Abstract
Background
Acrolein is a reactive aldehyde present in high amounts in coal, wood, paper, and tobacco smoke. It is also generated endogenously by lipid peroxidation and the oxidation of amino acids by myeloperoxidase. In animals, acrolein exposure is associated with the suppression of circulating progenitor cells and increases in thrombosis and atherogenesis. The purpose of this study was to determine whether acrolein exposure in humans is also associated with increased cardiovascular disease (CVD) risk.
Methods and Results
Acrolein exposure was assessed in 211 participants of the Louisville Healthy Heart Study with moderate to high (CVD) risk by measuring the urinary levels of the major acrolein metabolite—3‐hydroxypropylmercapturic acid (3‐HPMA). Generalized linear models were used to assess the association between acrolein exposure and parameters of CVD risk, and adjusted for potential demographic confounders. Urinary 3‐HPMA levels were higher in smokers than nonsmokers and were positively correlated with urinary cotinine levels. Urinary 3‐HPMA levels were inversely related to levels of both early (AC133+) and late (AC133−) circulating angiogenic cells. In smokers as well as nonsmokers, 3‐HPMA levels were positively associated with both increased levels of platelet–leukocyte aggregates and the Framingham Risk Score. No association was observed between 3‐HPMA and plasma fibrinogen. Levels of C‐reactive protein were associated with 3‐HPMA levels in nonsmokers only.
Conclusions
Regardless of its source, acrolein exposure is associated with platelet activation and suppression of circulating angiogenic cell levels, as well as increased CVD risk.
Keywords: endothelium, epidemiology, inflammation, risk factors, smoking, thrombosis, tobacco
Introduction
Although hypertension, diabetes, and dyslipidemia are robust risk factors for cardiovascular disease (CVD), exposure to environmental chemicals, pharmaceuticals or endogenous toxins is also associated with increased CVD risk.1 Several chemicals and toxicants affect cardiovascular function and disease progression, as reflected by strong associations between CVD risk and exposure to ambient particulate matter (PM), traffic pollution, and tobacco smoke.2 While specific chemicals that impart CVD risk have not been identified, pollutant exposure remains a significant cause of cardiovascular morbidity and mortality and it has been estimated that acute PM2.5 (PM with an aerodynamic diameter of less than 2.5 microns) exposure results in premature mortality in tens of thousands of individuals in the United States alone.2 Likewise, worldwide cardiovascular deaths due to smoking (1.69 million) far exceed smoking‐related deaths due to lung cancer (0.97 million) or pulmonary disease (0.85 million).3 Tobacco smoke is a complex mixture of several thousand reactive chemicals, many of which can induce cardiovascular injury; however, recent risk estimates indicate that acrolein in cigarette smoke accounts for 80% to 90% of the noncancer risk of smoking.4
Acrolein is a reactive α,β‐unsaturated aldehyde, and in addition to cigarette smoke, cotton, wood, and coal smoke as well as gasoline and diesel exhaust contain high acrolein levels.5 Because of its ubiquitous presence, acrolein is classified by the United States Environmental Protection Agency as a high‐priority air and water toxin.6 Acrolein is also present in beverages and foods including coffee, alcohol, cheese, and donuts; and it has been shown that heating and cooking of fats, oils, and sugars increases their acrolein content.5 In addition, acrolein is generated endogenously by the degradation of polyamines or by myeloperoxidase7 present in neutrophils. As a result, high levels of protein–acrolein adducts accumulate at sites of inflammation.5 Moreover, along with other structurally related aldehydes, acrolein is a reactive end product of lipid peroxidation that readily forms covalent adducts with proteins.8 Acrolein‐modified proteins have been detected in oxidized low‐density lipoprotein6 and human atherosclerotic lesions, and the uptake of acrolein‐modified low‐density lipoprotein by the SR‐A1 receptors is implicated in foam cell formation.9 In atherosclerotic lesions, acrolein‐adducted proteins co‐localize with apoA‐1, and adduction of apoA‐1 by acrolein has been shown to impair reverse cholesterol transport.10
Animal studies have shown that exposure to acrolein results in extensive cardiovascular injury. In rats, exposure to acrolein compromises mesenteric bed and aortic reactivity11 and in mice, acrolein exposure leads to left ventricle dilation and dysfunction and dilated cardiomyopathy.12 Acute acrolein consumption has also been shown to exacerbate myocardial ischemic injury13 and to induce lipoprotein modification and systemic dyslipidemia, leading to an increase in plasma levels of low‐density lipoprotein and triglycerides.14 Chronic exposures to acrolein have also been shown to increase platelet activation and reduce bleeding time,15 as well as destabilize atherosclerotic lesions16 and accelerate atherogenesis in apoE‐null mice.17 In addition, we have recently reported that acrolein suppresses circulating levels of Flk+/Sca‐1+ cells, indicating that acrolein exposure could impair vascular repair capacity.18 Nevertheless, the cardiovascular toxicity of acrolein in humans has not been studied.
The present study was designed to examine whether acrolein exposure is associated with increased CVD risk in humans. In a cohort of smoking and nonsmoking individuals, we found significant associations between acrolein exposure and indices of cardiovascular injury, suggesting that acrolein from both environmental and endogenous sources could contribute to CVD risk.
Methods
The study was approved by the Institutional Review Board at the University of Louisville, and all subjects gave written informed consent. Individuals (≥18 years of age) were enrolled in the Louisville Healthy Heart Study from October 2009 to March 2011 at the University of Louisville Hospital and affiliated Clinic System. Patients who met the enrollment criteria gave informed consent and were administered a brief questionnaire to provide demographic information and baseline characteristics. Medical records were reviewed for medication history. To reduce selection bias, all consecutive subjects who were eligible for this study were recruited.
Inclusion Criteria: Eligible individuals were (1) 18 years of age or older at the time of enrollment; and (2) patients of the University of Louisville Hospital and/or Clinic System.
Exclusion Criteria: Ineligible individuals were (1) persons unwilling or unable to provide informed consent; (2) subjects with significant and/or severe comorbidities including the following conditions: significant chronic lung, liver, kidney, hematological, or neoplastic disease; chronic neurological or psychiatric illness; chronic infectious disease such as HIV or hepatitis; severe coagulopathies; drug/substance abuse; and chronic cachexia; (3) pregnant women; and (4) prisoners and other vulnerable populations.
Circulating angiogenic cells19 and hematopoietic cell populations in blood were characterized using a 7‐color flow cytometry protocol with established markers: CD34, CD45, CD31, and AC133 as previously described.20 The complete listing of circulating angiogenic cell populations identified is shown in Table 1. Markers of thrombosis included in the study were platelet–leukocyte aggregates and fibrinogen. Platelet–leukocyte aggregates were identified by flow cytometry and quantified as events when found to be double positive for CD41 (platelets) and CD45 (leukocytes), and expressed as a percentage of total events.20 Plasma fibrinogen was measured using the STA Fibrinogen Kit (Stago BNL), and high‐sensitivity C‐reactive protein was measured using the VITROS kit (VITROS Chemistry and Immunodiagnostic Products). Framingham Risk Scores (FRS) were derived using the National Heart, Lung, and Blood Institute calculator.21 Urinary levels of the major acrolein metabolite 3‐hydroxypropylmercapturic acid (3‐HPMA) were measured by gas chromatography–mass spectrometry using 13C‐HMPA as an internal standard.22 Urinary cotinine levels were measured by gas chromatography– mass spectrometry using d3‐cotinine as an internal standard.23 3‐HPMA levels were normalized to urinary creatinine.22
Table 1.
