±10.1 | 3.4±5.2* | 5.7±10.1 | 4.1±9.5* | 5.6±11.0 | 4.6±9.7 | 5.5±11.6 | 4.7±8.5 | 5.4±9.3 | 4.9±11.5 |
| Dietary factors* | ||||||||||
| SFA, %energy | 10.5±2.2 | 10.0±2.1* | 10.5±2.3 | 10.2±2.1 | 10.4±2.2 | 10.2±2.1 | 10.2±2.3 | 10.3±2.1 | 10.3±2.3 | 10.3±2.0 |
| MUFA, %energy | 11.6±2.3 | 11.4±2.4 | 11.5±2.4 | 11.6±2.4 | 11.5±2.4 | 11.5±2.4 | 11.4±2.4 | 11.7±2.4 | 11.6±2.4 | 11.7±2.2 |
| Carbohydrates, %energy | 51.3±7.5 | 53.4±7.9* | 51.5±7.9 | 52.8±7.6* | 51.8±7.5 | 53.2±7.9* | 52.3±7.7 | 52.6±7.9 | 52.1±8.0 | 52.0±7.1 |
| Fiber, g/day | 28.9±12.5 | 30.3±12.2 | 29.1±12.3 | 29.1±12.3 | 29.2±12.0 | 29.6±12.4 | 30.0±12.0 | 29.0±12.1 | 29.2±12.8 | 29.7±11.0 |
| Trans16:1, %energy | 0.08±0.03 | 0.07±0.03* | 0.08±0.03 | 0.07±0.03 | 0.08±0.03 | 0.07±0.03 | 0.07±0.03 | 0.08±0.03* | 0.08±0.03 | 0.08±0.03 |
| Trans18:1, %energy | 1.91±0.6 | 2.09±0.7* | 1.87±0.6 | 2.16±0.7* | 1.93±0.7 | 2.13±0.7* | 1.88±0.7 | 2.15±0.7* | 1.96±0.6 | 2.03±0.7 |
| Trans18:2, %energy | 0.15±0.05 | 0.16±0.06* | 0.10±0.05 | 0.20±0.06* | 0.15±0.06 | 0.16±0.06* | 0.14±0.06 | 0.17±0.06* | 0.15±0.06 | 0.16±0.06 |
| Plasma phospholipid biomarker | ||||||||||
| Linoleic acid | 18.8±2.6 | 20.3±2.4* | 18.5±2.5 | 20.7±2.3* | 18.9±2.5 | 20.2±2.4* | 19.1±2.6 | 19.9±2.4* | 19.5±2.6 | 19.5±2.4 |
| EPA+DHA | 3.8±1.2 | 3.5±1.3* | 4.0±1.4 | 3.2±1.0* | 4.1±1.4 | 3.1±0.9* | 4.1±1.4 | 3.2±1.0* | 3.5±1.1 | 3.8±1.3* |
Baseline characteristics among adults in the lowest and highest quintile categories for each trans–fatty acid level are presented (values for other quintiles are presented in Table S3). Values for continuous variable represent mean±standard deviation; values for categorical variables represent percentage. EPA+DHA indicates eicosapentaenoic acids plus docosahexaenoic acids; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; MUFA, monounsaturated fatty acids; SFA, saturated fatty acids.
P<0.01 for trend across quintile categories are highlighted by asterisk.
Total trans‐18:1 consisted of subtypes including trans‐18:1n6, n7, n8, n9, n10‐12 that were highly inter‐related (pairwise correlations=0.83 to 0.93). Total trans–fatty acids predominantly consisted of trans‐18:1 (r=0.99), findings for total fats were similar to findings for total trans‐18:1 (data not shown).
Diabetes mellitus was defined as a fasting glucose value ≥126 mg/dL or use of insulin or oral hypoglycemic agent.
Hypertension was defined as systolic and diastolic blood pressures ≥140/90 mm Hg or use of antihypertensive treatment.
Diet factors were assessed 3 years before baseline in year 1989–1990.
During 31 494 person‐years of follow‐up, 1735 deaths occurred. In multivariable‐adjusted analysis, t/t‐18:2 was associated with higher total mortality (Table 3), with 21% higher risk among participants in the top quintiles, compared with the bottom quintile (hazard ratio [HR]=1.21, 95% CI=1.04 to 1.32; P‐trend=0.01). Other TFA subtypes were not significantly associated with total mortality. Modeling alcohol consumption with quadratic terms or as categorical variables, or further adjusting for CRP, LDL cholesterol, and HDL cholesterol did not appreciably alter these results (data not shown). In restricted cubic splines analyses, none of the circulating TFA subtypes were significantly nonlinearly related to total mortality (P‐nonlinearity<0.05 for each).
Table 3.
Prospective Associations of Plasma Phospholipid Trans–Fatty Acids With Risk of Total Mortality (n=2742)
| Quintiles of Plasma Phospholipids Trans–Fatty Acid Levels | P Trend* | |||||
|---|---|---|---|---|---|---|
| I | II | III | IV | V | ||
| Trans‐16:1n9 | ||||||
| Cases (person‐years) | 328 (6464) | 328 (6478) | 336 (6345) | 306 (6089) | 346 (6118) | |
| Hazard ratio (95% CI)* | 1.00 (ref) | 1.00 (0.86, 1.17) | 0.99 (0.85, 1.15) | 0.89 (0.76, 1.04) | 1.02 (0.87, 1.19) | 0.88 |
| Hazard ratio (95% CI)* | 1.00 (ref) | 1.05 (0.90, 1.23) | 1.06 (0.89, 1.25) | 0.93 (0.77, 1.13) | 1.05 (0.85, 1.30) | 0.92 |
| Total trans‐18:1* | ||||||
| Cases (person‐years) | 344 (6162) | 311 (6411) | 314 (6476) | 321 (6348) | 354 (6097) | |
| Hazard ratio (95% CI)* | 1.00 (ref) | 0.83 (0.72, 0.97) | 0.81 (0.69, 0.94) | 0.93 (0.80, 1.09) | 0.90 (0.77, 1.06) | 0.75 |
| Hazard ratio (95% CI)* | 1.00 (ref) | 0.83 (0.70, 0.99) | 0.80 (0.66, 0.96) | 0.91 (0.73, 1.13) | 0.82 (0.64, 1.05) | 0.33 |
| Cis/trans‐18:2 | ||||||
| Cases (person‐years) | 354 (5953) | 306 (6375) | 307 (6752) | 345 (6511) | 332 (5903) | |
| Hazard ratio (95% CI)* | 1.00 (ref) | 0.83 (0.71, 0.97) | 0.83 (0.72, 0.97) | 0.91 (0.78, 1.06) | 0.98 (0.84, 1.15) | 0.73 |
| Hazard ratio (95% CI)* | 1.00 (ref) | 0.87 (0.74, 1.02) | 0.86 (0.71, 1.03) | 0.90 (0.72, 1.10) | 0.92 (0.72, 1.17) | 0.65 |
| Trans/cis‐18:2 | ||||||
| Cases (person‐years) | 345 (6354) | 317 (6304) | 310 (6475) | 320 (6282) | 352 (6078) | |
| Hazard ratio (95% CI)* | 1.00 (ref) | 0.89 (0.77, 1.04) | 0.92 (0.79, 1.07) | 1.01 (0.86, 1.18) | 1.08 (0.92, 1.26) | 0.07 |
| Hazard ratio (95% CI)* | 1.00 (ref) | 0.97 (0.82, 1.14) | 1.04 (0.87, 1.24) | 1.13 (0.93, 1.37) | 1.19 (0.96, 1.49) | 0.05 |
| Trans/trans‐18:2 | ||||||
| Cases (person‐years) | 328 (7137) | 302 (5898) | 341 (6475) | 330 (6113) | 343 (5870) | |
| Hazard ratio (95% CI)* | 1.00 (ref) | 1.04 (0.89, 1.21) | 1.07 (0.92, 1.24) | 1.09 (0.94, 1.27) | 1.21 (1.04, 1.42) | 0.01 |
| Hazard ratio (95% CI)* | 1.00 (ref) | 1.05 (0.90, 1.23) | 1.08 (0.92, 1.25) | 1.09 (0.93, 1.27) | 1.23 (1.04, 1.44) | 0.02 |
BMI indicates body mass index; EPA+DHA, eicosapentaenoic acids plus docosahexaenoic acids.
