Abstract
Background
Pentraxin 3 (PTX3) is a novel inflammatory marker produced by various cell types including those of the vasculature and the heart. The relationship between inflammatory markers and prognosis of patients with heart failure with normal ejection fraction (HFNEF) remains unknown. We investigated whether plasma PTX3 levels can predict future cardiovascular events in patients with HFNEF.
Methods and Results
Plasma PTX3, high‐sensitivity C‐reactive protein, and B‐type natriuretic peptide levels were measured prospectively in 360 stable patients with HFNEF. The subsequent incidence of cardiovascular events, including cardiovascular death, nonfatal myocardial infarction (MI), unstable angina pectoris, nonfatal ischemic stroke, hospitalization for heart failure decompensation, and coronary revascularization, was determined. During a mean 30‐month follow‐up, 106 patients experienced cardiovascular events. These events were more frequent in patients with high plasma PTX3 levels (>3.0 ng/mL) than low levels (≤3.0 ng/mL). Multivariable Cox hazard analysis showed that PTX3 (hazard ratio: 1.16; 95% CI: 1.05 to 1.27; P<0.01) and B‐type natriuretic peptide (hazard ratio: 1.08; 95% CI: 1.03 to 1.14; P<0.001), but not high‐sensitivity C‐reactive protein levels, were significant predictors of future cardiovascular events. Multivariable Cox analysis with the forced inclusion model, including 5 previously identified prognostic factors, found that PTX3 was a significant predictor of cardiovascular events (hazard ratio: 1.16; 95% CI: 1.06 to 1.27; P<0.01). The C‐statistics for cardiovascular events substantially increased from 0.617 to 0.683 when PTX3 was added to the 5 previously identified prognostic factors.
Conclusions
High plasma PTX3 levels, but not other inflammatory markers, are correlated with future cardiovascular events in patients with HFNEF. PTX3 may be a useful biomarker for assessment of risk stratification in HFNEF.
Clinical Trial Registration
URL: http://www.umin.ac.jp; Unique identifier: UMIN000002170.
Keywords: cardiovascular events, heart failure with normal ejection fraction, inflammation, left ventricular diastolic dysfunction, pentraxin 3
Introduction
Heart failure (HF) can result from cardiac overload or injury as well as from a complex interplay of genetic, neurohormonal, and inflammatory factors. HF is classified as being associated with a reduced ejection fraction (HFREF) or with a normal ejection fraction (HFNEF). The percentages of patients and the mortality rates are similar in patients with HFREF and HFNEF.1–2 The likelihood of survival is higher in patients with HFREF than in those with HFNEF, and the survival rate in HFNEF has not significantly improved in recent years.3 Various biomarkers, including those for inflammation and neurohormones, provide important information about the pathogenesis, risk stratification, and diagnosis of HF and are used to monitor response to therapy.4 Inflammatory markers are closely associated with pathogenesis, poor functional state, and adverse prognosis in patients with HF.
Pentraxin 3 (PTX3) is a newly identified member of the pentraxin superfamily, which includes C‐reactive protein (CRP) and serum amyloid P. In contrast to CRP, which is produced by the liver, PTX3 is produced by various cell types in various tissues, especially in the vasculature, in response to inflammatory stimuli.5 PTX3 may reflect local inflammatory status in tissues and thus may be a new biomarker of inflammation.6 We recently demonstrated that plasma levels of PTX3, but not high‐sensitivity CRP (hs‐CRP), were significantly higher in patients with HFNEF than in non‐HF patients.7 In addition, these elevated levels of PTX3 were significantly and independently correlated with the presence of HFNEF in patients with normal left ventricular ejection fraction (LVEF).7 Other studies have also shown that PTX3 is a predictor of adverse clinical outcome in patients with HFREF.8–9 Consequently, PTX3 may be a useful inflammatory marker in cardiovascular medicine.
Although much attention has been focused on HFREF, less is known regarding factors that correlate with clinical outcome in patients with HFNEF. The Irbesartan in Heart Failure With Preserved Ejection Fraction (I‐PRESERVE) study demonstrated that age, presence of diabetes mellitus, history of hospitalization for HF, New York Heart Association classification, N‐terminal pro‐B‐type natriuretic peptide levels, and LVEF were associated with adverse outcomes in patients with HFNEF.10 The relationships between inflammatory markers and prognosis in HFNEF, however, remain unknown. This study was designed to determine the ability of plasma PTX3 levels to predict future cardiovascular events in patients with HFNEF.
Methods
Patients and Protocol
We initially screened 747 chronic HF patients under a clinically stable condition with New York Heart Association functional class II to IV who were referred to Kumamoto University Hospital, Kumamoto, Japan. We did not include decompensated HF patients with acute HF. The diagnosis of HF was based on the Framingham criteria for HF.11 None of the patients had any noncardiac causes of HF‐like symptoms, especially lung disease (eg, chronic obstructive lung disease). The diagnosis of HFNEF was based on the criteria formulated by the European Working Group on HFNEF.12 HFREF was defined as reduced systolic left ventricular (LV) function (LVEF≤50%). HFNEF was defined as normal or mildly abnormal systolic LV function (LVEF>50%) with LV diastolic dysfunction. LV diastolic dysfunction for HFNEF was defined as (1) a ratio of mitral early diastolic peak flow velocity to tissue Doppler early mitral annular diastolic velocity (E/e’) ≥15; (2) E/e’ ≥8 and <15 and B‐type natriuretic peptide (BNP) levels >200 pg/mL; or (3) E/e’ ≥8 and <15 and LV mass index (LVMI) ≥122 g/m2 (females) or 149 g/m2 (males). Patients were excluded if they had active systemic inflammatory disease, chronic renal failure requiring hemodialysis, collagen disease, presence of malignancy, or acute coronary syndrome within 3 months preceding enrollment. Twenty‐three HF patients with normal LVEF who did not meet these criteria were excluded. The study finally enrolled 360 patients with HFNEF (Figure 1). The study complied with the Declaration of Helsinki regarding investigation in humans and was approved by the institutional review committee of each institution. The study was also conducted in accordance with the guidelines of the ethics committee of our institution. Written informed consent was obtained from all patients. This study was registered at the University Hospital Medical Information Network Clinical Trials Registry with the identification number UMIN000002170.
Figure 1.
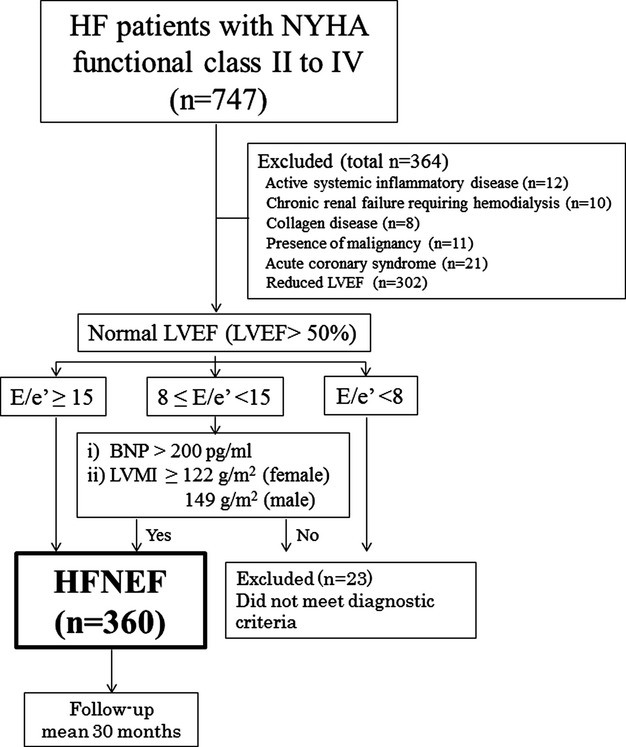
Flow chart showing the protocol that was used for enrollment of HFNEF patients in the present study. BNP indicates B‐type natriuretic peptide; E/e’, ratio of mitral early diastolic peak flow velocity to tissue Doppler early mitral annular diastolic velocity; HF, heart failure; HFNEF, HF with normal ejection fraction; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; NYHA, New York Heart Association.
