Abstract
Background
Remote ischemic preconditioning (RIPC) harnesses an innate defensive mechanism that protects against inflammatory activation and ischemia‐reperfusion injury, known sequelae of cardiac surgery with cardiopulmonary bypass. We sought to determine the impact of RIPC on clinical outcomes and physiological markers related to ischemia‐reperfusion injury and inflammatory activation after cardiac surgery in children.
Methods and Results
Overall, 299 children (aged neonate to 17 years) were randomized to receive an RIPC stimulus (inflation of a blood pressure cuff on the left thigh to 15 mm Hg above systolic for four 5‐minute intervals) versus a blinded sham stimulus during induction with a standardized anesthesia protocol. Primary outcome was duration of postoperative hospital stay, with serial clinical and laboratory measurements for the first 48 postoperative hours and clinical follow‐up to discharge. There were no significant baseline differences between RIPC (n=148) and sham (n=151). There were no in‐hospital deaths. No significant difference in length of postoperative hospital stay was noted (sham 5.4 versus RIPC 5.6 days; difference +0.2; adjusted P=0.91), with the 95% confidence interval (−0.7 to +0.9) excluding a prespecified minimal clinically significant differences of 1 or 1.5 days. There were few significant differences in other clinical outcomes or values at time points or trends in physiological markers. Benefit was not observed in specific subgroups when explored through interactions with categories of age, sex, surgery type, Aristotle score, or first versus second half of recruitment. Adverse events were similar (sham 5%, RIPC 6%; P=0.68).
Conclusions
RIPC is not associated with important improvements in clinical outcomes and physiological markers after cardiac surgery in children.
Clinical Trial Registration
URL: clinicaltrials.gov. Unique identifier: NCT00650507.
Keywords: congenital, heart defects, pediatrics, remote ischemic preconditioning, surgery
Introduction
Cardiopulmonary bypass incites a damaging systemic inflammatory response.1 Hence, multiorgan dysfunction is a common manifestation after cardiac surgery in adults and children. In addition, cessation and reinitiation of coronary artery circulation results in myocardial ischemia‐reperfusion injury.2 It has been shown that transient periods of ischemia induce an innate response that subsequently protects or preconditions against ischemia‐reperfusion injury associated with more profound periods of ischemia.3 In addition, this mechanism appears to operate when ischemia of one organ produces a protected state in a distant organ, known as remote ischemic preconditioning (RIPC).4–5 This response is mediated by a circulating factor, and intact neural pathways are required for its expression.5 The exact mechanisms of this protection are unknown, although upregulation of prosurvival intracellular kinase responses6–7 and downregulation of inflammatory pathways each play a part.8–9 Clinical studies in adults with a variety of ischemia‐reperfusion and inflammatory syndromes have shown promising results,10–13 and many, but not all, studies in adults undergoing cardiac surgery have shown beneficial effects of RIPC.14–15
Our group previously performed a preliminary clinical trial in children undergoing cardiac surgery with cardiopulmonary bypass, enrolling 37 participants, with 17 receiving 4 cycles of 5‐minute periods of lower limb ischemia induced by inflation of a blood pressure cuff, with a primary outcome of plasma troponin I levels.16 Significant reductions were noted in troponin I levels, as well as lower inotrope scores, at 3 and 6 hours after bypass. Reductions in the inflammatory marker tumor necrosis factor‐α and improved lung function were noted at 6 hours. Subsequently, similar benefits of RIPC in children undergoing cardiac surgery have been shown in some studies,17–18 whereas others have failed to show a response,19–20 and none were sufficiently powered to detect differences in clinical end points. Based on these disparate and inconclusive findings, we sought to determine the impact of RIPC on clinical outcomes and physiological markers related to ischemia‐reperfusion injury in a large‐scale randomized clinical trial of children undergoing open heart surgery.
Methods
Participants
Patients aged from birth (>36 weeks gestation) to 17 years were eligible for enrollment if they planned to undergo primary cardiac surgical repair involving the use of cardiopulmonary bypass with repair resulting in no anticipated residual shunting. Patients were excluded if they had any of the following issues: current or recent ischemic insult (vascular occlusion, episode of cardiorespiratory collapse) that was sustained within 7 days preceding anticipated repair, organ dysfunction requiring medical intervention, genetic or malformation syndrome associated with congenital heart disease (except for chromosome 22q11 microdeletion), current systemic anticoagulation treatment or the presence of a bleeding diathesis, pulmonary or airway disease requiring intervention, systemic corticosteroid use within 10 days preceding anticipated repair, documented systemic infection or sepsis within 7 days preceding anticipated repair, or no available uninstrumented limb for RIPC stimulus delivery. Potential participants were identified from surgical booking lists and preoperative clinic schedules and were approached by the study coordinators (N.A.C., S.K.) with the responsible cardiologist's permission either at the bedside if an inpatient or at the preoperative clinic visit if an outpatient. Once eligibility criteria and willingness to participate were confirmed, written informed consent was obtained (S.K., H.M.H., A.N.R.), as approved by the institutional research ethics board.
Trial Design
The study design was a parallel 2‐group, double‐blind, randomized clinical trial of RIPC stimulus versus an identical sham stimulus, with 1:1 allocation and no stratification. The randomization sequence was created using a random number generator using random blocks of 2 and 4 by an investigator not involved in application of the intervention or outcomes assessment (B.W.M.). Assignments were sealed in consecutively numbered tamperproof envelopes that were opened by the study coordinator (S.K.) immediately before application of the blinded study intervention.
Interventions
Participants deemed eligible and providing consent were randomized during induction of anesthesia and line placements, and the RIPC stimulus or sham maneuver was applied. For delivery of the study maneuver, the left lower limb was preferred; an appropriately sized blood pressure cuff was applied, and systolic blood pressure was measured using a hand aneroid sphygmomanometer with Doppler detection of the distal pulse. A second cuff was laid beside the limb, and the entire limb was draped. For those randomized to RIPC, the cuff placed on the limb was inflated to a pressure of 15 mm Hg above systolic pressure and maintained for 5 minutes, followed by 5‐minutes deflation and repeated for a total of 4 cycles. Participants randomized to the sham group had the second cuff inflated in an identical protocol. Only the study coordinator applying the stimulus had knowledge of the intervention assignment; blinding was maintained throughout the trial for the participant, research personnel, and clinical staff. Of note, participants who received the RIPC stimulus had no instances of unintentional unblinding due to bruising or petechiae of the limb.
To minimize factors either promoting or impairing preconditioning mechanisms, the anesthesia protocol was standardized to include induction with sevoflurane, with sevoflurane and fentanyl maintenance. Likewise, steroid therapy was used as standard practice in those participants aged <1 month or those undergoing circulatory arrest and limited to methylprednisolone given the night before surgery, at anesthesia induction, and in the pump prime. No patient received nitric oxide or sildenafil. Milrinone use was not precluded. Modified ultrafiltration was used for patients weighing <15 kg and for all Fontan procedure patients (no weight restriction). Continuous ultrafiltration was not used.