Antigenic Identity of Circulating Angiogenic Cells
| Circulating Angiogenic Cell Population | Cell Differentiation State |
|---|---|
| Cell type‐1 | CD31+/34+/45dim |
| Cell type‐2 | CD31+/34+/45+ |
| Cell type‐3 | CD31+/34+/45dim/AC133+ |
| Cell type‐4 | CD31+/34+/45+/AC133+ |
| Cell type‐5 | CD31+/AC133+ |
| Cell type‐6 | CD31+/34+ |
| Cell type‐7 | CD31+/34+/45dim/AC133− |
| Cell type‐8 | CD31+/34+/45+/AC133− |
| Cell type‐9 | CD34+ |
| Cell type‐10 | CD31+ |
| Cell type‐11 | AC133+ |
| Cell type‐12 | CD45+ |
| Cell type‐13 | CD34+/AC133+ |
| Cell type‐14 | CD34+/45+/AC133+ |
| Cell type‐15 | CD34+/45dim/AC133+ |
Statistical Analysis
Statistical analyses were performed using IBM SPSS Statistics version 21.0 for Windows (Armonk, NY). Population demographics, CVD risk factors, high‐sensitivity C‐reactive protein (hsCRP), fibrinogen, and platelet–leukocyte aggregates were compared across tertiles representing low, medium, and high 3‐HPMA levels using 1‐way ANOVA and χ2 tests. These associations were also assessed using 3‐HPMA as a continuous variable to determine whether adjustments were required for other potential demographic confounders in the final model. Demographic variables that were not normally distributed were transformed to their natural logarithms. Demographics were also assessed using 2 or 4 different levels of 3‐HPMA, but tertiles were found to most clearly reflect the associations identified in continuous data analysis.
To examine the relationship between 3‐HPMA and smoking, independent sample t test was used to test for bivariate associations between self‐reported smoking status and mean cotinine levels, as well as cotinine strata and mean 3‐HPMA levels. Cotinine was divided into 2 strata representing low (cotinine <200 μg/g creatinine) and high (cotinine ≥200 μg/g creatinine) to define empirically based smoking levels. These values are commonly used in the insurance industry to distinguish nonsmokers from smokers. Subsequently, 3‐HPMA levels were regressed against cotinine levels to assess the relationship between smoking and 3‐HPMA.
Generalized linear modeling (GLM) techniques were used to examine whether the circulating angiogenic cell levels, hsCRP, platelet–leukocyte aggregates, fibrinogen, and the FRS variables were associated with 3‐HPMA levels, after adjusting for ethnicity, self‐reported smoking status, body mass index (BMI), hypertension, diabetes, diuretics, angiotensin‐receptor blockers, and revascularization including coronary artery bypass graft (CABG)/percutaneous coronary intervention (PCI)/stents. The FRS variables were only adjusted for ethnicity, alcohol, and BMI (the remaining variables are reflected in the FRS). These variables were found to be potential confounders from the demographic analysis with 3‐HPMA tertiles or 3‐HPMA as a continuous variable. Circulating angiogenic cell levels were log transformed for normality and normalized to sample volume. Because the levels of these cells appeared to follow a gamma distribution, generalized linear modelings that assessed angiogenic cells as the dependent variable utilized the gamma probability distribution and the log link function. The binomial distribution was used for the dichotomized version of the FRS (described below) to describe CVD risk categories. All other generalized linear modelings utilized the normal probability distribution and the identity link function. Traditional model fit statistics (log‐likelihood) were used to develop the most parsimonious model. In addition we tested whether higher order modeling (eg, exponential, cubic) improved model fit using traditional model‐fit statistics ie, AIC (Akaike Information Criterion) and log likelihood. The final model included covariates that were significant at the 95% level and not highly correlated among themselves as determined by demographic comparison among 3‐HPMA tertiles or the regression of the continuous 3‐HPMA variable against demographic covariates.
To examine the relationship between 3‐HPMA and CVD risk, 3‐HPMA levels were regressed against individual FRS. The FRS was calculated only for individuals without clinically documented heart disease; however, to include those with existing heart disease, we divided the entire population into 2 CVD risk categories. The low CVD risk category includes individuals with an FRS <20, while the high CVD risk population includes those with an FRS ≥20 and those who had experienced a prior cardiovascular event. Three independent‐samples t tests were also used to examine differences in 3‐HPMA levels between the 2 CVD risk categories in the whole population, the subset of nonsmokers as defined by self‐report, and the population without CVD.
Results
Demographic Characteristics
There were no significant differences between 3‐HPMA tertiles and gender, hypertension, hyperlipidemia, diabetes mellitus, environmental tobacco smoke, myocardial infarction, stroke, heart failure, most medications (except diuretics), age, lymphocyte count, and median household income (Table 2). Whites were more likely to have higher 3‐HPMA levels than blacks and Hispanics (P=0.004). Obese participants had lower 3‐HPMA (P=0.015). Smoking was significantly associated with 3‐HPMA; high urinary 3‐HPMA levels were associated with self‐reported smoking (P<0.001) and cotinine levels (P<0.001). Participants with revascularization including CABG, PCI, or stents also had higher urinary 3‐HPMA levels (P=0.040). Participants taking diuretics had lower 3‐HPMA (P=0.005) (Table 2). When restricted to nonsmokers, obesity and diuretic use remained significantly associated with lower 3‐HPMA levels.
Table 2.