Measurement of Plasma PTX3 Levels and Other Blood Parameters
Venous blood samples were obtained from patients under clinically stable and fasting conditions. Serum and plasma samples were kept frozen at −80°C until later tests. The estimated glomerular filtration rate was calculated using the Japanese Society of Nephrology formula.13 PTX3 levels were measured using a high‐sensitivity enzyme‐linked immunosorbent assay system (values ranged from 0.1 to 20 ng/mL; Perseus Proteomics). Other factors that were measured included serum hs‐CRP levels and plasma tumor necrosis factor‐α, interleukin‐6, and BNP levels.
Doppler Echocardiography
Echocardiography was performed in standard parasternal and apical views by a specialized echocardiologist using commercially available ultrasound systems (Vivid 7, GE‐Vingmed Ultrasound; Aplio XG, Toshiba). LVEF was measured in biplane apical (2‐ and 4‐chamber) views using a modification of Simpson's method. The E/e’ ratio is used as an index of LV filling pressure and abnormal LV relaxation.14 To assess LV diastolic function, E/e’ was measured, as described previously,15 as was the medial annulus of E/e’. LV mass was calculated, as described previously,16 and the LVMI was expressed relative to body surface area.
Follow‐up
Patients with HFNEF were followed prospectively at the outpatient clinic until December 2012 or until an endpoint occurred. The endpoint was a composite of cardiovascular death, nonfatal MI, unstable angina pectoris, nonfatal ischemic stroke, hospitalization for HF decompensation, or coronary revascularization. Cardiovascular events were ascertained from a review of medical records and confirmed by direct contact with the patients, their families, and physicians. Patients were divided into low‐ and high‐PTX3 groups based on the median concentration of PTX3 (3.0 ng/mL).
Definition of Cardiovascular Events
Cardiovascular death was defined as death because of MI (within 28 days), congestive heart failure, or documented sudden death without apparent noncardiovascular causes. MI was diagnosed as a rise or fall in cardiac biomarkers (plasma creatine kinase‐MB or cardiac troponin‐T) greater than the 99th percentile of the upper limit of normal, together with evidence of myocardial ischemia with at least one of the following: ECG changes (recent ST‐T changes, left bundle branch block, or a pathological Q wave) or imaging evidence of new loss of viable myocardium or new regional wall motion abnormality.17 Unstable angina pectoris was diagnosed from new or accelerating symptoms of myocardial ischemia accompanied by recent ischemic ST‐T‐wave changes. Ischemic stroke was diagnosed by both neurological symptoms and radiological evidence, excluding intracranial hemorrhage. Hospitalization for HF decompensation was defined as admission for symptoms typical of HF with objective signs of worsening HF requiring intravenous drug administration. Coronary revascularization was defined as coronary artery bypass grafting or percutaneous coronary intervention in patients with stable angina pectoris. For subjects experiencing 2 or more cardiovascular events, only the first event was considered in the analysis.
Statistical Analysis
Parameters with a normal distribution, as assessed by the Shapiro‐Wilk test, are expressed as mean±SD. Parameters with a skewed distribution, such as PTX3, hs‐CRP, tumor necrosis factor‐α, interleukin‐6, and BNP levels, as well as E/e’ and LVMI, are shown as median values (interquartile range) and were logarithmically transformed before linear regression analysis. Differences between continuous variables were analyzed by the unpaired t test and the Mann‐Whitney U test, as appropriate. Differences between categorical variables were analyzed by the chi‐square test. Linear regression analysis was used to determine the associations between PTX3 and BNP levels, LVMI, inflammatory makers, and cardiovascular events. Survival data were analyzed by the Kaplan‐Meier method and assessed by the log‐rank test. Age‐ and sex‐adjusted event rates were calculated by multivariable logistic regression analyses. The ability of any marker to predict cardiovascular events was assessed by Cox proportional hazards regression analysis. In Cox proportional hazards regression analyses, associations between groups and all other parameters were first analyzed by univariate analysis followed by multivariable analysis after adjustment for factors that were significant with univariate analysis. Multivariable Cox proportional hazards regression analyses were also performed using forced inclusion models. Model 1 incorporated the inflammatory makers PTX3, hs‐CRP, tumor necrosis factor‐α, interleukin‐6, and BNP. Model 2 incorporated the 5 prognostic factors (PF5) that were identified during the I‐PRESERVE study10 in patients with HFNEF—age, presence of diabetes mellitus, previous hospitalization for HF, New York Heart Association classification, and LVEF—as well as BNP and PTX3. Model 3 incorporated PF5, BNP, and hs‐CRP. Model 4 incorporated PF5, BNP, hs‐CRP, and PTX3. Estimates of the C‐statistic for Cox proportional hazards regression models were calculated.18–20 C‐statistics were estimated after PTX3 and BNP levels were added to PF5.10 The proportional hazards assumption was confirmed by the Schoenfeld global test. Calibration of Cox regression models was also performed by the Grønnesby and Borgan calibration test.21 The incremental effects of addition of PTX3 to PF5 and BNP levels to predict future cardiovascular events were evaluated using the net classification index (NRI), as previously described.22 Patients were stratified into 1 of 3 risk categories based on PF5 and BNP levels measured during the mean 30‐month follow‐up period: low risk (0% to <10%), intermediate risk (10% to 20%), or high risk (>20%). PTX3 was subsequently used to reclassify the risk category for ascertaining whether there would be improvement in the NRI. The NRI was calculated using the following equation: NRI=([number of events reclassified as higher−number of events reclassified as lower]/number of events)−([number of nonevents reclassified as lower−number of nonevents reclassified as higher]/number of nonevents). A P value <0.05 was considered significant. All analyses were performed using SPSS version 19.0J for Windows (IBM Corporation), Stata version 11 (Stata Corporation), and SAS version 9.1.3 (SAS Institute Inc).
Results
Patient Characteristics
Table 1 shows the clinical characteristics of the participating patients. The median PTX3 level was 3.0 ng/mL. Mean age, New York Heart Association class, and BNP levels were higher and mean body mass index, waist circumference and estimated glomerular filtration rate were lower in the high‐PTX3 group (>3.0 ng/mL) than in the low‐PTX3 group (≤3.0 ng/mL). LVMI values were significantly higher in the high‐PTX3 group than in the low‐PTX3 group, whereas LVEF and E/e’ were similar in these 2 groups. Simple linear regression analysis showed a positive and significant correlation between Ln(PTX3) and Ln(BNP) (r=0.295, P<0.001) (Figure 2A) and a significant but weak positive correlation between Ln(PTX3) and Ln(LVMI) (r=0.114, P<0.05) (Figure 2B). Furthermore, simple linear regression analysis showed a significant but weak positive correlation between Ln(PTX3) and Ln(tumor necrosis factor‐α) (r=0.106, P<0.05) but not Ln(hs‐CRP) (P=0.11) or Ln(interleukin‐6) (P=0.80).
Table 1.