Outcomes
In addition to baseline medical record review, patients were monitored until hospital discharge for clinical events (mortality; duration of mechanical ventilation, postoperative intensive care unit [ICU] and hospital stay; reoperation; use of extracorporeal membrane oxygenation or other mechanical support; arrhythmia; infection). Physiological and laboratory measures were made over the first 48 hours after termination of bypass. These included measures of myocardial injury and hemodynamic stability (inotrope score, troponin I, lactate, mixed venous oxygen saturation), pulmonary insufficiency (duration of mechanical ventilation, ventilation variables), renal dysfunction (urea, creatinine, urine output, cystatin‐C), hepatic dysfunction (transaminases), hematological abnormalities (complete blood count, international normalized ratio), neurological injury (clinical assessment, S100B, phosphorylated neurofilament H), and metabolic dysfunction (cortisol, leptin, insulin, glucagon, glucose, glucose‐to‐insulin ratio). Markers of inflammation (interleukin‐6, ‐8, and 10; tumor necrosis factor‐α; C‐reactive protein; clusterin, β‐2 macroglobulin) were also serially assessed. Following repair, clinical events and measures were tracked until hospital discharge, and laboratory assessments were made for the first 48 hours. The study was monitored by an independent data and safety monitoring board, which evaluated all adverse events and blinded data after 50 participants and again after 200 participants had been enrolled. No participant was unblinded throughout the course of the study. No unblinded interim analyses were performed regarding trial outcomes other than safety outcomes.
Sample Size
Study sample size was based on the primary outcome of postoperative duration of hospital stay and was aimed to detect a hypothesized reduction of 1.5 days in the RIPC group. A review of prior repairs showed an average postoperative duration of stay of 12.6±15.9 days, and a normalizing logarithmic transformation of individual patient values showed a logarithmic mean of 8.7 days with a standard deviation of 4.2 days. With a 1.5‐day reduction, a logarithmic mean of 7.2 days was hypothesized, and for a Student t test with an α of 0.05 and power of 0.85, we estimated that 143 participants per group would be required, inflated to 150 per group for attrition.
Statistical Methods
All analyses were conducted per intention to treat; 1 patient whose surgery was halted was excluded from the analysis because no intraoperative or postoperative data could be collected on this patient. The group's characteristics were compared using the Student t test assuming unequal variance between groups and Fisher exact χ2 to determine any unintentional unbalancing related to randomization and unadjusted treatment effect. Several variables were highly skewed, and natural logarithmic transformations were applied. We created a propensity score for assignment to RIPC versus sham through a logistic regression model (forward entry of baseline variables with univariable P<0.10). Linear or logistic regression models (as appropriate, depending on level of measurement of the outcome) adjusted for the previously calculated propensity score, cross‐clamp time, and surgery Aristotle score were used to compare both groups while providing some adjustments for potential confounding. Linear regression models adjusted for repeated measures through a compound symmetry covariance structure were used to model change in laboratory and physiological measures for which serial data were available; both the group average and the slope of change of time were evaluated and are reported. Once again, these regression models were adjusted for propensity score, duration of aortic cross‐clamping, and surgery Aristotle score. A 2‐sided P<0.05 was considered statistically significant for all statistical analyses. All statistical analyses were performed using SAS version 9.3 (SAS Institute).
Results
Study Participation
From April 2008 through May 2011, 1136 participants were screened, 713 were deemed eligible, and 339 were enrolled. Changes in eligibility before repair resulted in subsequent exclusion of 39 participants, with 300 participants being randomized. One patient randomized to the RIPC group was withdrawn before study intervention when the planned surgery was aborted after an allergic reaction to anesthesia induction; no data could be collected for this patient. There were no dropouts or crossovers (Figure 1). Major protocol deviations occurred in 10 sham patients (all related to preoperative steroid use) and 19 RIPC patients (7 preoperative steroids, 1 extended isoflurane exposure, 11 incorrect blood pressure cuff inflation pressures [6 below target, 5 above target]). There were no study‐related adverse events throughout the study.
Figure 1.
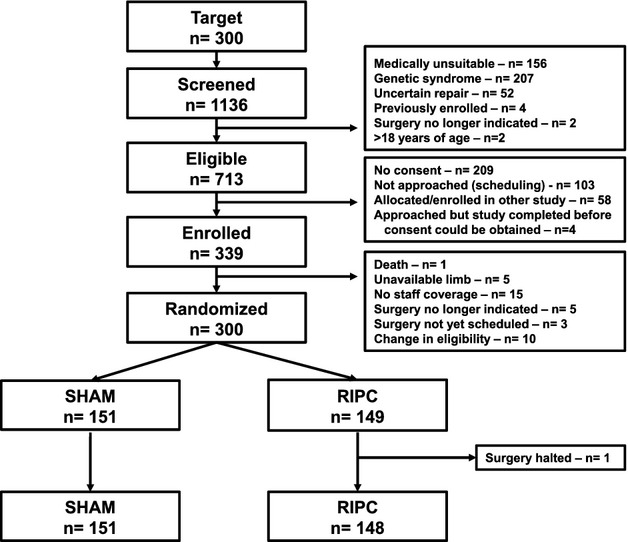
Consolidated Standards of Reporting Trials diagram: flow of patients through the study. RIPC indicates remote ischemic preconditioning; SHAM, sham stimulus.
Patient Characteristics
There were no significant differences in baseline characteristics between groups, although there was a tendency for fewer RIPC participants to be in Aristotle level IV and a tendency for longer duration of aortic cross‐clamping. These 2 variables were then included as adjustment factors in all analyses of outcomes. There were no significant differences between groups regarding underlying anatomy or type of repair, with septal defect repair, right heart lesion repair, left heart lesion repair, and Fontan procedure being the prominent repair classes (Table 1).
Table 1.