P‐trend was evaluated by assigning each participant the median value of each quintile category and evaluating this as a continuous variable.
Hazard ratios without mutual adjustment (95% CI) were adjusted for age (years), sex (male/female), race (white/nonwhite), education (<high school, high school, some college, college graduate), enrollment site, diabetes (yes/no), hypertension (yes/no), smoking (never/former/current), alcohol consumption (servings/week), physical activity (kcal/week), BMI (kg/m2), waist circumference (cm), dietary saturated fat (%energy), dietary monounsaturated fat (%energy), dietary fiber (g/day), plasma phospholipid EPA+DHA (%fatty acids), plasma phospholipid linoleic acid (%fatty acids).
Hazard ratios with mutual adjustment (95% CI) were adjusted for age (years), sex (male/female), race (white/nonwhite), education (<high school, high school, some college, college graduate), enrollment site, diabetes (yes/no), hypertension (yes/no), smoking (never/former/current), alcohol consumption (servings/week), physical activity (kcal/week), BMI (kg/m2), waist circumference (cm), dietary saturated fat (%energy), dietary monounsaturated fat (%energy), dietary fiber (g/day), plasma phospholipid EPA+DHA (% fatty acids), plasma phospholipid linoleic acid (% fatty acids), and other types of plasma phospholipid trans–fatty acids (% of fatty acids).
Total trans‐18:1 consisted of subtypes including trans‐18:1n6, n7, n8, n9, n10‐12 that were highly inter‐related (pairwise correlations=0.83 to 0.93).
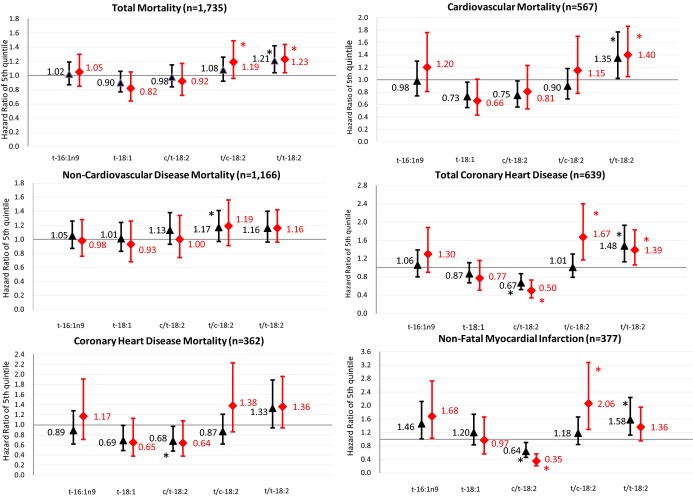
Due to intercorrelations of the TFA subtypes (Table 1), we performed analyses with and without mutual adjustment to evaluate independent associations (Figure 1). After mutual adjustment, t/t‐18:2 remained positively associated with total mortality (extreme quintile HR=1.23, P‐trend=0.01, Table 3). In addition, t/c‐18:2 was associated with a trend toward higher total mortality (HR=1.19, 95% CI=0.96 to 1.49, P‐trend=0.05). Other circulating TFAs were not significantly associated with total mortality either with or without mutual adjustment.
Figure 1.
Prospective associations of plasma phospholipid TFA with total mortality, CVD, and CHD before and after mutual adjustment. Black triangles indicate multivariate‐adjusted hazard ratios comparing adults in the top quintile category to adults in the bottom quintile category, before mutual adjustment for the 5 TFAs; red diamonds, after mutual adjustment for the 5 TFAs. Error bars represent 95% CI. All risk estimates were adjusted for age, sex, race, education, enrollment site, diabetes, hypertension, smoking, alcohol consumption, physical activity, BMI, waist circumference, dietary saturated fat, dietary monounsaturated fat, dietary fiber, plasma phospholipid EPA+DHA, plasma phospholipid linoleic acid. *P‐trend<0.05 across quintiles. BMI indicates body–mass index; CHD, coronary heart disease; CVD, cardiovascular disease; EPA+DHA, eicosapentaenoic acids plus docosahexaenoic acids; TFA, trans–fatty acid.
When we evaluated CVD and non‐CVD mortality and incident CHD after mutual adjustment, t/t‐18:2 was associated with 40% higher risk of CVD mortality (HR=1.40, 95% CI=1.05 to 1.86, P‐trend=0.02, Table 4 and Figure 1), but not with non‐CVD mortality (P‐trend=0.14); and with 39% higher incidence of total CHD (HR=1.39, 95% CI=1.06 to 1.83, P‐trend=0.01, Table 5 and Figure 1), with generally similar although not significant associations for fatal and nonfatal CHD (P‐trend>0.08 for each). No other TFAs were significantly related to CVD or non‐CVD mortality. t/c‐18:2 was positively associated with total CHD (HR=1.67, 95% CI=1.17 to 2.40, P‐trend=0.002) and nonfatal CHD (HR=2.06, 95% CI=1.29 to 3.28, P‐trend=0.002), but not fatal CHD (P‐trend=0.07). In contrast, c/t‐18:2 was inversely associated with total CHD (HR=0.50, 95% CI=0.34 to 0.73, P‐trend=0.001) and nonfatal CHD (HR=0.35, 95% CI=0.21 to 0.56, P‐trend<0.001), but not fatal CHD (P‐trend=0.10). Neither t‐16:1n9 nor t‐18:1 was significantly associated with fatal or nonfatal CHD (P‐trend>0.05 each).
Table 4.
Prospective Association of Plasma Phospholipid Trans–Fatty Acid With Cardiovascular and Noncardiovascular Mortality With Mutual Adjustment (n=2742)
| Quintiles of Plasma Phospholipid Trans–Fatty Acid Levels | P Value For Trend* | |||||
|---|---|---|---|---|---|---|
| I | II | III | IV | V | ||
| Cardiovascular mortality (n=567) | ||||||
| Trans16:1n9 | 1.00 (ref) | 1.37 (1.04, 1.80) | 1.21 (0.89, 1.65) | 1.16 (0.82, 1.63) | 1.20 (0.81, 1.76) | 0.65 |
| Total trans18: 1* | 1.00 (ref) | 0.72 (0.54, 0.96) | 0.64 (0.46, 0.89) | 0.71 (0.48, 1.04) | 0.66 (0.43, 1.01) | 0.14 |
| Cis/trans18:2 | 1.00 (ref) | 0.75 (0.57, 1.00) | 0.74 (0.54, 1.02) | 0.87 (0.61, 1.24) | 0.81 (0.53, 1.23) | 0.43 |
| Trans/cis18:2 | 1.00 (ref) | 1.02 (0.77, 1.34) | 1.02 (0.74, 1.39) | 1.12 (0.80, 1.57) | 1.15 (0.78, 1.70) | 0.40 |
| Trans/trans18:2 | 1.00 (ref) | 1.17 (0.89, 1.54) | 1.18 (0.89, 1.55) | 1.25 (0.95, 1.66) | 1.40 (1.05, 1.86) | 0.02 |
| Noncardiovascular disease mortality (n=1166) | ||||||
| Trans16:1n9 | 1.00 (ref) | 0.91 (0.75, 1.11) | 0.99 (0.80, 1.22) | 0.83 (0.66, 1.05) | 0.98 (0.76, 1.28) | 0.86 |
| Total trans18:1* | 1.00 (ref) | 0.90 (0.73, 1.11) | 0.90 (0.71, 1.14) | 1.03 (0.79, 1.35) | 0.93 (0.68, 1.26) | 0.91 |
| Cis/trans18:2 | 1.00 (ref) | 0.95 (0.77, 1.16) | 0.93 (0.74, 1.17) | 0.93 (0.72, 1.20) | 1.00 (0.74, 1.34) | 0.94 |
| Trans/cis18:2 | 1.00 (ref) | 0.94 (0.77, 1.15) | 1.04 (0.83, 1.29) | 1.11 (0.87, 1.41) | 1.19 (0.91, 1.56) | 0.09 |
| Trans/trans18:2 | 1.00 (ref) | 1.01 (0.83, 1.21) | 1.04 (0.86, 1.25) | 1.02 (0.85, 1.24) | 1.16 (0.96, 1.42) | 0.14 |
Models were adjusted for age (years), sex (male/female), race (white/nonwhite), education (<high school, high school, some college, college graduate), enrollment site, diabetes (yes/no), hypertension (yes/no), smoking (never/former/current), alcohol consumption (servings/week), physical activity (kcal/week), BMI (kg/m2), waist circumference (cm), dietary saturated fat (%energy), dietary monounsaturated fat (%energy), dietary fiber (g/day), plasma phospholipid EPA+DHA (% fatty acids), plasma phospholipid linoleic acid (% fatty acids), and other types of plasma phospholipid trans–fatty acids (% of fatty acids). BMI indicates body–mass index; EPA+DHA, eicosapentaenoic acids plus docosahexaenoic acids.