Demographic and Clinical Characteristics of Patients With HFNEF
| All HFNEF Patients (n=360) | PTX3 | |||
|---|---|---|---|---|
| Low‐PTX3 Group (n=180) | High‐PTX3 Group (n=180) | P Value | ||
| Age, y | 70.5±9.9 | 69.3±9.6 | 71.5±10.0 | <0.05 |
| Sex, male/female | 200/160 (55.6%/44.4%) | 107/73 (59.4%/41.6%) | 93/87 (51.7%/48.3%) | 0.17 |
| NYHA functional class II–III/IV | 259/101 | 152/28 | 107/73 | <0.001 |
| Body mass index, kg/m2 | 24.4±3.5 | 25.1±3.5 | 23.7±3.5 | <0.001 |
| Metabolic syndrome, n (%) | 157 (43.6) | 83 (46.1) | 74 (41.1) | 0.40 |
| Waist circumference, cm | 89.5±9.8 | 91.5±10.2 | 87.7±9.1 | <0.001 |
| Current smoking, n (%) | 50 (13.9) | 23 (12.8) | 27 (15.0) | 0.64 |
| Hypertension, n (%) | 274 (76.1) | 134 (74.4) | 140 (77.7) | 0.54 |
| Diabetes mellitus, n (%) | 171 (47.5) | 84 (46.7) | 87 (48.8) | 0.83 |
| Glucose, mg/dL | 106.2±29.1 | 104.7±25.6 | 107.8±32.2 | 0.32 |
| Hemoglobin A1c, % | 6.41±1.04 | 6.37±0.90 | 6.46±1.18 | 0.42 |
| Atrial fibrillation, n (%) | 63 (17.5) | 26 (14.4) | 37 (20.6) | 0.17 |
| CAD, n (%) | 240 (66.7) | 124 (68.9) | 116 (65.2) | 0.50 |
| HF hospitalization history, n (%) | 42 (11.7) | 16 (8.9) | 26 (14.4) | 0.14 |
| Dyslipidemia, n (%) | 181 (50.3) | 121 (67.2) | 60 (60.0) | 0.19 |
| Total cholesterol, mg/dL | 169.6±31.5 | 168.0±28.7 | 170.4±34.8 | 0.45 |
| HDL cholesterol, mg/dL | 51.5±13.3 | 50.7±12.6 | 52.3±13.9 | 0.23 |
| Triglycerides, mg/dL | 111.7±48.1 | 114.5±41.6 | 108.9±53.9 | 0.27 |
| LDL cholesterol, mg/dL | 98.0±26.2 | 97.1±24.7 | 98.9±27.7 | 0.50 |
| Estimated GFR, mL/min per 1.73 m2 | 63.3±17.4 | 65.1±17.2 | 61.5±17.5 | <0.05 |
| Inflammatory markers | ||||
| PTX3, ng/mL | 3.00 (1.80 to 3.85) | 1.80 (1.44 to 2.29) | 3.85 (3.22 to 4.76) | <0.001 |
| Hs‐CRP, mg/L | 0.80 (0.40 to 1.75) | 0.70 (0.30 to 1.60) | 0.85 (0.40 to 2.00) | 0.09 |
| TNF‐α, pg/mL | 1.60 (1.13 to 2.34) | 1.55 (1.13 to 2.30) | 1.66 (1.15 to 2.37) | 0.48 |
| IL‐6, pg/mL | 1.59 (1.03 to 2.46) | 1.56 (1.06 to 2.18) | 1.64 (1.00 to 2.85) | 0.49 |
| BNP, pg/mL | 85.4 (30.0 to 174.9) | 52.4 (25.4 to 110.5) | 122.9 (47.6 to 256.6) | <0.001 |
| Echocardiography | ||||
| LVEF, % | 63.2±6.7 | 63.3±6.6 | 63.1±6.9 | 0.73 |
| E/e’ | 15.5 (12.6 to 18.0) | 15.6 (12.9 to 18.1) | 15.3 (12.5 to 18.0) | 0.58 |
| LV mass index, g/m2 | 153.5 (128.0 to 183.4) | 147.8 (123.6 to 177.7) | 156.5 (131.5 to 187.0) | <0.05 |
| Medications | ||||
| Beta blockers, n (%) | 182 (50.6) | 96 (53.3) | 86 (47.8) | 0.34 |
| ACE‐Is or ARBs, n (%) | 251 (69.7) | 126 (70.0) | 125 (69.4) | 0.99 |
| Ca channel blockers, n (%) | 224 (62.2) | 114 (63.3) | 110 (61.1) | 0.74 |
| Statins, n (%) | 219 (60.8) | 117 (65.0) | 102 (56.7) | 0.13 |
| Aspirin, n (%) | 261 (72.5) | 134 (74.4) | 127 (60.6) | 0.20 |
| Diuretics, n (%) | 80 (22.2) | 33 (18.6) | 47 (26.1) | 0.10 |
| Spironolactone, n (%) | 39 (10.8) | 15 (8.3) | 24 (13.3) | 0.17 |
Data are mean±SD, number of patients (%), or median (interquartile range). ACE‐I indicates angiotensin‐converting enzyme inhibitors; ARB, angiotensin II receptor blocker; BNP, B‐type natriuretic peptide; CAD, coronary artery disease; E/e’, mitral early diastolic peak flow velocity to tissue Doppler early mitral annular diastolic velocity; GFR, glomerular filtration rate; HDL, high‐density lipoprotein; HF, heart failure; HFNEF, heart failure with normal ejection fraction; hs‐CRP, high‐sensitive C‐reactive protein; IL‐6, interleukin‐6; LDL, low‐density lipoprotein; LV, left ventricle; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PTX3, pentraxin 3; TNF‐α, tumor necrosis factor‐α.
Figure 2.
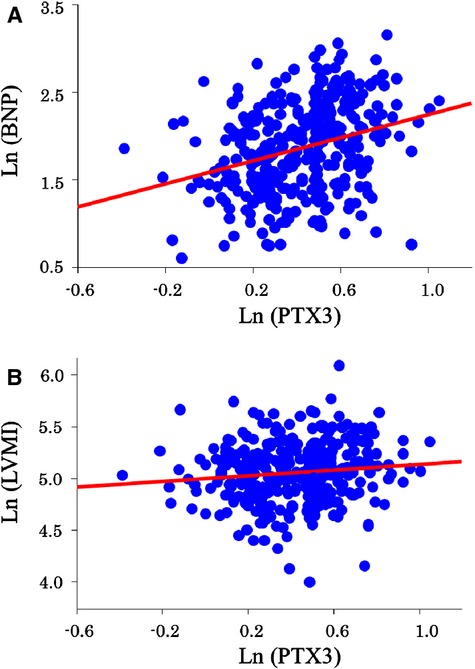
Relationships between PTX3 and BNP levels and PTX3 levels and LVMI. Simple linear regression analysis shows a positive and significant correlation between (A) Ln(PTX3) and Ln(BNP) (r=0.295, P<0.001) and (B) a significant but weak positive correlation between Ln(PTX3) and Ln(LVMI) (r=0.114, P<0.05). BNP indicates B‐type natriuretic peptide; LVMI; left ventricular mass index; PTX3, pentraxin 3.
Cardiovascular Events
Cardiovascular events were analyzed over time in 360 patients with HFNEF. The mean follow‐up period was 30 months (range: 1 to 59 months). During this time, 106 of these patients experienced cardiovascular events, including cardiovascular death (n=9), nonfatal MI (n=4), unstable angina pectoris (n=13), ischemic stroke (n=9), hospitalization for HF decompensation (n=41), and coronary revascularization (n=30). The frequency of cardiovascular events was significantly higher in the high‐PTX3 group (n=75) than in the low‐PTX3 group (n=31, P<0.001) (Table 2). HF‐related cardiovascular events, including cardiovascular death and hospitalization for HF decompensation, were significantly higher in the high‐PTX3 group (n=41) than in the low‐PTX3 group (n=9) (P<0.001) (Table 2). Kaplan‐Meier analysis showed that the probability of cardiovascular events was also significantly higher in the high‐PTX3 group than in the low‐PTX3 group (P<0.001, log‐rank test) (Figure 3A).