Patient and Surgical Characteristics Stratified by Study Intervention Group
| SHAM | RIPC | P Value | |
|---|---|---|---|
| Demographics and medical history | |||
| Sex, male | 85 (56%) | 79 (53%) | 0.64 |
| Height, cm | 92.5 (66.0 to 123.0) | 86.0 (64.0 to 113.5) | 0.21 |
| Weight, kg | 13.3 (6.6 to 23.5) | 11.1 (6.2 to 19.8) | 0.15 |
| Age at surgery, y | 3.1 (0.6 to 7.8) | 2.2 (0.5 to 5.9) | 0.23 |
| ≤31 days | 11 (7%) | 9 (6%) | |
| >31 days to 1 year | 38 (25%) | 51 (34%) | |
| 1 to 5 years | 52 (34%) | 44 (30%) | |
| >5 years | 50 (33%) | 44 (30%) | |
| Surgery type | |||
| Left heart lesion | 21 (14%) | 17 (12%) | 0.60 |
| Right heart lesion repair | 25 (17%) | 35 (24%) | 0.15 |
| Pulmonary venous anomaly repair | 11 (7%) | 8 (5%) | 0.64 |
| Septal defect repair | 52 (34%) | 53 (36%) | 0.81 |
| Conduit RV to PA | 6 (4%) | 4 (3%) | 0.75 |
| Fontan procedure | 20 (13%) | 11 (7%) | 0.13 |
| Transposition of great arteries repair | 11 (7%) | 13 (9%) | 0.68 |
| Thoracic veins and arteries repair | 4 (3%) | 6 (4%) | 0.54 |
| Miscellaneous | 1 (1%) | 1 (1%) | 1.00 |
| General operative data | |||
| Aristotle score | 7.2±2.0 | 6.9±2.2 | 0.29 |
| Level I (score 1.5 to 5.9) | 20 (13%) | 30 (20%) | 0.10 |
| Level II (score 6.0 to 7.9) | 63 (42%) | 52 (35%) | 0.24 |
| Level III (score 8.0 to 9.9) | 53 (35%) | 51 (34%) | 0.91 |
| Level IV (score 10 to 15) | 15 (10%) | 15 (10%) | 0.95 |
| Preoperative steroids | 10 (7%) | 7 (5%) | 0.62 |
| Cardiopulmonary bypass time, minutes | 78 (57 to 116) | 90 (59 to 126) | 0.17 |
| Aortic cross‐clamping time, minutes | 58 (27 to 83) | 69 (39 to 102) | 0.07 |
| Multiple cardiopulmonary bypass runs | 24 (16%) | 36 (24%) | 0.08 |
| Deep hypothermic circulatory arrest | 7 (5%) | 5 (3%) | 0.58 |
| Regional/selective cerebral perfusion | 2 (1%) | 1 (1%) | 1.00 |
| Induction anesthesia | |||
| Propofol | 40 (27%) | 27 (18%) | 0.10 |
| Fentanyl | 151 (100%) | 148 (100%) | 1.00 |
| Thiopentone | 9 (6%) | 9 (6%) | 1.00 |
| Sevoflurane | 150 (99%) | 144 (97%) | 0.21 |
| Isoflurane | 0 (0%) | 1 (1%) | 1.00 |
| Maintenance anesthesia | |||
| Sevoflurane | 147 (97%) | 144 (97%) | 1.00 |
| Isoflurane | 7 (5%) | 6 (4%) | 1.00 |
| Fentanyl | 149 (99%) | 149 (100%) | 0.50 |
| Propofol | 23 (15%) | 21 (14%) | 0.87 |
| Midazolam | 43 (28%) | 35 (24%) | 0.36 |
| Morphine | 74 (49%) | 58 (39%) | 0.10 |
| Diazepam | 4 (3%) | 2 (1%) | 0.68 |
| Thiopentone | 3 (2%) | 3 (2%) | 1.00 |
| Modified ultrafiltration | 91 (60%) | 96 (65%) | 0.47 |
| Propofol on transfer to ICU | 11 (7%) | 13 (9%) | 0.68 |
| Any perioperative propofol exposure | 59 (39%) | 52 (35%) | 0.55 |
| Intra‐ or postoperative milrinone | 103 (68%) | 103 (70%) | 0.80 |
| Intraoperative arrhythmia | 39 (26%) | 44 (30%) | 0.45 |
| Intraoperative cardiorespiratory arrest | 1 (1%) | 0 (0%) | 1.00 |
| Intraoperative death | 0 (0%) | 0 (0%) | 1.00 |
Median (25th to 75th percentiles), mean±SD. ICU indicates intensive care unit; kg, kilograms; PA, pulmonary artery; RIPC, remote ischemic preconditioning; RV, right ventricle; SHAM, sham stimulus.
Clinical Outcomes
The logarithmic mean postoperative duration of hospital stay was 5.4 days in the sham group and 5.6 days in the RIPC group, giving a relative absolute effect of RIPC of +0.2 days, with a 95% confidence interval ranging from −0.7 to +0.9 days, excluding a 1.5‐day and a 1‐day difference, with an adjusted P value of 0.75 (Figure 2). The median duration of mechanical ventilation was 19 hours in the sham group and 20 hours in the RIPC group, with an adjusted P value of 0.77 and with no significant differences at any time point or overall trend in ventilatory measures (Figure 3). Likewise, there was no difference in duration of ICU stay, with a median of 33 hours for both the sham and RIPC groups (P=0.21). There were a few encouraging trends regarding clinical events, with a significantly reduced likelihood of arrhythmia in the RIPC group and a trend toward reduced thrombotic complications. There were no significant differences regarding the prevalence of pleural effusions, systemic infections, or seizures (Table 2).
Figure 2.
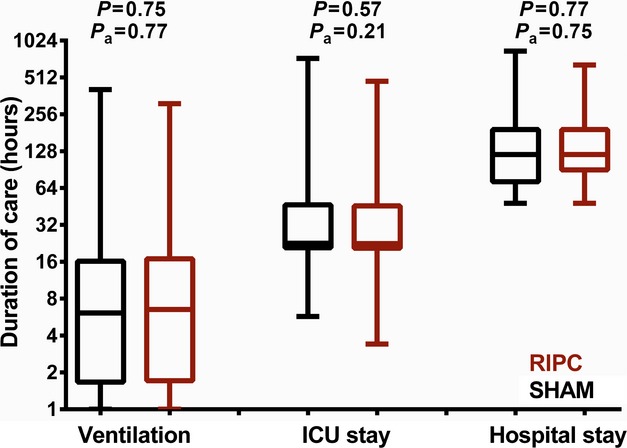
Duration of care (hours) for RIPC and SHAM patients. Unadjusted P values and P values adjusted for propensity score, duration of aortic cross‐clamping, and surgery Aristotle score are reported. ICU indicates intensive care unit; RIPC, remote ischemic preconditioning; SHAM, sham stimulus.
Figure 3.
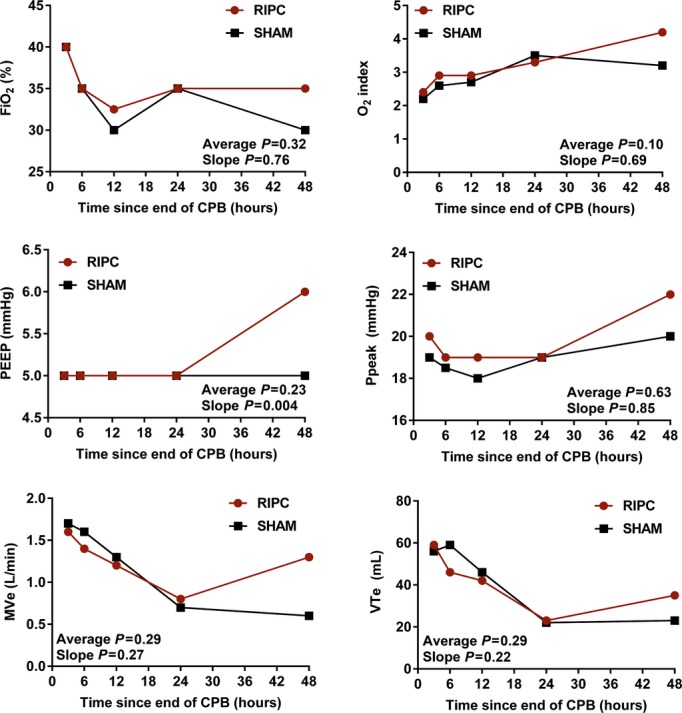
Serial ventilation parameters. P values are reported for the mean difference and the difference in slope between RIPC and SHAM. All P values are adjusted for propensity score, duration of aortic cross‐clamping, surgery Aristotle score, and proportion of patients extubated at each time point. Average indicates mean difference; CPB, cardiopulmonary bypass; FiO2, fraction of inspired oxygen; MVe, minute ventilation; PEEP, positive end‐expiratory pressure; Ppeak, peak inspiratory pressure; RIPC, remote ischemic preconditioning; SHAM, sham stimulus; slope, difference in slope; VTE, exhaled tidal volume.
Table 2.