Demographics of Louisville Healthy Heart Study Participants Stratified by Urinary 3‐Hydroxypropylmercapturic Acid (3‐HPMA) Levels
| Categorical Variable—n (%) | Total n=211 | Low 3‐HPMA (≤87.64 μg/g Creatinine) n=70 | Medium 3‐HPMA (>87.64 to 411.5 μg/g Creatinine) n=71 | High 3‐HPMA (>411.5 μg/g Creatinine) n=70 | P Value |
|---|---|---|---|---|---|
| Gender | 0.863 | ||||
| Female | 100 (47) | 32 (46) | 33 (47) | 35 (50) | |
| Male | 111 (53) | 38 (54) | 38 (54) | 35 (50) | |
| Ethnicity | 0.005 | ||||
| White | 120 (57) | 35 (50) | 34 (48) | 51 (73) | 0.004 |
| Black | 87 (41) | 32 (46) | 37 (52) | 18 (26) | 0.004 |
| Hispanic | 4 (1.9) | 3 (4.3) | 0 (0.0) | 1 (1.4) | 0.165 |
| CVD risk factors | |||||
| Hypertension | 168 (81) | 61 (88) | 55 (80) | 52 (75) | 0.137 |
| Hyperlipidemia | 131 (64) | 40 (58) | 46 (67) | 45 (66) | 0.492 |
| Diabetes | 55 (26) | 18 (26) | 23 (33) | 14 (20) | 0.243 |
| Obese | 118 (58) | 45 (66) | 44 (63) | 29 (43) | 0.015 |
| Current smoker (self‐reported) | 82 (39) | 8 (12) | 22 (32) | 52 (74) | <0.001 |
| Never smoked (self‐reported) | 56 (27) | 19 (27) | 28 (40) | 9 (13) | 0.001 |
| Former smoker (self‐reported) | 71 (34) | 42 (61) | 20 (28) | 9 (13) | <0.001 |
| Environmental smoke* | 41 (53) | 19 (31) | 13 (28) | 8 (44) | 0.425 |
| High CVD risk category* | 168 (80) | 49 (70) | 55 (78) | 64 (91) | 0.006 |
| Medical history | |||||
| Myocardial infarction | 73 (35) | 19 (28) | 25 (36) | 29 (41) | 0.225 |
| Stroke | 22 (11) | 7 (10) | 9 (13) | 6 (9) | 0.705 |
| CABG/PCI/stents | 58 (28) | 14 (20) | 17 (24) | 27 (39) | 0.040 |
| Heart failure | 36 (17) | 11 (16) | 17 (25) | 8 (12) | 0.120 |
| Medication | |||||
| Angiotensin‐converting‐enzyme inhibitor | 112 (54) | 35 (52) | 42 (59) | 35 (52) | 0.604 |
| Angiotensin‐receptor blockers | 12 (6) | 6 (9) | 4 (6) | 2 (3) | 0.328 |
| β‐blocker | 129 (63) | 40 (60) | 42 (59) | 47 (69) | 0.400 |
| Calcium‐channel blockers | 45 (23) | 17 (25) | 15 (21) | 13 (19.1) | 0.668 |
| Diuretics | 81 (39) | 32 (48) | 33 (47) | 16 (24) | 0.005 |
| Statins | 109 (53) | 33 (49) | 37 (52) | 39 (57) | 0.632 |
| Aspirin | 117 (57) | 37 (55) | 38 (54) | 42 (62) | 0.588 |
| Vasodilator* | 47 (23) | 10 (15) | 20 (28) | 17 (25) | 0.157 |
| Continuous Variable—Mean (SD) | Total | Low 3‐HPMA | Medium 3‐HPMA | High 3‐HPMA | P Value |
|---|---|---|---|---|---|
| Age (years)—median (IQR) | 51 (10) | 53 (11) | 50 (10) | 50 (10) | 0.196 |
| Cotinine (μg/g creatinine) | 516 (1052) | 37.1 (120) | 384 (785) | 1145 (1454) | <0.001 |
| Creatinine, mg/dL | 140 (90) | 138 (87) | 135 (82) | 148 (102) | 0.883 |
| Framingham Risk Score | 9 (8) | 7 (7) | 8 (7) | 13 (9) | 0.083 |
| Lymphocyte count×104* | 13 (9) | 12 (8) | 14 (10) | 14 (10) | 0.906 |
| Thrombosis | |||||
| Fibrinogen, mg/dL | 345 (108) | 334 (92) | 361 (141) | 340 (81) | 0.376 |
| Platelet–leukocyte Aggregates* | 10.8 (5.9) | 10.3 (5.6) | 11.1 (5.7) | 10.9 (6.3) | 0.503 |
| Inflammation | |||||
| hsCRP, mg/L | 4.7 (4.6) | 5.5 (5.1) | 4.8 (4.4) | 3.8 (4.3) | 0.198 |
| Median household income* | 30 774 (17 786) | 31 360 (18 222) | 30 188 (19 771) | 30 795 (15 032) | 0.880 |
CABG indicates coronary artery bypass graft; CVD, cardiovascular disease; hsCRP, high‐sensitivity C‐reactive protein; IQR, interquartile range; PCI, percutaneous coronary intervention.
Environmental smoke is secondhand smoke exposure in self‐reported non‐smokers (former smokers or never smokers).
Patients in the high CVD risk category were those with a Framingham Risk Score ≥20 or those who had experienced a cardiovascular event.
Vasodilators included nitrates and hydralazine.
The P‐value for the lymphocyte counts was calculated using the log‐transformed lymphocyte counts.
Platelet–leukocyte aggregates are presented as the percent total of cells CD41+/45+, and the P‐value is calculated for the log‐transformed platelet–leukocyte aggregate levels.
Median household income in United States dollars at the United States Census block group level.
Smoking and Urinary 3‐HPMA Levels
Because urinary 3‐HPMA levels were strongly associated with smoking, and tobacco smoke contains high acrolein levels,24 we examined the relationship between 3‐HPMA and smoking in greater detail. We found that the mean cotinine level was significantly higher in self‐reported smokers when compared with self‐reported nonsmokers (Figure 1A). Mean 3‐HPMA level was significantly higher in individuals with high (≥200 μg/g creatinine) cotinine than low (<200 μg/g creatinine) cotinine (Figure 1B). Mean values of 3‐HPMA in individuals with high (n=82, 757.2±79.4 μg/g creatinine) and low (n=126, 157.3±17.5 μg/g creatinine) cotinine levels are in good agreement with previously reported values,24 and suggest that ≈80% of the 3‐HPMA measured in the urine of smokers is derived from acrolein in cigarette smoke. Linear regression analysis indicated that despite wide variation, 3‐HPMA levels were significantly and positively related to urinary cotinine in the total population (β=0.260, R2=0.432, P<0.001, 95% CI=0.219 to 0.300) (Figure 1C) and in smokers (β=0.510, R2=0.476, P<0.001, 95% CI=0.390 to 0.630) (Figure 1D).
Figure 1.
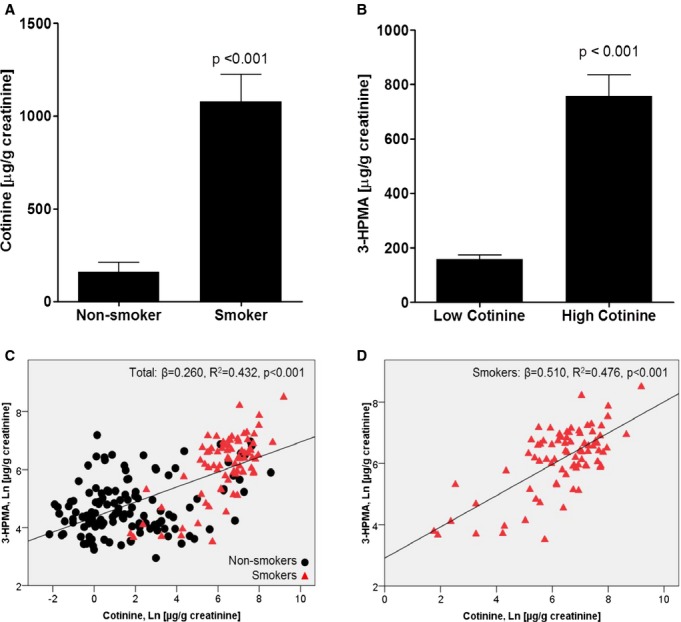
Association between smoking and 3‐hydroxypropylmercapturic acid (3‐HPMA) levels. A, Mean urine cotinine levels in self‐reported non‐smokers and smokers. B, Mean 3‐HPMA (μg/g creatinine) levels for low (<200 μg/g creatinine) and high (≥200 μg/g creatinine) cotinine strata. C and D, 3‐HPMA and cotinine regression in non‐smokers and smokers.
