Abstract
Background
The incidence of acute kidney injury (AKI) requiring dialysis in hospitalized patients is increasing; however, information on the long‐term incidence of stroke in patients surviving to discharge after recovering from AKI after dialysis has not been reported.
Methods and Results
Patients that survived after recovery from dialysis‐requiring AKI during index hospitalizations from 1999 to 2008 were identified in nationwide administrative registries. The risk of de novo stroke and death were analyzed with time‐varying Cox proportional hazard models. The results were validated by a critical care database. We enrolled 4315 patients in the AKI‐recovery group (men, 57.7%; mean age, 62.8±16.8 years) and matched 4315 control subjects as the non‐AKI group by propensity scores. After a median follow‐up period of 3.36 years, the incident stroke rate was 15.6 per 1000 person‐years. The AKI‐recovery group had higher risk (hazard ratio: 1.25; P=0.037) and higher severity of stroke events than the non‐AKI group, regardless of progression to subsequent chronic kidney disease. The rate of incident stroke was not statistically different in those with diabetes alone (without AKI) and in those with AKI alone (without diabetes) after hospital discharge (P=0.086). Furthermore, the risk of mortality in the AKI‐recovery group was higher than in the non‐AKI group (hazard ratio: 2.4; P<0.001).
Conclusions
The patients who recovered from AKI had a higher incidence of developing incident stroke and mortality than the patients without AKI, and the impact was similar to diabetes. Our results suggest that a public health initiative is needed to enhance postdischarge follow‐up of renal function and to control the subsequent incidence of stroke among patients who recover from AKI after dialysis.
Keywords: acute kidney injury, dialysis, kidney–brain interaction, mortality, recovery, stroke
Introduction
Acute kidney injury (AKI) is increasingly prevalent worldwide and is associated with higher morbidity and mortality.1 Recent studies in the developed world report AKI in 3.2% to 9.6% of admissions, resulting in in‐hospital mortality rates ranging from 20% to 50%.2 The incidence of AKI requiring dialysis is increasing at the rate of 10% per year in the United States and is higher than that of end‐stage renal disease (ESRD).3 Even though the recovery of kidney function following AKI is an important determinant of morbidity and has long‐term implications for the health and well‐being of patients, there is a lack of investigations on this topic.4
Cardiovascular disease is the main cause of death for patients on dialysis, and stroke is the third leading cause of cardiovascular disease–related death and disability.5 Rapidly declining renal function may augment the risk of cardiovascular events independently from baseline renal function.6 There are several potential pathophysiological mechanisms by which AKI may directly contribute to an increased risk of cardiovascular disease independent of the impact on chronic kidney disease (CKD) progression.7 AKI can lead to an increase in microvascular permeability in the brain, and acute uremia may induce proinflammatory chemokines that are mechanistically responsible for a leaky blood–brain barrier.8 Although both AKI and stroke may result from atheroembolic events and are independent predictors of mortality, it has been suggested that the 2 entities may interact in the pathophysiology.9–10 Few studies, however, have explored the impact of the development and severity of AKI on progression to de novo stroke.
We hypothesized that dialysis‐requiring AKI, even for only a temporary period, would have an adverse effect on first‐ever incident stroke among hospitalized patients. We used data from Taiwan's National Health Research Institute (NHRI) to examine whether recovery from AKI after dialysis is a risk factor for long‐term stroke and compared the impact on stroke with other known atherosclerotic risk factors such as diabetes mellitus (DM).11
Patients and Methods
Data Sources
The Taiwan National Health Insurance (NHI) program is a nationwide insurance program that covers outpatient visits, hospital admissions, prescriptions, intervention procedures, and disease profiles for >99% of the population of Taiwan (23.12 million people in 2009).12
The NHRI uses the original data of the NHI program to construct a longitudinal database of patients admitted between 1999 and 2008. This cohort includes 2 619 534 hospitalized patients, representing 10% of all NHI enrollees. This sampling fraction (a 3.4:1 ratio) is based on a regulation that limits the maximal amount of NHI data that can be extracted for research purposes. The NHRI database is one of the largest and most comprehensive databases in the world and has been used extensively in various studies on prescription use, diagnoses, and hospitalizations.13–15
Research Variables
The demographic and clinical characteristics of the participants at the index hospitalization were recorded. The parameters included age, sex, year of admission, hospital characteristics, prevalence of selected comorbid conditions, Charlson comorbidity index,16 organ dysfunction developing during the index hospitalization, the categories of major operations, intensive care unit admission, and outcomes. CKD codes were further validated, and the results showed a highly positive predictive value The anticoagulation prescriptions customarily used for ischemic stroke, including clopidogrel (B01AC04), acetylsalicylic acid (B01AA0), and vitamin K antagonists (B01AA0), were also identified. To determine preexisting comorbidities, we used a relatively strict criterion—at least 1 inpatient admission or 3 outpatient visits to treat a certain disease during the year prior to the index hospitalization—that was well validated with good predictive power.17–18 Septicemia was defined based on the International Classification of Diseases, 9th revision, clinical modification (ICD‐9‐CM) codes 003.1, 036.2, and 038.19 The identification of patients with severe sepsis was similar to that used by Angus et al20 and in our previous report21 and included all acute‐care hospitalizations with ICD‐9‐CM codes for bacterial or fungal infections and a diagnosis of acute organ dysfunction. Sepsis was defined according to the American College of Chest Physicians/Society of Critical Care Medicine guidelines as systemic inflammatory syndrome in response to an infection that, when associated with acute organ dysfunction, is said to be severe.22 Cigarette smoking is also likely to influence stroke; however, information on smoking is not available in the NHRI database. According to the surgeon general's reports,23 chronic obstructive pulmonary disease, coronary artery disease, DM, and lung cancer are highly related to smoking and could be used as proxies for cigarette smoking. Consequently, we used joint modeling of multiple diseases to capture the effect of smoking on the risk of stroke.24
Identification of Cases and Controls
The study group consisted of those aged ≥18 years with a first diagnosis of dialysis‐requiring AKI, according to procedural codes. The dialysis certificate data in Taiwan are highly reliable because they are used for insurance payments.25
We used a 1‐year period prior to the index hospitalization to identify preadmission comorbidities, AKI, and dialysis. Patients with preadmission AKI, ESRD, or stroke and those who had undergone kidney transplantation were excluded. Patients who underwent placement of a vascular access or peritoneal‐dialysis catheter were also excluded. Figure 1 shows the patient‐selection flow chart. Only those who survived for >30 days after discharge from the index hospitalization and who were not readmitted to hospital and did not receive further dialysis were enrolled as the AKI‐recovery group. A control cohort without AKI, dialysis, or incident stroke before and during the index hospitalization and matched for age, sex, the same calendar year of index hospitalization, and propensity score adjusted for acute dialysis during hospitalization with the study group (AKI‐recovery group) was selected for comparison (non‐AKI group). The patients were further divided into 4 mutually exclusive subgroups for further analysis: non‐DM/non‐AKI, AKI alone, DM alone, and AKI with DM.