Clinical Outcomes and Complications Stratified by Study Intervention Group
| SHAM | RIPC | P Value | P Value* | |
|---|---|---|---|---|
| Surgical outcomes | ||||
| Inotropic support at 3 hours postoperatively | 103 (69%) | 103 (70%) | 0.90 | 0.81 |
| Inotropic support at 6 hours postoperatively | 104 (69%) | 103 (70%) | 1.00 | 0.67 |
| Inotropic support at 12 hours postoperatively | 103 (69%) | 100 (68%) | 0.90 | 0.52 |
| Inotropic support at 24 hours postoperatively | 61 (41%) | 65 (44%) | 0.64 | 0.86 |
| Inotropic support at 48 hours postoperatively | 35 (23%) | 31 (21%) | 0.68 | 0.15 |
| Ventilation time after end of bypass (hours)* | 6.1 (1.7 to 16.2) | 6.5 (1.7 to 16.9) | 0.75 | 0.77 |
| Ventilated at 3 hours | 69/149 (46%) | 71/147 (48%) | 0.73 | 0.98 |
| Ventilated at 6 hours | 56/149 (38%) | 56/147 (38%) | 1.00 | 0.69 |
| Ventilated at 12 hours | 39/149 (26%) | 41/147 (28%) | 0.70 | 0.92 |
| Ventilated at 24 hours | 17/149 (11%) | 25/147 (17%) | 0.18 | 0.29 |
| Ventilated at 48 hours | 11/149 (7%) | 13/149 (9%) | 0.67 | 0.99 |
| ICU duration after end of bypass (hours)* | 22.7 (20.8 to 46.6) | 22.6 (20.6 to 45.8) | 0.57 | 0.21 |
| Hospitalization after surgery (days)* | 5 (3 to 8) | 5 (4 to 8) | 0.77 | 0.75 |
| Surgical complications | ||||
| Returned to ICU with opened chest | 5 (3%) | 8 (5%) | 0.41 | 0.43 |
| Sternal reopening | 1 (1%) | 1 (1%) | 1.00 | 0.91 |
| Cardiorespiratory assist | 15 (10%) | 17 (12%) | 0.71 | 0.86 |
| Reintubation | 3 (2%) | 2 (1%) | 1.00 | 0.56 |
| Reoperation | 0 (0%) | 2 (1%) | 0.25 | 0.96 |
| Extracorporeal membrane oxygenation | 1 (1%) | 0 (0%) | 1.00 | 0.96 |
| Arrhythmia | 39 (26%) | 24 (16%) | 0.05 | 0.02 |
| Pleural effusion | 19 (13%) | 12 (8%) | 0.26 | 0.21 |
| Pericardial effusion | 10 (7%) | 8 (5%) | 0.81 | 0.50 |
| Chylothorax | 19 (13%) | 9 (6%) | 0.07 | 0.09 |
| Lung atelectasis | 18 (12%) | 14 (10%) | 0.58 | 0.32 |
| Pneumothorax | 2 (1%) | 0 (0%) | 0.50 | 0.95 |
| Systemic infection | 6 (4%) | 5 (3%) | 1.00 | 0.66 |
| Surgical site infection | 4 (3%) | 3 (2%) | 1.00 | 0.09 |
| Renal failure | 0 (0%) | 0 (0%) | 1.00 | 1.00 |
| Thrombosis | 7 (5%) | 2 (1%) | 0.17 | 0.06 |
| Seizure | 2 (1%) | 1 (1%) | 1.00 | 0.47 |
| Cardiac arrest | 0 (0%) | 1 (1%) | 0.50 | 0.95 |
| Death | 0 (0%) | 0 (0%) | 1.00 | 1.00 |
Median (25th to 75th percentile). ICU indicates intensive care unit; RIPC, remote ischemic preconditioning; SHAM, sham stimulus.
Adjusted for propensity score, cross‐clamp time, and surgery Aristotle score.
P values based on values after natural log transformation.
Physiological and Laboratory Outcomes
Results of laboratory measures performed at 24 and 48 hours after the end of cardiopulmonary bypass are listed in Table 3 (excluding investigations performed serially). For markers of hematologic, hepatic, and renal dysfunction, no significant differences were observed at 24 hours and few were observed at 48 hours (lower white cell and neutrophil count in the RIPC group), albeit for a smaller proportion of patients with available data. There were no significant differences at any time point, and no difference in overall trend in measures of myocardial injury and hemodynamic stability (Figure 4) and metabolic markers (Figure 5). There were, likewise, no significant differences for serial assessments of inflammatory markers (Figure 6) and for C‐reactive protein, clusterin, and β2‐microglobulin measured at 24 and 48 hours (Table 3).
Table 3.
Laboratory Measures Stratified by Study Intervention Group
| N | SHAM | N | RIPC | P Value | P Value* | P Value* | |
|---|---|---|---|---|---|---|---|
| Laboratory investigations at 24 hours | |||||||
| Alanine transaminase, U/L | 123 | 27 (21 to 34) | 115 | 27 (20 to 35) | 0.66 | 0.45 | |
| Aspartate transaminase, U/L | 123 | 89 (55 to 137) | 114 | 87 (60 to 130) | 0.99 | 0.15 | |
| INR | 115 | 1.5±0.2 | 107 | 1.6±0.7 | 0.15 | 0.09 | |
| Activated partial thromboplastin time, seconds | 115 | 41 (36 to 52) | 107 | 40 (35 to 49) | 0.49 | 0.37 | |
| Urea nitrogen, mmol/L | 123 | 5.6 (4.4 to 7.0) | 113 | 5.3 (4.2 to 7.3) | 0.74 | 0.95 | |
| Serum creatinine, μmol/L | 123 | 38 (32 to 53) | 113 | 38 (31 to 47) | 0.19 | 0.18 | |