Circulating Angiogenic Cells
To examine whether acrolein exposure is associated with cardiovascular injury, we measured circulating angiogenic cell levels in peripheral blood. Pauperization of circulating progenitor cells is an indicator of CVD risk;19 nevertheless, it is unclear which specific progenitor cell phenotype best predicts CVD risk. Hence, we measured 13 antigenically defined populations of circulating angiogenic cells, as well as the total number of cells expressing surface markers of hematopoietic (CD45) and endothelial (CD31) origin. Analyses adjusted for age and gender revealed that levels of cell type‐2 (CD31+/34+/45+, P=0.001), cell type‐6 (CD31+/34+, P=0.024), cell type‐7 (CD31+/34+/45dim/AC133−, P=0.002), cell type‐8 (CD31+/34+/45+/AC133−, P<0.001), and cell type‐9 (CD34+, P=0.006) were significantly suppressed in smokers—a finding consistent with previous reports showing that smokers have reduced circulating angiogenic cell levels.25–26 Moreover, an unadjusted comparison of circulating angiogenic cell levels among 3‐HPMA tertiles revealed significantly lower levels of cell type‐2 (P=0.030) and cell type‐8 (P=0.035) in individuals with higher 3‐HPMA levels (data not shown).
To further examine the relationship between 3‐HPMA and circulating angiogenic cells, we adjusted for ethnicity, smoking status, BMI, hypertension, diuretics, angiotensin‐receptor blockers, and CABG/PCI/stents. After adjustment, the cell type‐2, cell type‐8, and cell type‐14 (CD34+/45+/AC133+) subgroups were negatively associated with 3‐HPMA levels (Table 3). In nonsmokers, cell type‐2, cell type‐8, and cell type‐14 remained inversely associated with 3‐HPMA (Table 3). In smokers, cell type‐5 (CD31+/AC133+, β=−0.001, P=0.012), cell type‐11 (AC133+, β=−0.001, P=0.001), and cell type‐14 (CD34/45+/AC133+, β=−0.002, P<0.001) were inversely associated with circulating angiogenic cell levels. The persistent association of HMPA with specific circulating angiogenic cell subpopulations, after adjustment for smoking and stratification into nonsmokers, suggests that acrolein from sources other than tobacco smoke also suppresses circulating angiogenic cell levels. Moreover, suppression of cell type‐2 and cell type‐8 in smokers is likely due to exposure to higher acrolein levels.
Table 3.
Association Between Urinary 3‐Hydroxypropylmercapturic Acid (3‐HPMA) Levels and Circulating Angiogenic Cells
| 3‐HPMA | Cell Type‐2 (CD31+/34+/45+) | Cell Type‐8 (CD31+/34+/45+/AC133−) | Cell Type‐14 (CD34+/45+/AC133+) |
|---|---|---|---|
| Total population (n=187) | |||
| β* (SEM) | −0.001 (0.0004) | −0.001 (0.0004) | −0.002 (0.0005) |
| P value | 0.005 | 0.007 | <0.001 |
| Non‐smokers (n=111) | |||
| β* (SEM) | −0.003 (0.0008) | −0.003 (0.0008) | −0.014 (0.0034) |
| P value | <0.001 | <0.001 | <0.001 |
Models were adjusted for ethnicity, body mass index, BMI, hypertension, diuretics, angiotensin‐receptor blockers, coronary artery bypass graft/percutaneous coronary intervention/stents, and smoking status. The β values are adjusted for 3‐HPMA.
Inflammation and Platelet Activation
To estimate in vivo platelet activation, we measured circulating levels of platelet–leukocyte aggregates, which reflect both systemic inflammation and increased platelet activity. The association of platelet–leukocyte aggregates with increased thrombosis and inflammation has been validated in several large clinical trials.27–28 In addition, our previous studies show that acrolein inhalation increases platelet–leukocyte aggregates in mice.15 After adjustment for ethnicity, smoking status, BMI, hypertension, diuretics, angiotensin‐receptor blockers, and CABG/PCI/stents, 3‐HPMA levels were positively associated with circulating levels of platelet–leukocyte aggregates, linking acrolein exposure with platelet activation in humans (Figure 2A). Additionally, we found a positive association between platelet–leukocyte aggregates and cotinine in smokers after adjustment for ethnicity, BMI, hypertension, diuretics, angiotensin‐receptor blockers, and CABG/PCI/stents (Figure 2B). Next, we measured plasma levels of fibrinogen, a marker of thrombosis, but found no association between 3‐HPMA and fibrinogen in this cohort. Urinary 3‐HPMA levels were not associated with serum hsCRP, a biomarker of inflammation,29 in the total population. However, 3‐HPMA level was inversely associated with hsCRP in the nonsmoking population (β=−0.002, P=0.023) after adjustment for ethnicity, BMI, hypertension, diuretics, angiotensin‐receptor blockers, and CABG/PCI/stents (data not shown). There was also a significant association between fibrinogen and hsCRP levels (β=0.093, P<0.001). No associations were observed between cotinine and hsCRP levels or between cotinine and fibrinogen levels.
Figure 2.
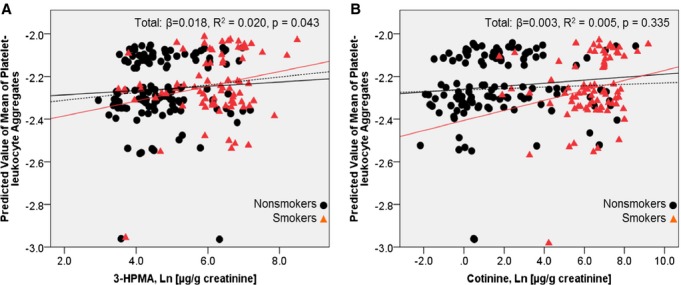
Adjusted association between platelet–leukocyte aggregates and 3‐hydroxypropylmercapturic acid (3‐HPMA) and cotinine. A, Scatterplot of the predicted mean of platelet–leukocyte aggregate levels and mean 3‐HPMA levels (log‐transformed μg/g creatinine). The model was adjusted for ethnicity, BMI, hypertension, diabetes, diuretics, CABG/PCI/stents, angiotensin‐receptor blockers, and smoking status. Non‐smokers (solid black line): β=0.010, R2=0.004, P=0.497. Smokers (solid red line): β=0.035, R2=0.055, P=0.035. B, Scatterplot of the predicted mean of platelet–leukocyte aggregate levels regressed against cotinine (log‐transformed μg/g creatinine) levels. The model was adjusted for ethnicity, BMI, hypertension, diabetes, diuretics, CABG/PCI/stents, and angiotensin‐receptor blockers. Non‐smokers (solid black line): β=0.007, R2=0.011, P=0.244. Smokers (solid red line): β=0.023, R2=0.048, P=0.049. BMI indicates body mass index; CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention.