Figure 1.
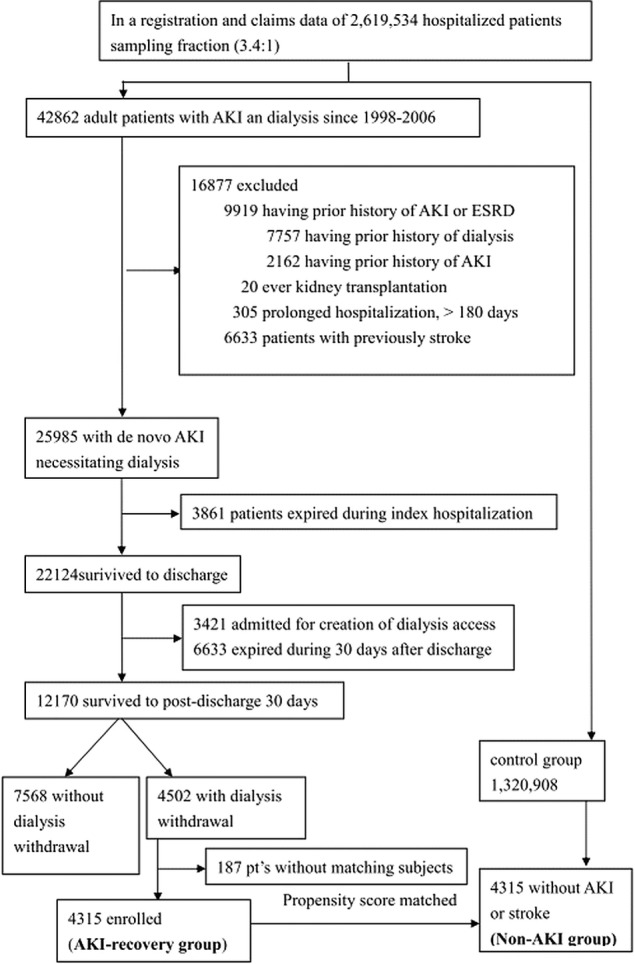
Flow diagram of the enrollees. AKI indicates acute kidney injury; ESRD, end stage renal disease.
Definition of De Novo Stroke
De novo stroke was defined as one of the following conditions in those who had no history of stroke at baseline:26 (1) records of emergency‐room service or hospitalization claim for >1 day or records of emergency‐room service with ICD‐9‐CM codes of 430 to 438 followed by claims for various brain‐imaging studies (computed tomography, magnetic resonance imaging, transcranial or carotid Doppler sonography) or claims for rehabilitation and anticoagulation prescriptions customarily used for ischemic stroke; (2) records of >3 consecutive outpatient visits with the codes and claims for examinations, services, or prescriptions as described for condition 1. In Taiwan, all medical facilities capable of admitting stroke patients are equipped with computed tomography or magnetic resonance imaging scanners, which considerably increase the validity of stroke diagnosis.27 Severe stroke was defined as those who were admitted to an intensive care unit within 30 days after the stroke event. A reproducibility study in Taiwan reported that the ICD‐9‐CM code for stroke from the hospital discharge data in the NHRI database was highly accurate.17–18,17–29 Each patient was followed from the date of discharge to the first documented date of de novo stroke, death, or the end of the study (December 31, 2007), whichever occurred first. The patients were enrolled from 1998 to 2006; therefore, there was a 1‐year latency period to ascertain that the patients were free of stroke after AKI.
The National Taiwan University Study Group on Acute Renal Failure (NSARF) 2002–2010
The NSARF,30–34 a multicenter prospective database, was used to validate baseline kidney function and the inclusion criteria for each risk group in this study to assess subsequent stroke events. CKD was defined according to an estimated glomerular filtration rate ≤45 mL/min per 1.73 m2,34 and AKI was defined according to the RIFLE (risk, injury, failure, loss, and end stage) criteria.35 We included NSARF 2002–2010 participants who were followed up to September 2012 with complete data on serum creatinine (measured following a standardized protocol).
Statistics
Continuous variables are described as mean±SD, and discrete variables are presented as counts or percentages. All data were analyzed using R software, version 2.8.1 (Free Software Foundation Inc, Boston, MA). A 2‐sided P<0.05 was considered to be statistically significant.
We calculated propensity score in an attempt to make an unbiased estimate of all the confounders predicting dialysis to balance the study and control groups during the index hospitalization, as a binary dependent variable, under a set of covariates. The predicted probability derived from the logistic equation was used as the propensity score for each individual.
To make comparisons comparable, each subject in the AKI‐recovery group was matched 1:1 with a subject in the control group. We calculated the Mahalanobis distances using of all the chosen important variables to find the nearest available subject within the specified propensity score caliper from the control group.
Due to the strong correlation among CKD, ESRD,36–37 anticoagulation agents, and incident stroke or mortality, we used a Cox proportional hazards model with time‐varying covariates to evaluate the impact of subsequent ESRD, CKD, and anticoagulation treatment after discharge on the risk of stroke events or mortality. This model assumed that changes in CKD or ESRD status or medications could appear at a subsequent time point after discharge. The medications used 14 days before incident stroke were identified. The Kaplan–Meier method was used to estimate survival curves, and the log‐rank test was used to test homogeneity between survival curves. For the outcome measurements, an individual was censored at death or at the end of the measured period.
Ethical Considerations
Informed consent was originally obtained by the NHRI, and because the patients were not identifiable in the present study, informed consent was not required. In addition, because the identification numbers of all individuals in the NHRI databases are encrypted to protect privacy, this study was exempt from full ethical review by the National Taiwan University Hospital Review Board.
Results
Demographic Characteristics of the Patients
Of 42 862 adult patients with AKI and dialysis from 1999 to 2008 who were screened, 3861 died during the index hospitalization, and 6633 died within 30 days after discharge (most were discharged with “do not resuscitate” orders). In total, 12 170 patients survived to 30 days after hospital discharge. Among them, 4502 patients withdrew from dialysis due to renal function recovery, and the rest (7568 patients) did not. During the matching procedure, 187 patients were excluded because no matching subjects were found. Consequently, 4315 patients were enrolled into the AKI‐recovery group, and 4315 patients were enrolled into the non‐AKI group (without AKI or dialysis or prior stroke) (men, 57.7%; mean age, 62.8±16.8 years in both groups; Figure 1, Tables 1 and 2).
Table 1.