P‐trend was evaluated by assigning each participant the median value of each quintile category and evaluating this as a continuous variable.
Total trans18:1 consisted of subtypes including trans‐18:1n6, n7, n8, n9, n10‐12 that were highly inter‐related (pairwise correlations=0.83 to 0.93).
Table 5.
Prospective Association of Plasma Phospholipid Trans–Fatty Acid With Total Coronary Heart Disease With Mutual Adjustment (n=2742)
| Quintiles of Plasma Phospholipid Trans–Fatty Acid Levels | P Value For Trend* | |||||
|---|---|---|---|---|---|---|
| I | II | III | IV | V | ||
| Total coronary heart disease (n=639) | ||||||
| Trans16:1n9 | 1.00 (ref) | 1.26 (0.96, 1.64) | 1.35 (1.01, 1.80) | 1.36 (0.99, 1.87) | 1.30 (0.90, 1.88) | 0.22 |
| Total trans18:1* | 1.00 (ref) | 0.89 (0.68, 1.18) | 0.83 (0.61, 1.14) | 0.78 (0.54, 1.13) | 0.77 (0.51, 1.16) | 0.23 |
| Cis/trans18:2 | 1.00 (ref) | 0.63 (0.48, 0.82) | 0.57 (0.43, 0.77) | 0.53 (0.38, 0.74) | 0.50 (0.34, 0.73) | 0.001 |
| Trans/cis18:2 | 1.00 (ref) | 1.13 (0.87, 1.48) | 1.16 (0.86, 1.56) | 1.65 (1.21, 2.25) | 1.67 (1.17, 2.40) | 0.002 |
| Trans/trans18:2 | 1.00 (ref) | 1.14 (0.88, 1.49) | 1.43 (1.11, 1.85) | 1.40 (1.07, 1.81) | 1.39 (1.06, 1.83) | 0.01 |
| Coronary heart disease mortality (n=362)* | ||||||
| Trans16:1n9 | 1.00 (ref) | 1.28 (0.91, 1.81) | 1.26 (0.86, 1.85) | 1.20 (0.79, 1.83) | 1.17 (0.71, 1.91) | 0.68 |
| Total trans18:1* | 1.00 (ref) | 0.70 (0.49, 1.00) | 0.65 (0.43, 0.96) | 0.60 (0.37, 0.98) | 0.65 (0.38, 1.13) | 0.20 |
| Cis/trans18:2 | 1.00 (ref) | 0.85 (0.61, 1.20) | 0.75 (0.50, 1.11) | 0.75 (0.48, 1.18) | 0.64 (0.38, 1.08) | 0.10 |
| Trans/cis18:2 | 1.00 (ref) | 0.92 (0.65, 1.31) | 0.89 (0.60, 1.32) | 1.32 (0.88, 1.98) | 1.38 (0.86, 2.23) | 0.07 |
| Trans/trans18:2 | 1.00 (ref) | 1.23 (0.87, 1.74) | 1.30 (0.91, 1.84) | 1.36 (0.96, 1.93) | 1.36 (0.94, 1.96) | 0.11 |
| Nonfatal coronary heart disease (n=377)* | ||||||
| Trans16:1n9 | 1.00 (ref) | 1.36 (0.94, 1.96) | 1.72 (1.17, 2.54) | 1.61 (1.05, 2.47) | 1.68 (1.03, 2.73) | 0.06 |
| Total trans18:1* | 1.00 (ref) | 1.09 (0.74, 1.59) | 1.15 (0.76, 1.74) | 1.00 (0.61, 1.61) | 0.97 (0.56, 1.66) | 0.72 |
| Cis/trans18:2 | 1.00 (ref) | 0.47 (0.33, 0.66) | 0.44 (0.30, 0.64) | 0.38 (0.25, 0.58) | 0.35 (0.21, 0.56) | <0.001 |
| Trans/cis18:2 | 1.00 (ref) | 1.37 (0.96, 1.95) | 1.52 (1.03, 2.24) | 2.11 (1.41, 3.16) | 2.06 (1.29, 3.28) | 0.002 |
| Trans/trans18:2 | 1.00 (ref) | 1.10 (0.77, 1.56) | 1.40 (1.00, 1.95) | 1.29 (0.92, 1.82) | 1.36 (0.95, 1.95) | 0.08 |
Models were adjusted for age (years), sex (male/female), race (white/nonwhite), education (<high school, high school, some college, college graduate), enrollment site, diabetes (yes/no), hypertension (yes/no), smoking (never/former/current), alcohol consumption (servings/week), physical activity (kcal/week), BMI (kg/m2), waist circumference (cm), dietary saturated fat (%energy), dietary monounsaturated fat (%energy), dietary fiber (g/day), plasma phospholipid EPA+DHA (% fatty acids), plasma phospholipid linoleic acid (% fatty acids), and other types of plasma phospholipid trans fatty acids (% of fatty acids). BMI indicates body–mass index; EPA+DHA, eicosapentaenoic acids plus docosahexaenoic acids.
P‐trend was evaluated by assigning each participant the median value of each quintile category and evaluating this as a continuous variable.
Total trans18:1 consisted of subtypes including trans‐18:1n6, n7, n8, n9, n10‐12 that were highly inter‐related (pairwise correlations=0.83 to 0.93).
Subtypes of total coronary heart disease.
Several of these associations were similar with (Tables 4, 5 and Figure 1) or without mutual adjustment (Tables 6 and 7). The main exceptions were for c/t‐ and t/c‐18:2 (intercorrelation r=0.78), for which associations were generally strengthened with mutual adjustment. There was little evidence that any of the observed relationship were modified by age, sex, or race (P‐interaction>0.001 each).
Table 6.