Table 2.
Cardiovascular Events in HFNEF Patients With Low and High Plasma PTX3 Levels
| Low‐PTX3 Group (n=180) | High‐PTX3 Group (n=180) | P Value | |
|---|---|---|---|
| Total cardiovascular events | 31 (17.2%) [17.5%] |
75 (41.7%) [42.4%] |
<0.001 |
| Cardiovascular death | 1 (0.6%) [0.4%] |
8 (4.4%) [2.5%] |
<0.05 |
| Nonfatal myocardial infarction | 2 (1.1%) [0.1%] |
2 (1.1%) [0.1%] |
0.99 |
| Unstable angina | 4 (2.2%) [2.0%] |
9 (5.0%) [4.7%] |
0.26 |
| Ischemic stroke | 2 (1.1%) [0.9%] |
7 (3.9%) [3.6%] |
0.17 |
| Hospitalization for HF decompensation | 8 (4.4%) [4.5%] |
33 (18.3%) [18.2%] |
<0.001 |
| Coronary revascularization | 14 (7.8%) [7.4%] |
16 (8.9%) [8.6%] |
0.85 |
| HF‐related cardiovascular events | 9 (5.0%) [5.0%] |
41 (22.8%) [21.9%] |
<0.001 |
Differences between the groups were assessed by the log‐rank test. Lower line in the angled bracket: Age‐ and sex‐adjusted event rates. HF indicates heart failure; HF‐related cardiovascular events, cardiovascular death and hospitalization for HF decompensation; HFNEF, heart failure with normal left ventricular ejection fraction; PTX3, pentraxin 3.
Figure 3.
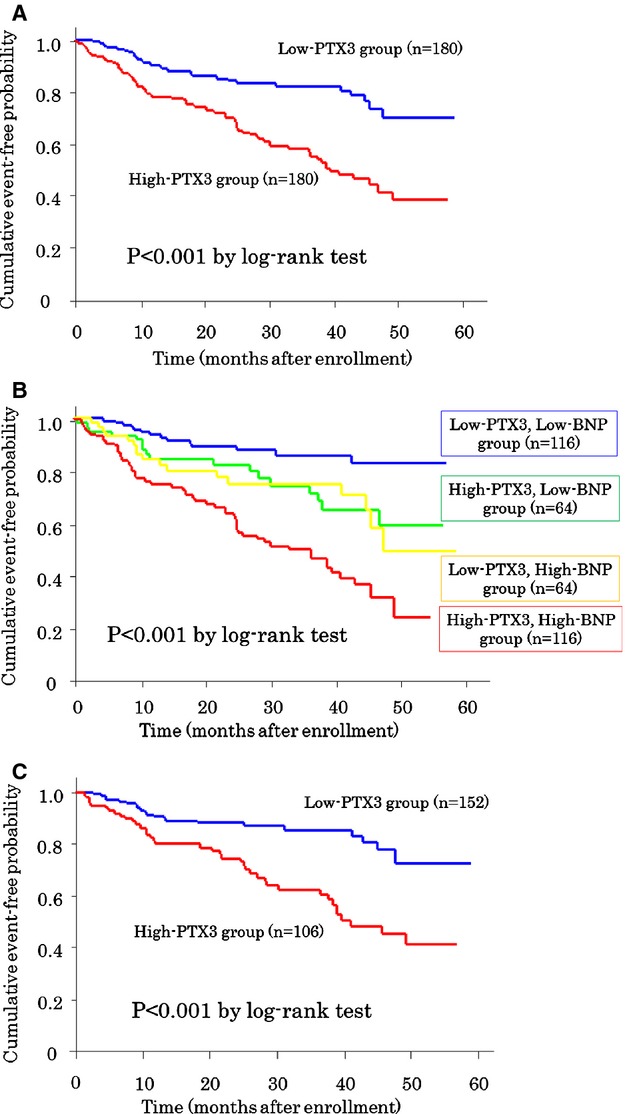
Kaplan‐Meier analysis for the probability of cardiovascular events. Patients with (A) high PTX3 levels and low PTX3 levels, (B) subgroups of patients with high and low PTX3 levels plus BNP, and (C) subgroups of New York Heart Association class II patients with high and low PTX3 levels are shown. BNP indicates B‐type natriuretic peptide; PTX3, pentraxin 3.
The combination of PTX3 and BNP levels led to identification of subgroups (n=116, high‐PTX3 and BNP group; n=64, high‐PTX3 and low‐BNP group; n=64, low‐PTX3 and high‐BNP group; and n=116, low‐PTX3 and BNP group) with significantly different probabilities of cardiovascular events (P<0.001, log‐rank test) (Figure 3B). In the low‐BNP (P=0.02) and high‐BNP (P<0.01) groups, the probability of cardiovascular events was significantly higher in those with high PTX3 levels than in those with low PTX3 levels. Even among patients with mild HF symptoms (New York Heart Association class II), the outcomes were poorer in patients with high PTX3 levels than in those with low PTX3 levels (P<0.001, log‐rank test) (Figure 3C).
Cox Proportional Hazards Analysis and C‐Statistics for Cardiovascular Events
The results of univariate and multivariable Cox proportional hazards analyses for cardiovascular events are shown in Tables 3 and 4. Multivariable Cox proportional hazards analysis identified PTX3 (hazard ratio [HR]: 1.16; 95% CI: 1.05 to 1.27; P<0.01), high‐density lipoprotein cholesterol (HR: 0.99; 95% CI: 0.97 to 0.99; P<0.05), LVMI (HR: 1.03; 95% CI: 1.05 to 1.27), and BNP (HR: 1.08; 95% CI: 1.03 to 1.14; P<0.001) as predictors of future cardiovascular events after adjustment for significant factors that were identified by univariate analysis (Table 3). Using the forced inclusion model, which evaluated levels of inflammatory markers and BNP (model 1) in multivariable Cox hazards analysis, PTX3, but not hs‐CRP, significantly predicted cardiovascular events (HR: 1.17; 95% CI: 1.07 to 1.28; P<0.001). In the forced inclusion model, which included PF5 and BNP, PTX3 significantly predicted cardiovascular events (model 2: HR: 1.16; 95% CI: 1.05 to 1.27; P<0.01; model 4: HR: 1.16; 95% CI: 1.06 to 1.27; P<0.01) (Table 4). Furthermore, multivariable Cox proportional hazards analysis identified PTX3 (HR: 1.16; 95% CI: 1.01 to 1.35; P<0.05) and BNP (HR: 1.21; 95% CI: 1.11 to 1.31; P<0.001) as predictors of future HF‐related cardiovascular events, including cardiovascular death and hospitalization for HF decompensation, after adjustment for significant factors identified by univariate analysis. We classified patients with HFNEF into 4 groups according to the levels of Ln(PTX3): first quartile (<0.59, PTX3: <1.80 ng/mL), second quartile (0.59 to 1.10, PTX3: 1.80 to 3.00 ng/mL), third quartile (1.10 to 1.35, PTX3: 3.00 to 3.85 ng/mL), and fourth quartile (>1.35, PTX3: >3.85 ng/mL); the relative risk of total cardiovascular events was 1 (reference), 1.36, 2.71 (P<0.001 versus the reference), and 3.17 (P<0.001 versus the reference), respectively. The log‐transformation values of PTX3 demonstrated a linear association with the occurrence of cardiovascular events in patients with HFNEF (r=0.965, P=0.03).
Table 3.