Demographics and Comorbidities Added Into a Nonparsimonious Propensity Model to Predict Dialysis at Index Hospitalization*
| OR | Lower 95% CI | Upper 95% CI | P Value | |
|---|---|---|---|---|
| Male | 1.11 | 1.04 | 1.18 | <0.001 |
| Age (per year) | 1.02 | 1.02 | 1.02 | <0.001 |
| Premorbid risk | ||||
| Charlson score | 1.25 | 1.21 | 1.29 | <0.001 |
| Congestive heart failure | 1.87 | 1.67 | 2.08 | <0.001 |
| Dementia | 0.67 | 0.54 | 0.82 | <0.001 |
| COPD | 0.55 | 0.50 | 0.61 | <0.001 |
| Rheumatologic disease | 2.23 | 1.73 | 2.83 | <0.001 |
| Hemiplegia | 0.69 | 0.52 | 0.91 | 0.009 |
| Tumor | 0.82 | 0.69 | 0.98 | 0.030 |
| Diabetes mellitus | 2.28 | 2.10 | 2.48 | <0.001 |
| Moderate or severe liver disease | 2.64 | 2.10 | 3.28 | <0.001 |
| Chronic kidney disease | 5.37 | 4.72 | 6.11 | <0.001 |
| Index hospital comobidity | ||||
| Respiratory | 2.69 | 2.46 | 2.94 | <0.001 |
| Neurologic | 2.41 | 1.89 | 3.04 | <0.001 |
| Hematologic | 2.99 | 2.30 | 3.84 | <0.001 |
| Metabolic | 14.08 | 11.27 | 17.51 | <0.001 |
| Operative categories | ||||
| Cardiothoracic | 1.23 | 1.02 | 1.48 | 0.027 |
| Upper GI | 0.49 | 0.35 | 0.67 | <0.001 |
| Hepatobiliary | 0.56 | 0.43 | 0.72 | <0.001 |
| ICU admission during index hospitalization | 19.79 | 18.48 | 21.20 | <0.001 |
The propensity score in an attempt to make an unbiased estimated of all the confounders to balance study and control groups and further add to the Cox regression model. AKI indicates acute kidney injury; COPD, chronic obstructive pulmonary disease; ESRD, end‐stage renal disease; GI, gastrointestinal; ICU, intensive care unit.
The propensity model for predicting the need for dialysis during index hospitalization in both groups had a high discrimination power (estimated area under the receiver operating characteristic curve: 0.937), and it fit well with the observed binary data (adjusted generalized R2=0.35).
Table 2.
Characteristics of the Patients in the Study Groups
| AKI‐Recovery Group (n=4315) | Non‐AKI Group (n=4315) | P Value | |
|---|---|---|---|
| Male | 2488 (57.7%) | 2488 (57.7%) | 0.999 |
| Age (per year) | 62.8±16.8 | 62.8±16.8 | 0.946 |
| Premorbid risk | |||
| Charlson score | 1.9±1.9 | 1.9±2.03 | 0.242 |
| Myocardial infarction | 163 (3.8%) | 161 (3.7%) | 0.955 |
| Congestive heart failure | 534 (12.4%) | 477 (11.1%) | 0.061 |
| Atrial fibrillation | 129 (3%) | 128 (3%) | 0.999 |
| Peripheral vascular disease | 69 (1.6%) | 81 (1.9%) | 0.365 |
| Dementia | 82 (1.9%) | 80 (1.9%) | 0.937 |
| COPD | 474 (11%) | 448 (10.4%) | 0.384 |
| Lung cancer | 15 (0.3%) | 31 (0.7%) | 0.026 |
| Diabetes mellitus | 720 (16.7%) | 713 (16.5%) | 0.862 |
| Coronary artery disease | 655 (15.2%) | 650 (15.1%) | 0.904 |
| Rheumatologic disease | 65 (1.5%) | 70 (1.6%) | 0.729 |
| Peptic ulcer | 562 (13%) | 561 (13%) | 0.999 |
| Hemiplegia | 17 (0.4%) | 14 (0.3%) | 0.720 |
| Tumor | 323 (7.5%) | 344 (8%) | 0.420 |
| Moderate or severe liver disease | 322 (7.5%) | 310 (7.2%) | 0.649 |
| Chronic kidney disease | 521 (12.1%) | 508 (11.8%) | 0.690 |
| Hypertension | 1806 (41.9%) | 1806 (41.9%) | 0.999 |
| Dyslipidemia | 406 (9.4%) | 415 (9.6%) | 0.769 |
| Index hospital comorbidity | |||
| Cardiovascular | 320 (7.4%) | 320 (7.4%) | 0.999 |
| Respiratory | 768 (17.8%) | 731 (16.9%) | 0.306 |
| Hepatic | 95 (2.2%) | 101 (2.3%) | 0.718 |
| Neurologic | 79 (1.8%) | 93 (2.2%) | 0.317 |
| Hematologic | 67 (1.6%) | 82 (1.9%) | 0.247 |
| Metabolic | 116 (2.7%) | 77 (1.8%) | 0.006 |
| Septicemia | 162 (3.8%) | 116 (2.7%) | 0.006 |
| Severe sepsis | 99 (2.3%) | 94 (2.2%) | 0.771 |
| Operative categories | |||
| Cardiothoracic | 177 (4.1%) | 187 (4.3%) | 0.630 |
| Upper GI | 30 (0.7%) | 32 (0.7%) | 0.899 |
| Lower GI | 96 (2.2%) | 102 (2.4%) | 0.719 |
| Hepatobiliary | 60 (1.4%) | 51 (1.2%) | 0.445 |
| ICU admission during index hospitalization | 2777 (64.4%) | 2817 (65.3%) | 0.379 |
| After discharge concomitant medication* | |||
| Acetylsalicylic acid | 832 (19.3%) | 972 (22.5%) | <0.001 |
| Clopidogrel | 496 (11.5%) | 510 (11.8%) | 0.663 |
| Vitamin K antagonists | 352 (8.2%) | 178 (4.1%) | <0.001 |
| Outcomes | |||
| Mortality | 2353 (54.5%) | 1306 (30.3%) | <0.001 |
| ESRD | 861 (20%) | 73 (1.7%) | <0.001 |
| Stroke events | 219 (5.1%) | 172 (4%) | 0.017 |
| Stroke with ventilator | 28 (0.6%) | 7 (0.2%) | <0.001 |
| Severe stroke* | 41 (1.0%) | 18 (0.4%) | 0.004 |
Number as percentage and, mean ± SD. AKI indicates acute kidney injury; COPD, chronic obstructive pulmonary disease; ESRD, end stage renal disease; GI, gastrointestinal; ICU, intensive care unit.
The medications used during 14 days before the incident stroke.
Severe stroke was defined as being admitted to an ICU within 30 days after the stroke event.
The mean Charlson score before the index admission was 1.9±1.9. The premorbid risks were not statistically different between the 2 groups; however, the non‐AKI group had a higher rate of metabolic comorbidities during the index hospitalization (P=0.006: Table 2). Most of the patients with hemiplegia had traffic accident–related cervical trauma (83%), followed by tumor‐related compression (13%).