Framingham Risk Score
To determine whether acrolein exposure is associated with CVD risk, we stratified the cohort into low (FRS <20) or high (FRS ≥20 or experienced a cardiovascular event) CVD risk strata. As shown in Figure 3A, individuals with low risk had significantly lower 3‐HPMA levels than those in the high‐risk category (181.5±30.0 μg 3‐HPMA/g [n=43] creatinine versus 453.7±46.7 μg 3‐HPMA/g creatinine [n=168], respectively [P<0.001]). To dissociate this risk from smoking, we examined this relationship only in nonsmokers. When stratified into the self‐reported nonsmoking population, 3‐HPMA was significantly lower (>50%) in individuals in the low‐risk category compared with those in the high‐risk category (low: 105.2±23.0 μg/g creatinine [n=28]; high: 220.9±26.7 μg/g creatinine [n=99], respectively [P=0.006]) (Figure 3B). These findings indicate that nonsmokers with lower levels of 3‐HPMA had lower CVD risk. In addition, after excluding individuals with pre‐existing CVD, we found that 3‐HPMA levels were significantly lower in individuals with low FRS compared to those with high FRS (low: 181.4±30.0 [n=43], high: 467.2±139.8 [n=11], respectively [P=0.035]) (Figure 3C). Both FRS as well as CVD risk strata were associated with 3‐HPMA even after adjustment for ethnicity, alcohol, and BMI. The FRS demonstrated a positive association with 3‐HPMA (β=0.011, P<0.001), whereas the low CVD risk category had lower 3‐HPMA (β=−0.002, P=0.004).
Figure 3.
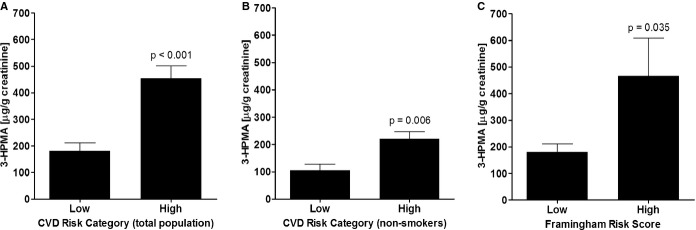
Association between CVD risk and 3‐hydroxypropylmercapturic acid (3‐HPMA). A, Mean 3‐HPMA levels for low risk (Framingham Risk Score [FRS]<20) and high risk (FRS≥20 or experienced a cardiovascular event) CVD risk categories. B, Mean 3‐HPMA levels for low and high CVD risk categories in self‐reported non‐smokers. C, Mean 3‐HPMA levels for low (FRS<20) and high (FRS≥20) FRS in patients without clinical manifestation of CVD. CVD indicates cardiovascular disease.
Discussion
The results of this study demonstrate that elevated urinary 3‐HPMA levels are associated with low abundance of specific circulating angiogenic cell subpopulations, increased circulating levels of platelet–leukocyte aggregates, and increased CVD risk. The association between platelet–leukocyte aggregates and HMPA levels persisted even after adjustment for smoking status. Similarly, even though circulating levels of angiogenic cells were suppressed in smokers, the association between 3‐HPMA and the angiogenic cell levels was independent of smoking. Taken together, these findings suggest that regardless of its source of exposure, acrolein induces increased CVD risk in conjunction with suppression of circulating angiogenic cells and increased platelet activation.
Acrolein is a reactive aldehyde that is rapidly detoxified by several metabolic pathways. Of these, conjugation with glutathione accounts for 60% to 70% of total acrolein metabolism.30 The acrolein–glutathione conjugate, after reduction and metabolism via the renal mercapturic acid pathway, is excreted in urine as 3‐HPMA.30 Previous studies have shown that urinary HMPA levels are higher in smokers than in nonsmokers, indicating that smoking is a major source of acrolein exposure in humans.24 Nevertheless, significant levels of 3‐HPMA were also detected in the urine of nonsmokers (at 1/3 the level of smokers), which is consistent with the view that in addition to smoking, there are other significant sources of acrolein exposure, including exposure to traffic, nontobacco smoke or air pollution, consumption of food and beverages, endogenous metabolism, lipid peroxidation, and inflammation.5 While our study was not designed to assess the contribution of specific sources, our results indicate that acrolein exposure is associated with increased CVD risk regardless of its source.
Our results show that general markers of inflammation were not associated with acrolein exposure in smokers. For example, neither hsCRP nor fibrinogen was associated with urinary 3‐HPMA (or cotinine) level. Although previous studies have reported positive correlations between smoking and inflammatory markers such as CRP and fibrinogen,31–34 these associations are weak and not replicated in other studies.35 Moreover, CRP levels do not decrease after smoking cessation,35 indicating that changes in CRP levels are not a direct consequence of smoking, but perhaps reflect general nonspecific injury.36 We speculate that in smokers the association between smoking and inflammatory markers may have been masked by a high CVD disease burden, BMI, and/or age. Nonetheless, the lack of a strong association between 3‐HPMA and markers of inflammation suggests that repeated acrolein exposure in smokers does not induce chronic low‐grade inflammation. This view is consistent with previous results showing that in apoE‐null mice, chronic acrolein exposure does not increase plasma levels of cytokines such as tumor necrosis factor‐α or interleukin (IL)‐6,17 and that in wild‐type C57BL/6 mice, exposure to acrolein after lipopolysaccharide challenge suppresses cytokine production.37 Similarly, acute exposure to tobacco smoke suppresses eosinophils as well several inflammatory cytokines such as tumor necrosis factor‐α, IL‐6 and IL‐2, and IFN‐γ, although it increases neutrophil and macrophage chemotaxis and activation.38 Thus, both human and animal data support the hypothesis that high‐dose acrolein exposure does not increase systemic inflammation and may even lead to suppression of pro‐inflammatory cytokine production.
In contrast to CRP, circulating platelet–leukocyte aggregates were significantly associated with acrolein exposure. Previous studies have shown that these aggregates correlate well with other markers of platelet activation, such as P‐selectin and CD40L,39 and in fact represent a more sensitive marker of in vivo platelet activation than platelet surface P‐selectin.40 Levels of platelet–leukocyte aggregates are increased in patients with stable coronary artery disease,41 and acute coronary syndromes,42–43 indicating that these aggregates are sensitive biomarkers of heightened thrombosis. Moreover, it has been shown that the binding of platelets induces IL‐1β, IL‐8, and MCP‐1 in monocytes,44 and promotes monocyte recruitment to atherosclerotic arteries and accelerates atherosclerotic lesion formation in apoE‐null mice.45 Collectively, these findings indicate that in addition to serving as a biomarker of platelet activation, these aggregates may be significant contributors to the initiation and progression of atherothrombosis. Thus, increased formation of platelet–leukocyte aggregates may be a mechanism underlying the pro‐atherogenic effects of acrolein observed in animal studies. While in humans this link remains speculative, our previous study showing that exposure to acrolein increases platelet–leukocyte aggregate formation in mice15 supports the biological plausibility of this association in humans.
Low circulating levels of circulating angiogenic cells have been shown to predict the occurrence of cardiovascular events and death from cardiovascular causes.46 Our previous studies have shown that in mice, acrolein suppresses the levels of circulating progenitor cells.18 Thus, lower levels of circulating angiogenic cells in smokers provide further evidence of increased cardiovascular risk. Previous studies have found that smoking reduces the number of circulating progenitor cells25–26 and that low levels of these cells are associated with increased CVD risk19 and predict CVD events.46 Smoking cessation is, however, linked with rapid recovery of circulating angiogenic cells.26 Consistent with this view, our study indicates that levels of several circulating angiogenic cell populations are suppressed in smokers. Specifically, we found a strong negative association between cell type‐2, cell type‐8, cell type‐11, and cell type‐14 subgroups with smoking, suggesting that these circulating angiogenic cell populations are particularly sensitive to acrolein exposure. Because cell type‐2 and cell type‐8 were also negatively associated with HPMA levels in nonsmokers, these results suggest that suppression of these circulating angiogenic cell subpopulations in smokers may be mediated, in part, by acrolein, while the suppression of cell type‐4 and cell type‐7 may be due to other tobacco smoke constituents.