Prospective Association of Plasma Phospholipid Trans–Fatty Acid With Cardiovascular and Noncardiovascular Mortality Without Mutual Adjustment (n=2742)
| Quintiles of Plasma Phospholipid Trans–Fatty Acid Levels | P Value For Trend* | |||||
|---|---|---|---|---|---|---|
| I | II | III | IV | V | ||
| Cardiovascular mortality (n=567) | ||||||
| Trans16:1n9 | 1.00 (ref) | 1.22 (0.94, 1.59) | 1.01 (0.77, 1.32) | 0.94 (0.71, 1.25) | 0.98 (0.74, 1.30) | 0.36 |
| Total trans18:1* | 1.00 (ref) | 0.75 (0.58, 0.97) | 0.67 (0.51, 0.87) | 0.75 (0.57, 0.99) | 0.73 (0.55, 0.96) | 0.08 |
| Cis/trans18:2 | 1.00 (ref) | 0.70 (0.54, 0.90) | 0.66 (0.50, 0.86) | 0.78 (0.60, 1.01) | 0.75 (0.56, 0.98) | 0.12 |
| Trans/cis18:2 | 1.00 (ref) | 0.86 (0.67, 1.11) | 0.81 (0.62, 1.06) | 0.91 (0.70, 1.19) | 0.90 (0.69, 1.18) | 0.74 |
| Trans/trans18:2 | 1.00 (ref) | 1.14 (0.87, 1.50) | 1.15 (0.88, 1.51) | 1.22 (0.93, 1.60) | 1.35 (1.02, 1.77) | 0.03 |
| Noncardiovascular disease mortality (n=1166) | ||||||
| Trans16:1n9 | 1.00 (ref) | 0.91 (0.75, 1.09) | 0.98 (0.82, 1.18) | 0.86 (0.71, 1.05) | 1.05 (0.87, 1.26) | 0.62 |
| Total trans18:1* | 1.00 (ref) | 0.89 (0.73, 1.07) | 0.89 (0.74, 1.08) | 1.04 (0.86, 1.26) | 1.01 (0.83, 1.24) | 0.36 |
| Cis/trans18:2 | 1.00 (ref) | 0.92 (0.77, 1.12) | 0.95 (0.78, 1.14) | 0.99 (0.82, 1.20) | 1.13 (0.93, 1.38) | 0.12 |
| Trans/cis18:2 | 1.00 (ref) | 0.91 (0.75, 1.09) | 0.98 (0.81, 1.18) | 1.06 (0.88, 1.28) | 1.17 (0.97, 1.41) | 0.02 |
| Trans/trans18:2 | 1.00 (ref) | 0.99 (0.82, 1.19) | 1.03 (0.86, 1.24) | 1.03 (0.86, 1.24) | 1.16 (0.96, 1.40) | 0.12 |
Models were adjusted for age (years), sex (male/female), race (white/nonwhite), education (<high school, high school, some college, college graduate), enrollment site, diabetes (yes/no), hypertension (yes/no), smoking (never/former/current), alcohol consumption (servings/week), physical activity (kcal/week), BMI (kg/m2), waist circumference (cm), dietary saturated fat (%energy), dietary monounsaturated fat (%energy), dietary fiber (g/day), plasma phospholipid EPA+DHA (% fatty acids), plasma phospholipid linoleic acid (% fatty acids). BMI indicates body–mass index; EPA+DHA, eicosapentaenoic acids plus docosahexaenoic acids.
P‐trend was evaluated by assigning each participant the median value of each quintile category and evaluating this as a continuous variable.
Total trans18:1 consisted of subtypes including trans‐18:1n6, n7, n8, n9, n10‐12 that were highly interrelated (pairwise correlations=0.83 to 0.93).
Table 7.
Prospective Association of Plasma Phospholipid Trans–Fatty Acid With Total Coronary Heart Disease Without Mutual Adjustment (n=2742)
| Quintiles of Plasma Phospholipid Trans–Fatty Acid Levels | P Value For Trend* | |||||
|---|---|---|---|---|---|---|
| I | II | III | IV | V | ||
| Total coronary heart disease (n=639) | ||||||
| Trans16:1n9 | 1.00 (ref) | 1.17 (0.91, 1.51) | 1.24 (0.96, 1.60) | 1.18 (0.90, 1.53) | 1.06 (0.80, 1.39) | 0.92 |
| Total trans18:1* | 1.00 (ref) | 0.95 (0.74, 1.22) | 0.89 (0.69, 1.15) | 0.87 (0.66, 1.13) | 0.87 (0.67, 1.15) | 0.29 |
| Cis/trans18:2 | 1.00 (ref) | 0.65 (0.51, 0.83) | 0.64 (0.50, 0.82) | 0.66 (0.51, 0.84) | 0.67 (0.52, 0.87) | 0.01 |
| Trans/cis18:2 | 1.00 (ref) | 0.90 (0.70, 1.15) | 0.83 (0.64, 1.07) | 1.08 (0.84, 1.38) | 1.01 (0.79, 1.30) | 0.45 |
| Trans/trans18:2 | 1.00 (ref) | 1.17 (0.90, 1.52) | 1.46 (1.14, 1.88) | 1.51 (1.17, 1.95) | 1.48 (1.13, 1.93) | <0.001 |
| Coronary heart disease mortality (n=362)* | ||||||
| Trans16:1n9 | 1.00 (ref) | 1.12 (0.80, 1.56) | 1.03 (0.73, 1.44) | 0.93 (0.65, 1.32) | 0.89 (0.62, 1.28) | 0.29 |
| Total trans18:1* | 1.00 (ref) | 0.73 (0.53, 1.01) | 0.69 (0.49, 0.95) | 0.64 (0.45, 0.91) | 0.69 (0.49, 0.99) | 0.06 |
| Cis/trans18:2 | 1.00 (ref) | 0.76 (0.55, 1.03) | 0.65 (0.47, 0.91) | 0.72 (0.52, 1.00) | 0.68 (0.48, 0.97) | 0.04 |
| Trans/cis18:2 | 1.00 (ref) | 0.77 (0.56, 1.06) | 0.67 (0.48, 0.95) | 0.94 (0.68, 1.29) | 0.87 (0.62, 1.21) | 0.92 |
| Trans/trans18:2 | 1.00 (ref) | 1.20 (0.85, 1.69) | 1.23 (0.88, 1.74) | 1.36 (0.97, 1.90) | 1.33 (0.93, 1.89) | 0.10 |
| Nonfatal coronary heart disease (n=377)* | ||||||
| Trans16:1n9 | 1.00 (ref) | 1.37 (0.96, 1.95) | 1.78 (1.26, 2.51) | 1.59 (1.11, 2.27) | 1.46 (1.01, 2.12) | 0.09 |
| Total trans18:1* | 1.00 (ref) | 1.20 (0.86, 1.68) | 1.30 (0.93, 1.82) | 1.18 (0.82, 1.69) | 1.20 (0.83, 1.74) | 0.50 |
| Cis/trans18:2 | 1.00 (ref) | 0.57 (0.41, 0.79) | 0.64 (0.47, 0.88) | 0.63 (0.46, 0.87) | 0.64 (0.46, 0.90) | 0.03 |
| Trans/cis18:2 | 1.00 (ref) | 1.06 (0.76, 1.47) | 1.05 (0.75, 1.46) | 1.30 (0.94, 1.80) | 1.18 (0.84, 1.66) | 0.20 |
| Trans/trans18:2 | 1.00 (ref) | 1.16 (0.82, 1.65) | 1.56 (1.12, 2.17) | 1.55 (1.11, 2.17) | 1.58 (1.12, 2.24) | <0.001 |
Models were adjusted for age (years), sex (male/female), race (white/nonwhite), education (<high school, high school, some college, college graduate), enrollment site, diabetes (yes/no), hypertension (yes/no), smoking (never/former/current), alcohol consumption (servings/week), physical activity (kcal/week), BMI (kg/m2), waist circumference (cm), dietary saturated fat (%energy), dietary monounsaturated fat (%energy), dietary fiber (g/day), plasma phospholipid EPA+DHA (% fatty acids), plasma phospholipid linoleic acid (% fatty acids). BMI indicates body–mass index; EPA+DHA, eicosapentaenoic acids plus docosahexaenoic acids.
P‐trend was evaluated by assigning each participant the median value of each quintile category and evaluating this as a continuous variable.
Total trans18:1 consisted of subtypes including trans‐18:1n6, n7, n8, n9, n10‐12 that were highly inter‐related (pairwise correlations=0.83 to 0.93).
Subtypes of total coronary heart disease.