| White blood cell count, ×109/L | 121 | 13.4 (10.5 to 15.4) | 122 | 12.8 (9.9 to 16.3) | 0.74 | 0.82 | |
| Red blood cell count, ×1012/L | 121 | 3.8±0.8 | 122 | 3.8±0.7 | 0.99 | 0.82 | |
| Hemoglobin, g/L | 121 | 112±21 | 122 | 111±21 | 0.66 | 0.88 | |
| Hematocrit (proportion of 1) | 121 | 0.32 (0.28 to 0.37) | 122 | 0.33 (0.29 to 0.37) | 0.33 | 0.53 | |
| Platelets, ×109/L | 119 | 192±61 | 120 | 194±66 | 0.86 | 0.92 | |
| Neutrophils, ×109/L | 119 | 9.2±3.4 | 118 | 9.1±3.7 | 0.66 | 0.99 | |
| Eosinophils, ×109/L | 117 | 0.02 (0.01 to 0.04) | 116 | 0.02 (0.01 to 0.04) | 0.36 | 0.47 | |
| Basophils, ×109/L | 116 | 0.02 (0.01 to 0.05) | 116 | 0.03 (0.01 to 0.05) | 0.29 | 0.25 | |
| C‐reactive protein, mg/L | 123 | 114±39 | 121 | 115±43 | 0.97 | 0.79 | |
| Cystatin‐C, μg/mL | 130 | 2.23±0.95 | 122 | 2.16±0.98 | 0.57 | 0.34 | |
| Clusterin, μg/mL | 130 | 169±59 | 122 | 176±65 | 0.38 | 0.19 | |
| β2‐microglobulin, μg/mL | 130 | 1.52 (1.08 to 2.19) | 122 | 1.92 (1.41 to 2.72) | 0.66 | 0.37 | |
| S100B, pg/mL | 131 | 77 (51 to 115) | 117 | 82 (60 to 130) | 0.65 | 0.92 | |
| pNF‐H, ng/mL | 113 | 0.14 (0.09 to 0.19) | 108 | 0.15 (0.08 to 0.21) | 0.89 | 0.50 | |
| Laboratory investigations at 48 hours | |||||||
| Alanine transaminase, U/L | 53 | 27 (20 to 40) | 48 | 29 (19 to 35) | 0.85 | 0.66 | 0.08 |
| Aspartate transaminase, U/L | 53 | 66 (46 to 90) | 48 | 61 (43 to 84) | 0.44 | 0.05 | 0.92 |
| INR | 54 | 1.4±0.4 | 49 | 1.3±0.3 | 0.39 | 0.60 | 0.16 |
| Activated partial thromboplastin time, seconds | 54 | 41 (35 to 51) | 49 | 43.0 (35 to 56) | 0.17 | 0.12 | 0.24 |
| Urea nitrogen, mmol/L | 55 | 6.3 (4.6 to 8.3) | 50 | 6.2 (5.2 to 7.5) | 0.56 | 0.07 | 0.27 |
| Serum creatinine, μmol/L | 55 | 34 (29 to 53) | 50 | 35 (28 to 44) | 0.08 | 0.05 | 0.40 |
| White blood cell count, ×109/L | 61 | 14.0 (10.9 to 15.7) | 55 | 12.8 (8.9 to 15.3) | 0.01 | 0.02 | 0.17 |
| Red blood cell count, ×1012/L | 61 | 4.0±0.8 | 55 | 3.9±0.7 | 0.70 | 0.43 | 0.76 |
| Hemoglobin, g/L | 61 | 117±22 | 55 | 116±19 | 0.72 | 0.51 | 0.86 |
| Hematocrit (proportion of 1) | 61 | 0.35±0.07 | 55 | 0.35±0.06 | 0.77 | 0.61 | 0.86 |
| Platelets, ×109/L | 61 | 183±53 | 54 | 176±65 | 0.45 | 0.80 | 0.30 |
| Neutrophils, ×109/L | 60 | 8.7±3.4 | 54 | 7.0±3.3 | 0.006 | 0.02 | 0.11 |
| Eosinophils, ×109/L | 57 | 0.08 (0.03 to 0.16) | 48 | 0.04 (0.01 to 0.12) | 0.11 | 0.51 | 0.03 |
| Basophils, ×109/L | 56 | 0.05 (0.02 to 0.11) | 47 | 0.03 (0.00 to 0.06) | 0.07 | 0.41 | 0.07 |
| C‐reactive protein, mg/L | 57 | 108 (72 to 153) | 55 | 102 (82 to 160) | 0.98 | 0.95 | 0.91 |
| Cyastatin‐C, μg/mL | 60 | 2.43±0.89 | 56 | 2.39±0.76 | 0.77 | 0.71 | 0.82 |
| Clusterin, μg/mL | 60 | 197±60 | 56 | 187±68 | 0.37 | 0.95 | 0.39 |
| β2‐microglobulin, μg/mL | 60 | 1.92 (1.36 to 2.58) | 56 | 1.84 (1.38 to 2.56) | 0.75 | 0.36 | 0.69 |
| S100B, pg/mL | 62 | 81 (51 to 114) | 53 | 83 (63 to 113) | 0.99 | 0.41 | 0.40 |
| pNF‐H, ng/mL | 49 | 0.19 (0.10 to 0.27) | 48 | 0.16 (0.10 to 0.30) | 0.51 | 0.32 | 0.48 |
Median (interquartile range), mean±SD. INR indicates international normalized ratio; pNF‐H, phosphorylated neurofilament H; RIPC, remote ischemic preconditioning; SHAM, sham stimulus.
Adjusted for propensity score, cross‐clamp time, and surgery Aristotle score.
Change between 24 and 48 hours.
Figure 4.
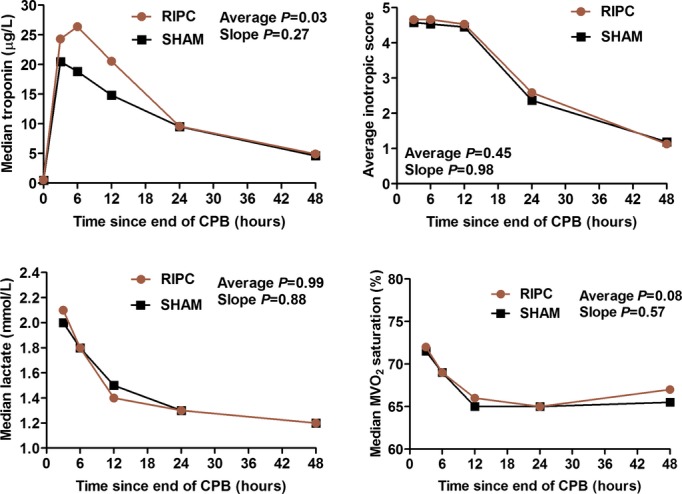
Serial measures of myocardial injury and hemodynamic stability. P values are reported for the mean difference and the difference in slope between RIPC and SHAM. All P values are adjusted for propensity score, duration of aortic cross‐clamping, and surgery Aristotle score. Average indicates mean difference; CPB, cardiopulmonary bypass; MVO2, mixed venous oxygen saturation; RIPC, remote ischemic preconditioning; SHAM, sham stimulus; slope, difference in slope.
Figure 5.
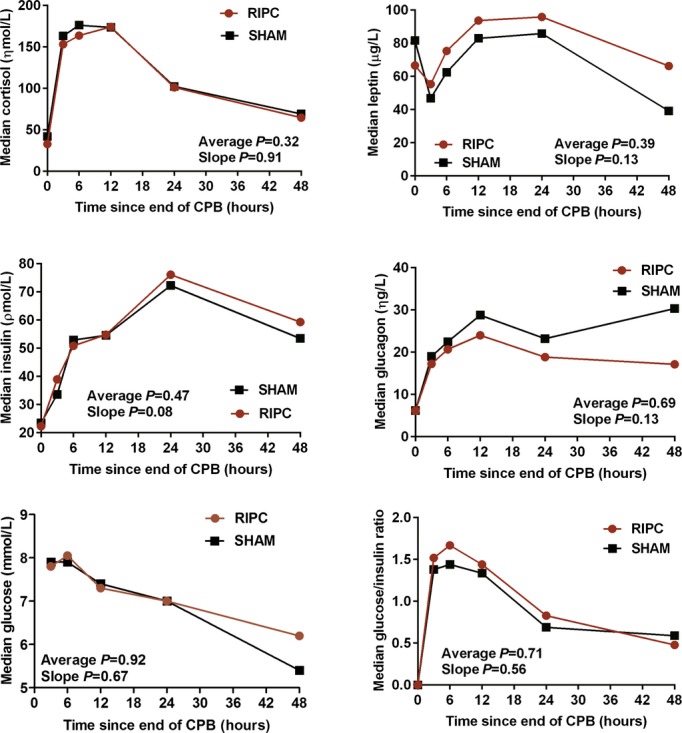
Serial measures of metabolic markers. P values are reported for the mean difference and the difference in slope between RIPC and SHAM. All P values are adjusted for propensity score, duration of aortic cross‐clamping, and surgery Aristotle score. Average indicates mean difference; CPB, cardiopulmonary bypass; RIPC, remote ischemic preconditioning; SHAM, sham stimulus; slope, difference in slope.
Figure 6.