Outcome Measurements
Long‐term incident stroke and all‐cause mortality
Our findings showed a higher rate of incident stroke in the AKI‐recovery group than in the non‐AKI group (P<0.05). After a mean follow‐up period of 3.36 years, the unadjusted rate of incident stroke was higher in the AKI‐recovery group than in the non‐AKI group (15.6 versus 11.5 per 1000 person‐years, respectively).
More patients in the AKI‐recovery group who had a stroke had used ventilators (P=0.001) and been admitted to critical care units (P<0.004) than the non‐AKI patients. Compared with the non‐AKI group, the AKI‐recovery group had a higher risk (P=0.037) of incident stroke after adjustment for other variables. The impact was independent of age (P<0.001), myocardial infarction (P<0.001), hemiplegia (P<0.001), DM (P=0.001), chronic obstructive pulmonary disease (P=0.012), atrial fibrillation (P=0.034), septicemia (P=0.003), subsequent ESRD (P<0.001), or CKD (P=0.00) after the index discharge. The final model had good discrimination (C‐index: 0.73) (Table 3). The proxy for cigarette smoking, lung cancer, was not independently related to de novo stroke. Kaplan–Meier curves showed that the AKI‐recovery group had a significantly higher risk of stroke compared with the non‐AKI group (Figure 2).
Table 3.
Factors Related to Developing Long‐term Incident Stroke*
| HR | Lower 95% CI | Upper 95% CI | P Value | |
|---|---|---|---|---|
| Age (per year) | 1.03 | 1.01 | 1.05 | <0.001 |
| Premorbid risk | ||||
| Hemiplegia | 4.67 | 1.92 | 11.33 | <0.001 |
| Diabetes mellitus | 1.54 | 1.19 | 1.98 | 0.001 |
| Hypertension | 1.49 | 1.20 | 1.86 | 0.004 |
| Atrial fibrillation | 1.69 | 1.04 | 2.74 | 0.034 |
| COPD | 1.69 | 1.12 | 2.55 | 0.012 |
| Myocardial infarction | 3.75 | 1.98 | 7.11 | <0.001 |
| AKI‐recovery vs non‐AKI | 1.25 | 1.10 | 1.65 | 0.037 |
| Septicemia | 2.31 | 1.87 | 2.75 | 0.003 |
| Varying‐time factors | ||||
| Varying‐time ESRD | 1.95 | 1.46 | 2.64 | <0.001 |
| Varying‐time CKD | 1.60 | 1.16 | 2.21 | 0.004 |
AKI indicates acute kidney injury; CI, confidence interval, CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; ESRD, end‐stage renal disease; HR, hazard ratio.
The final model had good discrimination (C‐index=0.73).
Figure 2.
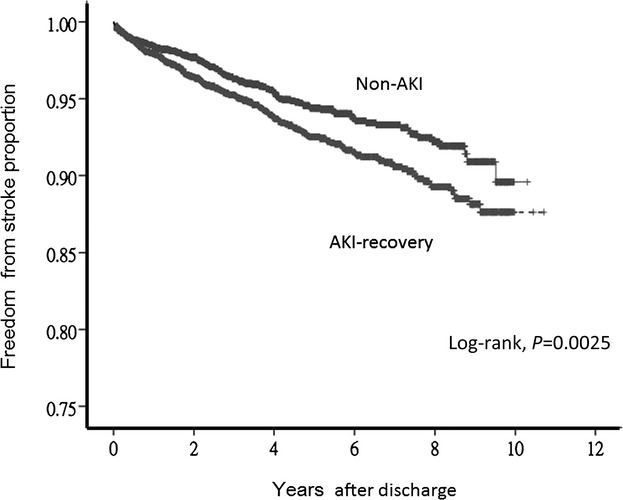
Kaplan–Meier curves of being free from stroke in the AKI‐recovery and non‐AKI groups (P=0.0025, log‐rank test). AKI indicates acute kidney injury.
There were 2353 deaths (54.5%) in the AKI‐recovery group and 1306 deaths (30.3%) in the non‐AKI group. The incidence of all‐cause mortality was 161.6 per 1000 person‐years in the AKI‐recovery group. The AKI‐recovery group had a higher risk of mortality than the non‐AKI group (hazard ratio: 2.38; 95% CI: 1.64 to 3.44; P<0.001).
Incident stroke stratified by diabetes and AKI status
There was no statistically significant difference in de novo stroke between the AKI‐alone group and the DM‐alone group (as reference) after adjusting for comorbidities and subsequent CKD, ESRD, and anticoagulation treatment after hospital discharge (P=0.086). The patients in the non‐DM/non‐AKI group had a lower risk of stroke than the DM‐alone group (hazard ratio: 0.56; 95% CI 0.38 to 0.84; P=0.005).
Sensitivity analysis
When considered as separate outcomes, the hospitalization rates for both ischemic and hemorrhagic strokes were also markedly higher in the AKI‐recovery group than in the non‐AKI group (ischemic: 4.7% versus 3.7%, P=0.017; hemorrhagic: 0.7% versus 0.3%, P=0.036). Further time‐varying Cox regression analysis by ischemic stroke also confirmed that the AKI‐recovery group had a higher risk of ischemic stroke than the non‐AKI group (hazard ratio: 1.23; 95% CI: 1.01 to 2.4; P=0.047).
Furthermore, the increased risk of long‐term stroke events following AKI recovery was consistent across the subgroups (Figure 3). This was especially true among the patients with or without traditional cardiovascular risk factors, prior DM, hypertension, or dyslipidemia.
Figure 3.
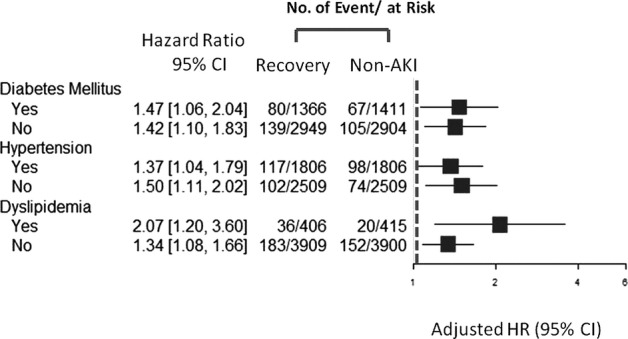
Hazard ratio of stroke stratified according to prior hypertension, diabetes, or dyslipidemia. AKI indicates acute kidney injury; HR, hazard ratio.
Patients with incident stroke and subsequent long‐term mortality
Further analysis using the time‐varying Cox regression model revealed that the AKI‐recovery group had a higher long‐term mortality rate after subsequent stroke events. The results indicated that long‐term mortality was also associated with temporary dialysis (hazard ratio: 2.38; 95% CI: 1.64 to 3.44; P<0.001) in patients after subsequent stroke events. This model showed good discrimination with a C‐index of 0.73.