In sensitivity analyses, total t‐18:2 (the sum of c/t, t/c, and t/t isomers) was positively associated with total mortality (HR=1.24, 95% CI=1.03 to 1.49, P‐trend=0.009) and non‐CVD mortality (HR=1.24, 95% CI=1.03 to 1.62, P‐trend=0.007), but not CVD mortality or CHD incidence (P‐trend>0.31 each). Excluding events within the first 2 years of follow‐up to minimize reverse causation had little effect on results, except for attenuation of the inverse associations between c/t‐18:2 and total (HR=1.02, 95% CI=0.87 to 1.21, P‐trend=0.36), fatal (HR=0.75, 95% CI=0.52 to 1.09, P‐trend=0.14), and nonfatal CHD (HR=0.83, 95% CI=0.62 to 1.10, P‐trend=0.33). Censoring follow‐up at 8 years to minimize exposure misclassification with increasing duration of follow‐up did not appreciably change the results (data not shown). We also corrected for regression dilution in TFA measurements; as would be expected, the magnitude of each of the associations was strengthened, with wider confidence intervals and unchanged results of tests for trend (Figure S2).
In sensitivity analysis of t/t‐18:2 without correction of laboratory drift, t/t‐18:2 levels were positively associated with total mortality and each of the other outcomes except for non‐CVD mortality (Figure 2). Magnitudes of these associations were larger compared with the original results correcting for laboratory drift. After excluding 285 participants in whom TFA levels were measured in 1999 (a subset enriched with early CHD cases), associations of t/t‐18:2 with total mortality and CVD events were attenuated and no longer statistically significant in models including mutual adjustment for other TFAs (Figure 3). In contrast, even with these exclusions, t/t‐18:2 remained associated with higher risk of total mortality and CVD outcomes in multivariable‐adjusted models that were not mutually adjusted for all TFAs.
Figure 2.
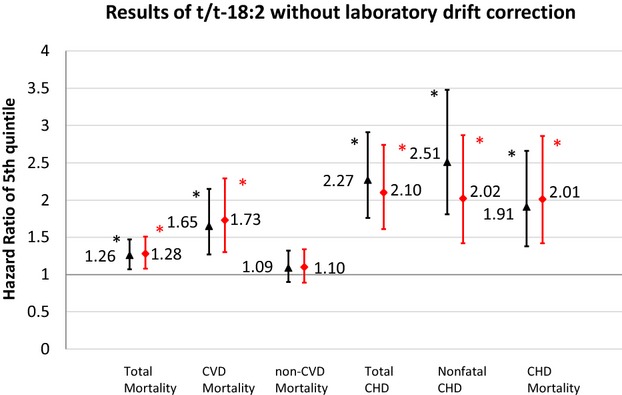
Prospective associations of plasma phospholipid trans/trans‐18:2 with total mortality, CVD and CHD before and after mutual adjustment without correction of laboratory drift. Black triangles indicate multivariate‐adjusted hazard ratios comparing adults in the top quintile category to adults in the bottom quintile category for t/t‐18:2 fatty acid levels, before mutual adjustment for the 5 TFAs and without correction of laboratory drift; red diamonds, after mutual adjustment for the 5 TFAs and without correction of laboratory drift. Error bars represent 95% CI. All risk estimates were adjusted for age, sex, race, education, enrollment site, diabetes, hypertension, smoking, alcohol consumption, physical activity, BMI, waist circumference, dietary saturated fat, dietary monounsaturated fat, dietary fiber, plasma phospholipid EPA+DHA, plasma phospholipid linoleic acid. *P‐trend<0.05 across quintiles. BMI indicates body–mass index; CHD, coronary heart disease; CVD, cardiovascular disease; EPA+DHA, EPA+DHA, eicosapentaenoic acids plus docosahexaenoic acids; TFA, trans–fatty acid.
Figure 3.
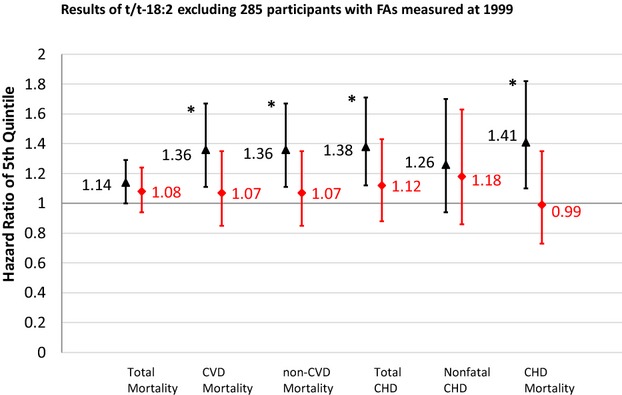
Prospective associations of plasma phospholipid trans/trans‐18:2 with total mortality, CVD, and CHD before and after mutual adjustment after excluding 285 participants with trans/trans‐18:2 levels measured in 1999. Black triangles indicate multivariate‐adjusted hazard ratios comparing adults in the top quintile category to adults in the bottom quintile category for t/t‐18:2 fatty acid levels, before mutual adjustment for the 5 TFAs and excluding 285 participants with t/t‐18:2 measured early; red diamonds, after mutual adjustment for the 5 TFAs and excluding 285 participants with t/t‐18:2 measured early. Error bars represent 95% CI. All risk estimates were adjusted for age, sex, race, education, enrollment site, diabetes, hypertension, smoking, alcohol consumption, physical activity, BMI, waist circumference, dietary saturated fat, dietary monounsaturated fat, dietary fiber, plasma phospholipid EPA+DHA, plasma phospholipid linoleic acid. *P‐trend<0.05 across quintiles. BMI indicates body–mass index; CHD, coronary heart disease; CVD, cardiovascular disease; EPA+DHA, eicosapentaenoic acids plus docosahexaenoic acids; TFA, trans–fatty acid.
Discussion
In this large, prospective study of older Americans, we found divergent associations of different circulating TFA subtypes with total mortality, CVD mortality, and incident CHD. t/t‐18:2 appeared most strongly associated with higher risk of total mortality, CVD mortality, and total CHD. t/c‐18:2 was also positively associated with risk, but primarily only after mutual adjustment for all TFAs. In contrast, t‐16:1n9, t‐18:1, and c/t‐18:2 levels were not significantly associated with outcomes, particularly after accounting for reverse causation.
To our knowledge, no prior study has evaluated how different t‐18:2 isomers relate to mortality or incident CHD. Prior studies, including from this cohort, have found that total t‐18:2 relates to higher risk of fatal ischemic heart disease (IHD),18 sudden cardiac arrest,19 total42 and nonfatal CHD,22 possibly related to adverse lipid effects,43 pro‐inflammatory responses,9–10 and abnormal heart rate variability.44 Our sensitivity analysis investigating total t‐18:2 also found a positive association with total mortality. Yet, our isomer‐specific analyses demonstrated substantial heterogeneity in associations of each t‐18:2 isomers with mortality and CHD, with most pronounced adverse associations for t/t‐18:2. Based on in vitro studies of human endothelial cells, t/t‐18:2 has multiple adverse effects: increasing NF‐кB, impairing endothelial insulin signaling and NO production, elevating superoxide production,45 inducing apoptosis and necrosis in a dose‐ and time‐dependent fashion.46 Yet, these studies did not compare effects of other t‐18:2 isomers. In addition, animal studies indicate that t/t‐18:2 is preferentially incorporated into sn‐1 position of phospholipids, similar to saturated fatty acids, whereas mono t‐18:2 isomers such as c/t‐ or t/c‐18:2 were acylated into sn‐2 position, similar to linoleic acid.47–48 The fundamental biological differences may result in varying cardiovascular effects of t‐18:2, as suggested in our study. Together with our findings, these experimental data support adverse cardiovascular effects of t/t‐18:2.
t/c‐18:2 was positively associated with total and nonfatal CHD after mutual adjustment for other TFAs. Because various TFA subtypes and isomers (in particular t/c‐ and c/t‐18:2) may be causally intercorrelated due to common dietary sources, the findings following mutual adjustment should be interpreted cautiously. For example, our prior work in CHS showed that fried and bakery foods are common food sources of both c/t‐ and t/c‐18:2.17 Whether the significant associations of t/c‐18:2 with CHD represent a true effect or an artifact of overadjustment in this cohort is not known. Of note, combined levels of t/c‐ and c/t‐18:2 have been shown to be associated with sudden cardiac arrest in this cohort.19
Unexpectedly, we observed c/t‐18:2 to be inversely associated with incidence of total and nonfatal CHD. Yet, these inverse associations were attenuated and no longer statistically significant after we excluded cases occurring in the first 2 years. Because the excluded cases were not younger than participants having events later in the follow‐up, this result could not be interpreted as a stronger association of c/t‐18:2 with premature CHD events. Instead, the findings suggested the possibility of reverse causation (ie, participants with substantial undiagnosed disease might have adapted healthier dietary habits or such disease could alter phospholipid c/t‐18:2 levels). However, excluding earlier cases also hindered our ability to incorporate the short‐term effects of c/t‐18:2 on CHD incidence into the final estimates, which could partially explain the attenuation of the association. Our interpretation of the findings after excluding earlier cases could be speculative, and additional investigation of the biologic effects of c/t‐18:2 are needed to confirm our findings.