Cox Proportional Hazards Analysis of Factors That Were Predictive of Future Cardiovascular Events in Patients With HFNEF After Adjustment for Significant Factors Identified by Univariate Analysis
| Factor | Univariate | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age, y | 1.04 (0.98 to 1.03) | 0.20 | ||
| Sex, male | 1.31 (0.88 to 1.93) | 0.18 | ||
| NYHA functional class III or IV, yes | 1.97 (1.34 to 2.91) | <0.001 | 1.08 (0.69 to 1.69) | 0.74 |
| Body mass index, kg/m2 | 0.96 (0.91 to 1.02) | 0.55 | ||
| Metabolic syndrome, yes | 1.01 (0.69 to 1.49) | 0.95 | ||
| Waist circumference, cm | 1.00 (0.98 to 1.02) | 0.72 | ||
| Current smoking | 1.40 (0.85 to 2.30) | 0.19 | ||
| Hypertension, yes | 0.93 (0.61 to 1.45) | 0.77 | ||
| Diabetes mellitus, yes | 1.45 (0.99 to 2.13) | 0.056 | ||
| Glucose, per mg/dL | 1.00 (0.99 to 1.01) | 0.08 | ||
| Hemoglobin A1c, per% | 1.17 (0.98 to 1.38) | 0.09 | ||
| Atrial fibrillation, yes | 1.76 (1.13 to 2.74) | <0.05 | 1.19 (0.75 to 1.90) | 0.46 |
| CAD, yes | 1.47 (0.95 to 2.27) | 0.08 | ||
| HF hospitalization history, yes | 1.16 (0.66 to 2.04) | 0.60 | ||
| Dyslipidemia, yes | 1.04 (0.70 to 1.55) | 0.85 | ||
| Total cholesterol, mg/dL | 0.99 (0.98 to 1.01) | 0.07 | ||
| HDL cholesterol, mg/dL | 0.98 (0.97 to 0.99) | <0.05 | 0.99 (0.97 to 0.99) | <0.05 |
| Triglycerides, mg/dL | 1.00 (0.99 to 1.01) | 0.62 | ||
| LDL cholesterol, mg/dL | 0.99 (0.98 to 1.01) | 0.10 | ||
| Ln(PTX 3), 0.1 | 1.23 (1.13 to 1.34) | <0.001 | 1.16 (1.05 to 1.27) | <0.01 |
| Ln(hs‐CRP), 0.1 | 0.99 (0.95 to 1.02) | 0.44 | ||
| Ln(TNF‐α), 0.1 | 1.01 (0.95 to 1.06) | 0.87 | ||
| Ln(IL‐6), 0.1 | 1.03 (0.97 to 1.08) | 0.34 | ||
| Ln(BNP), 0.1 | 1.12 (1.07 to 1.16) | <0.001 | 1.08 (1.03 to 1.14) | <0.001 |
| Estimated GFR, mL/min per 1.73 m2 | 0.98 (0.97 to 0.99) | <0.05 | 1.00 (0.99 to 1.01) | 0.86 |
| LVEF, % | 0.98 (0.95 to 1.01) | 0.10 | ||
| Ln(E/e’), 0.1 | 0.98 (0.92 to 1.05) | 0.57 | ||
| Ln(LV mass index), 0.1 | 1.08 (1.01 to 1.16) | <0.05 | 1.03 (1.05 to 1.27) | <0.01 |
BNP indicates B‐type natriuretic peptide; CAD, coronary artery disease; E/e’, mitral early diastolic peak flow velocity to tissue Doppler early mitral annular diastolic velocity; GFR, glomerular filtration rate; HDL, high‐density lipoprotein; HF, heart failure; HFNEF, heart failure with normal ejection fraction; HR, hazard ratio; hs‐CRP, high‐sensitivity C‐reactive protein; IL‐6, interleukin‐6; LDL, low‐density lipoprotein; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PTX3, pentraxin 3; TNF‐α, tumor necrosis factor‐α.
Table 4.
Cox Proportional Hazards Analysis of Factors That Were Associated With Future Cardiovascular Events in HFNEF Patients Using Forced Inclusion Models
| Factor | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age, y | 1.00 (0.98 to 1.02) | 0.95 | 1.01 (0.99 to 1.03) | 0.57 | 1.00 (0.98 to 1.02) | 0.98 | ||
| NYHA functional class III or IV, yes | 1.12 (0.72 to 1.75) | 0.63 | 1.27 (0.81 to 1.98) | 0.30 | 1.12 (0.73 to 1.77) | 0.58 | ||
| Diabetes mellitus, yes | 1.52 (1.04 to 2.24) | <0.05 | 1.50 (1.06 to 2.30) | <0.05 | 1.50 (1.02 to 2.21) | <0.05 | ||
| History of HF‐related hospitalization, yes | 1.60 (0.88 to 2.89) | 0.12 | 1.67 (0.92 to 3.04) | 0.10 | 1.65 (0.91 to 3.00) | 0.10 | ||
| LVEF, % | 0.99 (0.95 to 1.02) | 0.38 | 0.99 (0.96 to 1.02) | 0.39 | 0.99 (0.96 to 1.02) | 0.38 | ||
| Ln(PTX3), 0.1 | 1.17 (1.07 to 1.28) | <0.001 | 1.16 (1.05 to 1.27) | <0.01 | 1.16 (1.06 to 1.27) | <0.01 | ||
| Ln(hs‐CRP), 0.1 | 0.98 (0.94 to 1.02) | 0.26 | 0.99 (0.95 to 1.02) | 0.40 | 0.98 (0.94 to 1.02) | 0.22 | ||
| Ln(TNF‐α), 0.1 | 0.99 (0.94 to 1.04) | 0.67 | ||||||
| Ln(IL‐6), 0.1 | 1.00 (0.95 to 1.06) | 0.84 | ||||||
| Ln(BNP), 0.1 | 1.09 (1.05 to 1.14) | <0.001 | 1.10 (1.05 to 1.27) | <0.01 | 1.11 (1.06 to 1.17) | <0.001 | 1.10 (1.04 to 1.15) | <0.001 |
BNP indicates B‐type natriuretic peptide; CI, confidence interval; HF, heart failure; HFNEF, heart failure with normal ejection fraction; HR, hazard ratio; hs‐CRP, high‐sensitive C‐reactive protein; IL‐6, interleukin‐6; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PTX3, pentraxin 3; TNF‐α, tumor necrosis factor‐α.
We also estimated the C‐statistics of PF5 alone. Separate incorporation of PTX3 and BNP into PF5 increased the C‐statistics for the prediction of future cardiovascular events from 0.617 for PF5 alone to 0.656 for PF5 plus PTX3 and PF5 plus BNP (Table 5). Moreover, incorporation of PTX3 and BNP into PF5 increased the C‐statistics to 0.683 (P<0.05). In addition, the PF5 plus BNP plus PTX3 model performed better than the PF5 plus BNP model (C‐statistics: 0.683 versus 0.656). The Schoenfeld test indicated that the proportional hazards assumptions were appropriate (P=0.46). We also confirmed good calibration for the analysis by Grønnesby and Borgan statistics (P=0.19).
Table 5.
C‐Statistics for Cox Proportional Hazards Analysis Predicting Future Cardiovascular Events in Patients With Heart Failure With Normal Ejection Fraction
| Variable | C‐Statistic | 95% CI | Increment in C‐Statistic |
|---|---|---|---|
| PF5 | 0.617 | 0.555 to 0.681 | |
| PF5+PTX3 | 0.656 | 0.594 to 0.718 | 0.039 |
| PF5+BNP | 0.656 | 0.598 to 0.714 | |
| PF5+BNP+PTX3 | 0.683 | 0.624 to 0.742 | 0.027 |
BNP indicates B‐type natriuretic peptide; PF5, the 5 factors identified in the I‐PRESERVE study10 as prognostic in patients with heart failure with normal ejection fraction (age, presence of diabetes mellitus, previous hospitalization for heart failure, New York Heart Association classification, and left ventricular ejection fraction); PTX3, pentraxin 3.