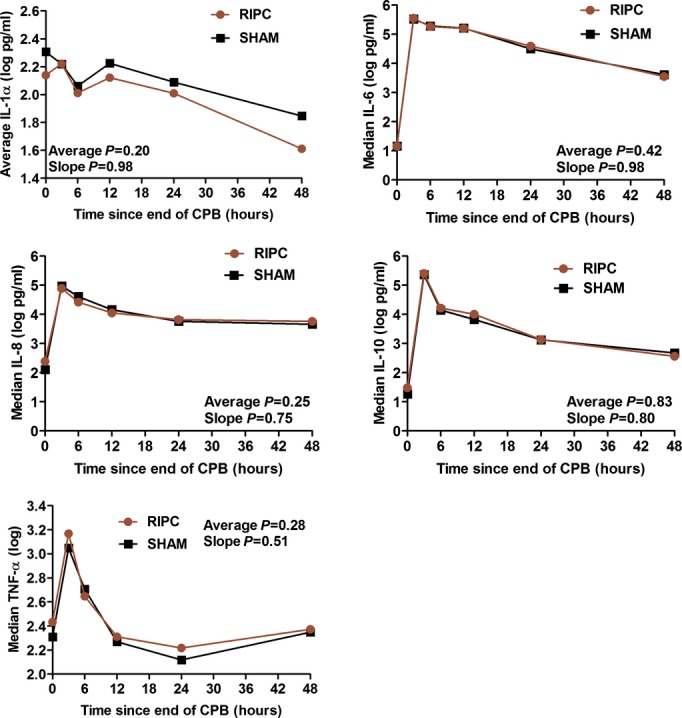
Serial measures of inflammatory markers. P values are reported for the mean difference and the difference in slope between RIPC and SHAM. All P values are adjusted for propensity score, duration of aortic cross‐clamping, and surgery Aristotle score. Average indicates mean difference; CPB, cardiopulmonary bypass; IL, interleukin; RIPC, remote ischemic preconditioning; SHAM, sham stimulus; slope, difference in slope; TNF, tumor necrosis factor.
Subgroup Analyses
Prespecified subgroup analyses using interaction terms were used for exploratory purposes only to determine if there was a differential benefit or harm associated with RIPC for postoperative duration of hospital stay. There were no significant differences regarding age category, sex, type of surgery, Aristotle level category IV, participants enrolled in the first half versus second half of the recruitment period, preoperative steroids, duration of cardiopulmonary bypass or aortic cross‐clamping, and perioperative use of propofol (during induction of anesthesia, during surgery, or during transfer to the ICU), for the primary outcome of postoperative duration of hospital stay (Figure 7) or any other secondary outcomes. Likewise, there was no significant differential effect of RIPC related to timing of propofol administration.
Figure 7.
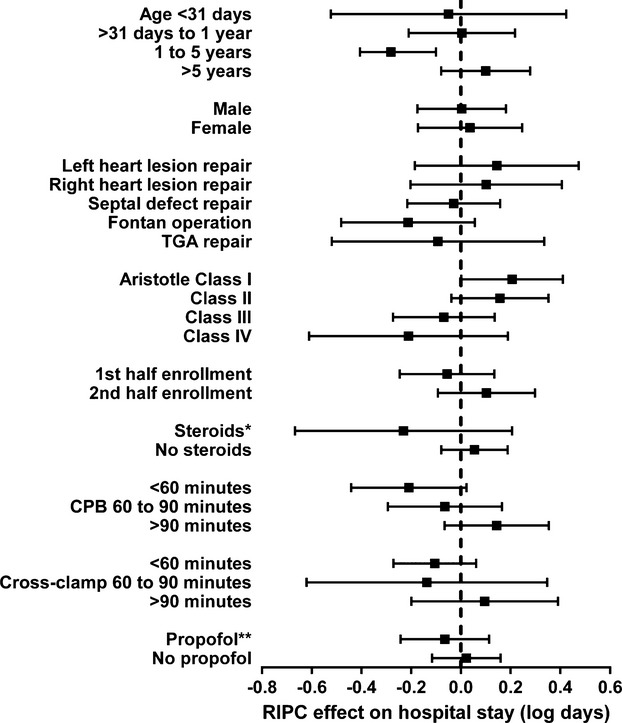
Unadjusted effect of RIPC (vs SHAM) on postoperative hospital length of stay (log transformed) among patient subgroups. The effect of RIPC (vs SHAM) on hospital length of stay is reported with 95% confidence interval for relevant patient subgroups. RIPC was not found to have any statistically significant effect in any of these subgroup analyses. *Steroids given 24 hours before surgery and/or in the pump prime. **Propofol given at induction of anesthesia, during surgery, or during transfer to the intensive care unit. CPB indicates cardiopulmonary bypass; RIPC, remote ischemic preconditioning; SHAM, sham stimulus; TGA, transposition of the great arteries.
Discussion
Summary
For infants and children undergoing cardiac surgery with cardiopulmonary bypass, RIPC did not significantly affect duration of mechanical ventilation, ICU, or hospital stay. RIPC was associated with lower prevalence of arrhythmia, higher overall troponin I levels, and lower white blood cell and neutrophil counts at 48 hours after bypass but with no significant impact on other clinical, physiologic, laboratory, and inflammatory markers. There were also no significant interactions in subgroup analyses.
Pediatric Clinical Trials of Ischemic Conditioning
Smaller pediatric clinical trials have explored the protective effects of ischemic conditioning in the setting of cardiac surgery with cardiopulmonary bypass. These trials have varied regarding the patient characteristics, the method and time of ischemic conditioning, and the outcomes studied. Zhou et al reported a randomized trial (n=60) in infants undergoing repair of ventricular septal defects to assess feasibility and safety of RIPC.18 They noted no limb complications and reduced markers of myocardial and pulmonary injury, with upregulation of heat shock protein 70 expression in myocytes. Jones et al performed a randomized trial (n=39) of RIPC in cyanosed neonates with transposition of the great arteries and hypoplastic left heart syndrome undergoing repair.21 They noted no significant benefit regarding myocardial, renal, or neuronal protection. A larger trial (n=105) of RIPC in children showed no benefit regarding the prevalence of acute kidney injury or impact on levels of renal biomarkers.20 A randomized trial of late RIPC (n=22) performed the day before cardiac surgery showed reduced levels of N‐terminal brain natriuretic peptide levels but no difference regarding troponin levels, with no difference in levels and gene expression of inflammatory markers.22 Pepe et al performed a randomized trial (n=40) of RIPC in children undergoing repair of tetralogy of Fallot; they examined cardiac tissue and leukocytes for protein expression and mitchondrial respiration and noted no significant differences.19 Zhong et al reported benefits of a different conditioning strategy.23 They performed a randomized trial (n=69) of remote ischemic postconditioning (transient limb ischemia performed at the time of aortic unclamping). They noted that postconditioning was associated with lower troponin and creatinine kinase‐MB levels, with shorter postoperative ICU and hospital stays (mean 8.6 versus 10.1 days) and no significant differences regarding inflammatory markers. Although this strategy superficially appears to be superior to preconditioning, in another randomized comparison of remote ischemic pre‐ versus postconditioning (n=60) in pediatric cardiac surgery, both preconditioning and postconditioning were associated with similar reductions in troponin and creatinine kinase‐MB levels in comparison to control participants.17 It is clear from these small proof‐of‐principle studies that the effect of remote conditioning in children undergoing cardiac surgery is inconsistent, even in terms of biochemical responses, and its effect on important physiological events and hard clinical end points cannot be assessed with the relatively small number of patients studied. Our larger clinical trial provides reliable evidence that the effects of RIPC on clinically relevant end points are minimal at best, at least in terms of the heterogeneous population included in our study. Consequently, although we cannot exclude a benefit of RIPC for particular subgroups of patients or some end‐organ effects (eg, a longer‐term study of neurodevelopmental outcomes), our study suggests that its role as a protective strategy for the general population of children undergoing open heart surgery is limited.