It is significant to point out that suppression of several circulating angiogenic cells in smokers was unrelated to cotinine levels, which might be reflective of the fact that depending upon the cigarette brand and smoking behavior, exposure to acrolein (or other chemicals) can vary independent of cotinine, such that even with similar nicotine delivery, smokers are exposed to varying acrolein levels. Regardless, in a fully adjusted model, the association between 3‐HPMA and circulating angiogenic cell levels was found to be independent of cotinine or smoking, reinforcing the idea that acrolein from sources other than tobacco could also suppress circulating angiogenic cell levels. Finally, our analysis of specific circulating angiogenic cell subsets indicates that the CD31+/CD34+ with or without AC133 are specifically affected by acrolein. Both of these cell types promote re‐endothelialization of vascular lesions when transplanted into nude mice47 and are predictive of cardiovascular events and mortality.46
While our results suggest a strong association between acrolein exposure and cardiovascular risk, the study has several limitations. Although our sample size is comparatively large for a study measuring circulating angiogenic cells, it is a relatively small in comparison with other studies in the area of CVD and environmental epidemiology. The cross‐sectional design of the study is limited in its ability to demonstrate causality; however, exposure to acrolein (through food substances, environmental exposures, and sporadic inflammation and oxidative stress) is episodic and, therefore, the effects of such exposures may be missed in a longitudinal prospective study if the exposure is not maintained throughout the observation period. Hence, the effects of contemporaneous exposure are likely to be more evident in a cross‐sectional design, although temporality and directionality may be difficult to establish. Finally, because of the exploratory nature of the study and a high level of correlation between the different angiogenic subpopulations, we did not correct for multiple comparisons. However, the relationship between acrolein exposure and CVD risk was not obtained from a post‐hoc analysis of the data, but was examined to test an a priori hypothesis developed on the basis of our previous experimental data showing that direct exposure to acrolein alone decreases the levels of circulating angiogenic cells in mice.18 Thus, the observed association between acrolein exposure and angiogenic cell levels in humans has strong experimental support and biological plausibility provided by data from animal studies.
To measure acrolein exposure, we quantified urinary levels of 3‐HPMA, which is derived from the glutathione conjugate of acrolein or related 3 carbon compounds. While most 3‐HPMA is likely to be derived directly from acrolein, it could also be generated by the metabolism of other allylic and aromatic compounds. However, for conversion to 3‐HPMA these compounds have to be first converted to acrolein, in which case, the penultimate metabolite will be acrolein itself, regardless of the parent compound (food, toxin, or drug). The most likely 3‐carbon pollutant that could directly generate HPMA is propylene oxide, but this compound generates 2‐HPMA rather than 3‐HPMA.48–50 In our measurements, 3‐HPMA was much more abundant than 2‐HPMA and the two were clearly separated during our gas chromatography–mass spectrometry analysis. Thus, it appears unlikely that propylene oxide is a major source of 3‐HPMA measured in this study.
Another potential limitation of our study is that only a single urine sample was collected from each individual and therefore, the values obtained may not reflect “total daily exposure.” However, it has been shown previously that values of tobacco exposure biomarkers obtained from spot urine correlate well with 24‐hour urine collection.51 Values of HMPA vary with time of day,51 perhaps due to its short half‐life and circadian rhythm of glutathione synthesis, but collections made in our study (mostly during the afternoon) reflect same‐day exposure and should be internally correlated with measurements of CVD risk because both blood and urine samples were collected at the same time. In addition, normalizing urinary metabolites by creatinine could be potentially problematic as adjustment for creatinine, while correcting for water dilution, introduces additional variations that relate to the dependence of creatinine excretion on muscle mass, gender, physical activity, diet, and disease.52 In any case, the relationships between HMPA and circulating angiogenic cells were not significantly affected even when 3‐HPMA levels are not normalized to creatinine (data not shown). Additional variability could also arise from individual differences in smoking behavior and metabolism of smoke constituents, which are not accounted for in our study. Finally, the study examined only CVD risk and not CVD per se. As indicated above, prospective evaluation of this relationship in a longitudinal study is difficult because exposure is episodic and sporadic. Nevertheless, the relationship between acrolein exposure and CVD risk is important because extensive studies have shown that FRS53–54 and circulating angiogenic cell levels19 are strongly predictive of cardiovascular events and mortality.
The findings of this study have significant public health implications as they indicate that in addition to tobacco smoke, exposure to acrolein‐containing pollutants such as automobile exhaust, burn‐pits, structural and forest fires could increase CVD risk. Indeed, several studies suggest that occupational exposure of firefighters to smoke is associated with increased CVD risk.55 Furthermore, if further substantiated, the association between acrolein and CVD risk would suggest that limiting acrolein exposure by regulating its levels in foods or polluting emissions or preventing exposure may decrease CVD prevalence in the general population. Likewise, regulation of the acrolein content in tobacco products could significantly reduce their cardiovascular toxicity. Given the high incidence of premature mortality due to smoking (3.5 million deaths annually worldwide), even marginal reduction in the acrolein content of cigarettes could save a substantial number of lives.
Sources of Funding
This work was supported in part by grants from the WellPoint Foundation and the NIH (HL120163 and GM103492). This work was supported in part by grants provided by the Center for Environmental Genomics and Integrative Biology (P30ES014443) and the National Institute of Environmental Health Sciences (ES11860, ES19217).
Disclosures
None.
Acknowledgments
The authors thank the phlebotomists at the University of Louisville Ambulatory Care and University Medical Associates for biological sample collection and Duane Bolanowski, Dave Young, Melissa Peak, and Imtiaz Ismail for their technical assistance.