Unmatched group
There were 187 participants (4.15%) with AKI and dialysis who could not be matched with a suitable control. Their incidence rates of stroke events and all‐cause mortality were 23.6 and 302 per 1000 person‐years, respectively, which were higher than those of the enrollees.
Validation
Among the NSARF 2002–2010 participants, 503 nonstroke patients in the AKI‐recovery group and 114 384 patients without acute dialysis and who survived to hospital discharge were examined as the non‐AKI group. The baseline CKD ratio was 1.4% among the non‐AKI group and 16.7% among the AKI‐recovery group.
Consistent with our previous findings, after adjusting for baseline comorbidities including CKD status, the AKI‐alone group had a similar long‐term risk of stroke events as those with DM alone (P=0.745) after a median follow‐up of 4.03 years (range: 1.57 to 6.21 years).
Discussion
In this population‐based cohort study, we found that dialysis‐requiring AKI, even if only temporary dialysis, was associated with an increased risk of developing stroke, and its impact was similar to DM. This suggests that all patients with AKI, regardless of the presence or extent of traditional cardiovascular risk factors, should be closely monitored for subsequent stroke.
AKI is now a global health problem,38 and the findings of the current study are noteworthy from the perspective of a clinician caring for a patient with AKI requiring dialysis. In recent decades, increased understanding of AKI epidemiology39 and improvements in posthospitalization resource utilization40–41 have allowed clinicians to examine the impact of AKI on brain disease and identify a large vulnerable population at increased risk of stroke and all‐cause mortality.42
AKI Related to De Novo Stroke and Mortality
Even after being adjusted for progression to subsequent CKD or ESRD, AKI still independently contributed to long‐term stroke events or all‐cause mortality. The patients who received temporary dialysis had higher severity of subsequent stroke in terms of ventilator use and critical care unit admission than the non‐AKI group. Furthermore, accumulating evidence suggests that the presence of underlying CKD modifies the outcomes of AKI but does not completely account for the impact of AKI.43 With detailed matching, we adjusted for traditional cardiovascular risks using uniform baseline comorbidities between the AKI‐recovery and non‐AKI groups. Consequently, the risk factors for kidney injury leading to an AKI event may persist44 and eventually lead to future stroke without a direct causal association with preexisting CKD. AKI may thus amplify the risk of long‐term incident stroke and mortality.
The effect of long‐term kidney–brain interactions was less clear. Our findings raise the possibility that AKI may trigger a cascade of perturbations that never completely resolve. The pathogenic mechanisms involved in this association may be related to nontraditional risk factors such as endothelial dysfunction, impaired endothelial progenitor cells, oxidative stress, inflammation, hyperhomocysteinemia, and thrombogenic factors during AKI.45–47 Each of these factors may play a role in accelerating atherosclerosis in the arteries of both the kidney and the brain, making AKI a nonmodifiable entity. The sudden decrease in systemic blood pressure during dialysis can cause a reduction in cerebral perfusion, leading to endothelial injury and subsequent stroke events. In addition, the dialysis hemofiltrate from patients with AKI has been shown to inhibit polymorphonuclear leukocyte chemotaxis, oxidative metabolism, and apoptosis in vitro, which could favorably influence the development of stroke.48 This indicates that AKI, in addition to traditional cardiovascular risk factors, is a “kidney specific” risk factor that has a causal relationship with future stroke. Increased serum concentrations of proinflammatory cytokines are putative mediators of remote organ effects during AKI and are believed to increase, in part, from subsequent impaired filtration and clearance of uremic toxins.49 An animal study demonstrated increased vascular permeability and an altered blood–brain barrier with inflammation including elevated levels of cytokines and granulocyte colony‐stimulating factor in mice undergoing renal ischemia.8 Furthermore, brain histology revealed increased microglial cells (brain macrophages) and pyknotic neuronal cells after AKI.8
Our results examining how much adjustment for interim CKD and ESRD attenuated the strength of the AKI–brain event association and how much adjustment for interim stroke attenuated the strength of the AKI–mortality association are important. Because AKI is gradually being recognized as a contributing factor to late‐stage CKD, it is possible that early kidney changes associated with endothelial phenotypic transition may have already taken place during acute events,50 leading to progressive deterioration. Recently, fibroblast growth factor‐23, a novel regulator of mineral metabolism, has been reported to be markedly elevated in AKI51 and to be an index of subclinical cardiovascular pathology in the community associated with adverse cardiovascular outcomes.52–53 Likewise, the expression of neutrophil gelatinase‐associated lipocalin, a marker of renal tubular injury, has also been associated with increased cardiovascular and all‐cause mortality independent of traditional cardiovascular risk factors.54
Disruption of the blood–brain barrier not only results in cerebral edema but also allows metabolites and toxins that are normally impermeable to the blood–brain barrier to cause changes to the central nervous system. Supporting this, numerous other reports have been published of encephalopathic events associated with AKI that are relevant to the known pathomechanisms of cerebral ischemia.55–56
DM and AKI Related to Stroke
The associated higher risk of stroke can only partly be explained by traditional risk factors such as DM, hypertension or dyslipidemia, and the kidney‐specific risks underlying AKI involved in the pathogenesis (Figure 3). This novel finding adds to the growing body of evidence suggesting that patients with AKI are at increased risk of stroke.
The kidney and brain share unique susceptibilities to vascular injury; therefore, some vascular risk factors may cause similar vascular injuries in both organs.57–58 No data on stroke have been reported with regard to the occurrence of AKI imposed on the course of DM, a factor known to have an impact on subsequent stroke.59 After a long‐term follow‐up period, we found that temporary dialysis had a similar effect to DM in causing de novo stroke independent of preexisting comorbidities.
The current study showed that the risk of developing stroke in the patients who had recovered from AKI was not statistically significantly associated with DM. In addition, the patients with AKI had a substantially higher mortality rate after stroke than the non‐AKI group, emphasizing the potential value of preventing stroke events in AKI patients. Recommendations regarding DM in the risk categorization for stroke could also be applied to patients who recover from AKI after dialysis. The US National Cholesterol Education Program Adult Treatment Panel III reported that 30% of cardiovascular disease events are accompanied by stroke and included DM as a risk equivalent to cardiovascular disease.60–61 Our results support the risk of stroke in patients with AKI, and the impact was similar to DM. Importantly, the patients with AKI had a higher mortality rate after a stroke event than those without AKI, further emphasizing the potential value of preventing stroke by targeting these conditions. In light of these findings, a comprehensive treatment strategy during admission or even after discharge for AKI patients is required to reduce the risk of ensuing stroke events and subsequent mortality. Taken together, AKI patients who undergo temporary dialysis are at a higher risk of subsequent stroke events compared with patients without AKI, supporting AKI as a cause of mortality.62
Study Limitations
This study has several limitations. First, validation of stroke hospitalization was not possible from the data sources available for this study; however, the procedural and prescription codes used to identify stroke in our study have been shown to have high sensitivity and specificity for identifying true stroke hospitalizations in the general population.18 Second, the baseline glomerular filtration rate was not available from the ICD‐9‐CM codes; therefore, misclassification was possible. Third, approximately 4.15% of the patients who recovered from AKI were excluded because no matched controls could be identified. Because the excluded unmatched individuals had higher rates of stroke and mortality than the enrollees, their exclusion attenuated the reported effect sizes. Finally, cigarette smoking is likely to influence stroke; however, direct information on smoking was not available in the source data. Although we did not have any variables directly reflecting tobacco use, we believe that we controlled for the effect of tobacco use by including DM, myocardial infarction, lung cancer, and chronic obstructive pulmonary disease as proxy indicators for tobacco use (past and current use). AKI was significantly independent from these proxies for de novo stroke.