Neither total t‐18:1 nor t‐16:1n9 were significantly associated with total mortality, CVD mortality, or CHD. These findings are consistent with prior biomarker studies showing no significant associations of erythrocyte membrane or adipose t‐18:1 levels with risk of sudden cardiac arrest or nonfatal CHD.19–20,22 In a prior nested case–control study in CHS, we found an inverse association between plasma phospholipid t‐18:1 and fatal CHD;18 this was no longer significant in this present analysis with more cases and longer follow‐up time. Animal experiments suggest different t‐18:1 isomers could have opposing influences on risk;45,49–52 therefore, effects may be masked when their sums are evaluated. Yet, t‐18:1 isomers were highly intercorrelated in our cohort, precluding their separate evaluation.
The absence of association between circulating t‐18:1 and CVD outcomes is puzzling, given that this fatty acid is the major contributor to total TFA consumption from PHVO, which has been consistently linked to adverse lipid effects in trials,4,53 and elevated CHD risk when estimated from self‐reported dietary questionnaires.53 This lack of association could be attributable, at least partly, to a survival effect, in that this cohort included elderly individuals who may already have survived higher exposure to TFA due to other characteristics protecting them from adverse outcomes. In addition, although t‐18:1 is the major component of total TFA from PHVO, the correlation between circulating and dietary t‐18:1 was low (r=0.16) in this cohort, consistent with the not high correlation between plasma and dietary TFAs in the Nurses’ Health Study (r=0.30).54 Circulating t‐18:1 levels reflect not only the dietary intake, but also the yet unknown incorporation and metabolic processes that may result in different associations for circulating versus dietary TFAs. On the other hand, blood levels of dietary fatty acids generally provide more reliable estimates than self‐reported intakes. The overall low laboratory coefficients of variation and the reasonable reliability of the exposures indicate the overall good measurements of circulating TFAs, despite the difficulty in quantifying TFAs with small peaks. Our results highlight the need to further investigate the determinants of t‐18:1 biomarker levels and to confirm their relations to disease risk and the related mechanisms.
Our study has important public health implications. We identified positive associations of t/c‐ and t/t‐18:2 with mortality and CVD. These results provide support for the recent US Food and Drug Administration proposal to remove PHVO from the list of additives that are generally recognized as safe.1 In addition, the specific associations of these t‐18:2 isomers with risks call attention to potential additional priorities for food policy. The recent US Food and Drug Administration proposal,1 current food labeling laws,55 and state and national regulations56–57 are all focused on PHVO and based on total TFA, which is predominantly t‐18:1 and t‐16:1n9. Yet, c/t‐ and t/c‐18:2 can be produced via processes unrelated to partial hydrogenation, such as vegetable oil deodorization and high‐temperature frying.58–60 If t‐18:2 isomers are especially harmful for CVD, even at low exposure levels such as seen in our study, then current policy measures may not fully capture all major sources of these fatty acids. On the global level, while a limited number of countries such as Denmark,57 Argentina,61 and the United States1 have national regulations limiting TFAs, the majority of the world does not. Many countries such as Mexico,62 Egypt,63 and Iran59 still have high consumption of TFAs, with little active proposed regulation. Other countries such as India have only proposed but not enforced regulation.64 Our findings provide useful insights to inform policy priorities not only in the United States, but also in these additional countries. In addition to policy implication, our findings inform scientific knowledge of how different TFA subtypes may influence total and cause‐specific mortality, an area that is not well understood and with many remaining questions. Our results also highlight the need for further investigation of dietary determinants, other drivers, and independent disease, physiologic, and molecular effects of t‐18:2 isomers and other circulating TFA subtypes.
Our study has several strengths. We utilized a well‐established prospective cohort with little loss to follow‐up, minimizing selection bias. TFA levels were assessed using biomarkers, providing objective measures of exposure and allowing assessment of individual TFA subtypes and isomers. Careful follow‐up and adjudication of events minimized the potential for missed or misclassified outcomes, and the large number of cases increased statistical power to detect associations. Information on other risk factors was prospectively collected using standard methods, increasing our ability to adjust for confounding. We performed several sensitivity analyses to evaluate the robustness of each finding.
Potential limitations deserve consideration. TFA levels were measured at baseline, and changes in exposure over time would result in misclassification and may attenuate true associations. We partly accounted for this using regression dilution correction, but this may incompletely account for within‐person variation over time. Laboratory drift was observed for t/t‐18:2, possibly related to reduced assay stability for fatty acids with very low concentrations. In sensitivity analyses excluding participants with fatty acids measured in 1999, t/t‐18:2 was no longer significantly associated with mortality or CHD after mutual adjustment for all TFAs, but remained significantly positively associated prior to such mutual adjustment, which would be unrelated to laboratory drift. In addition, our main analyses corrected for laboratory drift using calibration, decreasing its influence on our findings. The cohort comprised older, largely white Americans, and survival bias could partly explain the absence of associations for t‐18:1 and t‐16:1n9. Although the great majority of CVD occurs after age 65, which is the minimum enrollment age in CHS, the associations of TFA subtypes may differ in a much younger population.
In conclusion, our findings suggest that later in life, circulating t/t‐18:2 levels are associated with higher risk of total mortality, mainly due to increased CVD mortality and higher risk of total CHD; and t/c‐18:2 with higher total mortality and total CHD. These findings highlight the potentially varying health effects of different TFAs, with implications for both future research and for designing and evaluating measures to reduce population risk due to TFA exposures.
Supplementary Material
Supporting Information
Sources of Funding
The authors express their gratitude to all CHS participants, CHS investigators, and institutions; see https://chsnhlbi.org/PI.htm. This investigation was supported by the National Heart, Lung, and Blood Institute (NHLBI) and the Office of Dietary Supplements, National Institutes of Health (2R01‐HL‐085710, R01‐HL‐085710). CHS was supported by contracts HHSN268201200036C, HHSN268200800007C, N01 HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grant HL080295 from NHLBI, with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided by AG023629 from the National Institute on Aging. Dr Imamura was funded by Medical Research Council Epidemiology Unit Core Support (MC_UU_12015/5). The funders had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Disclosures
Dr Mozaffarian reports ad hoc travel reimbursement or honoraria from Bunge, Pollock Institute, Quaker Oats, and Life Sciences Research Organization; ad hoc consulting fees from McKinsey Health Systems Institute, Foodminds, Nutrition Impact, Amarin, Omthera, and Winston and Strawn LLP; membership, Unilever North America Scientific Advisory Board; royalties from UpToDate; and research grants from GlaxoSmithKline, Sigma Tau, Pronova, the Gates Foundation, the Sackler Institute of Nutrition, and the National Institutes of Health.
References
- 1.Department of Health and Human Services FDA. Tentative determination regarding partially hydrogenated oils; request for comments and for scientific data and information Available at: https://federalregister.gov/a/2013-26854. Accessed November 8, 2013.