References
- 1.Bhatnagar A. Environmental cardiology: studying mechanistic links between pollution and heart disease. Circ Res. 2006; 99:692-705. [DOI] [PubMed] [Google Scholar]
- 2.Brook RD, Rajagopalan S, Pope CA, III, Brook JR, Bhatnagar A, Diez‐Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC, Jr, Whitsel L, Kaufman JD. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010; 121:2331-2378. [DOI] [PubMed] [Google Scholar]
- 3.Ezzati M, Lopez AD. Estimates of global mortality attributable to smoking in 2000. Lancet. 2003; 362:847-852. [DOI] [PubMed] [Google Scholar]
- 4.Haussmann HJ. Use of hazard indices for a theoretical evaluation of cigarette smoke composition. Chem Res Toxicol. 2012; 25:794-810. [DOI] [PubMed] [Google Scholar]
- 5.Ghilarducci DP, Tjeerdema RS. Fate and effects of acrolein. Rev Environ Contam Toxicol. 1995; 144:95-146. [DOI] [PubMed] [Google Scholar]
- 6.De Woskin RS. Toxicological Review of Acrolein (Cas No. 107‐02‐08) in Support of Summary Information on the Integrated Risk Information System (Iris). 2003Washington, DC: U.S. Environmental Protection Agency [Google Scholar]
- 7.Anderson MM, Hazen SL, Hsu FF, Heinecke JW. Human neutrophils employ the myeloperoxidase‐hydrogen peroxide‐chloride system to convert hydroxy‐amino acids into glycolaldehyde, 2‐hydroxypropanal, and acrolein: A mechanism for the generation of highly reactive alpha‐hydroxy and alpha, beta‐unsaturated aldehydes by phagocytes at sites of inflammation. J Clin Invest. 1997; 99:424-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uchida K. Current status of acrolein as a lipid peroxidation product. Trends Cardiovasc Med. 1999; 9:109-113. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe K, Nakazato Y, Saiki R, Igarashi K, Kitada M, Ishii I. Acrolein‐conjugated low‐density lipoprotein induces macrophage foam cell formation. Atherosclerosis. 2013; 227:51-57. [DOI] [PubMed] [Google Scholar]
- 10.Shao B, Fu X, McDonald TO, Green PS, Uchida K, O'Brien KD, Oram JF, Heinecke JW. Acrolein impairs ATP binding cassette transporter A1‐dependent cholesterol export from cells through site‐specific modification of apolipoprotein A‐I. J Biol Chem. 2005; 280:36386-36396. [DOI] [PubMed] [Google Scholar]
- 11.Tsakadze NL, Srivastava S, Awe SO, Adeagbo AS, Bhatnagar A, D'Souza SE. Acrolein‐induced vasomotor responses of rat aorta. Am J Physiol Heart Circ Physiol. 2003; 285:H727-H734. [DOI] [PubMed] [Google Scholar]
- 12.Ismahil MA, Hamid T, Haberzettl P, Gu Y, Chandrasekar B, Srivastava S, Bhatnagar A, Prabhu SD. Chronic oral exposure to the aldehyde pollutant acrolein induces dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2011; 301:H2050-H2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang GW, Guo Y, Vondriska TM, Zhang J, Zhang S, Tsai LL, Zong NC, Bolli R, Bhatnagar A, Prabhu SD. Acrolein consumption exacerbates myocardial ischemic injury and blocks nitric oxide‐induced PKCepsilon signaling and cardioprotection. J Mol Cell Cardiol. 2008; 44:1016-1022. [DOI] [PubMed] [Google Scholar]
- 14.Conklin DJ, Barski OA, Lesgards JF, Juvan P, Rezen T, Rozman D, Prough RA, Vladykovskaya E, Liu S, Srivastava S, Bhatnagar A. Acrolein consumption induces systemic dyslipidemia and lipoprotein modification. Toxicol Appl Pharmacol. 2010; 243:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sithu SD, Srivastava S, Siddiqui MA, Vladykovskaya E, Riggs DW, Conklin DJ, Haberzettl P, O'Toole TE, Bhatnagar A, D'Souza SE. Exposure to acrolein by inhalation causes platelet activation. Toxicol Appl Pharmacol. 2010; 248:100-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Toole TE, Zheng YT, Hellmann J, Conklin DJ, Barski O, Bhatnagar A. Acrolein activates matrix metalloproteinases by increasing reactive oxygen species in macrophages. Toxicol Appl Pharmacol. 2009; 236:194-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srivastava S, Sithu SD, Vladykovskaya E, Haberzettl P, Hoetker DJ, Siddiqui MA, Conklin DJ, D'Souza SE, Bhatnagar A. Oral exposure to acrolein exacerbates atherosclerosis in apoE‐null mice. Atherosclerosis. 2011; 215:301-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wheat LA, Haberzettl P, Hellmann J, Baba SP, Bertke M, Lee J, McCracken J, O'Toole TE, Bhatnagar A, Conklin DJ. Acrolein inhalation prevents vascular endothelial growth factor‐induced mobilization of Flk‐1+/Sca‐1+ cells in mice. Arterioscler Thromb Vasc Biol. 2011; 31:1598-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fadini GP, Losordo D, Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res. 2012; 110:624-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Toole TE, Hellmann J, Wheat L, Haberzettl P, Lee J, Conklin DJ, Bhatnagar A, Pope CA., III Episodic exposure to fine particulate air pollution decreases circulating levels of endothelial progenitor cells. Circ Res. 2010; 107:200-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.NIH. Risk Assessment Tool for Estimating Your 10‐year Risk of Having a Heart Attack. Bethesda, MD: NIH; 2013. [Google Scholar]
- 22.Conklin DJ, Haberzettl P, Lesgards JF, Prough RA, Srivastava S, Bhatnagar A. Increased sensitivity of glutathione S‐transferase P‐null mice to cyclophosphamide‐induced urinary bladder toxicity. J Pharmacol Exp Ther. 2009; 331:456-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Man CN, Gam LH, Ismail S, Lajis R, Awang R. Simple, rapid and sensitive assay method for simultaneous quantification of urinary nicotine and cotinine using gas chromatography‐mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2006; 844:322-327. [DOI] [PubMed] [Google Scholar]
- 24.Carmella SG, Chen M, Zhang Y, Zhang S, Hatsukami DK, Hecht SS. Quantitation of acrolein‐derived (3‐hydroxypropyl)mercapturic acid in human urine by liquid chromatography‐atmospheric pressure chemical ionization tandem mass spectrometry: effects of cigarette smoking. Chem Res Toxicol. 2007; 20:986-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001; 89:E1-E7. [DOI] [PubMed] [Google Scholar]
- 26.Kondo T, Hayashi M, Takeshita K, Numaguchi Y, Kobayashi K, Iino S, Inden Y, Murohara T. Smoking cessation rapidly increases circulating progenitor cells in peripheral blood in chronic smokers. Arterioscler Thromb Vasc Biol. 2004; 24:1442-1447. [DOI] [PubMed] [Google Scholar]
- 27.Freedman JE, Loscalzo J. Platelet‐monocyte aggregates: bridging thrombosis and inflammation. Circulation. 2002; 105:2130-2132. [DOI] [PubMed] [Google Scholar]
- 28.Cerletti C, Tamburrelli C, Izzi B, Gianfagna F, de Gaetano G. Platelet‐leukocyte interactions in thrombosis. Thromb Res. 2012; 129:263-266. [DOI] [PubMed] [Google Scholar]