Net Reclassification Index
We reclassified the risk of PF5 and BNP for patients with HFNEF. The NRI was significant after PTX3 was included: 12.2% for patients without cardiovascular events, 0% for those with cardiovascular events, and 12.2% overall (P<0.01) (Table 6).
Table 6.
Reclassification of the Risk of PF5 and BNP for Cardiovascular Events After the Addition of PTX3
| Original Risk Category | Reclassification | ||
|---|---|---|---|
| PF5+BNP+PTX3 Low Risk | PF5+BNP+PX3 Intermediate Risk | PF5+BNP+PTX3 High Risk | |
| Patients without cardiovascular events | |||
| PF5+BNP low risk | 0 | 1 | 0 |
| PF5+BNP intermediate risk | 4 | 59 | 12 |
| PF5+BNP high risk | 0 | 40 | 138 |
| Patients with cardiovascular events | |||
| PF5+BNP low risk | 0 | 0 | 0 |
| PF5+BNP intermediate risk | 0 | 10 | 4 |
| PF5+BNP high risk | 0 | 4 | 88 |
BNP indicates B‐type natriuretic peptide; PF5, the 5 factors identified in the I‐PRESERVE study10 as prognostic in patients with heart failure with normal ejection fraction (age, presence of diabetes mellitus, previous hospitalization for heart failure, New York Heart Association classification, and left ventricular ejection fraction); PTX3, pentraxin 3.
Discussion
To the best of our knowledge, this report is the first to show a significant association between plasma levels of PTX3, a marker of inflammation, and adverse cardiovascular outcomes in patients with HFNEF. High plasma PTX3 levels can significantly predict future cardiovascular events in HFNEF patients. The addition of high PTX3 levels to the PF5 and BNP levels, which were previously found to be prognostic in patients with HFNEF, improved their risk stratification, as indicated by a substantial increase in the C‐statistics and significance of NRI.
Activation of the inflammatory process is important in the pathogenesis of HF and in adverse prognosis of patients with this condition. Several studies have investigated the role of inflammation as a therapeutic target, although initial trials had limited success.23 Consequently, specific anti‐inflammatory approaches for the different types and stages of HF (ie, HFNEF and HFREF) remain a priority, as does a better understanding of the mechanisms underlying HF‐related inflammation. The established inflammatory marker hs‐CRP is an independent predictor of morbidity and mortality in patients with HF.24–26 Elevated CRP levels predict hospitalization of HF patients, although the association between CRP and HF events is no longer significant after adjustment for the presence of LV diastolic dysfunction.27 Consequently, the relationships between inflammatory markers and the prognosis of patients with HFNEF remain unclear. Myocardial remodeling differs in HFNEF and HFREF.28 In HFREF, remodeling is driven by the loss of cardiomyocytes, whereas in HFNEF, a systemic inflammatory state induces oxidative stress in the coronary microvascular endothelium, which drives myocardial dysfunction and remodeling.28 Other studies have shown that serum levels of inflammatory cytokines are high in patients with HFNEF29 and that interleukin‐16, a cytokine considered an important mediator in inflammation, promotes cardiac fibrosis and myocardial stiffening in HFNEF.30 Consequently, investigating the relationships between inflammatory markers and prognosis in HFNEF is important. We observed a significant correlation between plasma levels of PTX3, a novel inflammatory maker, and future cardiovascular events in patients with HFNEF. Furthermore, considerable evidence suggests that PTX3 may better reflect local inflammatory status in tissues than does liver‐derived CRP.6 We previously reported that PTX3 was partly produced in the coronary circulation in patients with HFNEF.7 These results suggest that PTX3, rather than CRP, reflects vascular and cardiac inflammation in patients with HFNEF and might be a cardiovascular biomarker for the assessment and management of HF. In the present study, we showed that increased inflammation as assessed by PTX3 measurement was significantly associated with poor prognosis for HFNEF patients by multivariable Cox hazards analysis. Furthermore, the addition of 2 biomarkers—PTX3 as an inflammation maker and BNP as a myocardial stress marker—to the previously established prognostic factors in the I‐PRESERVE study10 of HFNEF patients improved their risk stratification performance, as indicated by an increase in the C‐statistics. These results suggest the possible relative merits of these 2 biomarkers for future risk management in HFNEF. In NRI analyses, the addition of PTX3 was able to provide a more appropriate risk assessment in patients who were evaluated as having a high risk state by previously described HFNEF prognostic factors (PF5 plus BNP) in patients without cardiovascular events. Furthermore, the addition of PTX3 never resulted in a worse risk classification in patients with cardiovascular events. Consequently, the significance of NRI was shown by incorporating PTX3 into the new risk‐assessment model.
The site of PTX3 production in patients with HFNEF remains unclear. Under normal physiological conditions, PTX3 mRNA is not expressed in the heart.31 Under pathological conditions, PTX3 is expressed in atherosclerotic lesions,32 adipose tissue,33 and the heart with acute and sub‐acute‐phase HF34; however, the types of cells that express PTX3 in failing hearts of patients with HFNEF and chronic HF have not been determined. HFNEF may result from the accumulation of extracellular matrix material within myocardial fibrosis.35–36 Accumulation of the extracellular matrix (ie, fibrosis) may also be pathophysiologically important in progression of the HFNEF disease process and be a contributor to subsequent events.37 HFNEF may also be caused by structural and molecular abnormalities of the cardiovascular system, including cardiac and noncardiac factors, such as those associated with vascular functions.38 We recently demonstrated significant involvement of endothelial dysfunction in the prognosis of patients with HFNEF, suggesting that improvement in endothelial function could be a potential therapeutic target in patients with HFNEF.39 PTX3 is produced by fibrous tissues and endothelial cells in response to inflammatory stimuli5 and is considered an important marker of vascular pathology.40 In patients with acute MI and infectious myocarditis, PTX3 is produced by macrophages, endothelial cells, and, to a lesser extent, myocardiocytes and is localized in the interstitium.34 The interstitial localization of PTX3 in human failing hearts suggests that PTX3 is produced locally by fibroblasts in the cardiac interstitium. Consequently, in patients with HFNEF, PTX3 might be produced by myocardial fibroblasts and/or endothelial cells rather than in subclinical atherosclerotic lesions in patients with HFNEF. We found that the frequency of HF‐related cardiovascular events, including cardiovascular death and hospitalization for HF decompensation, was significantly higher in patients with high PTX3 levels than in those with low PTX3 levels. This finding suggests that PTX3 is more predictive of future HF‐related cardiovascular events than of vascular events in patients with HFNEF.
We used the median value of PTX3 (3.0 ng/mL) as the cutoff point in the present study. Several studies have investigated the association between PTX3 values and HF. Suzuki et al demonstrated that the median value of PTX3 was 3.7 ng/mL in patients with HFREF and split the patients into 2 groups: those above and those below 4.0 ng/mL.8 In this study, patients with HFREF were targeted, and it has already been demonstrated that patients with HFREF have higher inflammatory activity than those with HFNEF. We previously demonstrated that the 75% range value of PTX3 was 2.90 ng/mL in non‐HF patients with risk factors (hypertension, 67.8%; diabetes mellitus, 37.4%; dyslipidemia, 57.1%; and coronary artery disease, 55.0%).7 Furthermore, Inoue et al showed that PTX3 levels were <2.28 ng/mL in healthy volunteers.41 In previous studies of cardiovascular events in stable patients with coronary risk factors, the cutoff points of PTX3 were 2.8342 and 3.24 ng/mL.43 Thus, we consider that the median value of 3.0 ng/mL for PTX3 was a clinically meaningful cutoff point in our study.