Conclusion
The current study found that AKI requiring dialysis, even if only temporary dialysis, was associated with an increased risk of de novo stroke, and the impact was similar to DM. Our results suggest that a public health initiative is needed to monitor and control subsequent stroke among patients with dialysis‐requiring AKI, even among those whose kidney function has recovered after discharge, because dialysis‐requiring AKI may hasten subsequent long‐term stroke events and mortality.
Sources of Funding
This study was supported by grants: National Science Council (NSC) 102‐2314‐B‐002‐140‐MY2, NSC 101‐2314‐B‐002‐132‐MY3, NSC 101‐2314‐B‐002‐085‐MY3, NSC 100‐2314‐B‐002‐119, NSC 100‐2314‐B‐002‐147‐MY3, National Taiwan University Hospital (NTUH) 103‐082, NTUH‐103‐S‐2467, NTUH‐102‐CGN03, NTUH‐102‐S2097, NTUH‐101‐M1953, NTUH‐100‐N1776, NHRI‐PH‐101‐SP‐09 and NHRI‐PH‐102‐SP‐09.
Disclosures
None.
Acknowledgments
The authors would like to thank the staff of the Second, Seventh, and Eighth Core Laboratory of the Department of Medical Research at National Taiwan University Hospital for technical assistance. This study is based partly on data provided by the Bureau of National Health Insurance, Department of Health, Taiwan. The interpretation and conclusions shown in this paper do not represent those of the Bureau of National Health Insurance, Department of Health, National Health Research Institutes, or National Taiwan University Hospital.
The National Taiwan University Hospital Study Group for Acute Renal Failure (NSARF) includes Wen‐Je Ko, MD, PhD, (NTUH, Director of Coordinating Center);Vin‐Cent Wu, MD (NTUH, Core PI of Committee), Chih‐Chung Shiao, MD, (Saint Mary's Hospital, PI of Committee) Chun‐Fu Lai, MD, (NTUH, PI of Committee), Tao‐Min Huang, MD (NTUH, PI of Committee), Yung‐Ming Chen, MD (NTUH, PI of Committee),Wei‐Jie Wang, MD (NTUH, PI of Committee), Cheng‐Yi Wang, MD (Cardinal Tien Hospital, Site Investigator), Pei‐Chen Wu, MD, (Mackay Memorial Hospital, Site Investigator), Pi‐Ru Tsai, RN (NTUH, Site Investigator), Yu‐Chang Yeh, MD, (NTUH, Site Investigator), Fu‐Chang Hu, MS, ScD, (Harvard statistics, Site Investigator), and Kwan‐Dun Wu, MD, PhD (NTUH, Advisory Committee).
References
- 1.Hsu CY, McCulloch CE, Fan D, Ordonez JD, Chertow GM, Go AS. Community‐based incidence of acute renal failure. Kidney Int. 2007; 72:208-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lafrance JP, Miller DR. Acute kidney injury associates with increased long‐term mortality. J Am Soc Nephrol. 2010; 21:345-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ. Acute kidney injury increases risk of esrd among elderly. J Am Soc Nephrol. 2009; 20:223-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagshaw SM. Epidemiology of renal recovery after acute renal failure. Curr Opin Crit Care. 2006; 12:544-550. [DOI] [PubMed] [Google Scholar]
- 5.U.S. Renal Data System. USRDS 2012 annual data report: atlas of chronic kidney disease and end‐stage renal disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases National Institutes of Health. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2012. [Google Scholar]
- 6.Shlipak MG, Katz R, Kestenbaum B, Siscovick D, Fried L, Newman A, Rifkin D, Sarnak MJ. Rapid decline of kidney function increases cardiovascular risk in the elderly. J Am Soc Nephrol. 2009; 20:2625-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu VC, Wu CH, Huang TM, Wang CY, Lai CF, Shiao CC, Chang CH, Lin SL, Chen YY, Chen YM, Chu TS, Chiang WC, Wu KD, Tsai PR, Chen L, Ko WJ. Long‐term risk of coronary events after aki. J Am Soc Nephrol. 2014; 25:595-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu M, Liang Y, Chigurupati S, Lathia JD, Pletnikov M, Sun Z, Crow M, Ross CA, Mattson MP, Rabb H. Acute kidney injury leads to inflammation and functional changes in the brain. J Am Soc Nephrol. 2008; 19:1360-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryden L, Ahnve S, Bell M, Hammar N, Ivert T, Holzmann MJ. Acute kidney injury following coronary artery bypass grafting: early mortality and postoperative complications. Scand Cardiovasc J. 2012; 46:114-120. [DOI] [PubMed] [Google Scholar]
- 10.Mangano CM, Diamondstone LS, Ramsay JG, Aggarwal A, Herskowitz A, Mangano DT. Renal dysfunction after myocardial revascularization: risk factors, adverse outcomes, and hospital resource utilization. The multicenter study of perioperative ischemia research group. Ann Intern Med. 1998; 128:194-203. [DOI] [PubMed] [Google Scholar]
- 11.Bulugahapitiya U, Siyambalapitiya S, Sithole J, Idris I. Is diabetes a coronary risk equivalent? Systematic review and meta‐analysis. Diabet Med. 2009; 26:142-148. [DOI] [PubMed] [Google Scholar]
- 12.Chen PC, Muo CH, Lee YT, Yu YH, Sung FC. Lung cancer and incidence of stroke: a population‐based cohort study. Stroke. 2011; 42:3034-3039. [DOI] [PubMed] [Google Scholar]
- 13.Ou SM, Chen YT, Chao PW, Lee YJ, Liu CJ, Yeh CM, Chen TJ, Chen TW, Yang WC, Li SY. Nonsteroidal anti‐inflammatory drug use is associated with cancer risk reduction in chronic dialysis patients. Kidney Int. 2013; 84:198-205. [DOI] [PubMed] [Google Scholar]
- 14.Chen YC, Lin HY, Li CY, Lee MS, Su YC. A nationwide cohort study suggests that hepatitis C virus infection is associated with increased risk of chronic kidney disease. Kidney Int. 2014; 85:1200-1207. [DOI] [PubMed] [Google Scholar]
- 15.Wu VC, Hu YH, Wu CH, Kao CC, Wang SM, Chen L, Wu KD. Administrative data on diagnosis and mineralocorticoid receptor antagonist prescription identified patients with primary aldosteronism in Taiwan. J Clin Epidemiol. 2014 [DOI] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987; 40:373-383. [DOI] [PubMed] [Google Scholar]