Reasons for Failure of RIPC in Pediatric Trials
The reported effects of RIPC in adult cardiac surgical trials range from strongly beneficial to adverse, with recent meta‐analyses suggesting an overall benefit in short‐term biochemical markers of injury but of uncertain long‐term impact.14–15 The reasons for these variable reported effects are incompletely understood, but some data are emerging regarding the effectiveness of RIPC in the complex milieu of cardiac surgery. Several aspects of our study are noteworthy in this regard. Although by far the largest pediatric cohort so far studied, the absolute number of patients in each subgroup was relatively small, and important modifying factors such as age, pre‐existing hypoxemia, anesthetic protocol, and degree of insult may all influence the effectiveness of RIPC.
With regard to the latter point regarding degree of insult, overall and regardless of RIPC, the outcomes for our study population were substantially better than predicted. There were no deaths among the 299 patients studied, and the median hospital stay of ≈5 days was substantially shorter than the 12 days predicted from previous studies and that formed the basis of our power calculation. Not only does this raise the possibility of a type 2 error, but the relative well‐being of the patients may influence the impact of RIPC itself. In a clinical trial of RIPC in patients with evolving myocardial infarction, for example, the benefit of RIPC was proportional to the potential size of the infarct, with more than 50% improvement in salvage by emergency angioplasty in those with potentially large infarcts in the left anterior descending territory but limited or no benefit seen in those experiencing smaller infarcts.8 Conversely, in the pediatric population, the nature and severity of the underlying disease may in itself modify the effects of RIPC. In a recent study reported by Jones et al, there was no benefit of RIPC in neonates undergoing surgical correction of transposition of the great arteries or stage 1 palliation of hypoplastic left heart syndrome.21 The same group, however, has recently shown that children with prior hypoxemia have evidence of pre‐existing “preconditioning” manifest by high concentrations of phosphorylated proteins in their myocardium characteristic of the preconditioned state.19 Additional RIPC did not lead to a further increase in phosphorylation, suggesting that protective pathways were maximally stimulated. It is clear then that if RIPC is to have a role for children undergoing cardiac surgery, it may be limited to certain anatomic and physiological subtypes.
It is also becoming apparent that cointerventions (anesthesia, analgesia) may have important modulating effects on the RIPC stimulus. Midazolam has been reported to block and flumazenil to mimic preconditioning in a rabbit model of ischemia‐reperfusion,24 although this observation has not yet been reported in the clinical setting. It has long been known that halogenated inhalational anesthetic agents, such as isoflurane, are known to precondition. A randomized trial of RIPC on a background of isoflurane anesthesia for patients undergoing on‐pump coronary artery bypass graft surgery failed to show an additional benefit of RIPC on myocardial injury, cerebral injury, or inflammatory markers.25 In addition, patients in the RIPC group had a higher prevalence of new perioperative arrhythmia and myocardial infarctions. For our trial, the inhalational anesthesia protocol was standardized to use only sevoflurane. Sevoflurane has been shown not have a significant preconditioning effect when administered for a short period26 but may be cardioprotective when given throughout the surgery.27 Nonetheless, most of the positive clinical trials in adults and children, including our earlier study,16 have shown benefit over and above the effects of inhalational anesthesia. Indeed, in a recent study of adults undergoing coronary revascularization surgery,24 there was a reduction in troponin release in patients randomized to receive RIPC plus isoflurane anesthesia versus isoflurane alone. This study, however, used a further “control” group of patients randomized to receive RIPC plus intravenous general anesthesia with propofol or propofol alone. Patients in these groups had postoperative troponin levels similar to those undergoing surgery with isoflurane alone, and there was no benefit of additional RIPC, suggesting that propofol blocked the effectiveness of RIPC. The same group subsequently showed that propofol inhibited the increase in patients' myocardial STAT5 expression,23 which has previously been shown to be a crucial element of the RIPC response in humans.28 About one‐third of patients in our trial received propofol at some point during their surgery. The use of propofol during induction, during surgery or during transfer to the ICU, or at any perioperative time point was not associated with a differential effect of RIPC on postoperative duration of hospital stay. Nonetheless, we cannot conclusively exclude the potential confounding effect of propofol use in the interpretation of our results. Likewise, we cannot exclude the potential confounding effect of other factors, both measured and unmeasured.
Conclusions and Implications
We have shown that RIPC does not significantly improve clinical outcomes in a large‐scale randomized trial in infants and children undergoing cardiopulmonary bypass. Furthermore, subgroup analyses failed to support hypothesized clinical variables that may be used to target future clinical trials, but the small numbers and the post hoc nature of these analyses preclude definitive recommendations. Multiple confounding factors, both physical and pharmacological, may have precluded a beneficial effect of RIPC in this clinical scenario.
Sources of Funding
Supported by the Canadian Institutes for Health Research, the Heart and Stroke Foundation of Ontario and the Fondation Leducq.
Disclosures
Brian McCrindle: Shareholder of CellAegis Devices Inc which produces the autoRIC® remote ischemic conditioning system. Nadia Clarizia: None. Svetlana Khaikin: None. Helen Holtby: None. Cedric Manlhiot: Shareholder of CellAegis Devices Inc which produces the autoRIC® remote ischemic conditioning system. Steven Schwartz: None. Christopher Caldarone: Shareholder of CellAegis Devices Inc which produces the autoRIC® remote ischemic conditioning system. John Coles: Shareholder of CellAegis Devices Inc which produces the autoRIC® remote ischemic conditioning system. Glen Van Arsdell: Shareholder of CellAegis Devices Inc which produces the autoRIC® remote ischemic conditioning system. Stephen Scherer: None. Andrew Redington: Shareholder of CellAegis Devices Inc which produces the autoRIC® remote ischemic conditioning system.