The present study has several limitations. First, our study included only a relatively small number of patients in a single center. Second, the population of this study was relatively young and predominantly male; had a low prevalence of atrial fibrillation, a higher frequency of mild HF, relatively lower BNP levels; and had less frequent use of diuretics. Because our institution is an educational hospital and the design was a single‐center study in Japan, the recruited patients with HFNEF might have had differences in characteristics compared with those in Western studies. Consequently, a large, multiracial, multicenter study is required to confirm our results. Third, the pathogenetic role of high PTX3 levels in HFNEF remains unclear. Recent studies in mouse models have shown that PTX3 has a cardioprotective effect,44–45 suggesting that PTX3 might protect the cardiovascular system by modulating the immune–inflammatory balance. Further in vivo and in vitro experiments are required to determine the exact role of high PTX3 levels in HFNEF.
Despite these limitations, our study provided the first evidence for the prognostic significance of PTX3 in HFNEF. A large‐scale multicenter trial is warranted to further examine the pathological role and clinical significance of PTX3 in HFNEF.
Conclusion
High plasma PTX3 levels, but not other inflammatory markers including hs‐CRP, are significantly correlated with future cardiovascular events in patients with HFNEF. Determination of PTX3 levels may provide incremental prognostic information in patients with HFNEF.
Sources of Funding
This study was supported in part by a Grant‐in‐Aid for Scientific Research (Nos. C22590786 and C25461086 for Sugiyama) from the Ministry of Education, Culture, Sports, Science and Technology in Japan.
Disclosures
.O. received lecture fees and research grants from Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Chugai, Daiichi Sankyo, Dainippon Sumitomo Pharma, Eisai, Kowa, Kyowa Hakko Kirin, Mitsubishi Tanabe, MSD, Novartis, Otsuka, Pfizer, Sanofi, Sionogi, Takeda, and Mochida.
Acknowledgments
We are grateful to Megumi Nagahiro and Saeko Tokunaga of the Department of Cardiovascular Medicine, Faculty of Life Sciences, Kumamoto University, Kumamoto, Japan, for their skillful technical assistance.
References
- 1.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. JAMA. 2006; 296:2209-2216. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population‐based study. N Engl J Med. 2006; 355:260-269. [DOI] [PubMed] [Google Scholar]
- 3.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006; 355:251-259. [DOI] [PubMed] [Google Scholar]
- 4.Braunwald E. Biomarkers in heart failure. N Engl J Med. 2008; 358:2148-2159. [DOI] [PubMed] [Google Scholar]
- 5.Garlanda C, Bottazzi B, Bastone A, Mantovani A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu Rev Immunol. 2005; 23:337-366. [DOI] [PubMed] [Google Scholar]
- 6.Mantovani A, Garlanda C, Bottazzi B, Peri G, Doni A, Martinez de la Torre Y, Latini R. The long pentraxin PTX3 in vascular pathology. Vascul Pharmacol. 2006; 45:326-330. [DOI] [PubMed] [Google Scholar]
- 7.Matsubara J, Sugiyama S, Nozaki T, Sugamura K, Konishi M, Ohba K, Matsuzawa Y, Akiyama E, Yamamoto E, Sakamoto K, Nagayoshi Y, Kaikita K, Sumida H, Kim‐Mitsuyama S, Ogawa H. Pentraxin 3 is a new inflammatory marker correlated with left ventricular diastolic dysfunction and heart failure with normal ejection fraction. J Am Coll Cardiol. 2011; 57:861-869. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki S, Takeishi Y, Niizeki T, Koyama Y, Kitahara T, Sasaki T, Sagara M, Kubota I. Pentraxin 3, a new marker for vascular inflammation, predicts adverse clinical outcomes in patients with heart failure. Am Heart J. 2008; 155:75-81. [DOI] [PubMed] [Google Scholar]
- 9.Latini R, Gullestad L, Masson S, Nymo SH, Ueland T, Cuccovillo I, Vardal M, Bottazzi B, Mantovani A, Lucci D, Masuda N, Sudo Y, Wikstrand J, Tognoni G, Aukrust P, Tavazzi L. Pentraxin‐3 in chronic heart failure: the CORONA and GISSI‐HF trials. Eur J Heart Fail. 2012; 14:992-999. [DOI] [PubMed] [Google Scholar]
- 10.Komajda M, Carson PE, Hetzel S, McKelvie R, McMurray J, Ptaszynska A, Zile MR, Demets D, Massie BM. Factors associated with outcome in heart failure with preserved ejection fraction: findings from the Irbesartan in Heart Failure with Preserved Ejection Fraction Study (I‐PRESERVE). Circ Heart Fail. 2011; 4:27-35. [DOI] [PubMed] [Google Scholar]
- 11.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971; 285:1441-1446. [DOI] [PubMed] [Google Scholar]
- 12.Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite‐Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007; 28:2539-2550. [DOI] [PubMed] [Google Scholar]
- 13.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009; 53:982-992. [DOI] [PubMed] [Google Scholar]
- 14.Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997; 30:1527-1533. [DOI] [PubMed] [Google Scholar]
- 15.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009; 10:165-193. [DOI] [PubMed] [Google Scholar]
- 16.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005; 18:1440-1463. [DOI] [PubMed] [Google Scholar]
- 17.Thygesen K, Alpert JS, White HD, Joint ESCAAHAWHFTFftRoMI. Jaffe AS, Apple FS, Galvani M, Katus HA, Newby LK, Ravkilde J, Chaitman B, Clemmensen PM, Dellborg M, Hod H, Porela P, Underwood R, Bax JJ, Beller GA, Bonow R, Van der Wall EE, Bassand JP, Wijns W, Ferguson TB, Steg PG, Uretsky BF, Williams DO, Armstrong PW, Antman EM, Fox KA, Hamm CW, Ohman EM, Simoons ML, Poole‐Wilson PA, Gurfinkel EP, Lopez‐Sendon JL, Pais P, Mendis S, Zhu JR, Wallentin LC, Fernandez‐Aviles F, Fox KM, Parkhomenko AN, Priori SG, Tendera M, Voipio‐Pulkki LM, Vahanian A, Camm AJ, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck‐Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Widimsky P, Zamorano JL, Morais J, Brener S, Harrington R, Morrow D, Lim M, Martinez‐Rios MA, Steinhubl S, Levine GN, Gibler WB, Goff D, Tubaro M, Dudek D, Al‐Attar N. Universal definition of myocardial infarction. Circulation. 2007; 116:2634-2653. [DOI] [PubMed] [Google Scholar]
- 18.Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007; 115:928-935. [DOI] [PubMed] [Google Scholar]
- 19.Pencina MJ, D'Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004; 23:2109-2123. [DOI] [PubMed] [Google Scholar]
- 20.Nozaki T, Sugiyama S, Koga H, Sugamura K, Ohba K, Matsuzawa Y, Sumida H, Matsui K, Jinnouchi H, Ogawa H. Significance of a multiple biomarkers strategy including endothelial dysfunction to improve risk stratification for cardiovascular events in patients at high risk for coronary heart disease. J Am Coll Cardiol. 2009; 54:601-608. [DOI] [PubMed] [Google Scholar]
- 21.Gronnesby JK, Borgan O. A method for checking regression models in survival analysis based on the risk score. Lifetime Data Anal. 1996; 2:315-328. [DOI] [PubMed] [Google Scholar]