- 17.Chu YT, Wu SC, Lee YC, Lai MS, Tam SC. Assessing measures of comorbidity using national health insurance databases. Taiwan J Public Health. 2010; 29:191-200. [Google Scholar]
- 18.Cheng CL, Kao YH, Lin SJ, Lee CH, Lai ML. Validation of the national health insurance research database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf. 2011; 20:236-242. [DOI] [PubMed] [Google Scholar]
- 19.Lee JT, Chung WT, Lin JD, Peng GS, Muo CH, Lin CC, Wen CP, Wang IK, Tseng CH, Kao CH, Hsu CY. Increased risk of stroke after septicaemia: a population‐based longitudinal study in Taiwan. PLoS ONE. 2014; 9:e89386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Angus DC, Linde‐Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the united states: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001; 29:1303-1310. [DOI] [PubMed] [Google Scholar]
- 21.Lai TS, Wang CY, Pan SC, Huang TM, Lin MC, Lai CF, Wu CH, Wu VC, Chien KLon behalf of the National Taiwan University Hospital Study Group on Acute Renal Failure. Risk of developing severe sepsis after acute kidney injury: a population‐based cohort study. Crit Care. 2013; 17:R231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM consensus conference committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992; 101:1644-1655. [DOI] [PubMed] [Google Scholar]
- 23.Surgeon general's report: The health consequences of smoking—50 years of progress. Available at: http://www.cdc.gov/tobacco/data_statistics/sgr/50th-anniversary/index.htm. Accessed June 6, 2014. [DOI] [PMC free article] [PubMed]
- 24.Best N, Hansell AL. Geographic variations in risk: adjusting for unmeasured confounders through joint modeling of multiple diseases. Epidemiology. 2009; 20:400-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chao CT, Hou CC, Wu VC, Lu HM, Wang CY, Chen L, Kao TW. The impact of dialysis‐requiring acute kidney injury on long‐term prognosis of patients requiring prolonged mechanical ventilation: nationwide population‐based study. PLoS One. 2012; 7:e50675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen HJ, Bai CH, Yeh WT, Chiu HC, Pan WH. Influence of metabolic syndrome and general obesity on the risk of ischemic stroke. Stroke. 2006; 37:1060-1064. [DOI] [PubMed] [Google Scholar]
- 27.Chen YH, Kang JH, Lin HC. Patients with traumatic brain injury: population‐based study suggests increased risk of stroke. Stroke. 2011; 42:2733-2739. [DOI] [PubMed] [Google Scholar]
- 28.Chang L, Chen YM, Chuang JH. A study of validation on comorbidity derived from claims data National Yangx2010Ming University, Master Thesis 2004;6. Available at: http://ndltd.ncl.edu.tw/cgi-bin/gs32/gsweb.cgi?o=dnclcdr&s=id=%22092YM005058012%005058022.&searchmode=basic Accessed June 6, 2014.
- 29.Leu HB, Chung CM, Chuang SY, Bai CH, Chen JR, Chen JW, Pan WH. Genetic variants of connexin37 are associated with carotid intima‐medial thickness and future onset of ischemic stroke. Atherosclerosis. 2011; 214:101-106. [DOI] [PubMed] [Google Scholar]
- 30.Huang TM, Wu VC, Young GH, Lin YF, Shiao CC, Wu PC, Li WY, Yu HY, Hu FC, Lin JW, Chen YS, Lin YH, Wang SS, Hsu RB, Chang FC, Chou NK, Chu TS, Yeh YC, Tsai PR, Huang JW, Lin SL, Chen YM, Ko WJ, Wu KD. Preoperative proteinuria predicts adverse renal outcomes after coronary artery bypass grafting. J Am Soc Nephrol. 2011; 22:156-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu VC, Wang CH, Wang WJ, Lin YF, Hu FC, Chen YW, Chen YS, Wu MS, Lin YH, Kuo CC, Huang TM, Chen YM, Tsai PR, Ko WJ, Wu KD. Sustained low‐efficiency dialysis versus continuous veno‐venous hemofiltration for postsurgical acute renal failure. Am J Surg. 2009; 199:466-476. [DOI] [PubMed] [Google Scholar]
- 32.Wu VC, Ko WJ, Chang HW, Chen YW, Lin YF, Shiao CC, Chen YM, Chen YS, Tsai PR, Hu FC, Wang JY, Lin YH, Wu KD. Risk factors of early redialysis after weaning from postoperative acute renal replacement therapy. Intensive Care Med. 2008; 34:101-108. [DOI] [PubMed] [Google Scholar]
- 33.Shiao CC, Wu VC, Li WY, Lin YF, Hu FC, Young GH, Kuo CC, Kao TW, Huang DM, Chen YM, Tsai PR, Lin SL, Chou NK, Lin TH, Yeh YC, Wang CH, Chou A, Ko WJ, Wu KD. Late initiation of renal replacement therapy is associated with worse outcomes in acute kidney injury after major abdominal surgery. Crit Care. 2009; 13:R171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu VC, Huang TM, Lai CF, Shiao CC, Lin YF, Chu TS, Wu PC, Chao CT, Wang JY, Kao TW, Young GH, Tsai PR, Tsai HB, Wang CL, Wu MS, Chiang WC, Tsai IJ, Hu FC, Lin SL, Chen YM, Tsai TJ, Ko WJ, Wu KD. Acute‐on‐chronic kidney injury at hospital discharge is associated with long‐term dialysis and mortality. Kidney Int. 2011; 80:1222-1230. [DOI] [PubMed] [Google Scholar]
- 35.Bihorac A, Yavas S, Subbiah S, Hobson CE, Schold JD, Gabrielli A, Layon AJ, Segal MS. Long‐term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg. 2009; 249:851-858. [DOI] [PubMed] [Google Scholar]
- 36.Chou CC, Lien LM, Chen WH, Wu MS, Lin SM, Chiu HC, Chiou HY, Bai CH. Adults with late stage 3 chronic kidney disease are at high risk for prevalent silent brain infarction: a population‐based study. Stroke. 2011; 42:2120-2125. [DOI] [PubMed] [Google Scholar]
- 37.Seliger SL, Gillen DL, Tirschwell D, Wasse H, Kestenbaum BR, Stehman‐Breen CO. Risk factors for incident stroke among patients with end‐stage renal disease. J Am Soc Nephrol. 2003; 14:2623-2631. [DOI] [PubMed] [Google Scholar]
- 38.Li PK, Burdmann EA, Mehta RL. World kidney day 2013: acute kidney injury‐global health alert. Acta Nephrologica. 2013; 27:5-10. [DOI] [PubMed] [Google Scholar]