- 2.Stampfer MJ, Sacks FM, Salvini S, Willett WC, Hennekens CH. A prospective study of cholesterol, apolipoproteins, and the risk of myocardial infarction. N Engl J Med. 1991; 325:373-381. [DOI] [PubMed] [Google Scholar]
- 3.Ascherio A, Katan MB, Zock PL, Stampfer MJ, Willett WC. Trans fatty acids and coronary heart disease. N Engl J Med. 1999; 340:1994-1998. [DOI] [PubMed] [Google Scholar]
- 4.Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta‐analysis of 60 controlled trials. Am J Clin Nutr. 2003; 77:1146-1155. [DOI] [PubMed] [Google Scholar]
- 5.Mauger JF, Lichtenstein AH, Ausman LM, Jalbert SM, Jauhiainen M, Ehnholm C, Lamarche B. Effect of different forms of dietary hydrogenated fats on LDL particle size. Am J Clin Nutr. 2003; 78:370-375. [DOI] [PubMed] [Google Scholar]
- 6.Baer DJ, Judd JT, Clevidence BA, Tracy RP. Dietary fatty acids affect plasma markers of inflammation in healthy men fed controlled diets: a randomized crossover study. Am J Clin Nutr. 2004; 79:969-973. [DOI] [PubMed] [Google Scholar]
- 7.de Roos NM, Bots ML, Katan MB. Replacement of dietary saturated fatty acids by trans fatty acids lowers serum HDL cholesterol and impairs endothelial function in healthy men and women. Arterioscler Thromb Vasc Biol. 2001; 21:1233-1237. [DOI] [PubMed] [Google Scholar]
- 8.Han SN, Leka LS, Lichtenstein AH, Ausman LM, Schaefer EJ, Meydani SN. Effect of hydrogenated and saturated, relative to polyunsaturated, fat on immune and inflammatory responses of adults with moderate hypercholesterolemia. J Lipid Res. 2002; 43:445-452. [PubMed] [Google Scholar]
- 9.Mozaffarian D, Rimm EB, King IB, Lawler RL, McDonald GB, Levy WC. Trans fatty acids and systemic inflammation in heart failure. Am J Clin Nutr. 2004; 80:1521-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mozaffarian D, Pischon T, Hankinson SE, Rifai N, Joshipura K, Willett WC, Rimm EB. Dietary intake of trans fatty acids and systemic inflammation in women. Am J Clin Nutr. 2004; 79:606-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oomen CM, Ocke MC, Feskens EJ, van Erp‐Baart MA, Kok FJ, Kromhout D. Association between trans fatty acid intake and 10‐year risk of coronary heart disease in the Zutphen Elderly Study: a prospective population‐based study. Lancet. 2001; 357:746-751. [DOI] [PubMed] [Google Scholar]
- 12.Willett WC, Stampfer MJ, Manson JE, Colditz GA, Speizer FE, Rosner BA, Sampson LA, Hennekens CH. Intake of trans fatty acids and risk of coronary heart disease among women. Lancet. 1993; 341:581-585. [DOI] [PubMed] [Google Scholar]
- 13.Hu FB, Stampfer MJ, Manson JE, Rimm E, Colditz GA, Rosner BA, Hennekens CH, Willett WC. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med. 1997; 337:1491-1499. [DOI] [PubMed] [Google Scholar]
- 14.Oh K, Hu FB, Manson JE, Stampfer MJ, Willett WC. Dietary fat intake and risk of coronary heart disease in women: 20 years of follow‐up of the nurses’ health study. Am J Epidemiol. 2005; 161:672-679. [DOI] [PubMed] [Google Scholar]
- 15.Mozaffarian D, Willett WC. Trans fatty acids and cardiovascular risk: a unique cardiometabolic imprint? Curr Atheroscler Rep. 2007; 9:486-493. [DOI] [PubMed] [Google Scholar]
- 16.Pietinen P, Ascherio A, Korhonen P, Hartman AM, Willett WC, Albanes D, Virtamo J. Intake of fatty acids and risk of coronary heart disease in a cohort of Finnish men. The Alpha‐Tocopherol, Beta‐Carotene Cancer Prevention Study. Am J Epidemiol. 1997; 145:876-887. [DOI] [PubMed] [Google Scholar]
- 17.Micha R, King IB, Lemaitre RN, Rimm EB, Sacks F, Song X, Siscovick DS, Mozaffarian D. Food sources of individual plasma phospholipid trans fatty acid isomers: the Cardiovascular Health Study. Am J Clin Nutr. 2010; 91:883-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemaitre RN, King IB, Mozaffarian D, Sotoodehnia N, Rea TD, Kuller LH, Tracy RP, Siscovick DS. Plasma phospholipid trans fatty acids, fatal ischemic heart disease, and sudden cardiac death in older adults: the Cardiovascular Health Study. Circulation. 2006; 114:209-215. [DOI] [PubMed] [Google Scholar]
- 19.Lemaitre RN, King IB, Raghunathan TE, Pearce RM, Weinmann S, Knopp RH, Copass MK, Cobb LA, Siscovick DS. Cell membrane trans‐fatty acids and the risk of primary cardiac arrest. Circulation. 2002; 105:697-701. [DOI] [PubMed] [Google Scholar]
- 20.Roberts TL, Wood DA, Riemersma RA, Gallagher PJ, Lampe FC. Trans isomers of oleic and linoleic acids in adipose tissue and sudden cardiac death. Lancet. 1995; 345:278-282. [DOI] [PubMed] [Google Scholar]
- 21.Thomas LH, Winter JA, Scott RG. Concentration of 18:1 and 16:1 transunsaturated fatty acids in the adipose body tissue of decedents dying of ischaemic heart disease compared with controls: analysis by gas liquid chromatography. J Epidemiol Community Health. 1983; 37:16-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baylin A, Kabagambe EK, Ascherio A, Spiegelman D, Campos H. High 18:2 trans‐fatty acids in adipose tissue are associated with increased risk of nonfatal acute myocardial infarction in costa rican adults. J Nutr. 2003; 133:1186-1191. [DOI] [PubMed] [Google Scholar]
- 23.Hølmer GK. In: J.L Sebedio, W.W Christie. (eds.). Biochemistry of trans‐monoenoic fatty acids. Trans Fatty Acids in Human Nutrition. 1998Dundee: The Oily Press; 163-189. [Google Scholar]
- 24.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, O'Leary DH, Psaty BM, Rautaharju P, Tracy RP, Weiler PG. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991; 1:263-276. [DOI] [PubMed] [Google Scholar]
- 25.Mozaffarian D, Lemaitre RN, King IB, Song X, Spiegelman D, Sacks FM, Rimm EB, Siscovick DS. Circulating long‐chain omega‐3 fatty acids and incidence of congestive heart failure in older adults: the Cardiovascular Health Study: a cohort study. Ann Intern Med. 2011; 155:160-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957; 226:497-509. [PubMed] [Google Scholar]
- 27.Mozaffarian D, Cao H, King IB, Lemaitre RN, Song X, Siscovick DS, Hotamisligil GS. Trans‐palmitoleic acid, metabolic risk factors, and new‐onset diabetes in U.S. adults: a cohort study. Ann Intern Med. 2010; 153:790-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mozaffarian D, de Oliveira Otto MC, Lemaitre RN, Fretts AM, Hotamisligil G, Tsai MY, Siscovick DS, Nettleton JA. Trans‐palmitoleic acid, other dairy fat biomarkers, and incident diabetes: the Multi‐Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. 2013; 97:854-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002; 360:1903-1913. [DOI] [PubMed] [Google Scholar]
- 30.Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J, Qizilbash N, Peto R, Collins R. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta‐analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007; 370:1829-1839. [DOI] [PubMed] [Google Scholar]
- 31.Aviles RJ, Martin DO, Apperson‐Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, Tracy RP, Van Wagoner DR, Psaty BM, Lauer MS, Chung MK. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003; 108:3006-3010. [DOI] [PubMed] [Google Scholar]