- 22.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008; 27:157-172.‐ [DOI] [PubMed] [Google Scholar]
- 23.Heymans S, Hirsch E, Anker SD, Aukrust P, Balligand JL, Cohen‐Tervaert JW, Drexler H, Filippatos G, Felix SB, Gullestad L, Hilfiker‐Kleiner D, Janssens S, Latini R, Neubauer G, Paulus WJ, Pieske B, Ponikowski P, Schroen B, Schultheiss HP, Tschope C, Van Bilsen M, Zannad F, McMurray J, Shah AM. Inflammation as a therapeutic target in heart failure? A scientific statement from the Translational Research Committee of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2009; 11:119-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamblin N, Mouquet F, Hennache B, Dagorn J, Susen S, Bauters C, de Groote P. High‐sensitivity C‐reactive protein: potential adjunct for risk stratification in patients with stable congestive heart failure. Eur Heart J. 2005; 26:2245-2250. [DOI] [PubMed] [Google Scholar]
- 25.Anand IS, Latini R, Florea VG, Kuskowski MA, Rector T, Masson S, Signorini S, Mocarelli P, Hester A, Glazer R, Cohn JN. C‐reactive protein in heart failure: prognostic value and the effect of valsartan. Circulation. 2005; 112:1428-1434. [DOI] [PubMed] [Google Scholar]
- 26.Vasan RS, Sullivan LM, Roubenoff R, Dinarello CA, Harris T, Benjamin EJ, Sawyer DB, Levy D, Wilson PW, D'Agostino RB. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation. 2003; 107:1486-1491. [DOI] [PubMed] [Google Scholar]
- 27.Williams ES, Shah SJ, Ali S, Na BY, Schiller NB, Whooley MA. C‐reactive protein, diastolic dysfunction, and risk of heart failure in patients with coronary disease: Heart and Soul Study. Eur J Heart Fail. 2008; 10:63-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013; 62:263-271. [DOI] [PubMed] [Google Scholar]
- 29.Collier P, Watson CJ, Voon V, Phelan D, Jan A, Mak G, Martos R, Baugh JA, Ledwidge MT, McDonald KM. Can emerging biomarkers of myocardial remodelling identify asymptomatic hypertensive patients at risk for diastolic dysfunction and diastolic heart failure? Eur J Heart Fail. 2011; 13:1087-1095. [DOI] [PubMed] [Google Scholar]
- 30.Tamaki S, Mano T, Sakata Y, Ohtani T, Takeda Y, Kamimura D, Omori Y, Tsukamoto Y, Ikeya Y, Kawai M, Kumanogoh A, Hagihara K, Ishii R, Higashimori M, Kaneko M, Hasuwa H, Miwa T, Yamamoto K, Komuro I. Interleukin‐16 promotes cardiac fibrosis and myocardial stiffening in heart failure with preserved ejection fraction. PLoS One. 2013; 8:e68893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Introna M, Alles VV, Castellano M, Picardi G, De Gioia L, Bottazzai B, Peri G, Breviario F, Salmona M, De Gregorio L, Dragani TA, Srinivasan N, Blundell TL, Hamilton TA, Mantovani A. Cloning of mouse ptx3, a new member of the pentraxin gene family expressed at extrahepatic sites. Blood. 1996; 87:1862-1872. [PubMed] [Google Scholar]
- 32.Rolph MS, Zimmer S, Bottazzi B, Garlanda C, Mantovani A, Hansson GK. Production of the long pentraxin PTX3 in advanced atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2002; 22:e10-e14. [DOI] [PubMed] [Google Scholar]
- 33.Alberti L, Gilardini L, Zulian A, Micheletto G, Peri G, Doni A, Mantovani A, Invitti C. Expression of long pentraxin PTX3 in human adipose tissue and its relation with cardiovascular risk factors. Atherosclerosis. 2009; 202:455-460. [DOI] [PubMed] [Google Scholar]
- 34.Nebuloni M, Pasqualini F, Zerbi P, Lauri E, Mantovani A, Vago L, Garlanda C. PTX3 expression in the heart tissues of patients with myocardial infarction and infectious myocarditis. Cardiovasc Pathol. 2011; 20:e27-e35. [DOI] [PubMed] [Google Scholar]
- 35.Martos R, Baugh J, Ledwidge M, O'Loughlin C, Conlon C, Patle A, Donnelly SC, McDonald K. Diastolic heart failure: evidence of increased myocardial collagen turnover linked to diastolic dysfunction. Circulation. 2007; 115:888-895. [DOI] [PubMed] [Google Scholar]
- 36.Borbely A, Papp Z, Edes I, Paulus WJ. Molecular determinants of heart failure with normal left ventricular ejection fraction. Pharmacol Rep. 2009; 61:139-145. [DOI] [PubMed] [Google Scholar]
- 37.Krum H, Elsik M, Schneider HG, Ptaszynska A, Black M, Carson PE, Komajda M, Massie BM, McKelvie RS, McMurray JJ, Zile MR, Anand IS. Relation of peripheral collagen markers to death and hospitalization in patients with heart failure and preserved ejection fraction: results of the I‐PRESERVE collagen substudy. Circ Heart Fail. 2011; 4:561-568. [DOI] [PubMed] [Google Scholar]
- 38.Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD, Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010; 56:845-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akiyama E, Sugiyama S, Matsuzawa Y, Konishi M, Suzuki H, Nozaki T, Ohba K, Matsubara J, Maeda H, Horibata Y, Sakamoto K, Sugamura K, Yamamuro M, Sumida H, Kaikita K, Iwashita S, Matsui K, Kimura K, Umemura S, Ogawa H. Incremental prognostic significance of peripheral endothelial dysfunction in patients with heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol. 2012; 60:1778-1786. [DOI] [PubMed] [Google Scholar]
- 40.Garlanda C, Bottazzi B, Moalli F, Deban L, Molla F, Latini R, Mantovani A. Pentraxins and atherosclerosis: the role of PTX3. Curr Pharm Des. 2011; 17:38-46. [DOI] [PubMed] [Google Scholar]
- 41.Inoue K, Sugiyama A, Reid PC, Ito Y, Miyauchi K, Mukai S, Sagara M, Miyamoto K, Satoh H, Kohno I, Kurata T, Ota H, Mantovani A, Hamakubo T, Daida H, Kodama T. Establishment of a high sensitivity plasma assay for human pentraxin3 as a marker for unstable angina pectoris. Arterioscler Thromb Vasc Biol. 2007; 27:161-167. [DOI] [PubMed] [Google Scholar]
- 42.Wang Z, Sato A, Akiyama D, Kimura T, Tajiri K, Hoshi T, Sakai S, Koike A, Miyauchi T, Aonuma K. Clinical value of plasma pentraxin 3 levels for predicting cardiac troponin elevation after percutaneous coronary intervention. Life Sci. 2014; 95:40-44. [DOI] [PubMed] [Google Scholar]
- 43.Koga S, Ikeda S, Yoshida T, Nakata T, Takeno M, Masuda N, Koide Y, Kawano H, Maemura K. Elevated levels of systemic pentraxin 3 are associated with thin‐cap fibroatheroma in coronary culprit lesions: assessment by optical coherence tomography and intravascular ultrasound. JACC Cardiovasc Interv. 2013; 6:945-954. [DOI] [PubMed] [Google Scholar]
- 44.Salio M, Chimenti S, De Angelis N, Molla F, Maina V, Nebuloni M, Pasqualini F, Latini R, Garlanda C, Mantovani A. Cardioprotective function of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2008; 117:1055-1064. [DOI] [PubMed] [Google Scholar]
- 45.Norata GD, Marchesi P, Pulakazhi Venu VK, Pasqualini F, Anselmo A, Moalli F, Pizzitola I, Garlanda C, Mantovani A, Catapano AL. Deficiency of the long pentraxin PTX3 promotes vascular inflammation and atherosclerosis. Circulation. 2009; 120:699-708. [DOI] [PubMed] [Google Scholar]