- 39.Hsu RK, McCulloch CE, Dudley RA, Lo LJ, Hsu CY. Temporal changes in incidence of dialysis‐requiring aki. J Am Soc Nephrol. 2012; 24:37-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liangos O, Wald R, O'Bell JW, Price L, Pereira BJ, Jaber BL. Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol. 2006; 1:43-51. [DOI] [PubMed] [Google Scholar]
- 41.Waikar SS, Curhan GC, Wald R, McCarthy EP, Chertow GM. Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol. 2006; 17:1143-1150. [DOI] [PubMed] [Google Scholar]
- 42.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta‐analysis. Kidney Int. 2012; 81:442-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pannu N. Bidirectional relationships between acute kidney injury and chronic kidney disease. Curr Opin Nephrol Hypertens. 2013; 22:351-356. [DOI] [PubMed] [Google Scholar]
- 44.Rifkin DE, Coca SG, Kalantar‐Zadeh K. Does AKI truly lead to CKD? J Am Soc Nephrol. 2012; 23:979-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonventre JV. Pathophysiology of AKI: injury and normal and abnormal repair. Contrib Nephrol. 2010; 165:9-17. [DOI] [PubMed] [Google Scholar]
- 46.Wu VC, Young GH, Huang PH, Lo SC, Wang KC, Sun CY, Liang CJ, Huang TM, Chen JH, Chang FC, Chen YL, Kuo YS, Chen JB, Chen JW, Chen YM, Ko WJ, Wu KD. The Ng. In acute kidney injury, indoxyl sulfate impairs human endothelial progenitor cells: modulation by statin. Angiogenesis. 2013; 16:609-624. [DOI] [PubMed] [Google Scholar]
- 47.Sun J, Shannon M, Ando Y, Schnackenberg LK, Khan NA, Portilla D, Beger RD. Serum metabolomic profiles from patients with acute kidney injury: a pilot study. J Chromatogr B Analyt Technol Biomed Life Sci. 2012; 893–894:107-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen G, Horl WH. Retinol binding protein isolated from acute renal failure patients inhibits polymorphonuclear leucocyte functions. Eur J Clin Invest. 2004; 34:774-781. [DOI] [PubMed] [Google Scholar]
- 49.Herget‐Rosenthal S, Glorieux G, Jankowski J, Jankowski V. Uremic toxins in acute kidney injury. Semin Dial. 2009; 22:445-448. [DOI] [PubMed] [Google Scholar]
- 50.Basile DP, Friedrich JL, Spahic J, Knipe N, Mang H, Leonard EC, Changizi‐Ashtiyani S, Bacallao RL, Molitoris BA, Sutton TA. Impaired endothelial proliferation and mesenchymal transition contribute to vascular rarefaction following acute kidney injury. Am J Physiol Renal Physiol. 2011; 300:F721-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leaf DE, Wolf M, Stern L. Elevated FGF‐23 in a patient with rhabdomyolysis‐induced acute kidney injury. Nephrol Dial Transplant. 2010; 25:1335-1337. [DOI] [PubMed] [Google Scholar]
- 52.Kendrick J, Cheung AK, Kaufman JS, Greene T, Roberts WL, Smits G, Chonchol M. FGF‐23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol. 2011; 22:1913-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arnlov J, Carlsson AC, Sundstrom J, Ingelsson E, Larsson A, Lind L, Larsson TE. Serum FGF23 and risk of cardiovascular events in relation to mineral metabolism and cardiovascular pathology. Clin J Am Soc Nephrol. 2013; 8:781-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Helmersson‐Karlqvist J, Larsson A, Carlsson AC, Venge P, Sundstrom J, Ingelsson E, Lind L, Arnlov J. Urinary neutrophil gelatinase‐associated lipocalin (NGAL) is associated with mortality in a community‐based cohort of older Swedish men. Atherosclerosis. 2013; 227:408-413. [DOI] [PubMed] [Google Scholar]
- 55.Adachi N, Lei B, Deshpande G, Seyfried FJ, Shimizu I, Nagaro T, Arai T. Uraemia suppresses central dopaminergic metabolism and impairs motor activity in rats. Intensive Care Med. 2001; 27:1655-1660. [DOI] [PubMed] [Google Scholar]
- 56.Arieff AI, Massry SG. Calcium metabolism of brain in acute renal failure. Effects of uremia, hemodialysis, and parathyroid hormone. J Clin Investig. 1974; 53:387-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nickolas TL, Khatri M, Boden‐Albala B, Kiryluk K, Luo X, Gervasi‐Franklin P, Paik M, Sacco RL. The association between kidney disease and cardiovascular risk in a multiethnic cohort: findings from the northern manhattan study (NOMAS). Stroke. 2008; 39:2876-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ. Chronic kidney disease as a risk factor for cardiovascular disease and all‐cause mortality: a pooled analysis of community‐based studies. J Am Soc Nephrol. 2004; 15:1307-1315. [DOI] [PubMed] [Google Scholar]
- 59.Yatsuya H, Iso H, Yamagishi K, Kokubo Y, Saito I, Suzuki K, Sawada N, Inoue M, Tsugane S. Development of a point‐based prediction model for the incidence of total stroke: Japan public health center study. Stroke. 2013; 44:1295-1302. [DOI] [PubMed] [Google Scholar]
- 60.Collins AJ, Foley RN, Herzog C, Chavers B, Gilbertson D, Ishani A, Johansen K, Kasiske B, Kutner N, Liu J, St Peter W, Ding S, Guo H, Kats A, Lamb K, Li S, Roberts T, Skeans M, Snyder J, Solid C, Thompson B, Weinhandl E, Xiong H, Yusuf A, Zaun D, Arko C, Chen SC, Daniels F, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers P, Agodoa L. US Renal Data System 2012 Annual Data Report. Am J Kidney Dis. 2013; 61:e1‐476. [DOI] [PubMed] [Google Scholar]
- 61.Grundy SM. Diabetes and coronary risk equivalency: what does it mean? Diabetes Care. 2006; 29:457-460. [DOI] [PubMed] [Google Scholar]
- 62.Liano F, Felipe C, Tenorio MT, Rivera M, Abraira V, Saez‐de‐Urturi JM, Ocana J, Fuentes C, Severiano S. Long‐term outcome of acute tubular necrosis: a contribution to its natural history. Kidney Int. 2007; 71:679-686. [DOI] [PubMed] [Google Scholar]