References
- 1.Levy JH, Tanaka KA. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 2003; 75:S715-S720. [DOI] [PubMed] [Google Scholar]
- 2.Ungerleider RM. Optimizing response of the neonate and infant to cardiopulmonary bypass. Cardiol Young. 2005; 15suppl 1:142-148. [DOI] [PubMed] [Google Scholar]
- 3.Hausenloy DJ, Yellon DM. The therapeutic potential of ischemic conditioning: an update. Nat Rev Cardiol. 2011; 8:619-629. [DOI] [PubMed] [Google Scholar]
- 4.Kharbanda RK, Mortensen UM, White P, Kristiansen SB, Schmidt MR, Hoschtitzky JA, Vogel M, Sorensen K, Redington AN, MacAllister R. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation. 2002; 106:2881-2883. [DOI] [PubMed] [Google Scholar]
- 5.Saxena P, Newman MA, Shehatha JS, Redington AN, Konstantinov IE. Remote ischemic conditioning: evolution of the concept, mechanisms, and clinical application. J Card Surg. 2010; 25:127-134. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Xuan W, Yan R, Tropak MB, Jean‐St‐Michel E, Liang W, Glasdstone R, Backx PH, Kharbanda RK, Redington AN. Remote preconditioning provides potent cardioprotection via pi3k/akt activation and is associated with nuclear accumulation of beta‐catenin. Clin Sci (Lond). 2011; 120:451-462. [DOI] [PubMed] [Google Scholar]
- 7.Hausenloy DJ, Iliodromitis EK, Andreadou I, Papalois A, Gritsopoulos G, Anastasiou‐Nana M, Kremastinos DT, Yellon DM. Investigating the signal transduction pathways underlying remote ischemic conditioning in the porcine heart. Cardiovasc Drugs Ther. 2012; 26:87-93. [DOI] [PubMed] [Google Scholar]
- 8.Konstantinov IE, Arab S, Kharbanda RK, Li J, Cheung MM, Cherepanov V, Downey GP, Liu PP, Cukerman E, Coles JG, Redington AN. The remote ischemic preconditioning stimulus modifies inflammatory gene expression in humans. Physiol Genomics. 2004; 19:143-150. [DOI] [PubMed] [Google Scholar]
- 9.Konstantinov IE, Arab S, Li J, Coles JG, Boscarino C, Mori A, Cukerman E, Dawood F, Cheung MM, Shimizu M, Liu PP, Redington AN. The remote ischemic preconditioning stimulus modifies gene expression in mouse myocardium. J Thorac Cardiovasc Surg. 2005; 130:1326-1332. [DOI] [PubMed] [Google Scholar]
- 10.Botker HE, Kharbanda R, Schmidt MR, Bøttcher M, Kaltoft AK, Terkelsen CJ, Munk K, Andersen NH, Hansen TM, Trautner S, Lassen JF, Christiansen EH, Krusell LR, Kristensen SD, Thuesen L, Nielsen SS, Rehling M, Sørensen HT, Redington AN, Nielsen TT. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010; 375:727-734. [DOI] [PubMed] [Google Scholar]
- 11.Alreja G, Bugano D, Lotfi A. Effect of remote ischemic preconditioning on myocardial and renal injury: meta‐analysis of randomized controlled trials. J Invasive Cardiol. 2012; 24:42-48. [PubMed] [Google Scholar]
- 12.Hoole SP, Heck PM, Sharples L, Khan SN, Duehmke R, Densem CG, Clarke SC, Shapiro LM, Schofield PM, O'Sullivan M, Dutka DP. Cardiac Remote Ischemic Preconditioning in Coronary Stenting (CRISP Stent) Study: a prospective, randomized control trial. Circulation. 2009; 119:820-827. [DOI] [PubMed] [Google Scholar]
- 13.Hausenloy DJ, Mwamure PK, Venugopal V, Harris J, Barnard M, Grundy E, Ashley E, Vichare S, Di Salvo C, Kolvekar S, Hayward M, Keogh B, MacAllister RJ, Yellon DM. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet. 2007; 370:575-579. [DOI] [PubMed] [Google Scholar]
- 14.Takagi H, Manabe H, Kawai N, Goto SN, Umemoto T. Review and meta‐analysis of randomized controlled clinical trials of remote ischemic preconditioning in cardiovascular surgery. Am J Cardiol. 2008; 102:1487-1488. [DOI] [PubMed] [Google Scholar]
- 15.Pilcher JM, Young P, Weatherall M, Rahman I, Bonser RS, Beasley RW. A systematic review and meta‐analysis of the cardioprotective effects of remote ischaemic preconditioning in open cardiac surgery. J Royal Soc Med. 2012; 105:436-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheung MM, Kharbanda RK, Konstantinov IE, Shimizu M, Frndova H, Li J, Holtby HM, Cox PN, Smallhorn JF, Van Arsdell GS, Redington AN. Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery: first clinical application in humans. J Am Coll Cardiol. 2006; 47:2277-2282. [DOI] [PubMed] [Google Scholar]
- 17.Luo W, Zhu M, Huang R, Zhang Y. A comparison of cardiac post‐conditioning and remote pre‐conditioning in paediatric cardiac surgery. Cardiol Young. 2011; 21:266-270. [DOI] [PubMed] [Google Scholar]
- 18.Zhou W, Zeng D, Chen R, Liu J, Yang G, Liu P, Zhou X. Limb ischemic preconditioning reduces heart and lung injury after an open heart operation in infants. Pediatr Cardiol. 2010; 31:22-29. [DOI] [PubMed] [Google Scholar]
- 19.Liaw NY, Hepponstall M, Sheeran FL, Yong MS, d'Udekem Y, Cheung MM, Konstantinov IE. Effect of remote ischemic preconditioning on phosphorylated protein signaling in children undergoing tetralogy of fallot repair: a randomized controlled trial. J Am Heart Assoc. 2013; 2:e000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pedersen KR, Ravn HB, Povlsen JV, Schmidt MR, Erlandsen EJ, Hjortdal VE. Failure of remote ischemic preconditioning to reduce the risk of postoperative acute kidney injury in children undergoing operation for complex congenital heart disease: a randomized single‐center study. J Thorac Cardiovasc Surg. 2012; 143:576-583. [DOI] [PubMed] [Google Scholar]
- 21.Jones BO, Pepe S, Sheeran FL, Donath S, Hardy P, Shekerdemian L, Penny DJ, McKenzie I, Horton S, Brizard CP, d'Udekem Y, Konstantinov IE, Cheung MM. Remote ischemic preconditioning in cyanosed neonates undergoing cardiopulmonary bypass: a randomized controlled trial. J Thorac Cardiovasc Surg. 2013; 146:1334-1340. [DOI] [PubMed] [Google Scholar]
- 22.Pavione MA, Carmona F, de Castro M, Carlotti AP. Late remote ischemic preconditioning in children undergoing cardiopulmonary bypass: a randomized controlled trial. J Thorac Cardiovasc Surg. 2012; 144:178-183. [DOI] [PubMed] [Google Scholar]
- 23.Zhong H, Gao Z, Chen M, Zhao J, Wang F, Li L, Dong H, Liu L, Wang Q, Xiong L. Cardioprotective effect of remote ischemic postconditioning on children undergoing cardiac surgery: a randomized controlled trial. Paediatr Anaesth. 2013; 23:726-733. [DOI] [PubMed] [Google Scholar]
- 24.Rivo J, Raphael J, Drenger B, Berenshtein E, Chevion M, Gozal Y. Flumazenil mimics whereas midazolam abolishes ischemic preconditioning in a rabbit heart model of ischemia‐reperfusion. Anesthesiology. 2006; 105:65-71. [DOI] [PubMed] [Google Scholar]
- 25.Lucchinetti E, Bestmann L, Feng J, Freidank H, Clanachan AS, Finegan BA, Zaugg M. Remote ischemic preconditioning applied during isoflurane inhalation provides no benefit to the myocardium of patients undergoing on‐pump coronary artery bypass graft surgery: lack of synergy or evidence of antagonism in cardioprotection? Anesthesiology. 2012; 116:296-310. [DOI] [PubMed] [Google Scholar]
- 26.Piriou V, Mantz J, Goldfarb G, Kitakaze M, Chiari P, Paquin S, Cornu C, Lecharny JB, Aussage P, Vicaut E, Pons A, Lehot JJ. Sevoflurane preconditioning at 1 mac only provides limited protection in patients undergoing coronary artery bypass surgery: a randomized bi‐centre trial. Br J Anaesth. 2007; 99:624-631. [DOI] [PubMed] [Google Scholar]
- 27.De Hert SG, Van der Linden PJ, Cromheecke S, Meeus R, Nelis A, Van Reeth V, ten Broecke PW, De Blier IG, Stockman BA, Rodrigus IE. Cardioprotective properties of sevoflurane in patients undergoing coronary surgery with cardiopulmonary bypass are related to the modalities of its administration. Anesthesiology. 2004; 101:299-310. [DOI] [PubMed] [Google Scholar]
- 28.Heusch G, Musiolik J, Kottenberg E, Peters J, Jakob H, Thielmann M. Stat5 activation and cardioprotection by remote ischemic preconditioning in humans: short communication. Circulation Res. 2012; 110:111-115. [DOI] [PubMed] [Google Scholar]