- 32.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972; 18:499-502. [PubMed] [Google Scholar]
- 33.Kumanyika S, Tell GS, Shemanski L, Polak J, Savage PJ. Eating patterns of community‐dwelling older adults: the Cardiovascular Health Study. Ann Epidemiol. 1994; 4:404-415. [DOI] [PubMed] [Google Scholar]
- 34.Kumanyika SK, Tell GS, Shemanski L, Martel J, Chinchilli VM. Dietary assessment using a picture‐sort approach. Am J Clin Nutr. 1997; 65:1123S-1129S. [DOI] [PubMed] [Google Scholar]
- 35.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, Cruise RG, Theroux S. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995; 5:278-285. [DOI] [PubMed] [Google Scholar]
- 36.Mozaffarian D, Lemaitre RN, Kuller LH, Burke GL, Tracy RP, Siscovick DS. Cardiac benefits of fish consumption may depend on the type of fish meal consumed: the Cardiovascular Health Study. Circulation. 2003; 107:1372-1377. [DOI] [PubMed] [Google Scholar]
- 37.Corrao G, Rubbiati L, Bagnardi V, Zambon A, Poikolainen K. Alcohol and coronary heart disease: a meta‐analysis. Addiction. 2000; 95:1505-1523. [DOI] [PubMed] [Google Scholar]
- 38.Bryson CL, Mukamal KJ, Mittleman MA, Fried LP, Hirsch CH, Kitzman DW, Siscovick DS. The association of alcohol consumption and incident heart failure: the Cardiovascular Health Study. J Am Coll Cardiol. 2006; 48:305-311. [DOI] [PubMed] [Google Scholar]
- 39.Baron RM, Kenny DA. The moderator‐mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986; 51:1173-1182. [DOI] [PubMed] [Google Scholar]
- 40.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989; 8:551-561. [DOI] [PubMed] [Google Scholar]
- 41.Rosner B, Spiegelman D, Willett WC. Correction of logistic regression relative risk estimates and confidence intervals for random within‐person measurement error. Am J Epidemiol. 1992; 136:1400-1413. [DOI] [PubMed] [Google Scholar]
- 42.Sun Q, Ma J, Campos H, Hankinson SE, Manson JE, Stampfer MJ, Rexrode KM, Willett WC, Hu FB. A prospective study of trans fatty acids in erythrocytes and risk of coronary heart disease. Circulation. 2007; 115:1858-1865. [DOI] [PubMed] [Google Scholar]
- 43.Mozaffarian D, Aro A, Willett WC. Health effects of trans‐fatty acids: experimental and observational evidence. Eur J Clin Nutr. 2009; 63suppl 2:S5-S21. [DOI] [PubMed] [Google Scholar]
- 44.Soares‐Miranda L, Stein PK, Imamura F, Sattelmair J, Lemaitre RN, Siscovick DS, Mota J, Mozaffarian D. Trans‐fatty acid consumption and heart rate variability in 2 separate cohorts of older and younger adults. Circ Arrhythm Electrophysiol. 2012; 5:728-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iwata NG, Pham M, Rizzo NO, Cheng AM, Maloney E, Kim F. Trans fatty acids induce vascular inflammation and reduce vascular nitric oxide production in endothelial cells. PLoS One. 2011; 6:e29600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bin Q, Rao H, Hu JN, Liu R, Fan YW, Li J, Deng ZY, Zhong X, Du FL. The caspase pathway of linoelaidic acid (9t, 12t‐c18:2)‐induced apoptosis in human umbilical vein endothelial cells. Lipids. 2013; 48:115-126. [DOI] [PubMed] [Google Scholar]
- 47.Sebedio JL, Vermunt SH, Chardigny JM, Beaufrere B, Mensink RP, Armstrong RA, Christie WW, Niemela J, Henon G, Riemersma RA. The effect of dietary trans alpha‐linolenic acid on plasma lipids and platelet fatty acid composition: the TransLinE study. Eur J Clin Nutr. 2000; 54:104-113. [DOI] [PubMed] [Google Scholar]
- 48.Privett OS, Nutter LJ, Lightly FS. Metabolism of trans acids in the rat: influence of the geometric isomers of linoleic acid on the structure of liver triglycerides and lecithins. J Nutr. 1966; 89:257-264. [DOI] [PubMed] [Google Scholar]
- 49.Fournier N, Attia N, Rousseau‐Ralliard D, Vedie B, Destaillats F, Grynberg A, Paul JL. Deleterious impact of elaidic fatty acid on ABCA1‐mediated cholesterol efflux from mouse and human macrophages. Biochim Biophys Acta. 2012; 1821:303-312. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Lu J, Ruth MR, Goruk SD, Reaney MJ, Glimm DR, Vine DF, Field CJ, Proctor SD. Trans‐11 vaccenic acid dietary supplementation induces hypolipidemic effects in JCR:LA‐cp rats. J Nutr. 2008; 138:2117-2122. [DOI] [PubMed] [Google Scholar]
- 51.Blewett HJ, Gerdung CA, Ruth MR, Proctor SD, Field CJ. Vaccenic acid favourably alters immune function in obese JCR:LA‐cp rats. Br J Nutr. 2009; 102:526-536. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y, Jacome‐Sosa MM, Ruth MR, Goruk SD, Reaney MJ, Glimm DR, Wright DC, Vine DF, Field CJ, Proctor SD. Trans‐11 vaccenic acid reduces hepatic lipogenesis and chylomicron secretion in JCR:LA‐cp rats. J Nutr. 2009; 139:2049-2054. [DOI] [PubMed] [Google Scholar]
- 53.Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Trans fatty acids and cardiovascular disease. N Engl J Med. 2006; 354:1601-1613. [DOI] [PubMed] [Google Scholar]
- 54.Sun Q, Ma J, Campos H, Hankinson SE, Hu FB. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am J Clin Nutr. 2007; 86:74-81. [DOI] [PubMed] [Google Scholar]
- 55.Food and Drug Administration, HHS. Food labeling: trans fatty acids in nutrition labeling, nutrient content claims, and health claims. Final rule. Fed Reg. 2003; 68:41433-41506. [PubMed] [Google Scholar]
- 56.Hygiene NYCDoHaM. Article 81: food preparation and food establishments Available at: www.nyc.gov/html/doh/downloads/pdf/rii/article81-book.pdf. Accessed July 12, 2012.
- 57.Astrup A. The trans fatty acid story in Denmark. Atheroscler Suppl. 2006; 7:43-46. [DOI] [PubMed] [Google Scholar]
- 58.Allison DB, Egan SK, Barraj LM, Caughman C, Infante M, Heimbach JT. Estimated intakes of trans fatty and other fatty acids in the US population. J Am Diet Assoc. 1999; 99:166-174.‐ [DOI] [PubMed] [Google Scholar]
- 59.Mozaffarian D, Abdollahi M, Campos H, Houshiarrad A, Willett WC. Consumption of trans fats and estimated effects on coronary heart disease in Iran. Eur J Clin Nutr. 2007; 61:1004-1010. [DOI] [PubMed] [Google Scholar]
- 60.Kemeny ZRK, Henon G, Kovari K, Zwobada F. Deodorization of vegetable oils: prediction of trans polyunsaturated fatty acid content. J Am Oil Chem Soc. 2001; 78:973-979. [Google Scholar]
- 61. Article no. 155 tris, chapter iii, the Argentina food code. 2009.
- 62.Villalpando S. Grasas, dieta y salud: tablas de composición de ácidos grasos de alimentos frecuentes en la dieta [Google Scholar]
- 63.Mahfouz MM, Osman MY. Evaluation of Trans and Essential Fatty Acid Content in Some Egyptian Consumer‐Available Hydrogenated Fats. Fette, Seifen, Anstrichm. 1983; 85:283-28910.1002/lipi.19830850708 [Google Scholar]
- 64.Downs SM, Thow AM, Ghosh‐Jerath S, McNab J, Reddy KS, Leeder SR. From Denmark to Delhi: the multisectoral challenge of regulating trans fats in India. Public Health Nutr. 2013; 16:2273-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information