- 29.Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, Gao P, Walker M, Thompson A, Sarwar N, Caslake M, Butterworth AS, Amouyel P, Assmann G, Bakker SJ, Barr EL, Barrett‐Connor E, Benjamin EJ, Bjorkelund C, Brenner H, Brunner E, Clarke R, Cooper JA, Cremer P, Cushman M, Dagenais GR, D'Agostino RB, Sr, Dankner R, Davey‐Smith G, Deeg D, Dekker JM, Engstrom G, Folsom AR, Fowkes FG, Gallacher J, Gaziano JM, Giampaoli S, Gillum RF, Hofman A, Howard BV, Ingelsson E, Iso H, Jorgensen T, Kiechl S, Kitamura A, Kiyohara Y, Koenig W, Kromhout D, Kuller LH, Lawlor DA, Meade TW, Nissinen A, Nordestgaard BG, Onat A, Panagiotakos DB, Psaty BM, Rodriguez B, Rosengren A, Salomaa V, Kauhanen J, Salonen JT, Shaffer JA, Shea S, Ford I, Stehouwer CD, Strandberg TE, Tipping RW, Tosetto A, Wassertheil‐Smoller S, Wennberg P, Westendorp RG, Whincup PH, Wilhelmsen L, Woodward M, Lowe GD, Wareham NJ, Khaw KT, Sattar N, Packard CJ, Gudnason V, Ridker PM, Pepys MB, Thompson SG, Danesh J. C‐reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012; 367:1310-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parent RA, Paust DE, Schrimpf MK, Talaat RE, Doane RA, Caravello HE, Lee SJ, Sharp DE. Metabolism and distribution of [2,3‐14C]acrolein in Sprague‐Dawley rats. II. Identification of urinary and fecal metabolites. Toxicol Sci. 1998; 43:110-120. [DOI] [PubMed] [Google Scholar]
- 31.Yanbaeva DG, Dentener MA, Creutzberg EC, Wesseling G, Wouters EF. Systemic effects of smoking. Chest. 2007; 131:1557-1566. [DOI] [PubMed] [Google Scholar]
- 32.Levitzky YS, Guo CY, Rong J, Larson MG, Walter RE, Keaney JF, Jr, Sutherland PA, Vasan A, Lipinska I, Evans JC, Benjamin EJ. Relation of smoking status to a panel of inflammatory markers: the Framingham offspring. Atherosclerosis. 2008; 201:217-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, III, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003; 107:499-511. [DOI] [PubMed] [Google Scholar]
- 34.Rohde LE, Hennekens CH, Ridker PM. Survey of C‐reactive protein and cardiovascular risk factors in apparently healthy men. Am J Cardiol. 1999; 84:1018-1022. [DOI] [PubMed] [Google Scholar]
- 35.Asthana A, Johnson HM, Piper ME, Fiore MC, Baker TB, Stein JH. Effects of smoking intensity and cessation on inflammatory markers in a large cohort of active smokers. Am Heart J. 2010; 160:458-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hastie CE, Haw S, Pell JP. Impact of smoking cessation and lifetime exposure on C‐reactive protein. Nicotine Tob Res. 2008; 10:637-642. [DOI] [PubMed] [Google Scholar]
- 37.Kasahara DI, Poynter ME, Othman Z, Hemenway D, van der Vliet A. Acrolein inhalation suppresses lipopolysaccharide‐induced inflammatory cytokine production but does not affect acute airways neutrophilia. J Immunol. 2008; 181:736-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Vaart H, Postma DS, Timens W, ten Hacken NH. Acute effects of cigarette smoke on inflammation and oxidative stress: a review. Thorax. 2004; 59:713-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burdess A, Michelsen AE, Brosstad F, Fox KA, Newby DE, Nimmo AF. Platelet activation in patients with peripheral vascular disease: reproducibility and comparability of platelet markers. Thromb Res. 2012; 129:50-55. [DOI] [PubMed] [Google Scholar]
- 40.Michelson AD, Barnard MR, Krueger LA, Valeri CR, Furman MI. Circulating monocyte‐platelet aggregates are a more sensitive marker of in vivo platelet activation than platelet surface P‐selectin: studies in baboons, human coronary intervention, and human acute myocardial infarction. Circulation. 2001; 104:1533-1537. [DOI] [PubMed] [Google Scholar]
- 41.Furman MI, Benoit SE, Barnard MR, Valeri CR, Borbone ML, Becker RC, Hechtman HB, Michelson AD. Increased platelet reactivity and circulating monocyte‐platelet aggregates in patients with stable coronary artery disease. J Am Coll Cardiol. 1998; 31:352-358. [DOI] [PubMed] [Google Scholar]
- 42.Sarma J, Laan CA, Alam S, Jha A, Fox KA, Dransfield I. Increased platelet binding to circulating monocytes in acute coronary syndromes. Circulation. 2002; 105:2166-2171. [DOI] [PubMed] [Google Scholar]
- 43.Brambilla M, Camera M, Colnago D, Marenzi G, De Metrio M, Giesen PL, Balduini A, Veglia F, Gertow K, Biglioli P, Tremoli E. Tissue factor in patients with acute coronary syndromes: expression in platelets, leukocytes, and platelet‐leukocyte aggregates. Arterioscler Thromb Vasc Biol. 2008; 28:947-953. [DOI] [PubMed] [Google Scholar]
- 44.Neumann FJ, Marx N, Gawaz M, Brand K, Ott I, Rokitta C, Sticherling C, Meinl C, May A, Schomig A. Induction of cytokine expression in leukocytes by binding of thrombin‐stimulated platelets. Circulation. 1997; 95:2387-2394. [DOI] [PubMed] [Google Scholar]
- 45.Huo Y, Schober A, Forlow SB, Smith DF, Hyman MC, Jung S, Littman DR, Weber C, Ley K. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med. 2003; 9:61-67. [DOI] [PubMed] [Google Scholar]
- 46.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Bohm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005; 353:999-1007. [DOI] [PubMed] [Google Scholar]
- 47.Friedrich EB, Walenta K, Scharlau J, Nickenig G, Werner N. CD34‐/CD133+/VEGFR‐2+ endothelial progenitor cell subpopulation with potent vasoregenerative capacities. Circ Res. 2006; 98:e20-e25. [DOI] [PubMed] [Google Scholar]
- 48.Schettgen T, Musiol A, Kraus T. Simultaneous determination of mercapturic acids derived from ethylene oxide (HEMA), propylene oxide (2‐HPMA), acrolein (3‐HPMA), acrylamide (AAMA) and N,N‐dimethylformamide (AMCC) in human urine using liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2008; 22:2629-2638. [DOI] [PubMed] [Google Scholar]
- 49.Eckert E, Drexler H, Göen T. Determination of six hydroxyalkyl mercapturic acids in human urine using hydrophilic interaction liquid chromatography with tandem mass spectrometry (HILIC–ESI‐MS/MS). J Chromatogr B. 2010; 878:2506-2514. [DOI] [PubMed] [Google Scholar]
- 50.Eckert E, Schmid K, Schaller B, Hiddemann‐Koca K, Drexler H, Göen T. Mercapturic acids as metabolites of alkylating substances in urine samples of German inhabitants. Int J Hyg Environ Health. 2011; 214:196-204. [DOI] [PubMed] [Google Scholar]
- 51.Sarkar M, Muhammad‐Kah R, Liang Q, Kapur S, Feng S, Roethig H. Evaluation of spot urine as an alternative to 24 h urine collection for determination of biomarkers of exposure to cigarette smoke in adult smokers. Environ Toxicol Pharmacol. 2013; 36:108-114. [DOI] [PubMed] [Google Scholar]
- 52.Boeniger MF, Lowry LK, Rosenberg J. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am Ind Hyg Assoc J. 1993; 54:615-627. [DOI] [PubMed] [Google Scholar]
- 53.Wilson PWF, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998; 97:1837-1847. [DOI] [PubMed] [Google Scholar]
- 54.D'Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham heart study. Circulation. 2008; 117:743-753. [DOI] [PubMed] [Google Scholar]
- 55.Institute of Medicine. Long‐Term Health Consequences of Exposure to Burn Pits in Iraq and Afghanistan. 2011Washington, DC: The National Academies Press [Google Scholar]


